A Pharmacokinetic Study of Ephedrine and Pseudoephedrine after Oral Administration of Ojeok-San by Validated LC-MS/MS Method in Human Plasma
Abstract
1. Introduction
2. Results and Discussion
2.1. Method Development
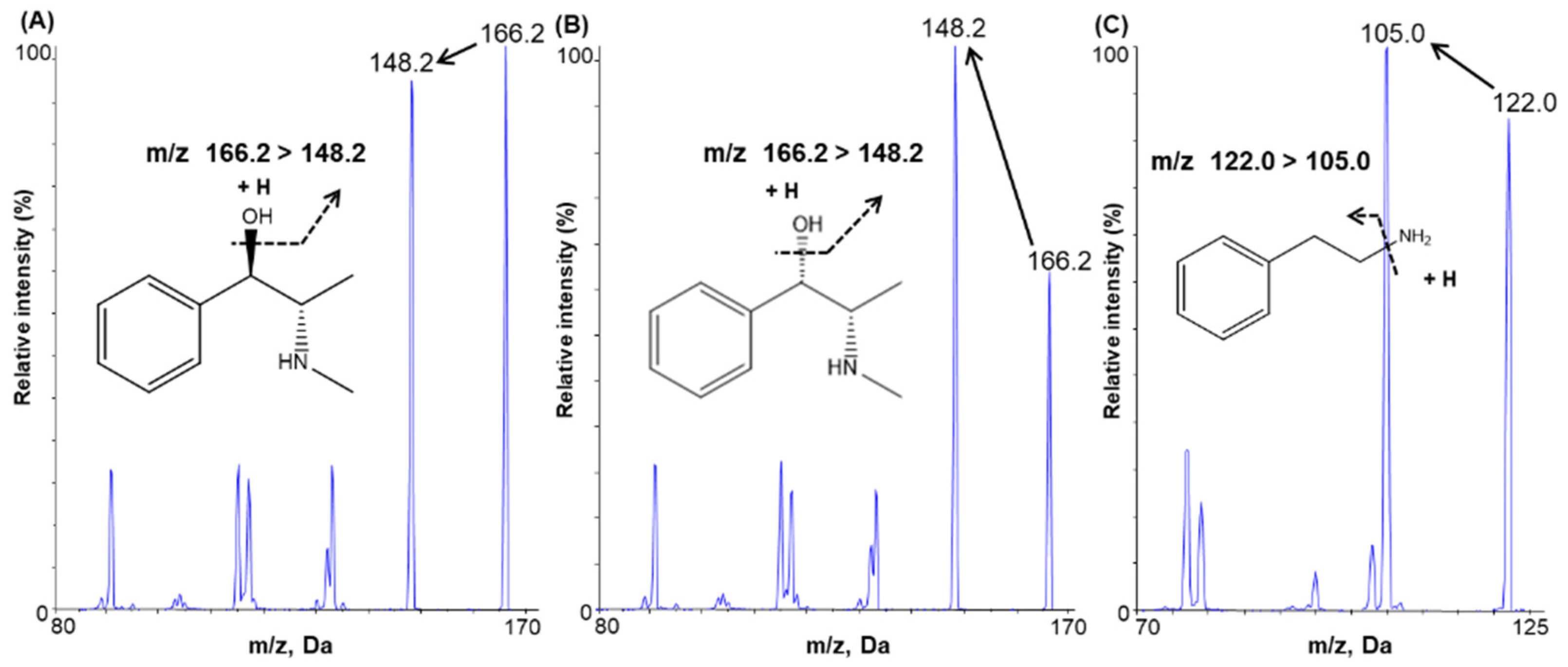
2.1.1. Mass Spectrometry
2.1.2. Chromatographic Conditions
2.1.3. Sample Preparation
2.2. Method Validation
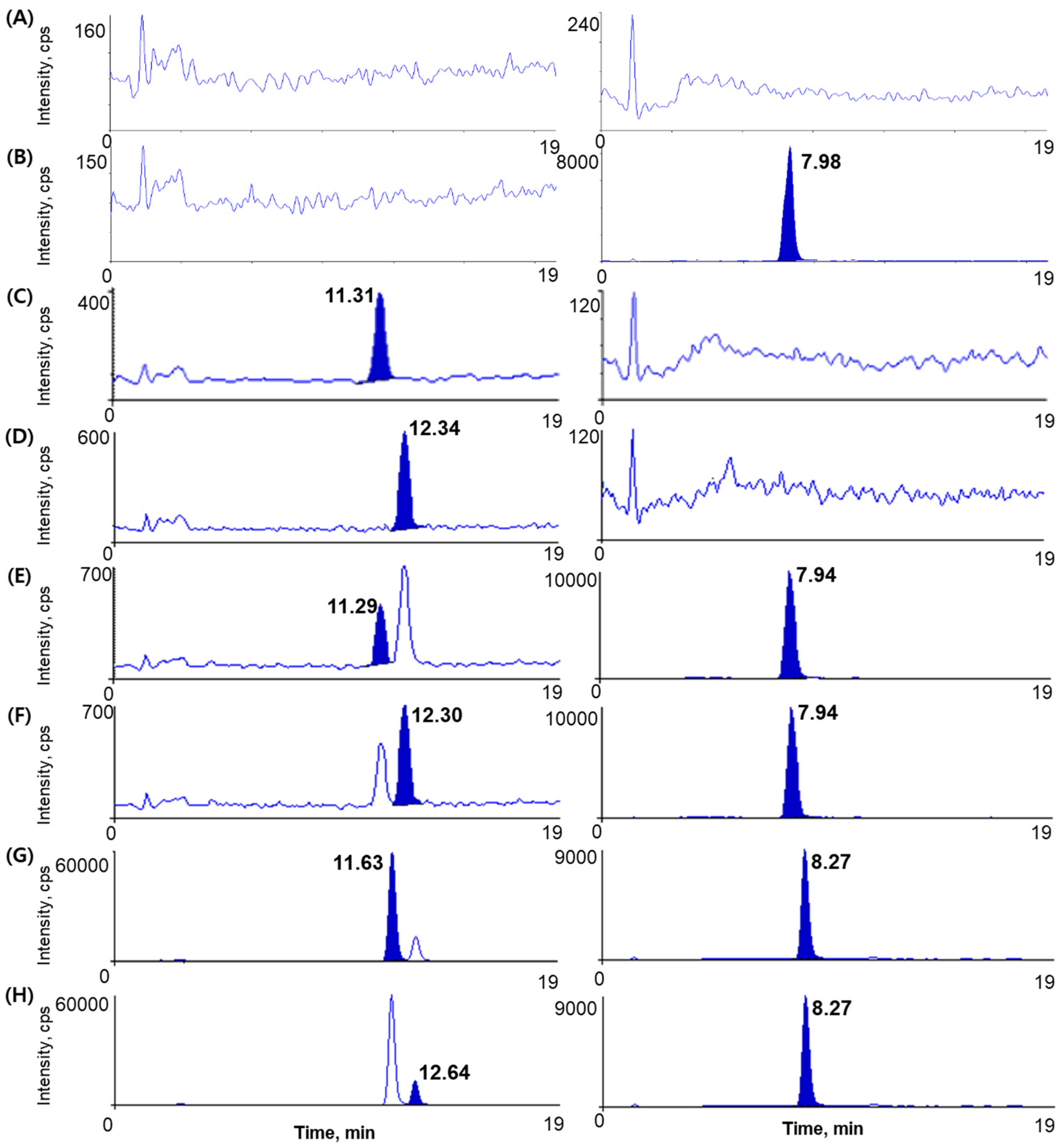
2.2.1. Specificity and Selectivity
2.2.2. Linearity and Lower Limit of Quantification
2.2.3. Precision and Accuracy
2.2.4. Recovery and Matrix Effect
2.2.5. Stability
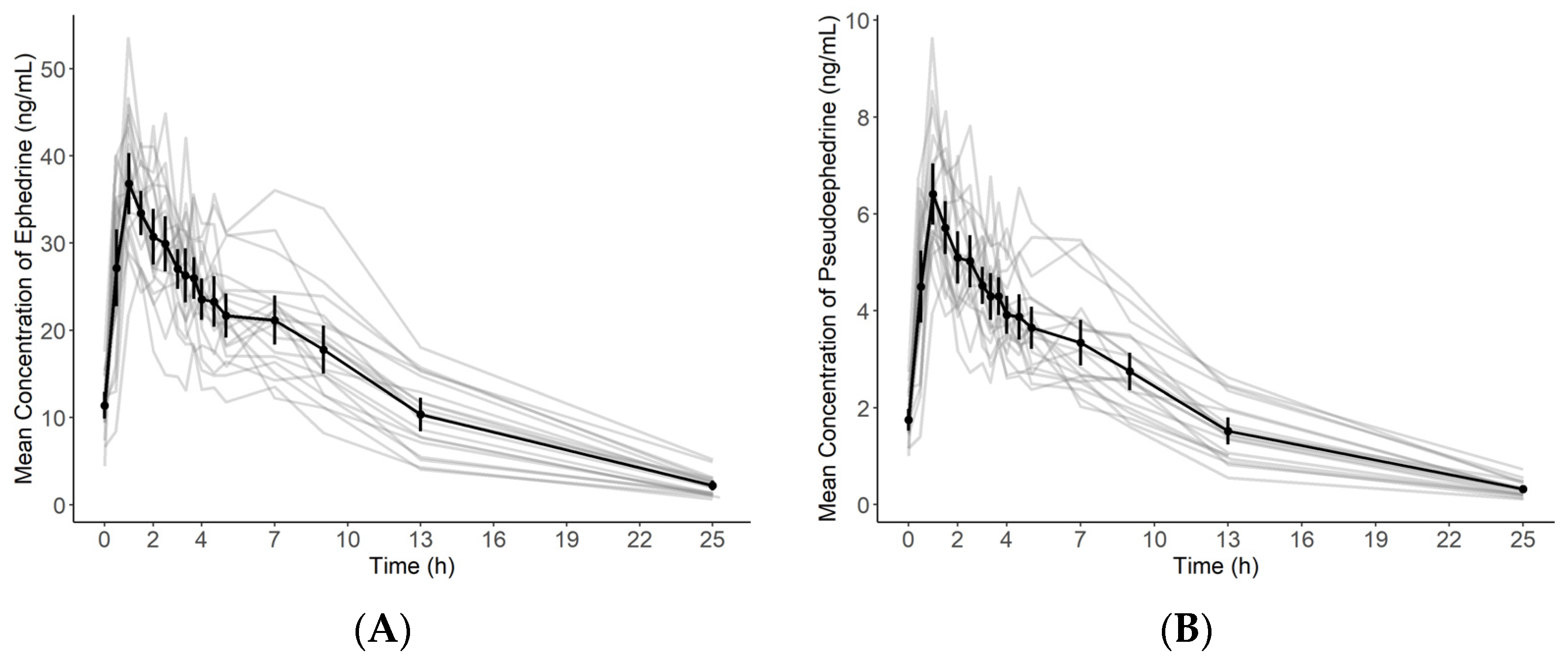
2.3. Application to a Pharmacokinetic Study
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation and Chromatographic Conditions
3.3. Preparation of Calibration Standards and Quality Control Samples
3.4. Plasma Sample Preparation
3.5. Method Validation
3.5.1. Specificity and Selectivity
3.5.2. Linearity and Lower Limits of Quantification
3.5.3. Precision and Accuracy
3.5.4. Extraction Recovery and Matrix Effect
3.5.5. Stability
3.6. Application to a Pharmacokinetic Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Woo, J.-M.; Park, E.-J.; Lee, M.; Ahn, M.; Kwon, S.; Koo, K.H. Changes in attitudes toward and patterns in traditional Korean medicine among the general population in South Korea: A comparison between 2008 and 2011. BMC Complement. Altern. Med. 2014, 14, 436. [Google Scholar] [CrossRef][Green Version]
- Lee, N.-H.; Ha, H.-K.; Lee, H.-Y.; Jung, D.-Y.; Choi, J.-Y.; Lee, J.-K.; Shin, H.-K. Analysis of studies on Ojeok-san for establishment of evidence based medicine. Herb. Formula Sci. 2008, 16, 1–9. [Google Scholar]
- Kim, J.-H.; Lee, D.H.; Kim, J.H.; Jung, S.E.; Ham, S.H.; Yang, W.M.; Kwon, B.-I. Anti-inflammatory Effects of Ojeok-san in LPS-induced Inflammatory Rat Model. J. Korean Med. 2021, 42, 21–30. [Google Scholar] [CrossRef]
