Leucosceptoside A from Devil’s Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes
Abstract
:1. Introduction
2. Results
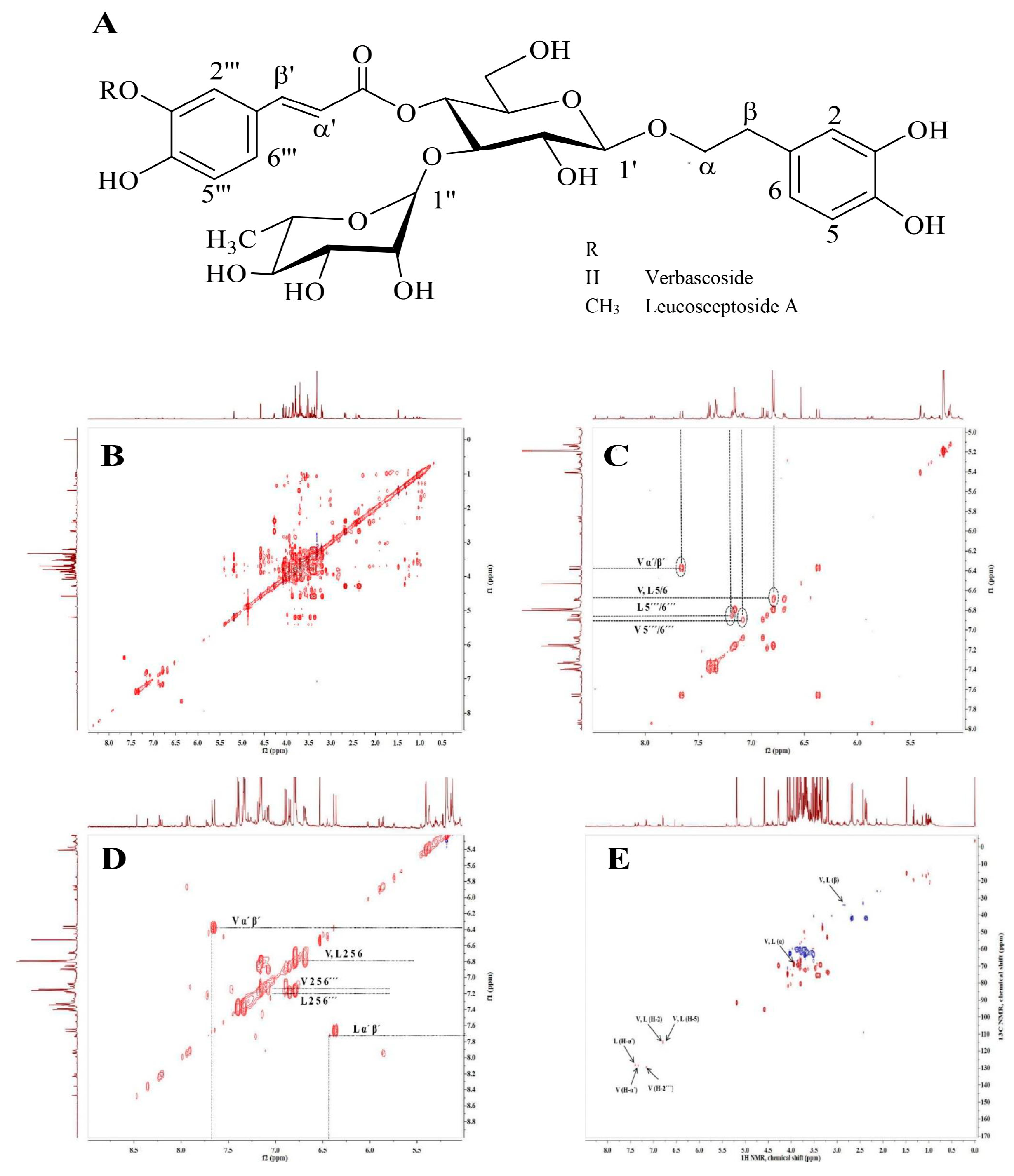
2.1. Phytochemical Analysis of H. procumbens Cell Suspension Extract
2.2. In Silico Docking Simulation
2.3. H. procumbens Extract and Its Constituents VER and LEU Modulate the Gene Expression Profile of IFN-γ/IL-17A/IL-22-Stimulated Keratinocytes
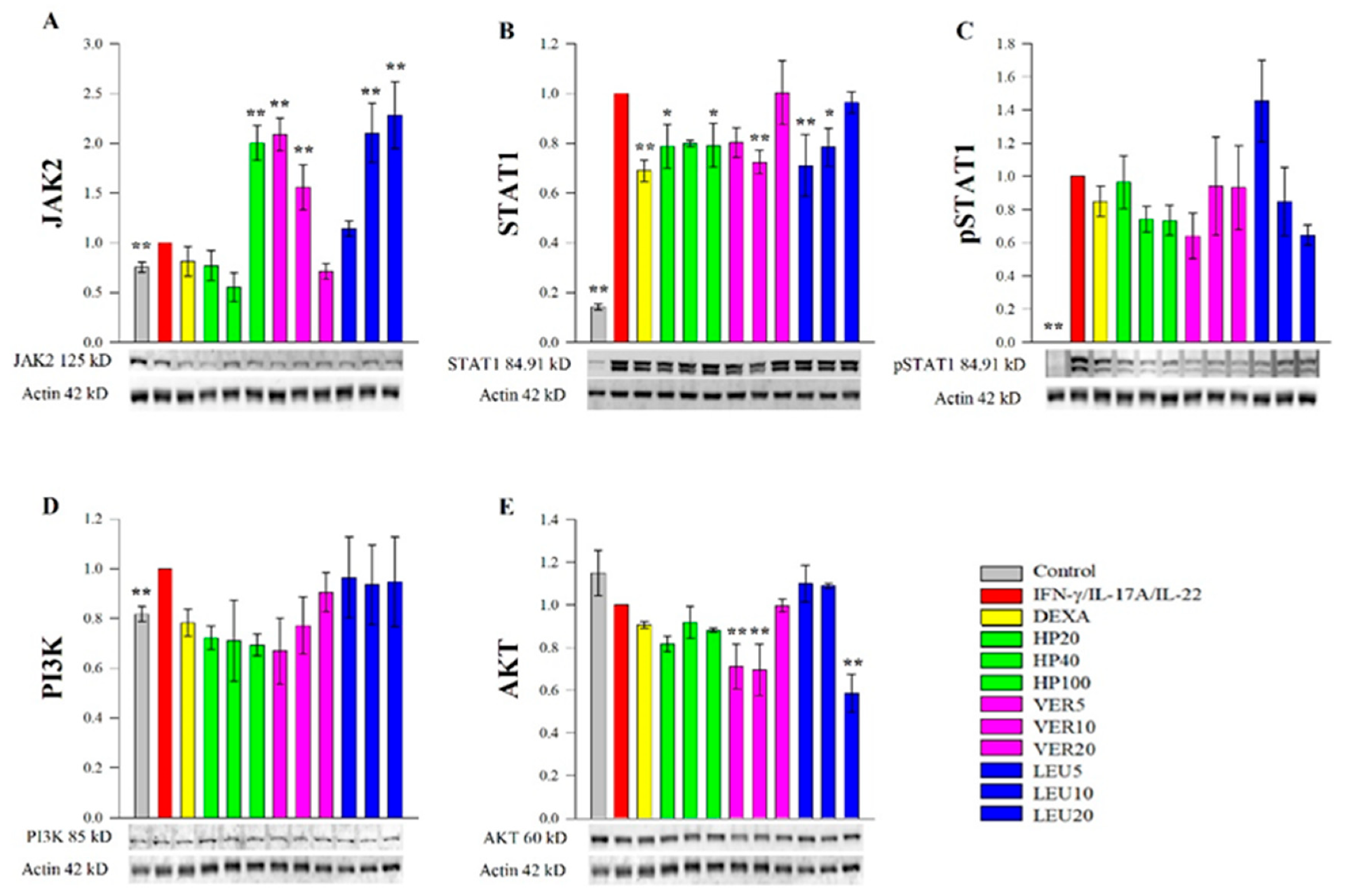
2.4. Effect of H. procumbens Extract and Pure VER and LEU on Expression of Key Psoriasis-Related Proteins
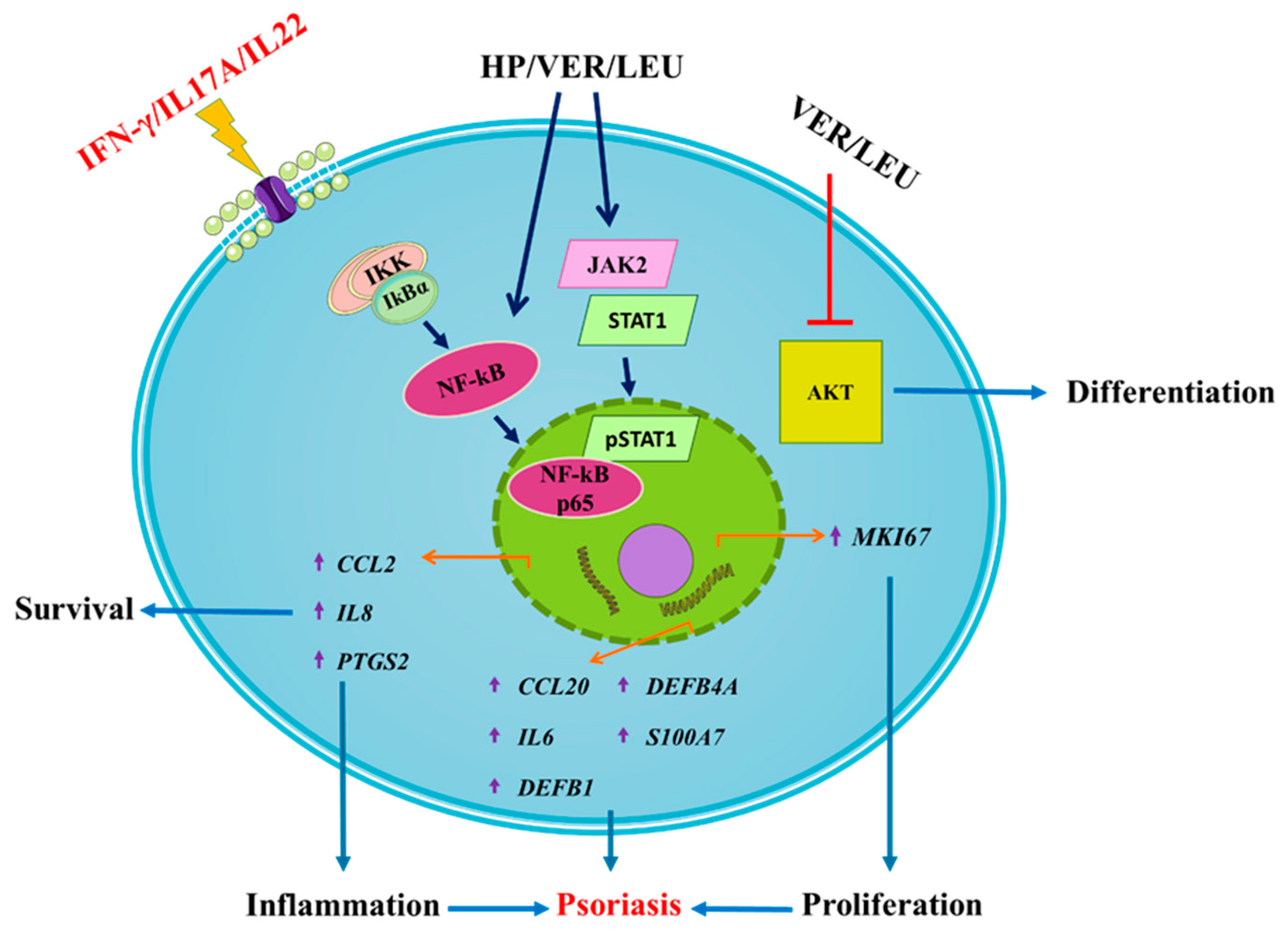
2.5. Proposed Mechanism of the Anti-Psoriatic Action of H. procumbens Extract, VER and LEU in Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cultivation and Extraction of H. procumbens Cell Suspension
4.3. Nuclear Magnetic Resonance (NMR)-Based Metabolite Profiling and Chromatographic Analysis
4.4. Cell Culture and Treatment
4.5. In Silico Molecular Docking
4.6. Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
4.7. Western Blot Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greb, J.E.; Goldminz, A.M.; Elder, J.T.; Lebwohl, M.G.; Gladman, D.D.; Wu, J.J.; Mehta, N.N.; Finlay, A.Y.; Gottlieb, A.B. Psoriasis. Nat. Rev. Dis. Primers 2016, 2, 16082. [Google Scholar] [CrossRef]
- Cai, Y.; Fleming, C.; Yan, J. New insights of T cells in the pathogenesis of psoriasis. Cell. Mol. Immunol. 2019, 9, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Lynde, C.W.; Poulin, Y.; Vender, R.; Bourcier, M.; Khalil, S. Interleukin 17A: Toward a new understanding of psoriasis pathogenesis. J. Am. Acad. Dermatol. 2014, 71, 141–150. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Senra, L.; Boehncke, W.-H. The IL-17 family of cytokines in psoriasis: IL-17A and beyond. Front. Immunol. 2018, 9, 1682. [Google Scholar] [CrossRef] [Green Version]
- Madonna, S.; Scarponi, S.; Pallotta, S.; Cavani, A.; Albanesi, C. Anti-apoptotic effects of suppressor of cytokine signaling 3 and 1 in psoriasis. Cell Death Dis. 2012, 3, 334. [Google Scholar] [CrossRef] [Green Version]
- Albanesi, C.; Madonna, S.; Gisondi, P.; Girolomoni, G. The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front. Immunol. 2018, 9, 1549. [Google Scholar] [CrossRef] [Green Version]
- Sharma, M.; Levenson, C.; Clements, I.; Castella, P.; Gebauer, K.; Cox, M.E. East Indian sandalwood oil (EISO) alleviates inflammatory and proliferative pathologies of psoriasis. Front. Pharmacol. 2017, 8, 125. [Google Scholar] [CrossRef] [Green Version]
