Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl−/H+ Antiporter
Abstract
1. Introduction
2. Results
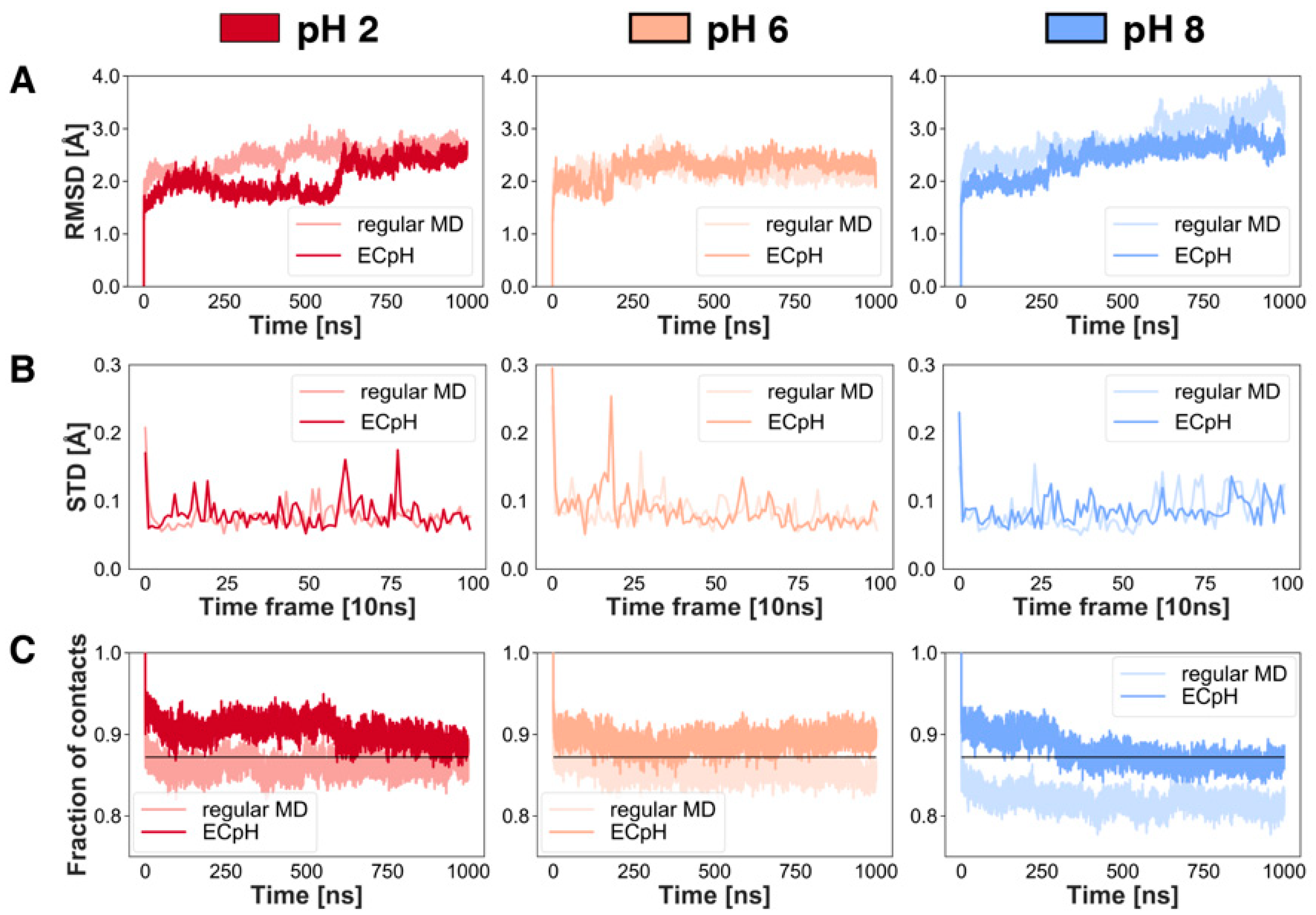
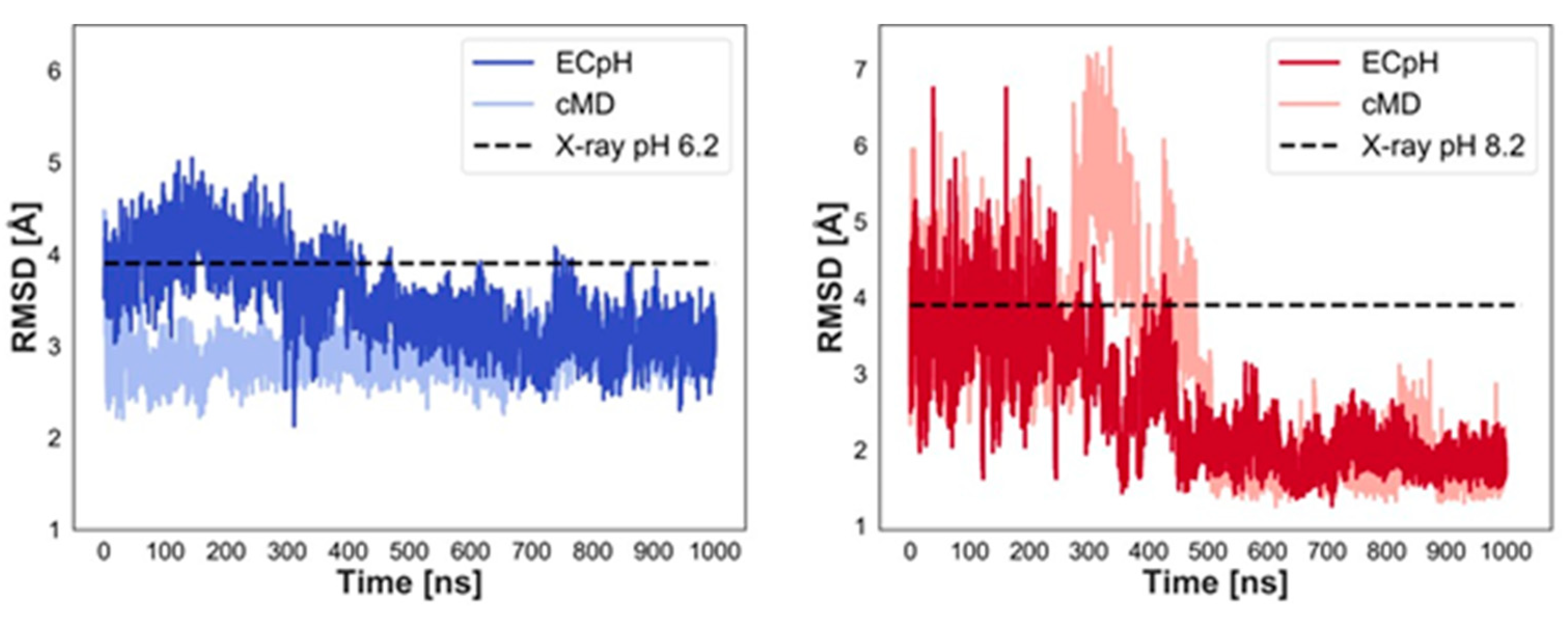
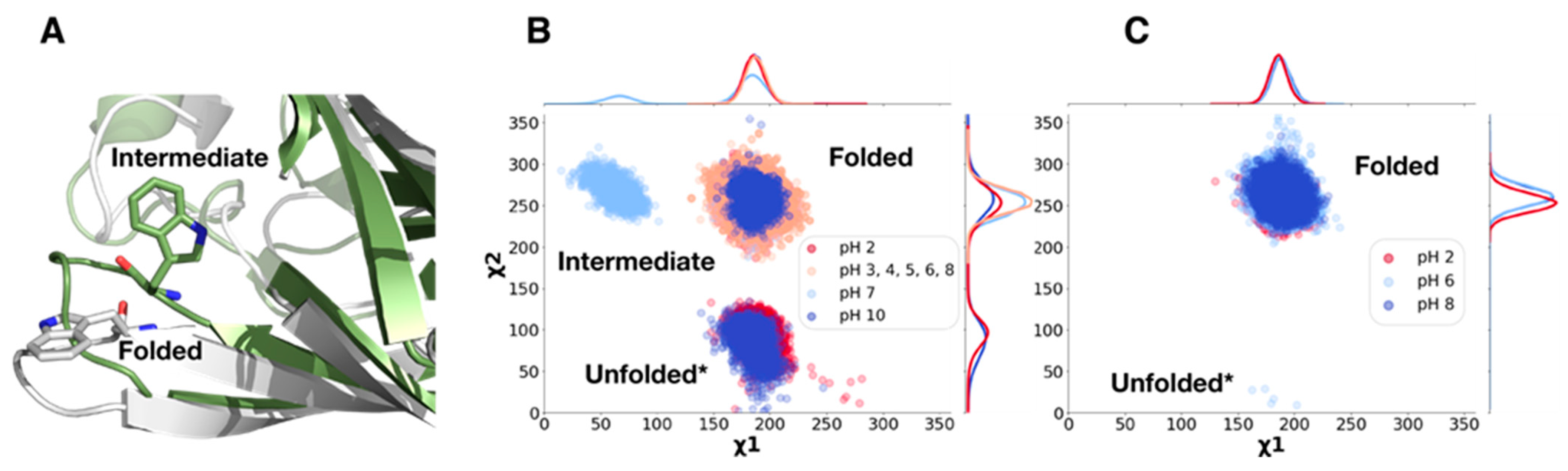
2.1. Analysis of ECpH Trajectories Brings to Light the Mechanisms of Structural Changes Detected Experimentally
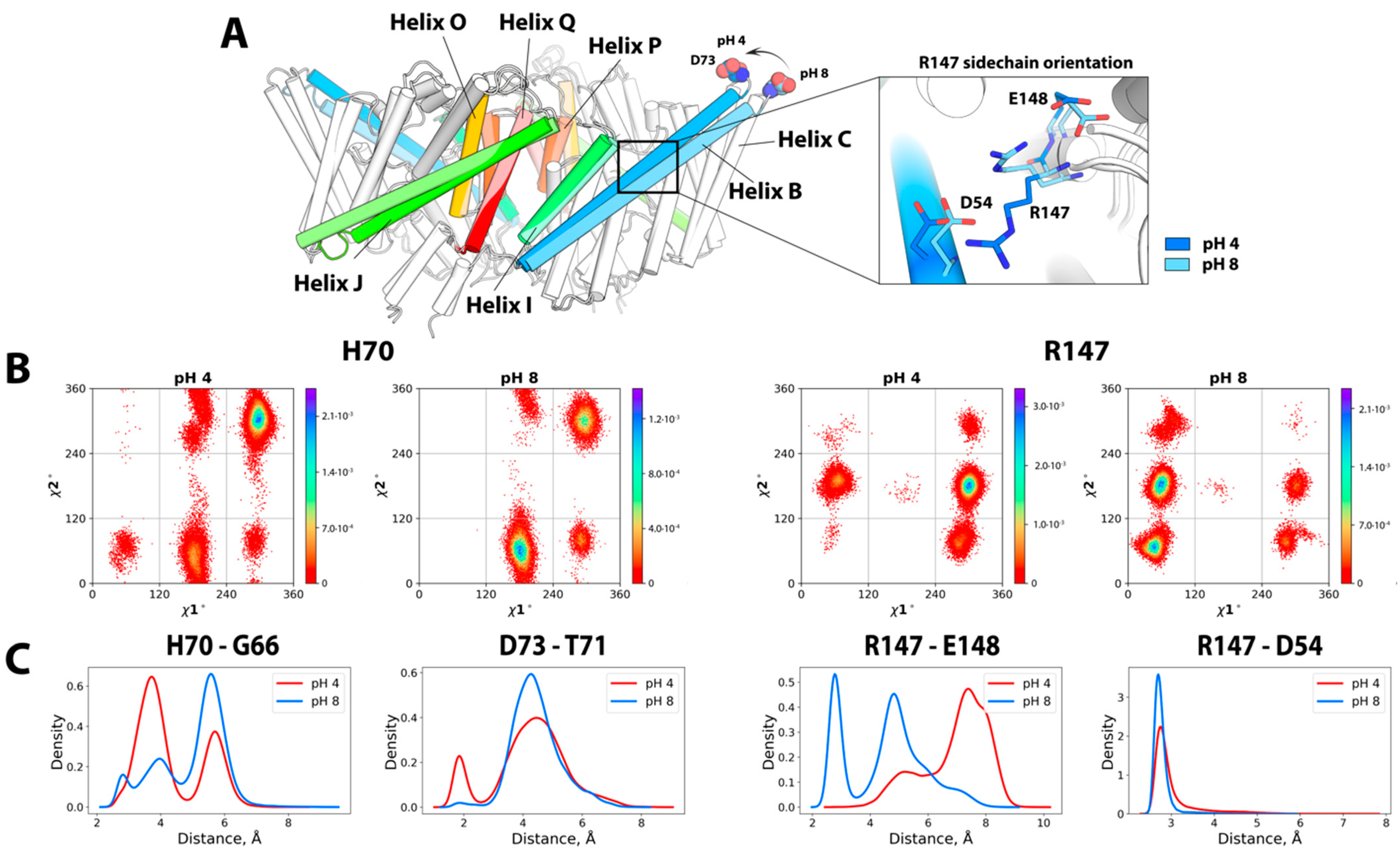
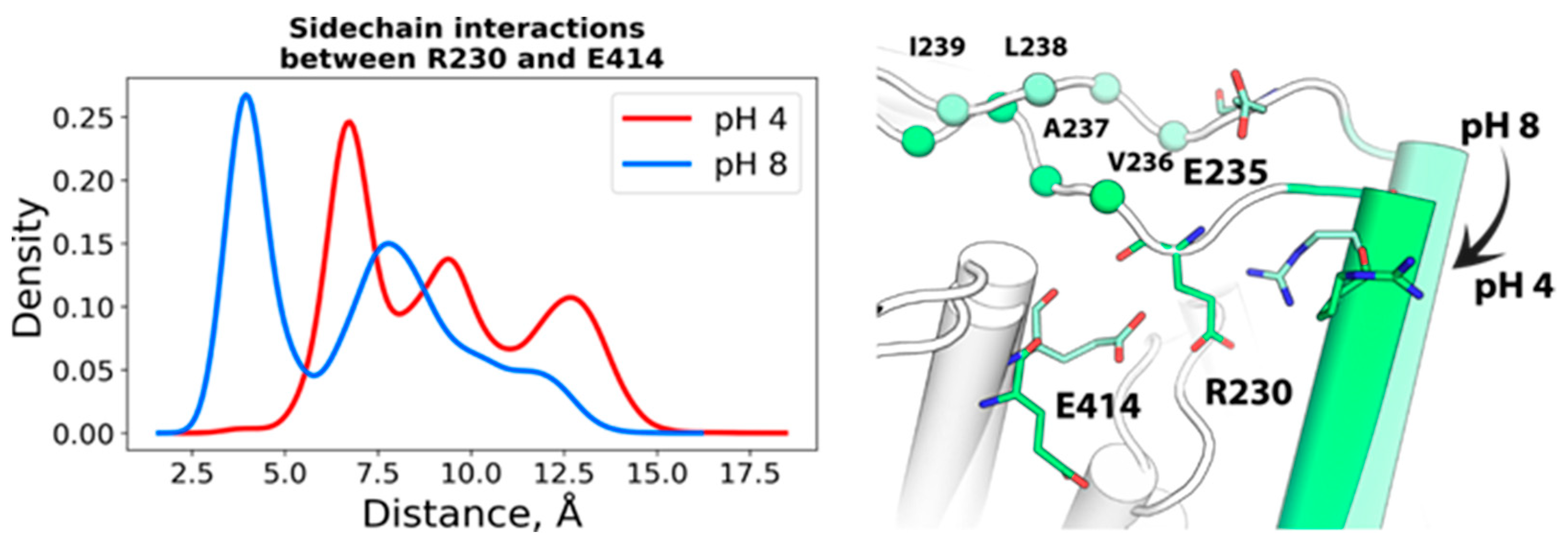
2.1.1. Spatial Reorganization of Helices B and C
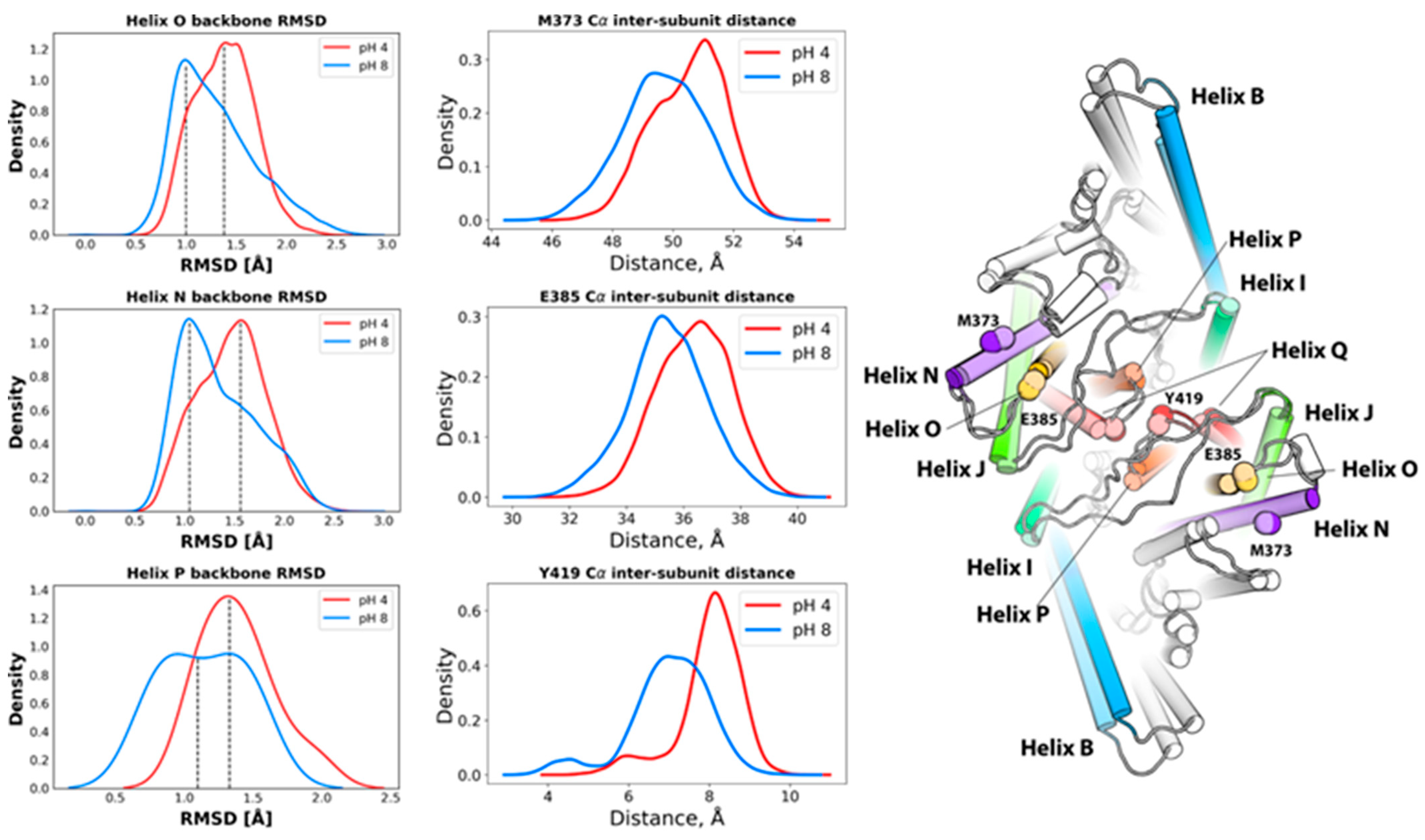
2.1.2. pH-Dependent Conformational Rearrangement of Helices N, P and O
3. Discussion
4. Materials and Methods
4.1. Definition of the ECpH Framework
4.1.1. Individual pKa Definition
4.1.2. Protonation State Representation
4.1.3. The Protonation Scheme: Definition of The Effective Forcefield
4.2. Parameters Used in the cMD and ECpH Simulations
4.2.1. Individual pKa Assignment in CLC-ec1 Homodimer
4.2.2. ECpH and cMD Simulation Protocols for MD Trajectories of CLC-ec1 System
