Experimental Research on NO2 Viscosity and Absorption for (1-Ethyl-3-methylimidazolium Trifluoroacetate + Triethanolamine) Binary Mixtures
Abstract
:1. Introduction
2. Results and Discussion
2.1. Viscosity and Model
2.2. Absorption Capacity
2.3. Theoretical Analysis
2.4. Reusability of ([EMIM] [TFA] + TEA)
3. Materials and Methods
3.1. Materials
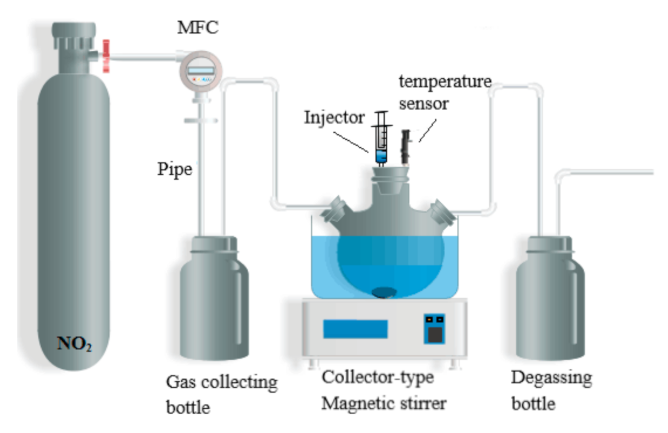
3.2. Instruments and Procedures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, C.; Liu, Y.; Zheng, S.; Jiang, A. Optimizing combustion of coal fired boilers for reducing NOx emission using Gaussian Process. Energy 2018, 153, 149–158. [Google Scholar] [CrossRef]
- Duan, E.; Guo, B.; Zhang, D.; Shi, L.; Sun, H.; Wang, Y. Absorption of NO and NO2 in Caprolactam Tetrabutyl Ammonium Halide Ionic Liquids. J. Air Waste Manag. Assoc. 2011, 61, 1393–1397. [Google Scholar] [CrossRef]
- Li, Y.; Yi, H.; Tang, X.; Liu, X.; Wang, Y.; Cui, B.; Zhao, S. Study on the performance of simultaneous desulfurization and denitrification of Fe3O4-TiO2 composites. Chem. Eng. J. 2016, 304, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Deng, Q.; Lu, C.; Li, Y.; Sundell, J.; Norbäck, D. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ. Res. 2016, 150, 119–127. [Google Scholar] [CrossRef]
- Norbäck, D.; Hashim, J.H.; Hashim, Z.; Ali, F. Volatile organic compounds (VOC), formaldehyde and nitrogen dioxide (NO2 ) in schools in Johor Bahru, Malaysia: Associations with rhinitis, ocular, throat and dermal symptoms, headache and fatigue. Sci. Total Environ. 2017, 592, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Park, C.; Kim, C.; Kim, K.; Lee, D.; Song, Y.; Moriyoshi, Y. The influence of hydrogen-enriched gas on the performance of lean NOx trap catalyst for a light-duty diesel engine. Int. J. Hydrog. Energy 2010, 35, 1789–1796. [Google Scholar] [CrossRef]
- Kameoka, Y.; Pigford, R.L. Absorption of Nitrogen Dioxide into Water, Sulfuric Acid, Sodium Hydroxide, and Alkaline Sodium Sulfite Aqueous Solutions. Ind. Eng. Chem. Fundam. 1977, 16, 163–169. [Google Scholar] [CrossRef]
- Tang, N.; Liu, Y.; Wang, H.; Xiao, L.; Wu, Z. Enhanced absorption process of NO2 in CaSO3 slurry by the addition of MgSO4. Chem. Eng. J. 2010, 160, 145–149. [Google Scholar] [CrossRef]
- Fine, N.A.; Rochelle, G.T. Absorption of Nitrogen Oxides in Aqueous Amines. Energy Procedia 2014, 63, 830–847. [Google Scholar] [CrossRef] [Green Version]
- Shoukat, U.; Baumeister, E.; Pinto, D.D.; Knuutila, H.K. Thermal stability and corrosion of tertiary amines in aqueous amine and amine-glycol-water solutions for combined acid gas and water removal. J. Nat. Gas Sci. Eng. 2019, 62, 26–37. [Google Scholar] [CrossRef]
- Vichi, F.; De Santis, F. The measurement of the sink properties of triethanolamine (TEA) as a coating for collecting NO2by using annular diffusion denuders. Environ. Technol. 2012, 33, 1065–1069. [Google Scholar] [CrossRef]