- Damen, M.; Wirtz, L.; Soroka, E.; Khatif, H.; Kukat, C.; Benjamin, D.; Simons, B.D.; Bazzi, H. High proliferation and delamination during skin epidermal stratification. Nat. Commun. 2021, 12, 3227. [Google Scholar] [CrossRef]
- Lowes, M.A.; Suárez-Fariñas, M.; Krueger, J.G. Immunology of psoriasis. Annu. Rev. Immunol. 2014, 32, 227–255. [Google Scholar] [CrossRef] [Green Version]
- Tsoi, L.C.; Stuart, P.E.; Tian, C.; Gudjonsson, J.E.; Das, S.; Zawistowski, M.; Ellinghaus, E.; Barker, J.N.; Chandran, V.; Dand, N.; et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat. Commun. 2017, 8, 15382. [Google Scholar] [CrossRef] [Green Version]
- Nograles, K.E.; Zaba, L.C.; Guttman-Yassky, E.; Fuentes-Duculan, J.; Suárez-Farinas, M.; Cardinale, I.; Khatcherian, A.; Gonzalez, J.; Pierson, K.C.; White, T.R.; et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br. J. Dermatol. 2008, 159, 1092–1102. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Chen, P.; Li, C.; Li, D.; Wang, J.; Xue, R.; Zhang, S.; Ruan, J.; Zhang, X. IL-22 downregulates Cx43 expression and decreases gap junctional intercellular communication by activating the JNK pathway in psoriasis. J. Invest. Dermatol. 2019, 139, 400–411. [Google Scholar] [CrossRef]
- Li, H.-J.; Wu, N.-L.; Pu, C.-M.; Hsiao, C.-Y.; Chang, D.C.; Hung, C.F. Chrysin alleviates imiquimod induced psoriasis-like skin inflammation and reduces the release of CCL20 and antimicrobial peptides. Sci. Rep. 2020, 10, 2932. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.; Zhang, L.; Li, X.; Liu, W.; Wang, P.; Lin, Y.; Han, X.; Li, P. Liangxue jiedu formula improves psoriasis and dyslipidemia comorbidity via PI3K/Akt/mTOR pathway. Front. Pharmacol. 2021, 12, 591608. [Google Scholar] [CrossRef]
- Schwartz, D.M.; Kanno, Y.; Villarino, A.; Ward, M.; Gadina, M.; O’Shea, J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat. Rev. Drug Discov. 2017, 16, 843–862. [Google Scholar] [CrossRef]
- Buerger, C. Epidermal mTORC1 signalling contributes to the pathogenesis of psoriasis and could serve as a therapeutic target. Front. Immunol. 2018, 9, 2786. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Zhang, X.; Xu, M.; Zhang, F.; Tian, F.; Cui, J.; Xia, Y.; Liang, C.; Zhou, S.; Wei, H.; et al. Berberine downregulates CDC6 and inhibits proliferation via targeting JAK-STAT3 signaling in keratinocytes. Cell Death Dis. 2019, 10, 274. [Google Scholar] [CrossRef]
