Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet
Abstract
:1. Introduction
2. Results
2.1. The Quantity of Rutin in Red Quinoa and the Extract Ratio
2.2. Body Weight, Liver Weight and Liver Weight/Body Weight Ratio
2.3. Serum AST, ALT, and ALP Activities
2.4. Serum and Hepatic Lipid Contents
2.5. Hepatic Pathological Changes
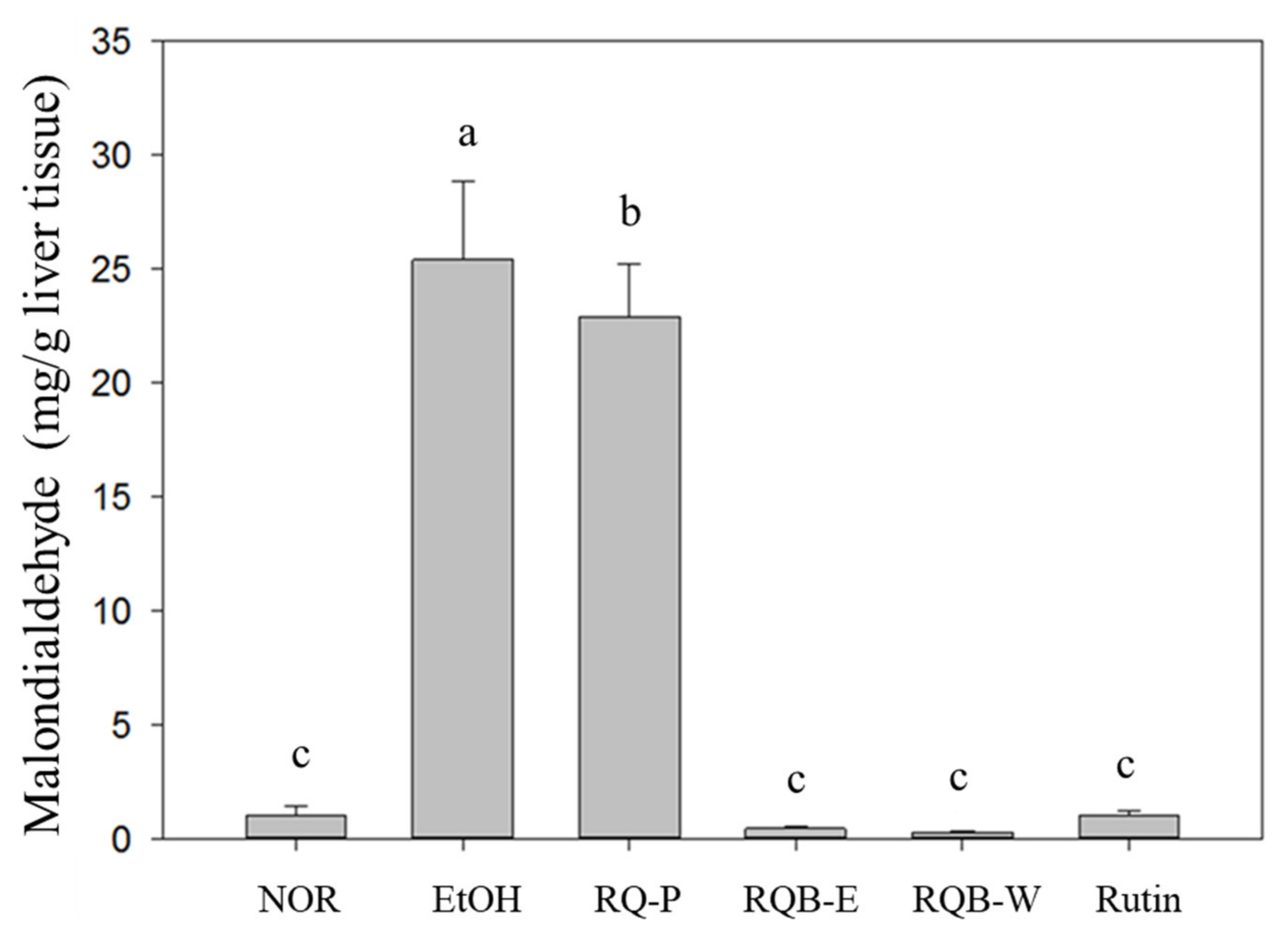
2.6. Lipid Peroxidation in the Liver
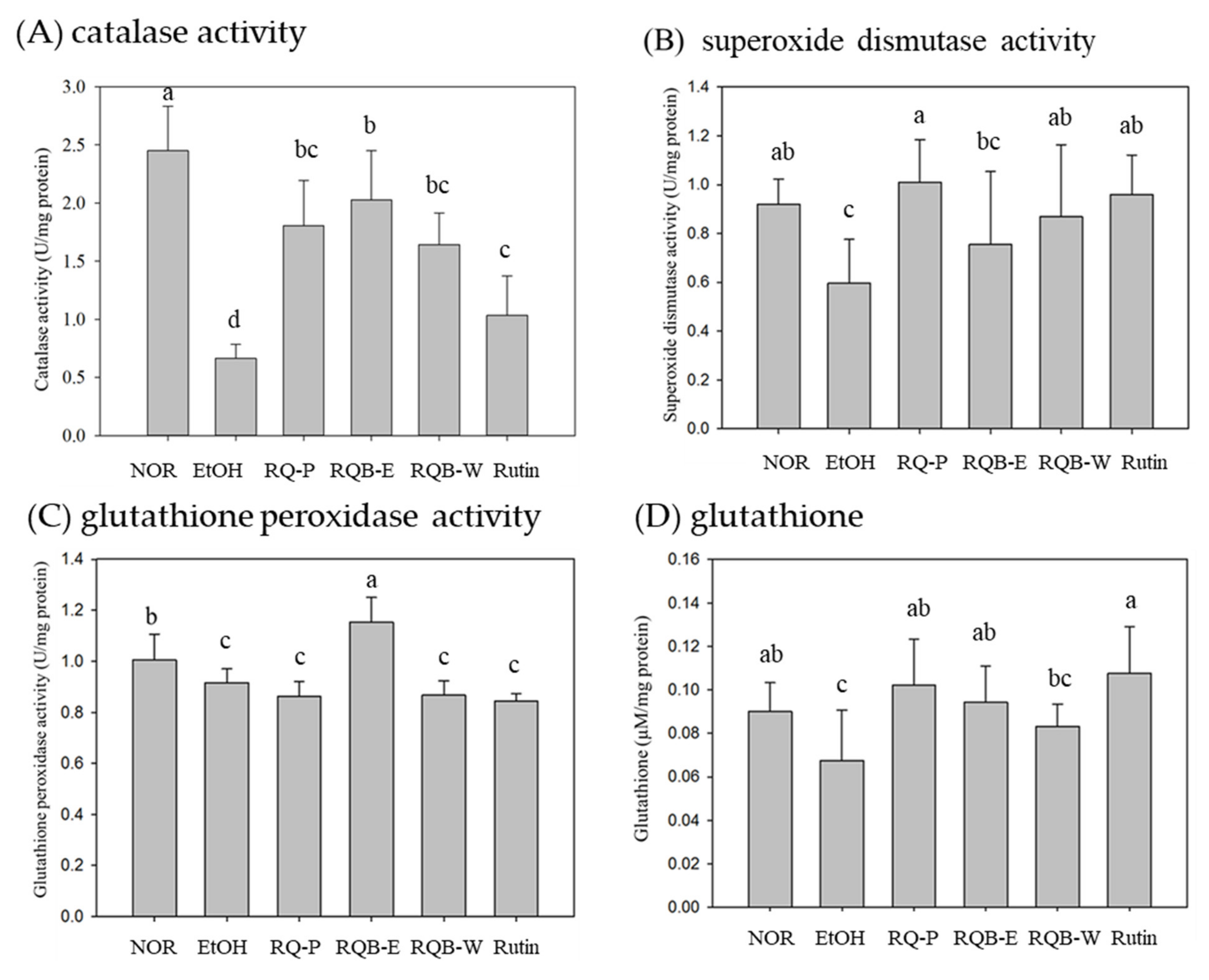
2.7. Activities of Antioxidative System
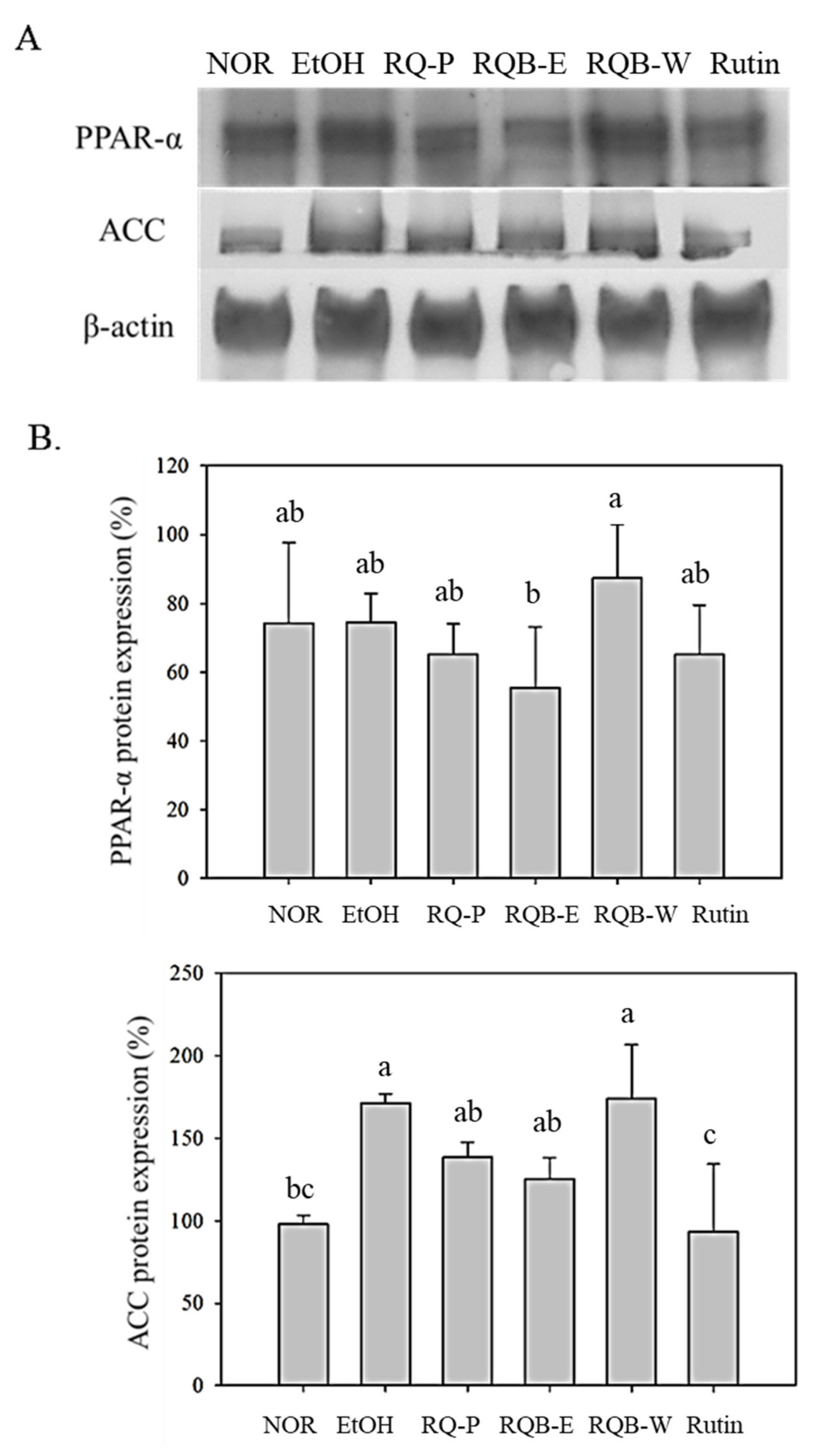
2.8. Mediation for Fatty Acid Metabolism
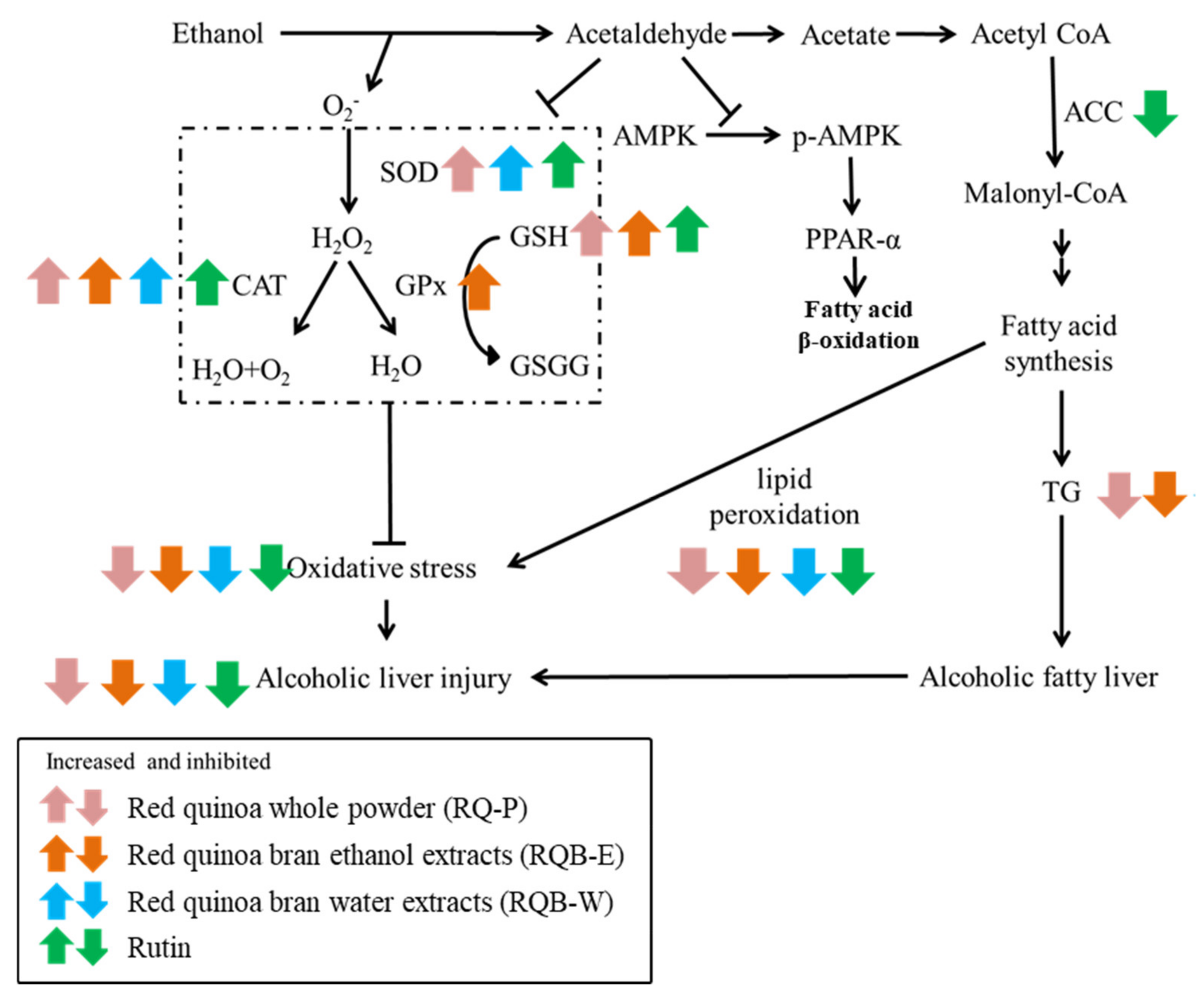
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Sample Preparation
4.3. Animal Model and Grouping
4.4. Serum Biochemistry Parameters
4.5. Liver Lipids Content
4.6. Lipid Peroxidation and Oxidative Stress in the Liver
4.7. Hematoxyline and Eosin Stain (H&E Stain)
4.8. Immunoblotting
4.9. Statistic Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Addolorato, G.; Russell, M.; Albano, E.; Haber, P.S.; Wands, J.R.; Leggio, L. Understanding and Treating Patients With Alcoholic Cirrhosis: An Update. Alcohol. Clin. Exp. Res. 2009, 33, 1136–1144. [Google Scholar] [CrossRef] [PubMed]
- Rouach, H.; Fataccioli, V.; Gentil, M.; French, S.W.; Morimoto, M.; Nordmann, R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology 1997, 25, 351–355. [Google Scholar] [CrossRef]
- Gao, B.; Bataller, R. Alcoholic Liver Disease: Pathogenesis and New Therapeutic Targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef] [Green Version]