- Hawrylak, B.; Burke, S.E.; Palepu, R. Partial Molar and Excess Volumes and Adiabatic Compressibilities of Binary Mixtures of Ethanolamines with Water. J. Solut. Chem. 2000, 29, 575–594. [Google Scholar] [CrossRef]
- Soosaiprakasam, I.R.; Veawab, A. Corrosion and polarization behavior of carbon steel in MEA-based CO2 capture process. Int. J. Greenh. Gas Control 2008, 2, 553–562. [Google Scholar] [CrossRef]
- Islam, M.S.; Yusoff, R.; Ali, B.S.; Islam, M.N. Degradation studies of amines and alkanolamines during sour gas treatment process. Int. J. Phys. Sci. 2011, 6, 5883. [Google Scholar] [CrossRef]
- Zheng, S.; Tao, M.; Liu, Q.; Ning, L.; He, Y.; Shi, Y. Capturing CO2 into the Precipitate of a Phase-Changing Solvent after Absorption. Environ. Sci. Technol. 2014, 48, 8905–8910. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Park, J.H.; Yun, S.H.; Nam, S.C.; Jeong, S.K.; Yoon, Y.I. Carbon dioxide absorption using a phase transitional alkanolamine–alcohol mixture. J. Ind. Eng. Chem. 2014, 20, 1486–1492. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. Novel water-free biphasic absorbents for efficient CO2 capture. Int. J. Greenh. Gas Control 2017, 60, 100–109. [Google Scholar] [CrossRef]
- Ren, H.; Lian, S.; Wang, X.; Zhang, Y.; Duan, E. Exploiting the hydrophilic role of natural deep eutectic solvents for greening CO2 capture. J. Clean. Prod. 2018, 193, 802–810. [Google Scholar] [CrossRef]
- Li, M.; Guan, J.; Han, J.; Liang, W.; Wang, K.; Duan, E.; Guo, B. Absorption and oxidation of H2S in triethylamine hydrochloride·ferric chloride ionic liquids. J. Mol. Liq. 2015, 209, 58–61. [Google Scholar] [CrossRef]
- Duan, E.; Guo, B.; Zhang, M.; Guan, Y.; Sun, H.; Han, J. Efficient capture of SO2 by a binary mixture of caprolactam tetrabutyl ammonium bromide ionic liquid and water. J. Hazard. Mater. 2011, 194, 48–52. [Google Scholar] [CrossRef]
- Kunov-Kruse, A.; Thomassen, P.L.; Riisager, A.; Mossin, A.P.S.; Fehrmann, R. Absorption and Oxidation of Nitrogen Oxide in Ionic Liquids. Chem. A Eur. J. 2016, 22, 11745–11755. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.; Zhang, F.; Geng, J.; Wu, Y.-T. Highly efficient reversible adsorption of NO2 in imidazole sulfonate room temperature ionic liquids. RSC Adv. 2014, 4, 39572–39575. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Zhang, S.; Zhou, G.; Liu, X.; Chen, X. Structure, interaction and property of amino-functionalized imidazolium ILs by molecular dynamics simulation and Ab initio calculation. AIcHE J. 2007, 53, 3210–3221. [Google Scholar] [CrossRef]
- Haider, B.; Hussain, Z.; Kumar, R. CO2 absorption and kinetic study in ionic liquid amine blends. J. Mol. Liq. 2016, 224, 1025–1031. [Google Scholar] [CrossRef]