- Teng, Y.; Fan, Y.; Ma, J.; Lu, W.; Liu, N.; Chen, Y.; Pan, W.; Tao, X. The PI3K/Akt pathway: Emerging roles in skin homeostasis and a group of non-malignant skin disorders. Cells 2021, 10, 1219. [Google Scholar] [CrossRef]
- Blair, H.A. Risankizumab: A review in moderate to severe plaque psoriasis. Drugs 2020, 80, 1235–1245. [Google Scholar] [CrossRef]
- Xie, J.; Huang, S.; Huang, H.; Deng, X.; Yue, P.; Lin, J.; Yang, M.; Han, L.; Zhang, D.-K. Advances in the application of natural products and the novel drug delivery systems for psoriasis. Front. Pharmacol. 2021, 12, 644952. [Google Scholar] [CrossRef]
- Farahnik, B.; Sharma, D.; Alban, J.; Sivamani, R.K. Topical botanical agents for the treatment of psoriasis: A systematic review. Am. J. Clin. Dermatol. 2017, 18, 451–468. [Google Scholar] [CrossRef]
- Gamret, A.C.; Price, A.; Fertig, R.M.; Lev-Tov, H.; Nichols, A.J. Complementary and alternative medicine therapies for psoriasis a systematic review. Dermatology 2018, 154, 1330–1337. [Google Scholar] [CrossRef]
- Koycheva, I.K.; Vasileva, L.V.; Amirova, K.M.; Marchev, A.S.; Balcheva-Sivenova, Z.P.; Georgiev, M.I. Biotechnologically produced Lavandula angustifolia Mill. extract rich in rosmarinic acid resolves psoriasis-related inflammation through Janus kinase/signal transducer and activator of transcription signaling. Front. Pharmacol. 2021, 12, 680168. [Google Scholar] [CrossRef]
- Georgiev, M.; Alipieva, K.; Pashova, S.; Denev, P.; Angelova, M.; Kerns, G.; Bley, T. Antioxidant activity of Devil’s claw cell biomass and its active constituents. Food Chem. 2010, 121, 967–972. [Google Scholar] [CrossRef]
- Weckessera, S.; Engela, K.; Simon-Haarhausa, B.; Wittmerb, A.; Pelzb, K.; Schempp, C.M. Screening of plant extracts for antimicrobial activity against bacteria and yeasts with dermatological relevance. Phytomedicine 2007, 14, 508–516. [Google Scholar] [CrossRef]
- Georgiev, M.; Pastore, S.; Lulli, D.; Alipieva, K.; Kostyuk, V.; Potapovich, A.; Panetta, M.; Korkina, L. Verbascum xanthophoeniceum-derived phenylethanoid glycosides are potent inhibitors of inflammatory chemokines in dormant and interferon-gamma-stimulated human keratinocytes. J. Ethnopharmacol. 2012, 144, 754–760. [Google Scholar] [CrossRef]
- Wachsmuth, L.; Lindhorst, E.; Wrubel, S.; Hadzhiyski, H.; Hudelmaier, M.; Eckstein, F.; Chrubasik, S. Micro-morphometrical assessment of the effect of Harpagophytum procumbens extract on articular cartilage in rabbits with experimental osteoarthritis using magnetic resonance imaging. Phytother. Res. 2011, 25, 1133–1140. [Google Scholar] [CrossRef]