- Zakhari, S. Overview: How Is Alcohol Metabolized by the Body? Alcohol Res. Health J. Natl. Inst. Alcohol Abus. Alcohol. 2006, 29, 245–254. [Google Scholar]
- Lu, Y.; Cederbaum, A.I. CYP2E1 and oxidative liver injury by alcohol. Free. Radic. Biol. Med. 2008, 44, 723–738. [Google Scholar] [CrossRef] [Green Version]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoek, J.B.; Pastorino, J.G. Ethanol, oxidative stress, and cytokine-induced liver cell injury. Alcohol 2002, 27, 63–68. [Google Scholar] [CrossRef]
- Pan, X.; Ma, X.; Jiang, Y.; Wen, J.; Yang, L.; Chen, D.; Cao, X.; Peng, C. A Comprehensive Review of Natural Products against Liver Fibrosis: Flavonoids, Quinones, Lignans, Phenols, and Acids. Evid.-Based Complement. Altern. Med. 2020, 2020, 1–19. [Google Scholar] [CrossRef]
- Tsai, P.-J.; Sheu, C.-H.; Wu, P.-H.; Sun, Y.-F. Thermal and pH Stability of Betacyanin Pigment of Djulis (Chenopodium formosanum) in Taiwan and Their Relation to Antioxidant Activity. J. Agric. Food Chem. 2010, 58, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Jiao, J.; Zhang, W.; Xu, J.; Wan, Z.; Zhang, W.; Gao, X.; Qin, L. Dietary fiber prevents obesity-related liver lipotoxicity by modulating sterol-regulatory element binding protein pathway in C57BL/6J mice fed a high-fat/cholesterol diet. Sci. Rep. 2015, 5, 15256. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Suo, T.; Andersson, R.; Cao, Y.; Wang, C.; Lu, J.; Chui, E. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst. Rev. 2017, 1, CD003430. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. The Inhibitory Effects of Djulis (Chenopodium formosanum) and Its Bioactive Compounds on Adipogenesis in 3T3-L1 Adipocytes. Molecules 2018, 23, 1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nafees, S.; Rashid, S.; Ali, N.; Hasan, S.K.; Sultana, S. Rutin ameliorates cyclophosphamide induced oxidative stress and inflammation in Wistar rats: Role of NFκB/MAPK pathway. Chem. Interact. 2015, 231, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Sikder, K.; Kesh, S.B.; Das, N.; Manna, K.; Dey, S. The high antioxidative power of quercetin (aglycone flavonoid) and its glycone (rutin) avert high cholesterol diet induced hepatotoxicity and inflammation in Swiss albino mice. Food Funct. 2014, 5, 1294–1303. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-Y.; Nam, S.-Y.; Hong, S.-W.; Kim, M.J.; Jeong, H.-J.; Kim, H.-M. Protective Effects of Rutin through Regulation of Vascular Endothelial Growth Factor in Allergic Rhinitis. Am. J. Rhinol. Allergy 2015, 29, e87–e94. [Google Scholar] [CrossRef]
- Hafez, M.M.; Al-Harbi, N.O.; Al-Hoshani, A.R.; Al-Hosaini, K.A.; Al Shrari, S.D.; Al Rejaie, S.S.; Sayed-Ahmed, M.M.; Al-Shabanah, O.A. Hepato-protective effect of rutin via IL-6/STAT3 pathway in CCl4-induced hepatotoxicity in rats. Biol. Res. 2015, 48, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Lee, J.; Lee, H.; Sung, J. Relative protective activities of quercetin, quercetin-3-glucoside, and rutin in alcohol-induced liver injury. J. Food Biochem. 2019, 43, e13002. [Google Scholar] [CrossRef]