- Bernard, F.L.; Vecchia, F.D.; Rojas, M.F.; Ligabue, R.; Vieira, M.O.; Costa, E.M.; Chaban, V.V.; Einloft, S. Anticorrosion Protection by Amine–Ionic Liquid Mixtures: Experiments and Simulations. J. Chem. Eng. Data 2016, 61, 1803–1810. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; An, L.; Tu, S.-T.; Yan, J. CO2 capture with the absorbent of a mixed ionic liquid and amine solution considering the effects of SO2 and O2. Appl. Energy 2017, 194, 9–18. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, L.; Gao, G. Using Ionic Liquid Mixtures to Improve the SO2 Absorption Performance in Flue Gas. Energy Fuels 2017, 31, 1771–1777. [Google Scholar] [CrossRef]
- Yu, G.; Fan, S.; Chen, X.; Abdeltawab, A.A.; Al-Deyab, S.S. CO2 absorption by binary mixture of ionic liquids-monoethanolamine at lower pressure. Int. J. Greenh. Gas Control 2016, 44, 52–58. [Google Scholar] [CrossRef]
- Chaban, V.V.; Prezhdo, O.V. Ionic and Molecular Liquids: Working Together for Robust Engineering. J. Phys. Chem. Lett. 2013, 4, 1423–1431. [Google Scholar] [CrossRef]
- Wolf, J.-C.; Niessner, R. High-capacity NO2 denuder systems operated at various temperatures (298–473 K). Anal. Bioanal. Chem. 2012, 404, 2901–2907. [Google Scholar] [CrossRef] [PubMed]
- Gold, A. Stoichiometry of nitrogen dioxide determination in triethanolamine trapping solution. Anal. Chem. 1977, 49, 1448–1450. [Google Scholar] [CrossRef]
- López, A.B.; García, A.B.L.; Gómez-Díaz, D.; La Rubia, M.D.; Navaza, J.M. Density, speed of sound, viscosity, refractive index and surface tension of N-methyl-2-pyrrolidone+diethanolamine (or triethanolamine) from T = (293.15 to 323.15)K. J. Chem. Thermodyn. 2013, 61, 1–6. [Google Scholar] [CrossRef]
- Maham, Y.; Liew, C.-N.; Mather, A.E. Viscosities and Excess Properties of Aqueous Solutions of Ethanolamines from 25 to 80 °C. J. Solut. Chem. 2002, 31, 743–756. [Google Scholar] [CrossRef]
- Li, X.-X.; Fan, G.-C.; Zhang, Z.-L.; Wang, Y.-W.; Lu, Y.-Q. Density and Viscosity for Binary Mixtures of Diethylene Glycol Monobutyl Ether with Monoethanolamine, Diethanolamine, and Triethanolamine from (293.15 to 333.15) K. J. Chem. Eng. Data 2013, 58, 1229–1235. [Google Scholar] [CrossRef]
- Golzar, K.; Amjad-Iranagh, S.; Modarress, H. Prediction of Thermophysical Properties for Binary Mixtures of Common Ionic Liquids with Water or Alcohol at Several Temperatures and Atmospheric Pressure by Means of Artificial Neural Network. Ind. Eng. Chem. Res. 2014, 53, 7247–7262. [Google Scholar] [CrossRef]
- Rodríguez, H.; Brennecke, J.F. Temperature and Composition Dependence of the Density and Viscosity of Binary Mixtures of Water + Ionic Liquid. J. Chem. Eng. Data 2006, 51, 2145–2155. [Google Scholar] [CrossRef]
- Seddon, K.R.; Stark, A.; Torres, M.-J. Influence of chloride, water, and organic solvents on the physical properties of ionic liquids. Pure Appl. Chem. 2000, 72, 2275–2287. [Google Scholar] [CrossRef]
- Domańska, U.; Laskowska, M. Temperature and Composition Dependence of the Density and Viscosity of Binary Mixtures of {1-Butyl-3-methylimidazolium Thiocyanate + 1-Alcohols}. J. Chem. Eng. Data 2009, 54, 2113–2119. [Google Scholar] [CrossRef]
