Regioisomers Salviprolin A and B, Unprecedented Rosmarinic Acid Conjugated Dinorditerpenoids from Salvia przewalskii Maxim
Abstract
:1. Introduction
2. Results
3. Discussion
4. Experimental
4.1. General Experimental Procedures
4.2. Plant Materials
4.3. Extraction and Isolation
4.4. Salviprolin A (1)
4.5. Salviprolin B (2)
4.6. Cytotoxic Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, N.; Niwa, M.; Luo, H.-W. Triterpenoids from Salvia przewalskii. Phytochemistry 1988, 27, 299–301. [Google Scholar] [CrossRef]
- Liu, J.-M.; Nan, P.; Tsering, Q.; Tsering, T.-S.; Bai, Z.-K.; Wang, L.; Liu, Z.-J.; Zhong, Y. Volatile constituents of the leaves and flowers of Salvia przewalskii Maxim. from Tibet. Flavour Fragr. J. 2006, 21, 421–438. [Google Scholar] [CrossRef]
- Wang, L.; Jiang, Y.-Y.; Zhang, L.; Wang, T.; Zhou, Y.-H. High-performance liquid chromatography fingerprints and simultaneous quantification of bioactive compounds in Salvia przewalskii Maxim. Acta Chromatogr. 2017, 29, 291–308. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-F.; Duo, D.-L.; Yan, Y.-J.; He, R.-Y.; Wu, X.-N. Bioactive constituents of Salvia przewalskii and the molecular mechanism of its antihypoxia effects determined using quantitative proteomics. Pharm. Biol. 2020, 58, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-M.; Dai, H.-X.; Li, X.-R.; Li, Y.-H.; Wang, L.-J.; Xue, M. Structural elucidation of metabolites of tanshinone I and its analogue dihydrotanshinone I in rats by HPLC–ESI-MSn. J. Chromatogr. B 2010, 878, 915–924. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Luo, L.; Cao, F.-L.; Yang, B.-Y.; Gao, J.; Yan, Y.-G.; Zhang, G.; Peng, L.; Hu, B.-X. Increased phenolic acid and tanshinone production and transcriptional responses of biosynthetic genes in hairy root cultures of Salvia przewalskii Maxim. treated with methyl jasmonate and salicylic acid. Mol. Biol. Rep. 2020, 47, 8565–8578. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Piasecka, A.; Gryszczyńska, A.; Sawikowska, A.; Opala, A.P.B.; Mikołajczak, P.; Kujawski, R.; Kachlicki, P.; Buchwald, W.; Mrozikiewicz, A.S. Determination of phenolic compounds and diterpenes in roots of Salvia miltiorrhiza and Salvia przewalskii by two LC–MS tools: Multi-stage and high resolution tandem mass spectrometry with assessment of antioxidant capacity. Phytochem. Lett. 2017, 20, 331–338. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.-W.; Wu, C.-Y.; Li, X.-N.; Zhao, Q.-S. Przewalskone: A cytotoxic adduct of a danshenol type terpenoid and an icetexane diterpenoid via hetero-Diels–Alder reaction from Salvia przewalskii. Chem. Commun. 2012, 48, 4438–4440. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Hou, A.-J.; Wang, R.-R.; Liang, G.-Y.; Zheng, Y.-T.; Liu, Z.-Y.; Li, X.-L.; Zhao, Y.; Huang, S.-X.; Peng, L.-Y.; et al. Przewalskin A: A New C23 Terpenoid with a 6/6/7 Carbon Ring Skeleton from Salvia przewalskii Maxim. Org. Lett. 2006, 8, 4453–4456. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Hou, A.-J.; Zheng, Y.-T.; Zhao, Y.; Li, X.-L.; Peng, L.-Y.; Zhao, Q.-S. Przewalskin B, a Novel Diterpenoid with an Unprecedented Skeleton from Salvia przewalskii Maxim. Org. Lett. 2006, 9, 291–293. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Duo, D.-L.; Yan, Y.-J.; He, R.-Y.; Wu, X.-N. Extract of Salvia przewalskii Repair Tissue Damage in Chronic Hypoxia Maybe through the RhoA–ROCK Signalling Pathway. Biol. Pharm. Bull. 2020, 43, 432–439. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Luo, X.; Wang, L.; Li, Y.; Xue, M. Acute and subacute toxicity of ethanol extracts from Salvia przewalskii Maxim in rodents. J. Ethnopharmacol. 2010, 131, 110–115. [Google Scholar] [CrossRef]