4.2.3. ECpH and cMD Simulation Protocols for MD Trajectories of BBL Protein
4.2.4. Native Contacts Analysis Procedure
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
Appendix B
References
- Miller, C. CLC chloride channels viewed through a transporter lens. Nature 2006, 440, 484–489. [Google Scholar] [CrossRef]
- Pusch, M.; Zifarelli, G.; Murgia, A.R.; Picollo, A.; Babini, E. Channel or transporter? The CLC saga continues. Exp. Physiol. 2006, 91, 149–152. [Google Scholar] [CrossRef]
- Jentsch, T.; Pusch, M. CLC chloride channels and transporters: Structure, function, physiology, and disease. Physiol. Rev. 2018, 98, 1493–1590. [Google Scholar] [CrossRef]
- Jentsch, T. CLC chloride channels and transporters: From genes to protein structure, pathology and physiology. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 3–36. [Google Scholar] [CrossRef]
- Steinmeyer, K.; Ortland, C.; Jentsch, T. Primary structure and functional expression of a developmentally regulated skeletal muscle chloride channel. Nature 1991, 354, 301–304. [Google Scholar] [CrossRef]
- Stölting, G.; Fischer, M.; Fahlke, C. CLC channel function and dysfunction in health and disease. Front. Physiol. 2014, 5, 378. [Google Scholar] [CrossRef]
- Maduke, M.; Pheasant, D.J.; Miller, C. High-level expression, functional reconstitution, and quaternary structure of a prokaryotic ClC-type chloride channel. J. Gen. Physiol. 1999, 114, 713–722. [Google Scholar] [CrossRef]
- Vien, M.; Basilio, D.; Leisle, L.; Accardi, A. Probing the conformation of a conserved glutamic acid within the Cl− pathway of a CLC H+/Cl− exchanger. J. Gen. Physiol. 2017, 149, 523–529. [Google Scholar] [CrossRef]
- Accardi, A.; Miller, C. Secondary active transport mediated by a prokaryotic homologue of CLC Cl- channels. Nature 2004, 427, 803–807. [Google Scholar] [CrossRef]
- Accardi, A.; Walden, M.; Nguitragool, W.; Jayaram, H.; Williams, C.; Miller, C. Separate ion pathways in a Cl−/H+ exchanger. J. Gen. Physiol. 2005, 126, 563–570. [Google Scholar] [CrossRef]