- Locatelli, M.; Ferrante, C.; Carradori, S.; Secci, D.; Leporini, L.; Chiavaroli, A.; Leone, S.; Recinella, L.; Orlando, G.; Martinotti, S.; et al. Optimization of aqueous extraction and biological activity of Harpagophytum procumbens root on ex vivo rat colon inflammatory model. Phytother. Res. 2017, 31, 937–944. [Google Scholar] [CrossRef]
- Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Cicala, C.; Brunetti, L.; Vladimir-Knežević, S.; Orlando, G.; Ferrante, C. Devil’s claw (Harpagophytum procumbens) and chronic inflammatory diseases: A concise overview on preclinical and clinical data. Phytother. Res. 2019, 33, 2152–2162. [Google Scholar] [CrossRef]
- Mariano, A.; Di Sotto, A.; Leopizzi, M.; Garzoli, S.; Di Maio, V.; Gullì, M.; Vedova, P.D.; Ammendola, S.; d’Abusco, A.S. Antiarthritic effects of a root extract from Harpagophytum procumbens DC: Novel insights into the molecular mechanisms and possible bioactive phytochemicals. Nutrients 2020, 12, 2545. [Google Scholar] [CrossRef]
- Recinella, L.; Chiavaroli, A.; Ronci, M.; Menghini, L.; Brunetti, L.; Leone, S.; Tirillini, B.; Angelini, P.; Covino, S.; Venanzoni, R.; et al. Multidirectional pharma-toxicological study on Harpagophytum procumbens DC. ex Meisn.: An IBD-focused investigation. Antioxidants 2020, 9, 168. [Google Scholar] [CrossRef] [Green Version]
- Gyurkovska, V.; Alipieva, K.; Maciuk, A.; Dimitrova, P.; Ivanovska, N.; Haas, C.; Bley, T.; Georgiev, M. Anti-inflammatory activity of Devil’s claw in vitro systems and their active constituents. Food Chem. 2011, 125, 171–178. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Wu, L.; Georgiev, M.I.; Cao, H.; Nahar, L.; El-Seedi, H.R.; Sarker, S.D.; Xiao, J.; Lu, B. Therapeutic potential of phenylethanoid glycosides: A systematic review. Med. Res. Rev. 2020, 40, 2605–2649. [Google Scholar] [CrossRef]
- Stancheva, N.; Weber, J.; Schulze, J.; Alipieva, K.; Ludwig-Muller, J.; Haas, C.; Georgiev, V.; Bley, T.; Georgiev, M. Phytochemical and flow cytometric analyses of Devil’s claw cell cultures. Plant Cell Tissue Organ Cult. 2011, 105, 79–84. [Google Scholar] [CrossRef]