- Dai, D.-S.; Liu, X.; Yang, Y.; Luo, X.-M.; Tang, R.-X.; Yin, Z.-C.; Ren, H.-Q. Protective Effect of Salvia przewalskii Extract on Puromycin-Induced Podocyte Injury. Am. J. Nephrol. 2015, 42, 216–227. [Google Scholar] [CrossRef]
- Alsirrag, M.; Ali, R. Determination of antimicrobial and antioxidants activity of Salvia przewalskii seed oil against pathogenic bacteria and fungi. J. Phys. Conf. Ser. 2018, 1032, 012070. [Google Scholar] [CrossRef]
- Bolchi, C.; Bavo, F.; Appiani, R.; Roda, G.; Pallavicini, M. 1,4-Benzodioxane, an evergreen, versatile scaffold in medicinal chemistry: A review of its recent applications in drug design. Eur. J. Med. Chem. 2020, 200, 112419–112435. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Park, Y.J.; Han, Y.B.; Lee, J.C.; Lee, S.L.; Park, H.J.; Lee, H.J.; Kim, K.H. Isoamericanoic Acid B from Acer tegmentosum as a Potential Phytoestrogen. Nutrients 2018, 10, 1915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Ueno, H.; Yoshida, N.; Ouchi, H.; Asakawaet, T.; Yoshimura, F.; Inai, M.; Kan, T. Diastereo- and Regiodivergent Total Synthesis of Princepin and Isoprincepin in Both (7″R, 8″R) and (7″S, 8″S) Isomers. J. Org. Chem. 2019, 84, 14227–14240. [Google Scholar] [CrossRef]
- Li, Y.; Xie, S.; Ying, J.; Wei, W.; Gao, K. Chemical Structures of Lignans and Neolignans Isolated from Lauraceae. Molecules 2018, 32, 3164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Y.; Wu, Y.; Zhu, H.; Li, X.-N.; Qian, J.-F.; Lai, Y.; Chen, C.; Yao, G.; Luo, Z.; Li, Y.; et al. Salviprzols A and B, C21- and C22-terpenoids from the roots of Salvia przewalskii Maxim. Fitoterapia 2014, 99, 204–210. [Google Scholar] [CrossRef]