- Miller, C.; White, M.M. Dimeric structure of single chloride channels from Torpedo electroplax. Proc. Natl. Acad. Sci. USA 1984, 81, 2772–2775. [Google Scholar] [CrossRef]
- Ludewig, U.; Pusch, M.; Jentsch, T. Two physically distinct pores in the dimeric CIC-0 chloride channel. Nature 1996, 383, 340–343. [Google Scholar] [CrossRef]
- Kuang, Z.; Mahankali, U.; Beck, T.L. Proton pathways and H+/Cl- stoichiometry in bacterial chloride transporters. Proteins 2007, 68, 26–33. [Google Scholar] [CrossRef]
- Han, W.; Cheng, R.C.; Maduke, M.C.; Tajkhorshid, E. Water access points and hydration pathways in CLC H+/Cl- transporters. Proc. Natl. Acad. Sci. USA 2014, 111, 1819–1824. [Google Scholar] [CrossRef]
- Lim, H.H.; Shane, T.; Miller, C. Intracellular proton access in a Cl-/H+ antiporter. PLoS Biol. 2012, 10, e1001441. [Google Scholar] [CrossRef]
- Dutzler, R.; Campbell, E.B.; MacKinnon, R. Gating the selectivity filter in ClC chloride channels. Science 2003, 300, 108–112. [Google Scholar] [CrossRef]