- Jiang, H.; Zhao, Y.; Wang, J.; Zhao, F.; Liu, R.; Hu, Y. Density and surface tension of pure ionic liquid 1-butyl-3-methyl-imidazolium l-lactate and its binary mixture with alcohol and water. J. Chem. Thermodyn. 2013, 64, 1–13. [Google Scholar] [CrossRef]
- Haghtalab, A.; Shojaeian, A. Volumetric and viscometric behaviour of the binary systems of N-methyldiethanolamine and diethanolamine with 1-butyl-3-methylimidazolium acetate at various temperatures. J. Chem. Thermodyn. 2014, 68, 128–137. [Google Scholar] [CrossRef]
- Abu-Daabes, M.A.; Awwad, A.M. Volumetric and viscometric properties of aqueous solutions of N-(2-hydroxyethyl)morpholine at T = (293.15, 303.15, 313.15, 323.15, 333.15)K. J. Chem. Thermodyn. 2008, 40, 874–878. [Google Scholar] [CrossRef]
- Noack, K.; Leipertz, A.; Kiefer, J. Molecular interactions and macroscopic effects in binary mixtures of an imidazolium ionic liquid with water, methanol, and ethanol. J. Mol. Struct. 2012, 1018, 45–53. [Google Scholar] [CrossRef]
- Anouti, M.; Vigeant, A.; Jacquemin, J.; Brigouleix, C.; Lemordant, D. Volumetric properties, viscosity and refractive index of the protic ionic liquid, pyrrolidinium octanoate, in molecular solvents. J. Chem. Thermodyn. 2010, 42, 834–845. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, T.; Yashiro, T. Analytical study of low-concentration gases: IV. Investigation of the reaction by trapping nitrogen dioxide in air using the triethanolamine method. J. Chromatogr. A 1983, 265, 69–78. [Google Scholar] [CrossRef]
- Soldatović, D.; Vuksanović, J.; Radovic, I.; Visak, Z.; Kijevcanin, M. Excess molar volumes and viscosity behaviour of binary mixtures of aniline/or N,N-dimethylaniline with imidazolium ionic liquids having triflate or bistriflamide anion. J. Chem. Thermodyn. 2017, 109, 137–154. [Google Scholar] [CrossRef] [Green Version]
- Chao, J.; Wilhoit, R.C.; Zwolinski, B.J. Gas phase chemical equilibrium in dinitrogen trioxide and dinitrogen tetroxide. Thermochim. Acta 1974, 10, 359–371. [Google Scholar] [CrossRef]
- Amoruso, A.; Crescentini, L.; Fiocco, G.; Volpe, M. New measurements of the NO2 absorption cross section in the 440- to 460-nm region and estimates of the NO2-N2O4 equilibrium constant. J. Geophys. Res. Space Phys. 1993, 98, 16857–16863. [Google Scholar] [CrossRef]
- Addison, C.C. Dinitrogen tetroxide, nitric acid, and their mixtures as media for inorganic reactions. Chem. Rev. 1980, 80, 21–39. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, Highly Conductive Ambient-Temperature Molten Salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Y. Properties for binary mixtures of (acetamide + KSCN) eutectic ionic liquid with ethanol at several temperatures. J. Chem. Thermodyn. 2016, 92, 1–7. [Google Scholar] [CrossRef]
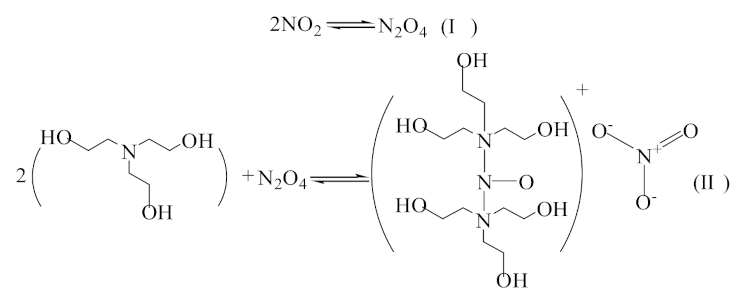
 ) [33,40]; (
) [33,40]; (  ) [34]; (
) [34]; (  ) [35,36].