- Zhu, H.; Chen, C.; Yang, J.; Li, X.-N.; Liu, J.; Sun, B.; Huang, S.-X.; Li, D.; Yao, G.; Luo, Z. Bioactive Acylphloroglucinols with Adamantyl Skeleton from Hypericum sampsonii. Org. Lett. 2014, 16, 6322–6325. [Google Scholar] [CrossRef]
- Qiao, Y.; Xu, Q.; Hu, Z.; Li, X.-N.; Xiang, M.; Liu, J.; Huang, J.; Zhu, H.; Wang, J.; Luo, Z. Diterpenoids of the Cassane Type from Caesalpinia decapetala. J. Nat. Prod. 2016, 79, 3134–3142. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, N.; Chen, C.; Huang, J.; Li, X.-N.; Liu, J.; Zhu, H.; Tong, Q.; Zhang, J.; Luo, Z. Tricyclic Polyprenylated Acylphloroglucinols from St John’s Wort, Hypericum perforatum. J. Nat. Prod. 2017, 80, 1493–1504. [Google Scholar] [CrossRef]
- Wu, Y.; Xie, S.-S.; Hu, Z.-X.; Wu, Z.-D.; Guo, Y.; Zhang, J.-W.; Wang, J.-P.; Xue, Y.-B.; Zhang, Y.-H. Triterpenoids from Whole Plants of Phyllanthus urinaria. Chin. Herb. Med. 2017, 9, 193–196. [Google Scholar] [CrossRef]
- Yin, Z.-K.; Liu, Z.-Z.; Yuan, Z.; Feng, Z.-M.; Jiang, J.-S.; Zhang, X.; Zhang, P.-C.; Yang, Y.-N. Thirteen undescribed diterpenoid quinones derived from the rhizomes of Salvia miltiorrhiza and their anti-tumor activities. Phytochemistry 2021, 191, 112902. [Google Scholar] [CrossRef]
- Seo, Y.-H.; Trinh, T.-A.; Ryu, S.-M.; Kim, H.-S.; Choi, G.; Moon, B.-C.; Shim, S.-H.; Jang, D.-S.; Lee, D.-H.; Kang, K.-S.; et al. Chemical Constituents from the Aerial Parts of Elsholtzia ciliata and Their Protective Activities on Glutamate-Induced HT22 Cell Death. J. Nat. Prod. 2020, 83, 3149–3155. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-F.; Lee, S.-F. Neolignans as xanthine oxidase inhibitors from Hyptis rhomboides. Phytochemistry 2014, 101, 121–127. [Google Scholar] [CrossRef]
- Yong, H.-C.; Shin, D.; Na, Z.; Lee, H.-S.; Kim, D.-D.; Oh, K.-B.; Shin, J. Dihydroxystyrene Metabolites from an Association of the Sponges Poecillastra wondoensis and Jaspis sp. J. Nat. Prod. 2008, 71, 779–783. [Google Scholar]