- Lim, H.H.; Miller, C. Intracellular proton-transfer mutants in a CLC Cl-/H+ exchanger. J. Gen. Physiol. 2009, 133, 131–138. [Google Scholar] [CrossRef][Green Version]
- Chavan, T.S.; Cheng, R.C.; Jiang, T.; Mathews, I.I.; Stein, R.A.; Koehl, A.; Mchaourab, H.S.; Tajkhorshid, E.; Maduke, M. A CLC-ec1 mutant reveals global conformational change and suggests a unifying mechanism for the CLC Cl−/H+ transport cycle. Elife 2020, 9, 53479. [Google Scholar]
- Heath, G.R.; Kots, E.; Robertson, J.L.; Lansky, S.; Khelashvili, G.; Weinstein, H.; Scheuring, S. Localization atomic force microscopy. Nature 2021, 594, 385–390. [Google Scholar] [CrossRef]
- Kots, E.; Shore, D.M.; Weinstein, H. An equilibrium constant pH molecular dynamics method for accurate prediction of pH-dependence in protein systems: Theory and application. bioRxiv 2020, 11, 394015. [Google Scholar] [CrossRef]
- Abraham, S.J.; Cheng, R.C.; Chew, T.A.; Khantwal, C.M.; Liu, C.W.; Gong, S.; Nakamoto, R.K.; Maduke, M. 13C NMR Detects Conformational Change in the 100-kD Membrane Transporter CLC-ec1. J. Biomol. NMR 2015, 61, 209–226. [Google Scholar] [CrossRef][Green Version]
- Basilio, D.; Noack, K.; Picollo, A.; Accardi, A. Conformational Changes Required for H+/Cl- Exchange Mediated by CLC Transporter. Nat. Struct. Mol. Biol. 2014, 21, 456–464. [Google Scholar] [CrossRef]
- Khantwal, C.M.; Abraham, S.J.; Han, W.; Jiang, T.; Chavan, T.S.; Cheng, R.C.; Elvington, S.M.; Liu, C.W.; Mathews, I.I.; Stein, R.A.; et al. Revealing an outward-facing open conformational state in a CLC Cl−/H+ exchange transporter. Elife 2016, 5, 11189. [Google Scholar] [CrossRef]
- Bell, S.P.; Curran, P.K.; Choi, S.; Mindell, J.A. Site-directed fluorescence studies of a prokaryotic CLC antiporter. Biochemistry 2006, 45, 6773–6782. [Google Scholar] [CrossRef] [PubMed]
- Dotson, D.; Alibay, I.; Sexton, R.; Fan, S.; Zijajo, A.; Beckstein, O. Zenodo. Becksteinlab/Propkatraj: 1.1.x. 2020. Available online: https://zenodo.org/record/3228447#.YZR3_p5Bw2w (accessed on 15 November 2021).