- Yoo, S.-R.; Jeong, S.-J.; Kim, Y.-J.; Lim, H.-S.; Jin, S.-E.; Jeon, W.-Y.; Shin, I.-S.; Shin, N.-R.; Kim, S.-S.; Kim, J.-H. Effects of water and ethanol extracts from Ojeok-san on inflammation and its related diseases. J. Intern. Korean Med. 2012, 33, 418–428. [Google Scholar]
- Ha, H.; Lee, J.K.; Lee, H.Y.; Seo, C.-S.; Kim, J.-H.; Lee, M.-Y.; Koh, W.S.; Shin, H.K. Evaluation of safety of the herbal formula Ojeok-san: Acute and sub-chronic toxicity studies in rats. J. Ethnopharmacol. 2010, 131, 410–416. [Google Scholar] [CrossRef]
- Park, S.; Kim, Y.; Oh, M. Prescriptionology; Young Lim Press: Seoul, Korea, 2003; pp. 268–269. [Google Scholar]
- Kim, D.; Kim, H.; Lyu, J.; Young, U.; Chan, K.; Ha, Y.; Jo, M.; Lim, J.; Hong, S. Pharmacology of Traditional Oriental Medicine; Shinilbooks: Seoul, Korea, 2005; pp. 348–353. [Google Scholar]
- Cupp, M.J. Herbal remedies: Adverse effects and drug interactions. Am. Fam. Phys. 1999, 59, 1239. [Google Scholar]
- Haller, C.A.; Benowitz, N.L. Adverse cardiovascular and central nervous system events associated with dietary supplements containing ephedra alkaloids. New Engl. J. Med. 2000, 343, 1833–1838. [Google Scholar] [CrossRef]
- Skalli, S.; Zaid, A.; Soulaymani, R. Drug interactions with herbal medicines. Ther. Drug Monit. 2007, 29, 679–686. [Google Scholar] [CrossRef]
- Wang, J.-W.; Chiang, M.-H.; Lu, C.-M.; Tsai, T.-H. Determination the active compounds of herbal preparation by UHPLC-MS/MS and its application on the preclinical pharmacokinetics of pure ephedrine, single herbal extract of Ephedra, and a multiple herbal preparation in rats. J. Chromatogr. B 2016, 1026, 152–161. [Google Scholar] [CrossRef]
- Shao, G.; Wang, D.-S.; Wu, F.; Chen, S.-J.; Luo, X. Separation and determination of (I)-ephedrine and (d)-pseudoephedrine in plasma by high-performance liquid chromatography with fluorescence detection. J. Liq. Chromatogr. Relat. Technol. 1995, 18, 2133–2145. [Google Scholar] [CrossRef]