- Szabo, G.; Mandrekar, P. Focus On: Alcohol and the Liver. Alcohol Res. Heal. J. Natl. Inst. Alcohol Abus. Alcohol. 2010, 33, 87–96. [Google Scholar]
- Orman, E.S.; Odena, G.; Bataller, R. Alcoholic liver disease: Pathogenesis, management, and novel targets for therapy. J. Gastroenterol. Hepatol. 2013, 28, 77–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goolsby, M.J. Screening, Diagnosis, and Monitoring of Hepatic Injury. J. Am. Acad. Nurse Pract. 2003, 15, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Ress, C. Mechanisms of intrahepatic triglyceride accumulation. World J. Gastroenterol. 2016, 22, 1664–1673. [Google Scholar] [CrossRef]
- Fadhel, Z.A.; Amran, S. Effects of black tea extract on carbon tetrachloride-induced lipid peroxidation in liver, kidneys, and testes of rats. Phytotherapy Res. 2002, 16, 28–32. [Google Scholar] [CrossRef]
- Yilmaz, F.M.; Yilmaz, G.; Erol, M.F.; Köklü, S.; Yücel, D. Nitric oxide, lipid peroxidation and total thiol levels in acute appendicitis. J. Clin. Lab. Anal. 2010, 24, 63–66. [Google Scholar] [CrossRef]
- Alberts, A.W.; Strauss, A.W.; Hennessy, S.; Vagelos, P.R. Regulation of synthesis of hepatic fatty acid synthetase: Binding of fatty acid synthetase antibodies to polysomes. Proc. Natl. Acad. Sci. USA 1975, 72, 3956–3960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misra, P.; Chakrabarti, R. The role of AMP kinase in diabetes. Indian J. Med. Res. 2007, 125, 389–398. [Google Scholar] [PubMed]