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent treatment of internal and surface residues in empirical pKa predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Dutzler, R.; Campbell, E.B.; Cadene, M.; Chait, B.T.; MacKinnon, R. X-ray structure of a CLC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 2002, 415, 287–294. [Google Scholar] [CrossRef]
- Elvington, S.M.; Liu, C.W.; Maduke, M.C. Substrate-driven conformational changes in CLC-ec1 observed by fluorine NMR. EMBO J. 2009, 28, 3090–3102. [Google Scholar] [CrossRef]
- Jiang, W.; Chipot, C.; Roux, B. Computing relative binding affinity of ligands to receptor: An effective hybrid single-dual-topology free-energy perturbation approach in NAMD. J. Chem. Inf. Model. 2019, 59, 3794–3802. [Google Scholar] [CrossRef]
- Henin, J.; Chipot, C. Overcoming free energy barriers using unconstrained molecular dynamics simulations. J. Chem. Phys. 2004, 121, 2904. [Google Scholar] [CrossRef] [PubMed]
- Radak, B.K.; Chipot, C.; Suh, D.; Jo, S.; Jiang, W.; Phillips, J.C.; Schulten, K.; Roux, B. Constant-pH molecular dynamics simulations for large biomolecular systems. J. Chem. Theory Comput. 2017, 13, 5933–5944. [Google Scholar] [CrossRef] [PubMed]
- Eastman, P.; Swails, J.; Chodera, J.D.; McGibbon, R.T.; Zhao, Y.; Beauchamp, K.A.; Wang, L.-P.; Simmonett, A.C.; Harrigan, M.P.; Stern, C.D.; et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLoS Comp. Biol. 2017, 13, 1005659. [Google Scholar] [CrossRef]
- Best, R.B.; Zhu, X.; Shim, J.; Lopes, P.E.M.; Mittal, J.; Feig, M.; MacKerell, A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone phi, psi and side-chain chi1 and chi2 dihedral angles. J. Chem. Theory Comput. 2012, 8, 3257–3273. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.; Kim, T.; Iyer, V.G.; Im, W. CHARMM-GUI: A web-based graphical user interface for CHARMM. J. Comput. Chem. 2008, 29, 1859–1865. [Google Scholar] [CrossRef]
- Wu, E.L.; Cheng, X.; Jo, S.; Rui, H.; Song, K.C.; Dàvila-Contreras, E.M.; Qi, Y.; Lee, J.; Monje-Galvan, V.; Venable, R.M.; et al. CHARMM-GUI membrane builder toward realistic biological membrane simulations. J. Comput. Chem. 2014, 35, 1997–2004. [Google Scholar] [CrossRef]
- Lee, J.; Cheng, X.; Swails, J.M.; Yeom, M.S.; Eastman, P.K.; Lemkul, J.A.; Wei, S.; Buckner, J.; Jeong, J.C.; Qi, Y.; et al. CHARMM-GUI input generator for NAMD, GROMACS, Amber, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016, 12, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.C.; Braun, R.; Wang, W.; Gumbart, J.; Tajkhorshid, E.; Villa, E.; Chipot, C.; Skeel, R.D.; Kalé, L.; Schulten, K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005, 26, 1781–1802. [Google Scholar] [CrossRef]
- Picollo, A.; Xu, Y.; Johner, N.; Bernèche, S.; Accardi, A. Synergistic substrate binding determines the stoichiometry of transport of a prokaryotic H+/Cl- exchanger. Nat. Struct. Mol. Biol. 2012, 19, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Farado-Gómez, J.D.; Roux, B. Electrostatics of ion stabilization in a CLC chloride channel homologue from escherichia coli. J. Mol. Biol. 2004, 339, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.Y.; Bewley, M.C.; Creamer, L.K.; Baker, H.M.; Baker, E.N.; Jameson, G.B. Structural basis of the tanford transition of bovine beta-lactoglobulin. Biochemistry 1998, 37, 14014–14023. [Google Scholar] [CrossRef]
- Tanford, C.; Bunville, L.G.; Nozaki, Y. The reversible transformation of β-lactoglobulin at pH 7.51. J. Am. Chem. Soc. 1959, 81, 4032–4036. [Google Scholar] [CrossRef]