- Alshana, U.; Göğer, N.G.; Ertaş, N. Ultrasound-assisted emulsification microextraction for the determination of ephedrines in human urine by capillary electrophoresis with direct injection. Comparison with dispersive liquid-liquid microextraction. J. Sep. Sci. 2012, 35, 2114–2121. [Google Scholar] [CrossRef]
- Hou, J.; Zheng, J.; Shamsi, S.A. Simultaneous chiral separation of ephedrine alkaloids by MEKC-ESI-MS using polymeric surfactant II: Application in dietary supplements. Electrophoresis 2007, 28, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P., III; Haller, C.A.; Duan, M.; Yu, L.; Peng, M.; Benowitz, N.L. Determination of ephedra alkaloid and caffeine concentrations in dietary supplements and biological fluids. J. Anal. Toxicol. 2004, 28, 152–159. [Google Scholar] [CrossRef]
- Cooper, S.D.; Fletcher, B.L.; Rehder Silinski, M.A.; Brown, S.S.; Lodge, J.W.; Fernando, R.A.; Collins, B.J. Determination of L-ephedrine, pseudoephedrine, and caffeine in rat plasma by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2011, 35, 341–348. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Q.; Chen, S.; Liu, A.; Wang, Y. Quantification of 10 bioactive components of Yazhangsan in rat plasma by LC-MS/MS and its application. Biomed. Chromatogr. 2020, 34, e4958. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Sahu, P.L.; Jethi, S.; Gosain, H.; Sahu, K. Method Development and Validation of Ephedrine and Pseudoephedrine in Horse Urine by Liquid Chromatography-Tandem Mass Spectrometry. Indian J. Pharm. Educ. Res. 2020, 54, S671–S676. [Google Scholar] [CrossRef]
- Beyer, J.; Peters, F.T.; Kraemer, T.; Maurer, H.H. Detection and validated quantification of nine herbal phenalkylamines and methcathinone in human blood plasma by LC-MS/MS with electrospray ionization. J. Mass Spectrom. 2007, 42, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Schänzer, W. Examples of doping control analysis by liquid chromatography-tandem mass spectrometry: Ephedrines, β-receptor blocking agents, diuretics, sympathomimetics, and cross-linked hemoglobins. J. Chromatogr. Sci. 2005, 43, 22–31. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.; Wang, Y. Validated quantification method for five ephedrines in dietary supplements using LC-MS/MS: Application to 503 cases. J. Chromatogr. B 2016, 1039, 1–7. [Google Scholar] [CrossRef]
- Food and Drug Administration. Bioanalytical Method Validationguidance for Industry. US Department of Health and Human Services. 2018. Available online: https://www.fda.gov/media/70858/download (accessed on 3 November 2021).
- Ministry of Food and Drug Safety. Guideline on Bioanalytical Method Validation. 2013. Available online: https://www.mfds.go.kr/brd/m210/down.do?brd_id=data0010&seq=13054data_tp=A&file_seq=1 (accessed on 3 November 2021).
- Park, S.I.; Park, J.Y.; Park, M.J.; Yim, S.V.; Kim, B.H. Effects of Ojeok-san on the Pharmacokinetics of Celecoxib at Steady-state in Healthy Volunteers. Basic Clin. Pharmacol. Toxicol. 2018, 123, 51–57. [Google Scholar] [CrossRef]
- Pickup, M.; May, C.; Ssendagire, R.; Paterson, J. The pharmacokinetics of ephedrine after oral dosage in asthmatics receiving acute and chronic treatment. Br. J. Clin. Pharmacol. 1976, 3, 123–134. [Google Scholar] [CrossRef]
- Dickerson, J.; Perrier, D.; Mayersohn, M.; Bressler, R. Dose tolerance and pharmacokinetic studies of L (+) pseudoephedrine capsules in man. Eur. J. Clin. Pharmacol. 1978, 14, 253–259. [Google Scholar] [CrossRef]
- Chan, K.H.; Pan, R.N.; Hsu, M.C. Simultaneous quantification of six ephedrines in a Mahwang preparation and in urine by high-performance liquid chromatography. Biomed. Chromatogr. 2005, 19, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Luo, J.; Chen, F.; Yu, L. Human pharmacokinetics of ephedrine and pseudoephedrine in Mahuang Tang by GC-MS. Tradit. Chin. Drug Res. Clin. Pharmacol. 2000, 5. [Google Scholar]