- Lee, D.H.; Cuendet, M.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Fong, H.H.S.; Pezzuto, J.M.; Kinghorn, A.D. A Novel Cyclooxygenase-Inhibitory Stilbenolignan from the Seeds of Aiphanes aculeata. Org. Lett. 2001, 3, 2169–2171. [Google Scholar] [CrossRef]
- Alley, M.C.; Scudiero, D.A.; Monks, A.; Hursey, M.L.; Czerwinski, M.J.; Fine, D.L.; Abbott, B.J.; Mayo, J.G.; Shoemaker, R.H.; Boyd, M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Cancer Res. 1988, 48, 589–601. [Google Scholar] [PubMed]
- Chatzopoulou, A.; Karioti, A.; Gousiadou, C.; Lax Vivancos, V.; Kyriazopoulos, P.; Golegou, S.; Skaltsaet, H. Depsides and Other Polar Constituents from Origanum dictamnus L. and Their in Vitro Antimicrobial Activity in Clinical Strains. J. Agric. Food Chem. 2010, 58, 6064–6068. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.-D.; Park, Y.-S.; Jin, Y.-H.; Park, C.-S. Production and applications of rosmarinic acid and structurally related compounds. Appl. Microbiol. Biotechnol. 2015, 99, 2083–2092. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-P.; Wei, K.-H.; Zhang, G.-J.; Lei, L.-J.; Yang, D.-W.; Wang, W.-L.; Han, Q.-H.; Xia, Y.; Bi, Y.-Q.; Yang, M.; et al. Ethnopharmacology, phytochemistry, and pharmacology of Chinese Salvia species: A review. J. Ethnopharmacol. 2018, 225, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.-J.; Zhou, P.-P.; Lin, C.-J.; Wang, W.-F.; Li, Y.; Gao, K. Diterpenoids from Salvia miltiorrhiza and Their Immune-Modulating Activity. J. Agric. Food Chem. 2017, 65, 5985–5993. [Google Scholar] [CrossRef] [PubMed]
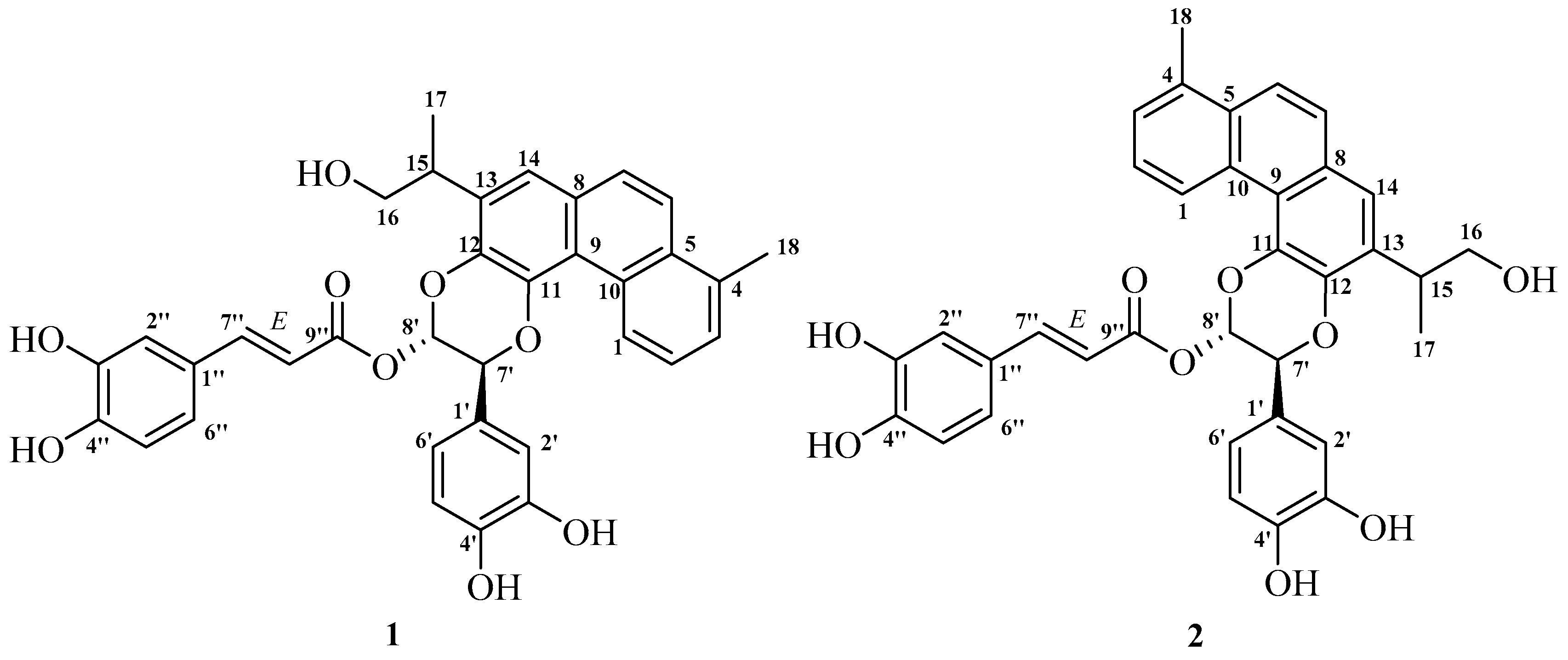
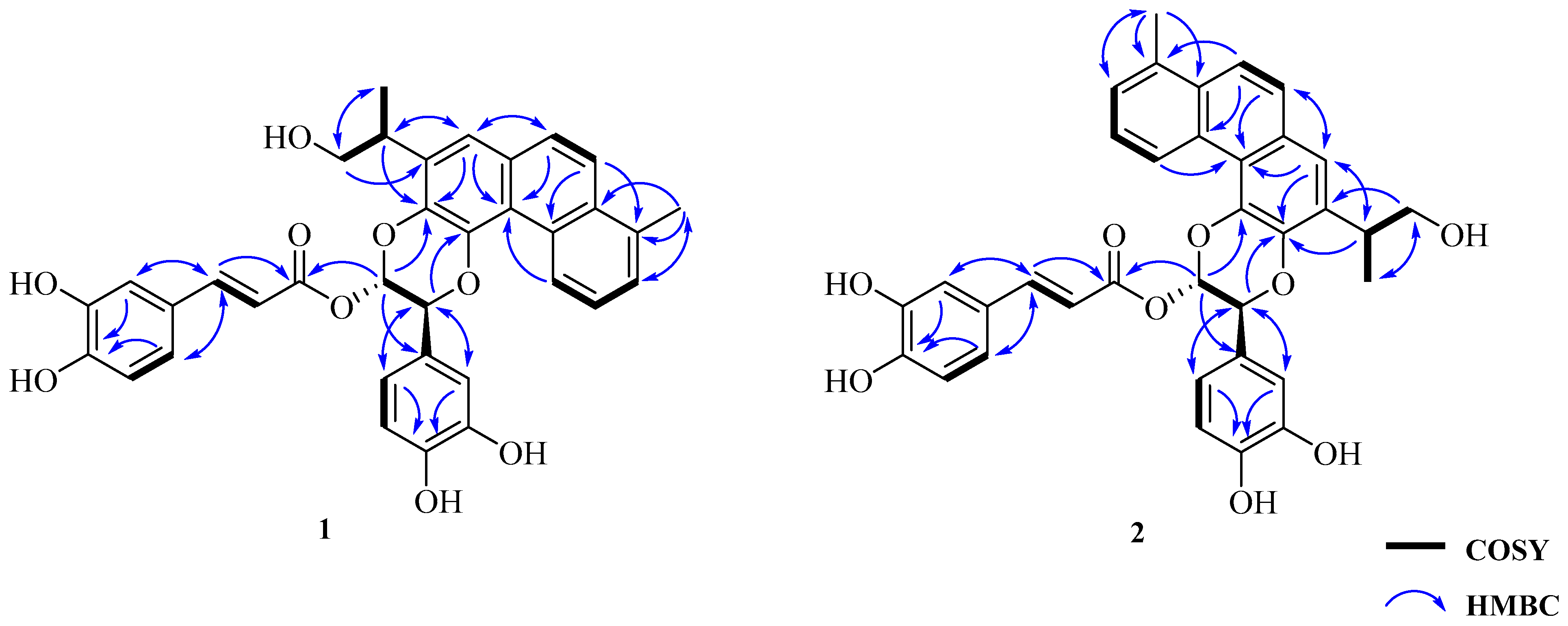
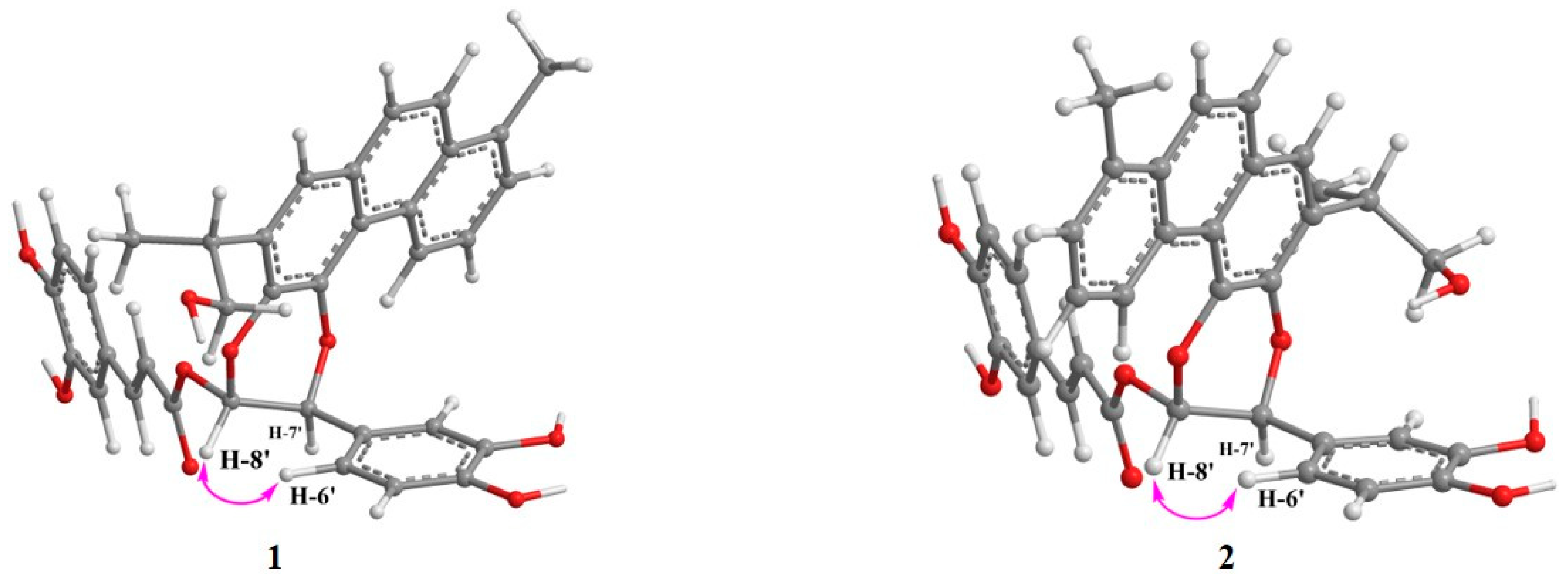