- Santos-Cruz, L.F.; Avila-Acevedo, J.G.; Ortega-Capitaine, D.; Ojeda-Duplancher, J.C.; Perdigyn-Moya, J.L.; Hernandez-Portilla, L.B.; Lopez-Dionicio, H.; Duran-Diaz, B.; Duenas-Garcia, I.E.; Castaneda-Partida, L.; et al. Verbascoside is not genotoxic in the ST and HB crosses of the drosophila wing spot test, and its constituent, caffeic acid, decreases the spontaneous mutation rate in the ST cross. Food Chem. Toxicol. 2012, 50, 1082–1090. [Google Scholar] [CrossRef]
- Burgos, C.; Muñoz-Mingarro, D.; Navarro, I.; Martín-Cordero, C.; Acero, N. Neuroprotective potential of verbascoside isolated from Acanthus mollis L. leaves through its enzymatic inhibition and free radical scavenging ability. Antioxidants 2020, 9, 1207. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Ebada, S.S.; Sobeh, M.; El-Beshbishy, H.A.; Singab, A.N.; Wink, M. Antihyperglycaemic activity of the methanol extract from leaves of Eremophila maculata (Scrophulariaceae) in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2017, 69, 733–742. [Google Scholar] [CrossRef]
- Andres, R.M.; Montesinos, M.C.; Navalon, P.; Paya, M.; Terencio, M.C. NF-κB and STAT3 inhibition as a therapeutic strategy in psoriasis: In vitro and in vivo effects of BTH. J. Invest. Dermatol. 2013, 133, 2362–2371. [Google Scholar] [CrossRef] [Green Version]
- Goldminz, A.M.; Au, S.C.; Kim, K.; Gottlieb, A.B.; Lizzul, P.F. NF-kB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013, 69, 89–94. [Google Scholar] [CrossRef]
- Kim, B.-H.; Lee, J.M.; Jung, Y.-G.; Kim, S.; Kim, T.-Y. Phytosphingosine derivatives ameliorate skin inflammation by inhibiting NF-κB and JAK/STAT signaling in keratincoytes and mice. J. Invest. Dermatol. 2014, 134, 1023–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-K.; Bae, M.J.; Lim, S.; Lee, W.; Kim, S. A water-soluble extract from Actinidia argute ameliorates psoriasis-like skin inflammation in mice by inhibition of neutrophil infiltration. Nutrients 2018, 10, 1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thatikonda, S.; Pooladanda, V.; Sigalapalli, D.K.; Godugu, C. Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis. 2020, 11, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Jin, L.; Dang, E.; Chang, T.; Feng, Z.; Liu, Y.; Wang, G. IL-17A upregulates keratin 17 expression in keratinocytes through STAT1- and STAT3- dependent mechanisms. J. Invest. Dermatol. 2011, 131, 2401–2408. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Huang, P.; Pan, Y.; Zhu, Z.; Zhou, R.; Yang, Z.; Wang, C. Weighted gene coexpression network and experimental analyses identify lncRNA SPRR2C as a regulator of the IL-22-stimulated HaCaT cell phenotype through the miR-330/STAT1/S100A7 axis. Cell Death Dis. 2021, 12, 86. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, H.; Zhang, J.; Tang, B.; Zhang, H.; Ma, C.; Tang, X.; Li, L.; Wu, J.; Wei, J.; et al. Fuzhenghefuzhiyang formula (FZHFZY) improves epidermal differentiation via suppression of the Akt/mTORC1/S6K1 signalling pathway in psoriatic models. Front. Pharmacol. 2021, 12, 650816. [Google Scholar] [CrossRef]
- Gangadevi, V.; Thatikonda, S.; Pooladanda, V.; Devabattula, G.; Godugu, C. Selenium nanoparticles produce a beneficial effect in psoriasis by reducing epidermal hyperproliferation and inflammation. J. Nanobiotechnol. 2021, 19, 101. [Google Scholar] [CrossRef]
- Choi, M.R.; Kim, H.D.; Cho, S.; Jeon, S.H.; Kim, D.H.; Wee, J.; Yang, Y.D. Anoctamin1 induces hyperproliferation of HaCaT keratinocytes and triggers miquimod-induced psoriasis-like skin injury in mice. Int. J. Mol. Sci. 2021, 22, 7145. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Afaq, F.; Syed, D.N.; Siddiqui, I.A.; Adhami, V.M.; Khan, N.; Singh, S.; Boylan, B.T.; Wood, G.S.; Mukhtar, H. Delphinidin, a dietary antioxidant, induces human epidermal keratinocyte differentiation but not apoptosis: Studies in submerged and three-dimensional epidermal equivalent models. Exp. Dermatol. 2013, 22, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Chamcheu, J.C.; Adhami, V.M.; Esnault, S.; Sechi, M.; Siddiqui, I.A.; Kenneth, A.S.; Syed, D.N.; Dodwad, S.-J.M.; Chaves-Rodriquez, M.-I.; Longley, B.J.; et al. Dual inhibition of PI3K/Akt and mTOR by the dietary antioxidant delphinidin ameliorates psoriatic features in vitro and in an imiquimod-induced psoriasis-like disease in mice. Antioxid. Redox Signal. 2016, 26, 49–69. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Zhang, T.; Zhang, C.; Xiao, C.; Bai, X.; Wang, G. Upregulated E3 ligase tripartite motif-containing protein 21 in psoriatic epidermis ubiquitylates nuclear factor-κB p65 subunit and promotes inflammation in keratinocytes. Transl. Res. 2020, 184, 111–122. [Google Scholar] [CrossRef]
- Bian, G.; Wang, L.; Xie, Q.; Wang, Y.; Feng, H.; Yu, Y.; Chen, Z.; Deng, S.; Li, Y. DGT, a novel heterocyclic diterpenoid, effectively suppresses psoriasis via inhibition of STAT3 phosphorylation. Br. J. Pharmacol. 2020, 178, 636–653. [Google Scholar] [CrossRef]