- Sever, P.; Dring, L.; Williams, R. The metabolism of (−)-ephedrine in man. Eur. J. Clin. Pharmacol. 1975, 9, 193–198. [Google Scholar] [CrossRef]
- Csajka, C.; Haller, C.; Benowitz, N.; Verotta, D. Mechanistic pharmacokinetic modelling of ephedrine, norephedrine and caffeine in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, F.; Debackere, M. The influence of diuretics on the excretion and metabolism of doping agents: Part VI. Pseudoephedrine. Biopharm. Drug Dispos. 1991, 12, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Chester, N.; Mottram, D.R.; Reilly, T.; Powell, M. Elimination of ephedrines in urine following multiple dosing: The consequences for athletes, in relation to doping control. Br. J. Clin. Pharmacol. 2004, 57, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Ihaka, R.; Gentleman, R. R: A language for data analysis and graphics. J. Comput. Graph. Stat. 1996, 5, 299–314. [Google Scholar]
- Buckeridge, C.; Duvvuri, S.; Denney, W.S. Simple, automatic noncompartmental analysis: The PKNCA R package. J. Pharmacokinet. Pharmacodyn. 2015, 42, 11–107. [Google Scholar]
| Compound | Nominal Concentration (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/mL) | Precision (CV, %) a | Accuracy (%) b | Mean ± SD (ng/mL) | Precision (CV, %) | Accuracy (%) | ||||||
| Ephedrine | 0.2 | 0.19 | ± | 0.03 | 14.99 | 97.10 | 0.19 | ± | 0.02 | 11.38 | 94.07 |
| 0.6 | 0.60 | ± | 0.05 | 8.85 | 100.57 | 0.55 | ± | 0.06 | 11.09 | 91.56 | |
| 10 | 10.26 | ± | 0.63 | 6.09 | 102.62 | 9.21 | ± | 1.01 | 10.97 | 92.09 | |
| 50 | 53.20 | ± | 3.13 | 5.88 | 106.40 | 46.20 | ± | 5.79 | 12.53 | 92.40 | |
| Pseudo- ephedrine | 0.2 | 0.21 | ± | 0.02 | 9.32 | 103.70 | 0.19 | ± | 0.03 | 13.74 | 93.00 |
| 0.6 | 0.55 | ± | 0.06 | 10.92 | 90.80 | 0.54 | ± | 0.05 | 8.77 | 89.88 | |
| 10 | 8.68 | ± | 0.72 | 8.33 | 86.78 | 9.15 | ± | 0.83 | 9.02 | 91.53 | |
| 50 | 43.47 | ± | 3.68 | 8.45 | 86.95 | 45.39 | ± | 4.47 | 9.84 | 90.78 | |