| No | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δC | δH (Mult, J, Hz) | HMBC | δC | δH (Mult, J, Hz) | HMBC | |
| 1 | 127.4 | 9.61 m | 3, 5, 9 | 127.3 | 9.44 m | 3, 5, 9 |
| 2 | 126.4 | 7.43 c | 4, 10 | 126.3 | 7.40 c | 4, 10 |
| 3 | 128.1 | 7.43 c | 1, 4, 5, 18 | 128.1 | 7.40 c | 1, 4, 5, 18 |
| 4 | 134.6 | - | 134.5 | - | ||
| 5 | 132.2 | - | 132.2 | - | ||
| 6 | 122.4 | 7.90 d (9.2) | 4, 8, 10 | 122.1 | 7.85 d (9.1) | 4, 8, 10 |
| 7 | 127.9 | 7.77 d (9.2) | 5, 6, 8, 9, 14 | 127.7 | 7.75 d (9.1) | 5, 8, 9, 14 |
| 8 | 129.3 | - | 128.9 | - | ||
| 9 | 120.3 | - | 120.5 | - | ||
| 10 | 130.6 | - | 130.3 | - | ||
| 11 | 141.3 | - | 139.1 | - | ||
| 12 | 138.3 | - | 140.4 | - | ||
| 13 | 134.2 | - | 134.0 | - | ||
| 14 | 120.9 | 7.51 s | 7, 9, 12, 15 | 121.4 | 7.56 s | 7, 9, 12, 15 |
| 15 | 36.6 | 3.42 m | 12, 13, 14, 16, 17 | 36.5 | 3.57 m | 12, 14, 16, 17 |
| 16 | 67.0 | 3.65 dd (10.4, 7.2) | 13, 15, 17 | 67.1 | 3.70 dd (10.4, 7.2) | 13, 15, 17 |
| 3.85 dd (10.4, 5.6) | 3.92 dd (10.4, 5.6) | |||||
| 17 | 17.1 | 1.35 d (5.6) | 13, 15, 16 | 17.3 | 1.42 d (6.8) | 13, 15, 16 |
| 18 | 20.5 | 2.73 s | 3, 4, 5 | 20.5 | 2.69 s | 3, 4, 5 |
| 1′ | 128.5 | - | 128.6 | - | ||
| 2′ | 114.8 | 7.12 d (1.9) | 1′, 3′, 4′, 6′, 7′ | 114.9 | 7.11 br s | 4′, 6′ |
| 3′ | 146.2 b | - | 146.1 b | - | ||
| 4′ | 146.3 b | - | 146.4 b | - | ||
| 5′ | 116.2 b | 6.84 c d (8.0) | 1′, 3′, 4′, 6′ | 116.1 b | 6.85 c | 1′, 3′ |
| 6′ | 119.5 | 6.99 dd (8.0, 1.9) | 1′, 2′, 4′, 7′ | 119.7 | 6.97 d (7.8) | 2′, 4′, 5′, 7′ |
| 7′ | 76.0 | 5.52 d (3.8) | 1′, 2′, 6′, 8′, 11 | 76.1 | 5.36 d (4.1) | 1′, 2′, 6′, 8′, 12 |
| 8′ | 90.4 | 6.77 d (3.8) | 1′, 7′, 9″, 12 | 90.8 | 6.74 d (4.1) | 1′, 7′, 9″, 11 |
| 1″ | 127.1 | - | 127.0 | - | ||
| 2″ | 115.5 | 7.16 d (1.5) | 4″, 6″, 7″ | 115.5 | 7.17 br s | 4″, 6″ |
| 3″ | 146.5 | - | 146.6 b | - | ||
| 4″ | 149.4 | - | 149.4 | - | ||