) [35,36].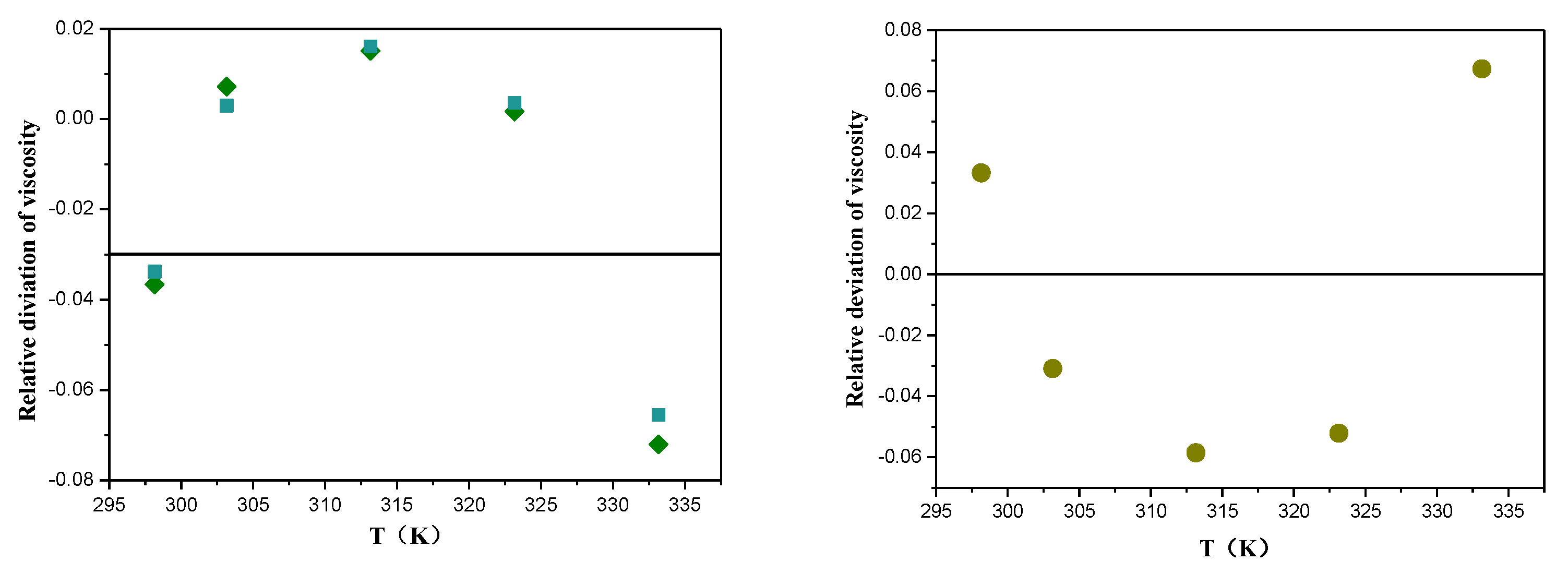



 ) and desorption quantities (
) and desorption quantities (  ) in ([EMIM] [TFA] + TEA) over six adsorption–desorption cycles.
) in ([EMIM] [TFA] + TEA) over six adsorption–desorption cycles.
 ) and desorption quantities (
) and desorption quantities (  ) in ([EMIM] [TFA] + TEA) over six adsorption–desorption cycles.
) in ([EMIM] [TFA] + TEA) over six adsorption–desorption cycles.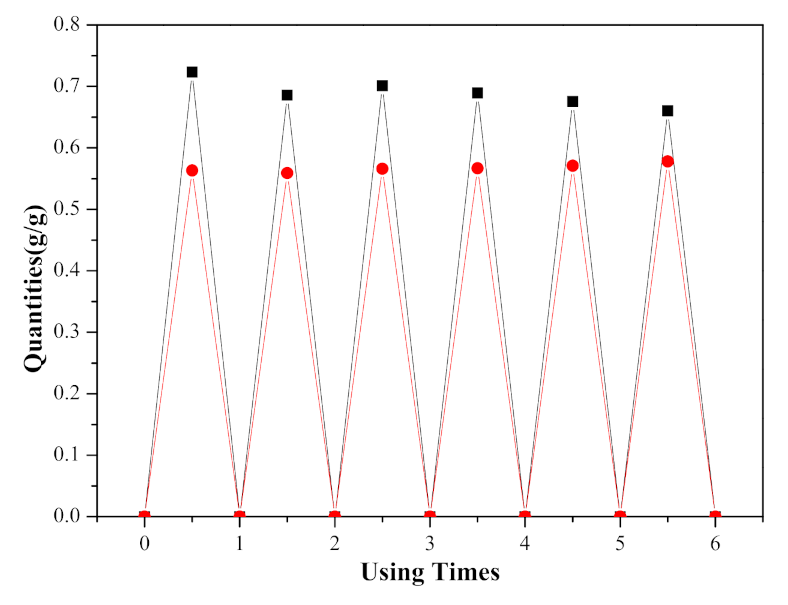

| T(K) | TEA Viscosity (mPa·s) | [EMIM] [TFA] Viscosity (mPa·s) | |||
|---|---|---|---|---|---|
| Expt | Lit [39,40] | Lit [34] | Expt | Lit [34,35] | |
| 298.15 | 584.98 | 606.4 | 604.8 | 33.10 | 32 |
| 303.15 | 405.92 | 403.0 | 404.7 | 26.56 | 27.38 |
| 313.15 | 207.24 | 204.1 | 203.9 | 17.24 | 18.25 |
| 323.15 | 108.69 | 108.50 | 108.3 | 13.04 | 13.72 |
| 333.15 | 60.25 | 64.6 | 64.2 | 10.39 | 11.09 |
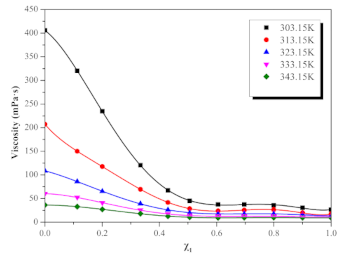
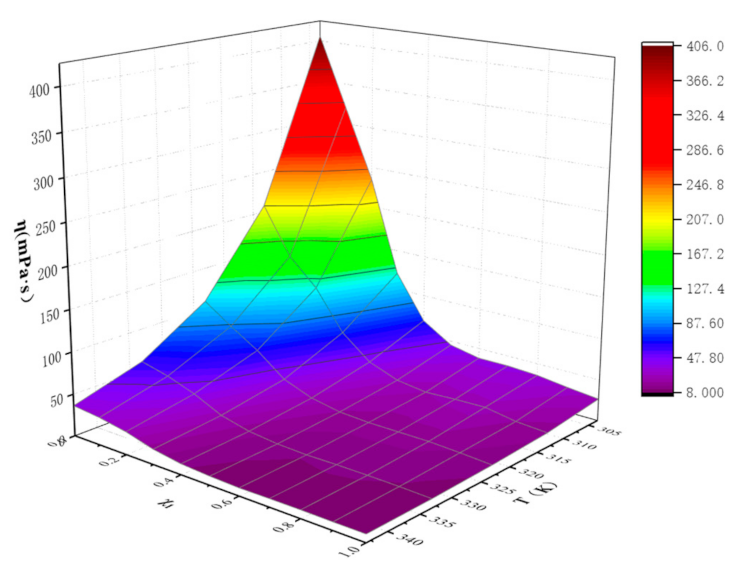
| χ1 | T(K) | ||||
|---|---|---|---|---|---|
| 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | |
| 0 | 405.92 | 207.24 | 108.69 | 60.25 | 36.29 |