- Lieber, C.S. Hepatic and Metabolic Effects of Ethanol: Pathogenesis and Prevention. Ann. Med. 1994, 26, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Mezey, E.; Caballeria, J.; Mitchell, M.C.; Pares, A.; Herlong, H.F.; Rodes, J. Effect of parenteral amino acid supplementation on short-term and long-term outcomes in severe alcoholic hepatitis: A randomized controlled trial. Hepatology 1991, 14, 1090–1096. [Google Scholar] [CrossRef]
- Jiménez-Cruz, A.; Loustaunau-López, V.M.; Bacardi-Gascon, M. The use of low glycemic and high satiety index food dishes in Mexico: A low cost approach to prevent and control obesity and diabetes. Nutr. Hosp. 2006, 21, 353–356. [Google Scholar] [PubMed]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant Activity of Betalains from Plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Rasineni, K.; Penrice, D.D.; Natarajan, S.K.; McNiven, M.A.; McVicker, B.L.; Kharbanda, K.K.; Casey, C.A.; Harris, E.N. Alcoholic vs non-alcoholic fatty liver in rats: Distinct differences in endocytosis and vesicle trafficking despite similar pathology. BMC Gastroenterol. 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dufour, D.R.; Lott, J.A.; Nolte, F.S.; Gretch, D.R.; Koff, R.S.; Seeff, L.B. Diagnosis and monitoring of hepatic injury. I. Performance characteristics of laboratory tests. Clin. Chem. 2000, 46, 2027–2049. [Google Scholar] [CrossRef]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Zhao, S.; Sadrzadeh, S.M.; Dannenberg, A.J.; Tahan, S.R.; Waxman, D.J. Markedly enhanced cytochrome P450 2E1 induction and lipid peroxidation is associated with severe liver injury in fish oil-ethanol-fed rats. Alcohol. Clin. Exp. Res. 1994, 18, 1280–1285. [Google Scholar] [CrossRef]