| Compound | Nominal Concentration (ng/mL) | Recovery (%) a | Matrix Effect (%) b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD (%) | CV (%) | Mean ± SD (%) | CV (%) | ||||||
| Ephedrine | 0.6 | 76.09 | ± | 4.97 | 6.53 | 100.48 | ± | 3.84 | 3.82 |
| 10 | 76.04 | ± | 2.17 | 2.86 | 101.99 | ± | 2.87 | 2.82 | |
| 40 | 73.31 | ± | 4.14 | 5.65 | 102.15 | ± | 2.03 | 1.98 | |
| Pseudo- ephedrine | 0.6 | 72.76 | ± | 4.14 | 5.69 | 105.17 | ± | 2.98 | 2.83 |
| 10 | 72.97 | ± | 2.43 | 3.34 | 101.60 | ± | 1.33 | 1.31 | |
| 40 | 71.44 | ± | 3.19 | 4.47 | 102.56 | ± | 1.83 | 1.79 | |
| IS | 5000 | 67.76 | ± | 10.13 | 14.95 | 103.70 | ± | 10.91 | 10.52 |
| Nominal Concentration (ng/mL) | Working Solutions (Mean ± SD, %) | Plasma Samples (Mean ± SD, %) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Room Temperature (7 h) | Room Temperature (7 h) | 4 °C (7 h) | −70 °C (7 h) | Freeze-Thaw Stability (5 Cycles) | Autosampler (97 h, 10 °C) | |||||||||||||
| Ephedrine | ||||||||||||||||||
| 0.6 | 96.26 | ± | 2.57 | 104.83 | ± | 6.95 | 100.61 | ± | 1.95 | 109.00 | ± | 10.58 | 92.28 | ± | 4.73 | 103.89 | ± | 10.00 |
| 10 | 113.09 | ± | 4.03 | 108.05 | ± | 4.45 | 112.71 | ± | 11.06 | 99.50 | ± | 3.67 | 87.22 | ± | 9.49 | |||
| 40 | 96.98 | ± | 1.61 | 102.04 | ± | 6.27 | 102.64 | ± | 4.78 | 103.25 | ± | 2.67 | 93.46 | ± | 3.32 | 109.60 | ± | 14.05 |
| Pseudoephedrine | ||||||||||||||||||
| 0.6 | 99.12 | ± | 1.42 | 98.39 | ± | 2.28 | 93.06 | ± | 7.42 | 96.83 | ± | 7.26 | 85.83 | ± | 7.78 | 104.11 | ± | 10.73 |
| 10 | 108.49 | ± | 6.78 | 103.17 | ± | 1.77 | 109.58 | ± | 10.87 | 95.13 | ± | 5.60 | 88.64 | ± | 7.12 | |||
| 40 | 96.93 | ± | 2.24 | 91.35 | ± | 5.95 | 91.73 | ± | 4.66 | 92.92 | ± | 4.33 | 91.73 | ± | 2.40 | 106.25 | ± | 16.45 |
| Parameter | Ephedrine (Mean ± SD) | Pseudoephedrine (Mean ± SD) | ||||
|---|---|---|---|---|---|---|
| Cmax,ss (ng/mL) | 39.24 | ± | 6.45 | 6.83 | ± | 1.19 |
| AUClast (ng·h/mL) | 336.88 | ± | 86.13 | 52.92 | ± | 13.48 |