| 0.1128 | 320.40 | 149.99 | 86.10 | 52.38 | 32.87 |
| 0.2006 | 234.79 | 117.78 | 65.22 | 40.93 | 27.13 |
| 0.3337 | 120.61 | 69.56 | 39.08 | 25.55 | 18.02 |
| 0.4299 | 67.35 | 41.65 | 26.19 | 17.46 | 12.69 |
| 0.5058 | 44.99 | 28.61 | 19.73 | 13.72 | 10.47 |
| 0.6051 | 37.59 | 23.83 | 17.74 | 12.32 | 9.27 |
| 0.6965 | 37.59 | 25.49 | 17.60 | 12.60 | 9.64 |
| 0.7994 | 35.58 | 26.28 | 17.44 | 12.36 | 9.74 |
| 0.9001 | 30.63 | 19.88 | 15.61 | 11.64 | 9.41 |
| 1.0000 | 26.56 | 17.24 | 13.04 | 10.39 | 9.34 |
| T (K) | A0 | A1 | A2 | A3 | A4 | A5 | A6 | R2 |
|---|---|---|---|---|---|---|---|---|
| 303.15 | 405.84 | −460.72 | −3557.9 | 9150.8 | −5717.9 | −1840.4 | 2047.1 | 1.0000 |
| 313.15 | 207.20 | −715.74 | 2963.5 | −12302 | 25291 | −23213 | 7786.5 | 1.0000 |
| 323.15 | 108.72 | −162.30 | −475.60 | 1078.0 | 157.78 | −1399.4 | 705.82 | 0.9999 |
| 343.15 | 60.272 | −13.505 | −674.36 | 1546.2 | −1021.8 | −125.96 | 239.58 | 0.9999 |
| 353.15 | 36.295 | −3.7401 | −271.20 | 206.93 | 807.81 | −1308.2 | 541.51 | 0.9999 |
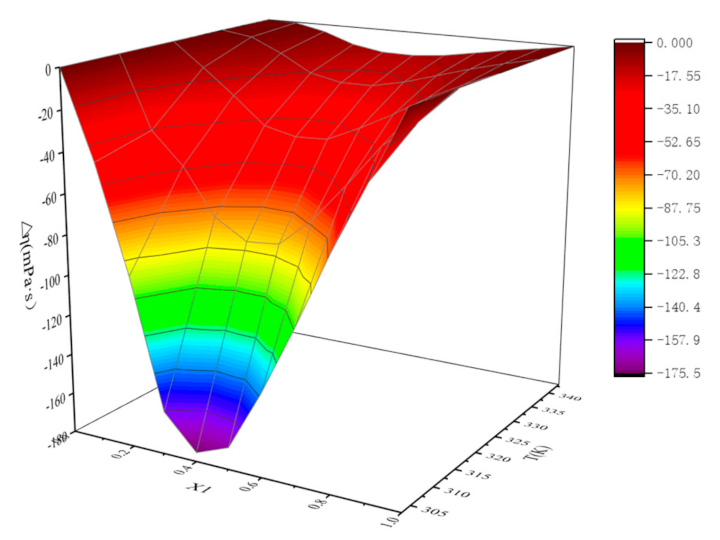
| χ1 | 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K |
|---|---|---|---|---|---|