- Chyau, C.-C.; Chu, C.-C.; Chen, S.-Y.; Duh, P.-D. Djulis (Chenopodiun formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. J. Funct. Foods 2015, 18, 159–170. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Hernandez, M.; Zhang, H.; Marcone, M.F.; Liu, R.; Tsao, R. Characterisation of fatty acid, carotenoid, tocopherol/tocotrienol compositions and antioxidant activities in seeds of three Chenopodium quinoa Willd. genotypes. Food Chem. 2015, 174, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Lieber, C.S.; DeCarli, L.M. The Feeding of Alcohol in Liquid Diets: Two Decades of Applications and 1982 Update. Alcohol. Clin. Exp. Res. 1982, 6, 523–531. [Google Scholar] [CrossRef]
- Anji, A.; Kumari, M. Supplementing the liquid alcohol diet with chow enhances alcohol intake in C57 BL/6 mice. Drug Alcohol Depend. 2008, 97, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Minamimoto, R.; Hamabe, Y.; Miyaoka, T.; Hara, T.; Yoshida, K.; Oka, T.; Inoue, T. Guidance for Industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. Ann. Nucl. Med. 2008, 22, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Kolakowska, A.; Deutry, J. Some comments on the usefulness of 2-thiobarbituric acid (TBA) test for the evaluation of rancidity in frozen fish. Food/Nahrung 1983, 27, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Bihaqi, S.W.; Singh, A.P.; Tiwari, M. Supplementation of Convolvulus pluricaulis attenuates scopolamine-induced increased tau and Amyloid precursor protein (AβPP) expression in rat brain. Indian J. Pharmacol. 2012, 44, 593–598. [Google Scholar] [CrossRef]