| 5″ | 116.4 b | 6.84 c d (8.0) | 1″, 3″, 4″, 6″ | 116.4 b | 6.85 c | 1″, 3″, 4″, 6″ |
| 6″ | 123.0 | 7.04 dd (8.0, 1.5) | 2″, 4″, 7″ | 123.0 | 7.02 d (7.8) | 2″, 4″, 5″ |
| 7″ | 148.0 | 7.64 d (15.8) | 1″, 2″, 6″, 9″ | 148.2 | 7.64 d (15.8) | 1″, 2″, 6″, 9″ |
| 8″ | 114.0 | 6.29 d (15.8) | 1″, 7″, 9″ | 113.8 | 6.30 d (15.8) | 1″, 9″ |
| 9″ | 165.9 | - | 165.9 | - | ||
| Compound | A-549 | HL-60 | MCF-7 | SMMC-7721 | SW-480 |
|---|---|---|---|---|---|
| 1 | >40 | >40 | >40 | >40 | >40 |
| 2 | >40 | >40 | >40 | >40 | >40 |
| cis-platin | 15.6 | 0.9 | 14.9 | 13.8 | 19.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, X.; Wu, Y.; Wu, M.; Lu, J.; Jia, S.; He, X.; Liu, S.; Zhou, Y.; Xing, H.; Xue, Y. Regioisomers Salviprolin A and B, Unprecedented Rosmarinic Acid Conjugated Dinorditerpenoids from Salvia przewalskii Maxim. Molecules 2021, 26, 6955. https://doi.org/10.3390/molecules26226955
Su X, Wu Y, Wu M, Lu J, Jia S, He X, Liu S, Zhou Y, Xing H, Xue Y. Regioisomers Salviprolin A and B, Unprecedented Rosmarinic Acid Conjugated Dinorditerpenoids from Salvia przewalskii Maxim. Molecules. 2021; 26(22):6955. https://doi.org/10.3390/molecules26226955
Chicago/Turabian StyleSu, Xiangdong, Yichuang Wu, Meifang Wu, Jielang Lu, Shujie Jia, Xin He, Shuna Liu, Yuyang Zhou, Hui Xing, and Yongbo Xue. 2021. "Regioisomers Salviprolin A and B, Unprecedented Rosmarinic Acid Conjugated Dinorditerpenoids from Salvia przewalskii Maxim" Molecules 26, no. 22: 6955. https://doi.org/10.3390/molecules26226955
APA StyleSu, X., Wu, Y., Wu, M., Lu, J., Jia, S., He, X., Liu, S., Zhou, Y., Xing, H., & Xue, Y. (2021). Regioisomers Salviprolin A and B, Unprecedented Rosmarinic Acid Conjugated Dinorditerpenoids from Salvia przewalskii Maxim. Molecules, 26(22), 6955. https://doi.org/10.3390/molecules26226955