| 0.0000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 0.1128 | −42.73 | −35.82 | −11.80 | −2.25 | −0.38 |
| 0.2006 | −95.03 | −51.35 | −24.28 | −9.32 | −3.75 |
| 0.3337 | −158.72 | −74.28 | −37.69 | −18.06 | −9.28 |
| 0.4299 | −175.48 | −83.91 | −41.38 | −21.36 | −12.01 |
| 0.5058 | −169.05 | −82.53 | −40.58 | −21.31 | −12.19 |
| 0.6051 | −138.78 | −68.44 | −33.07 | −17.76 | −10.71 |
| 0.6965 | −104.11 | −49.42 | −24.47 | −12.92 | −7.88 |
| 0.7994 | −67.08 | −29.07 | −14.79 | −8.03 | −5.01 |
| 0.9001 | −33.83 | −16.34 | −6.99 | −3.73 | −2.62 |
| 1.0000 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| T (K) | B0 | B1 | B2 | B3 | B4 | σ |
|---|---|---|---|---|---|---|
| 303.15 | −677.1505 | −346.9174 | 532.6040 | 536.1765 | −127.0621 | 0.7056 |
| 313.15 | −331.3255 | −125.2933 | 399.0103 | 17.7388 | −486.4350 | 0.2340 |
| 323.15 | −161.1407 | −80.3842 | 129.4112 | 89.9606 | −44.0274 | 0.2428 |
| 333.15 | −84.6904 | −32.6001 | 94.7237 | 74.1388 | −14.9678 | 0.1724 |
| 343.15 | −49.0278 | −7.6149 | 74.1177 | 39.5426 | −33.6108 | 0.0761 |
| χ1 | T (K) | ||||
|---|---|---|---|---|---|
| 303.15 | 313.15 | 323.15 | 333.15 | 343.15 | |