- Lee, C.-L.; Kuo, T.-F.; Wu, C.-L.; Wang, J.-J.; Pan, T.-M. Red Mold Rice Promotes Neuroprotective sAPPalpha Secretion Instead of Alzheimer’s Risk Factors and Amyloid Beta Expression in Hyperlipidemic Aβ40-Infused Rats. J. Agric. Food Chem. 2010, 58, 2230–2238. [Google Scholar] [CrossRef] [PubMed]

| Sample | Extract Ratio 1 (%) | Rutin (mg/g) |
|---|---|---|
| Whole powder (RQ-P) | - | 1.65 ± 0.16 |
| Ethanol extract (RQB-E) | 14.87 | 10.65 ± 1.34 |
| Water extract (RQB-W) | 19.70 | 2.45 ± 0.82 |
| Group | Liver Weight (g) | Body Weight (g) | Liver Wight/Body Weight (%) |
|---|---|---|---|
| NOR | 0.97 ± 0.09 a | 26.63 ± 1.60 a | 3.62 ± 0.23 b |
| EtOH | 0.88 ± 0.05 b | 23.00 ± 1.31 c | 3.83 ± 0.15 a |
| RQ-P | 0.86 ± 0.04 b | 22.38 ± 1.19 c | 3.85 ± 0.17 a |
| RQB-E | 0.81 ± 0.04 b | 23.13 ± 1.25 c | 3.52 ± 0.17 b |
| RQB-W | 0.82 ± 0.03 b | 23.25 ± 1.39 c | 3.53 ± 0.16 b |
| Rutin | 0.85 ± 0.08 b | 24.75 ± 1.49 b | 3.44 ± 0.17 b |
| Group | AST Activity (U/L) | ALT Activity (U/L) | ALP Activity (IU/L) |
|---|---|---|---|
| NOR | 40.5 ± 3.8 b | 14.5 ± 0.5 d | 65.0 ± 2.0 c |
| EtOH | 64.5 ± 18.7 a | 23.8 ± 5.3 a | 93.9 ± 11.7 a |
| RQ-P | 46.4 ± 4.9 b | 18.0 ± 2.1 bc | 100.4 ± 7.0 a |
| RQB-E | 40.0 ± 2.0 b | 15.9 ± 1.4 cd | 73.3 ± 4.9 b |
| RQB-W | 44.1 ± 2.9 b | 18.9 ± 1.6 bc | 79.3 ± 6.4 b |
| Rutin | 47.6 ± 2.4 b | 19.6 ± 4.2 b | 79.4 ± 5.0 b |