| AUCτ,ss (ng·h/mL) | 180.49 | ± | 31.59 | 30.08 | ± | 5.33 |
| Tmax (h) | 1.36 | ± | 1.21 | 1.44 | ± | 0.85 |
| t1/2 (h) | 5.98 | ± | 1.23 | 5.20 | ± | 0.65 |
| Compound | Ion Transition (m/z) | DP (V) | EP (V) | CE (V) | CXP (V) | RT (Min) |
|---|---|---|---|---|---|---|
| Ephedrine | 166.20 → 148.20 | 40.0 | 6.0 | 15.0 | 8.0 | 11.5 |
| Pseudoephedrine | 166.20 → 148.20 | 40.0 | 6.0 | 15.0 | 8.0 | 12.5 |
| IS (2-phenylethylamine) | 121.98 → 105.00 | 111.0 | 10.0 | 17.0 | 18.0 | 8.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Shim, W.-S.; Yoo, H.; Choi, S.; Yoon, J.; Lee, K.-Y.; Chung, E.-K.; Lee, B.-C.; Yim, S.-V.; Kim, B.-H.; et al. A Pharmacokinetic Study of Ephedrine and Pseudoephedrine after Oral Administration of Ojeok-San by Validated LC-MS/MS Method in Human Plasma. Molecules 2021, 26, 6991. https://doi.org/10.3390/molecules26226991
Lee S, Shim W-S, Yoo H, Choi S, Yoon J, Lee K-Y, Chung E-K, Lee B-C, Yim S-V, Kim B-H, et al. A Pharmacokinetic Study of Ephedrine and Pseudoephedrine after Oral Administration of Ojeok-San by Validated LC-MS/MS Method in Human Plasma. Molecules. 2021; 26(22):6991. https://doi.org/10.3390/molecules26226991
Chicago/Turabian StyleLee, Sooyoung, Wang-Seob Shim, Heejo Yoo, Sanghee Choi, Jiyoung Yoon, Kwang-Young Lee, Eun-Kyoung Chung, Byung-Cheol Lee, Sung-Vin Yim, Bo-Hyung Kim, and et al. 2021. "A Pharmacokinetic Study of Ephedrine and Pseudoephedrine after Oral Administration of Ojeok-San by Validated LC-MS/MS Method in Human Plasma" Molecules 26, no. 22: 6991. https://doi.org/10.3390/molecules26226991
APA StyleLee, S., Shim, W.-S., Yoo, H., Choi, S., Yoon, J., Lee, K.-Y., Chung, E.-K., Lee, B.-C., Yim, S.-V., Kim, B.-H., & Lee, K.-T. (2021). A Pharmacokinetic Study of Ephedrine and Pseudoephedrine after Oral Administration of Ojeok-San by Validated LC-MS/MS Method in Human Plasma. Molecules, 26(22), 6991. https://doi.org/10.3390/molecules26226991