- Castaneda, C.A.; Fitch, C.A.; Majumdar, A.; Khangulov, V.; Schlessman, J.L.; Garcia-Moreno, B.E. Molecular determinants of the pKa values of Asp and Glu residues in staphylococcal nuclease. Proteins 2009, 77, 570–588. [Google Scholar] [CrossRef] [PubMed]
- Webb, H.; Tynan-Connolly, B.M.; Lee, G.M.; Farrell, D.; O’Meara, F.; Sondergaard, C.R.; Teilum, K.; Hewage, C.; McIntosh, L.P.; Nielsen, J.E. Remeasuring HEWL pKa values by NMR spectroscopy: Methods, analysis, accuracy, and implications for theoretical pKa calculations. Proteins 2011, 79, 685–702. [Google Scholar] [CrossRef] [PubMed]
- Bogusz, S.; Cheatham, T.E., III; Brooks, B.R. Removal of pressure and free energy artifacts in charged periodic systems via net charge corrections to the ewald potential. J. Chem. Phys. 1998, 108, 7070–7084. [Google Scholar] [CrossRef]
- Hub, J.S.; de Groot, B.L.; Grubmuller, H.; Groenhof, G. Quantifying artifacts in ewald simulations of inhomogeneous systems with a net charge. J. Chem. Theory Comput. 2014, 10, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Goto, Y. Principal component analysis of the pH-dependent conformational transitions of bovine D-lactoglobulin monitored by heteronuclear NMR. Proc. Natl. Acad. Sci. USA 2007, 104, 15346–15351. [Google Scholar] [CrossRef]
- Swails, J.M.; York, D.M.; Roitberg, A.E. Constant pH replica exchange molecular dynamics in explicit solvent using discrete protonation states: Implementation, testing, and validation. J. Chem. Theory Comput. 2014, 10, 1341–1352. [Google Scholar] [CrossRef]
- Zhang, D.; Lazim, R. Application of conventional molecular dynamics simulation in evaluating the stability of apomyoglobin in urea solution. Sci. Rep. 2017, 7, 44651. [Google Scholar] [CrossRef]
- Taulier, N.; Chalikian, T.V. Characterization of pH-Induced Transitions of Beta-Lactoglobulin: Ultrasonic, Densimetric, and Spectroscopic Studies. J. Mol. Biol. 2001, 314, 873–889. [Google Scholar] [CrossRef]
- Kuwata, K.; Era, S.; Hoshino, M.; Forge, V.; Goto, Y.; Batt, C.A. Solution structure and dynamics of bovine d-lactoglobulin A. Protein Sci. 1999, 8, 2541–2545. [Google Scholar] [CrossRef]
- Uhrinova, S.; Smith, M.H.; Jameson, G.B.; Uhrin, D.; Sawyer, L.; Barlow, P.N. Structural changes accompanying pH-induced dissociation of the d-lactoglobulin dimer. Biochemistry 2020, 39, 3565–3574. [Google Scholar] [CrossRef]
- Schwaighofer, A.; Alcaraz, M.R.; Lux, L.; Lendl, B. pH Titration of d-lactoglobulin monitored by laser-based mid-IR transmission spectroscopy coupled to chemometric analysis. Spectrochim. Acta A 2019, 226, 117636. [Google Scholar] [CrossRef] [PubMed]
- Casal, H.L.; Köhler, U.; Mantsch, H.H. Structural and conformational changes of beta-lactoglobulin B: An infrared spectroscopic study of the effect of pH and temperature. Biochim. Biophys. Acta 1988, 957, 11–20. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kots, E.; Shore, D.M.; Weinstein, H. Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl−/H+ Antiporter. Molecules 2021, 26, 6956. https://doi.org/10.3390/molecules26226956
Kots E, Shore DM, Weinstein H. Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl−/H+ Antiporter. Molecules. 2021; 26(22):6956. https://doi.org/10.3390/molecules26226956
Chicago/Turabian StyleKots, Ekaterina, Derek M. Shore, and Harel Weinstein. 2021. "Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl−/H+ Antiporter" Molecules 26, no. 22: 6956. https://doi.org/10.3390/molecules26226956
APA StyleKots, E., Shore, D. M., & Weinstein, H. (2021). Simulation of pH-Dependent Conformational Transitions in Membrane Proteins: The CLC-ec1 Cl−/H+ Antiporter. Molecules, 26(22), 6956. https://doi.org/10.3390/molecules26226956