| Group | Serum TC (mg/dL) | Serum TG (mg/dL) | Liver TC (mg/g) | Liver TG (mg/g) | Liver Glycerol (mg/g) |
|---|---|---|---|---|---|
| NOR | 107.1 ± 8.4 cd | 84.3 ± 13.9 b | 1.51 ± 0.14 ab | 0.95 ± 0.24 ab | 0.70 ± 0.15 b |
| EtOH | 128.8 ± 8.6 a | 168.8 ± 21.2 a | 1.42 ± 0.08 b | 1.13 ± 0.42 a | 1.08 ± 0.18 a |
| RQ-P | 83.1 ± 3.1 e | 54.5 ± 6.3 d | 1.50 ± 0.04 ab | 0.60 ± 0.14 c | 0.40 ± 0.10 c |
| RQB-E | 100.9 ± 8.1 d | 68.3 ± 3.5 c | 1.60 ± 0.13 a | 0.80 ± 0.12 bc | 0.44 ± 0.08 c |
| RQB-W | 110.1 ± 7.0 bc | 75.4 ± 8.7 bc | 1.50 ± 0.07 ab | 0.94 ± 0.18 ab | 0.74 ± 0.15 b |
| Rutin | 116.3 ± 10.9 b | 89.0 ± 14.9 b | 1.44 ± 0.06 b | 0.93 ± 0.23 ab | 0.70 ± 0.16 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-A.; Ke, B.-J.; Cheng, S.-C.; Lee, C.-L. Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet. Molecules 2021, 26, 6973. https://doi.org/10.3390/molecules26226973
Lin T-A, Ke B-J, Cheng S-C, Lee C-L. Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet. Molecules. 2021; 26(22):6973. https://doi.org/10.3390/molecules26226973
Chicago/Turabian StyleLin, Ting-An, Bo-Jun Ke, Shih-Cheng Cheng, and Chun-Lin Lee. 2021. "Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet" Molecules 26, no. 22: 6973. https://doi.org/10.3390/molecules26226973
APA StyleLin, T.-A., Ke, B.-J., Cheng, S.-C., & Lee, C.-L. (2021). Red Quinoa Bran Extract Prevented Alcoholic Fatty Liver Disease via Increasing Antioxidative System and Repressing Fatty Acid Synthesis Factors in Mice Fed Alcohol Liquid Diet. Molecules, 26(22), 6973. https://doi.org/10.3390/molecules26226973