- Balkrishna, A.; Sakat, S.S.; Joshi, K.; Joshi, K.; Sharma, V.; Ranjan, R.; Bhattacharya, K.; Varshney, A. Cytokines driven anti-inflammatory and anti-psoriasis like efficacies of nutraceutical sea buckthorn (Hippophae rhamnoides) oil. Front. Pharmacol. 2019, 10, 1186. [Google Scholar] [CrossRef]
- Guo, W.; Xu, F.; Zhuang, Z.; Liu, Z.; Xie, J. Liping Bai Ebosin ameliorates psoriasis-like inflammation of mice via miR-155 targeting tnfaip3 on IL-17 pathway. Front. Immunol. 2021, 12, 662362. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xiao, C.; Dang, E.; Cao, J.; Zhu, Z.; Fu, M.; Yao, X.; Liu, Y.; Jin, B.; Wang, G.; et al. CD100-Plexin-B2 Promotes the Inflammation in Psoriasis by Activating NF-κB and the Inflammasome in Keratinocytes. J. Investig. Dermatol. 2018, 138, 375–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, J.-W.; Kwon, M.; Hwang, J.; Lee, S.-J.; Lee, J.-H.; Kim, H.-J.; Lee, K.; Lee, S.-J.; Jeong, E.M.; Chung, J.H.; et al. Keratinocyte transglutaminase 2 promotes CCR6+ γδT-cell recruitment by upregulating CCL20 in psoriatic inflammation. Cell Death Dis. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Bigas, J.; Sevilla, L.M.; Carceller, E.; Boix, J.; Pérez, P. Epidermal glucocorticoid and mineralocorticoid receptors act cooperatively to regulate epidermal development and counteract skin inflammation. Cell Death Dis. 2018, 9, 588. [Google Scholar] [CrossRef]
- Wang, L.; Yang, M.; Wang, X.; Cheng, B.; Ju, Q.; Eichenfield, D.Z.; Sun, B.K. Glucocorticoids promote CCL20 expression in keratinocytes. Br. J. Dermatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.; Puig, L.; Torres, T. JAK inhibitors for treatment of psoriasis: Focus on selective TYK2 inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- Banerjee, S.; Biehl, A.; Gadina, M.; Hasni, S.; Schwartz, D.M. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: Current and future prospects. Drugs 2017, 77, 521–546. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.H.; Shi, X.; Ji, J.; Zhan, F.Q.; Leng, H. Sortilin regulates keratinocyte proliferation and apoptosis through the PI3K-AKT signaling pathway. Life Sci. 2021, 278, 119630. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xiao, L.; Jia, J.; Li, F.; Wang, X.; Duan, Q.; Jing, H.; Yang, P.; Chen, C.; Wang, Q.; et al. Cornulin is induced in psoriasis lesions and promotes keratinocyte proliferation via phosphoinositide 3-kinase/AKT pathways. J. Investig. Dermatol. 2019, 139, 71–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, M.I.; Ali, K.; Alipieva, K.; Verpoorte, R.; Choi, Y.H. Metabolic differentiations and classification of Verbascum species by NMR-based metabolomics. Phytochemistry 2011, 72, 2045–2051. [Google Scholar] [CrossRef]
- Slivka, P.F.; Hsieh, C.-L.; Lipovsky, A.; Pratt, S.D.; Locklear, J.; Namovic, M.T.; McDonald, H.A.; Wetter, J.; Edelmayer, R.; Hu, M.; et al. Small molecule and pooled CRISPR screens investigating IL17 signaling identify BRD2 as a novel contributor to keratinocyte inflammatory responses. ACS Chem. Biol. 2019, 14, 857–872. [Google Scholar] [CrossRef]
- Vasileva, L.V.; Savova, M.S.; Amirova, K.M.; Balcheva-Sivenova, Z.; Ferrante, C.; Orlando, G.; Wabitsch, M.; Georgiev, M.I. Caffeic and chlorogenic acids synergistically activate browning program in human adipocytes: Implications of AMPK- and PPAR-mediated pathways. Int. J. Mol. Sci. 2020, 21, 9740. [Google Scholar] [CrossRef] [PubMed]
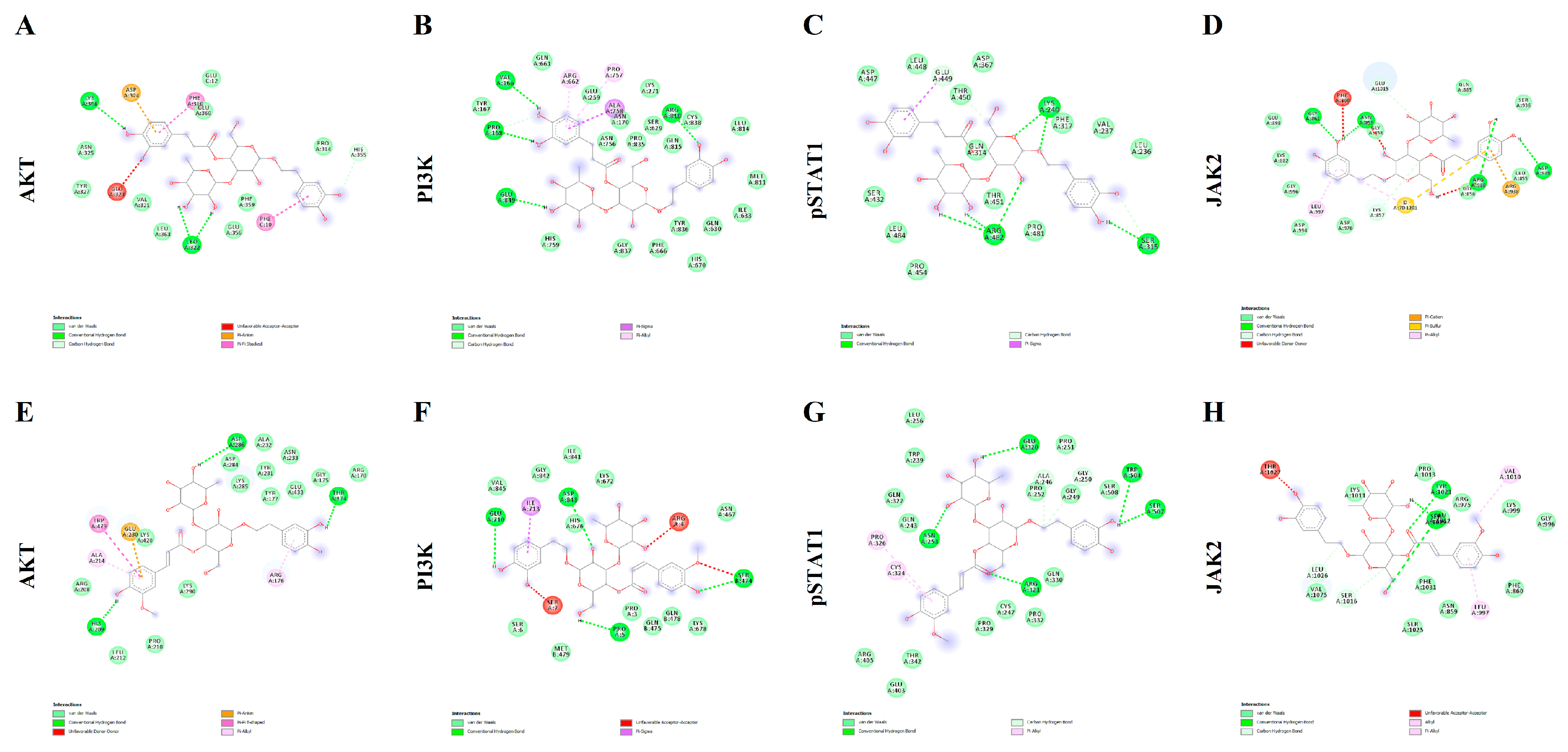

| Protein Target | Verbascoside | Leucosceptoside A |
|---|---|---|
| AKT | −7.5 kcal/M; 3.2 µM | −7.2 kcal/M; 5.4 µM |
| PI3K | −9.7 kcal/M; 0.08 µM | −8.8 kcal/M; 0.4 µM |
| pSTAT1 | −8.5 kcal/M; 0.6 µM | −8.2 kcal/M; 1.0 µM |
| JAK2 | −7.5 kcal/M; 3.2 µM | −7.2 kcal/M; 5.4 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koycheva, I.K.; Mihaylova, L.V.; Todorova, M.N.; Balcheva-Sivenova, Z.P.; Alipieva, K.; Ferrante, C.; Orlando, G.; Georgiev, M.I. Leucosceptoside A from Devil’s Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes. Molecules 2021, 26, 7014. https://doi.org/10.3390/molecules26227014
Koycheva IK, Mihaylova LV, Todorova MN, Balcheva-Sivenova ZP, Alipieva K, Ferrante C, Orlando G, Georgiev MI. Leucosceptoside A from Devil’s Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes. Molecules. 2021; 26(22):7014. https://doi.org/10.3390/molecules26227014
Chicago/Turabian StyleKoycheva, Ivanka K., Liliya V. Mihaylova, Monika N. Todorova, Zhivka P. Balcheva-Sivenova, Kalina Alipieva, Claudio Ferrante, Giustino Orlando, and Milen I. Georgiev. 2021. "Leucosceptoside A from Devil’s Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes" Molecules 26, no. 22: 7014. https://doi.org/10.3390/molecules26227014
APA StyleKoycheva, I. K., Mihaylova, L. V., Todorova, M. N., Balcheva-Sivenova, Z. P., Alipieva, K., Ferrante, C., Orlando, G., & Georgiev, M. I. (2021). Leucosceptoside A from Devil’s Claw Modulates Psoriasis-like Inflammation via Suppression of the PI3K/AKT Signaling Pathway in Keratinocytes. Molecules, 26(22), 7014. https://doi.org/10.3390/molecules26227014