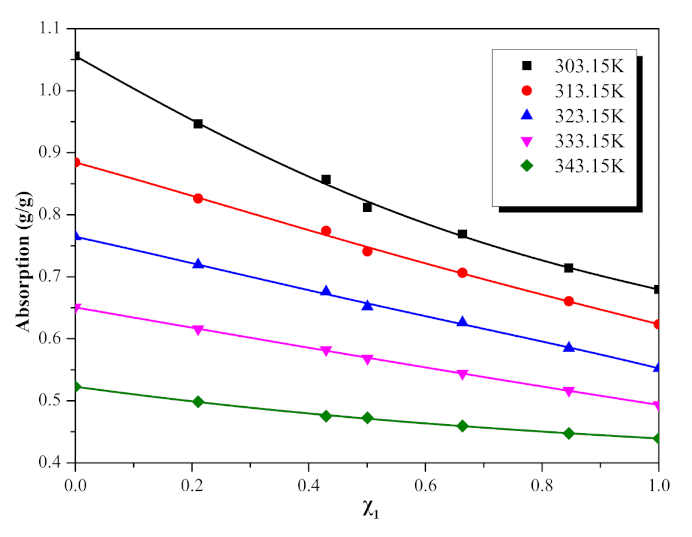
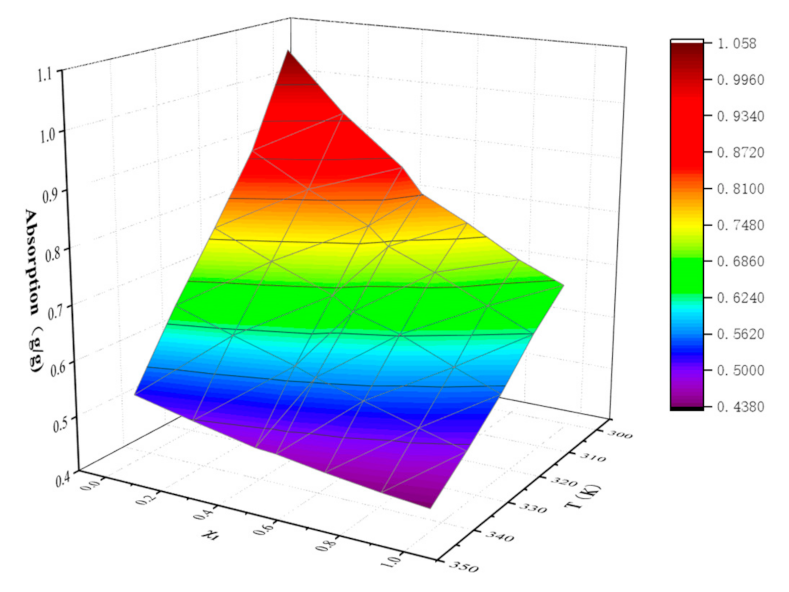
| 0.0000 | 1.0562 | 0.8846 | 0.7645 | 0.6506 | 0.5227 |
| 0.2106 | 0.9465 | 0.8261 | 0.7192 | 0.6159 | 0.4985 |
| 0.4299 | 0.8570 | 0.7739 | 0.6757 | 0.5820 | 0.4753 |
| 0.5006 | 0.8119 | 0.7409 | 0.6518 | 0.5683 | 0.4725 |
| 0.6636 | 0.7687 | 0.7062 | 0.6261 | 0.5440 | 0.4594 |
| 0.8458 | 0.7139 | 0.6605 | 0.5850 | 0.5163 | 0.4473 |
| 1.0000 | 0.6795 | 0.6233 | 0.5525 | 0.4933 | 0.4394 |
| χ1 | C0 | C1 (10−2) | C2 (10−5) | R2 |
|---|---|---|---|---|
| 0.0000 | 11.9481 | −5.622 | 6.6857 | 0.9949 |
| 0.2106 | 5.0106 | −1.549 | 0.6857 | 0.9987 |
| 0.4299 | 0.5809 | 1.016 | −3.05 | 0.9996 |
| 0.5006 | 0.1247 | 1.18 | −3.1429 | 0.9992 |
| 0.6636 | −0.2954 | 1.352 | −3.3 | 0.9991 |
| 0.8458 | 0.9571 | 0.449 | 1.7429 | 0.9971 |
| 1.0000 | 3.7355 | −1.358 | 1.1571 | 0.9971 |
| T(K) | D0 | D1 | D2 | D3 | D4 | R2 |
|---|---|---|---|---|---|---|
| 303.15 | 1.0560 | −0.5343 | 0.0549 | 0.2016 | −0.0987 | 0.9953 |
| 313.15 | 0.8844 | −0.2580 | −0.0812 | 0.1287 | −0.0504 | 0.9941 |
| 323.15 | 0.7645 | −0.2040 | −0.0712 | 0.137 | −0.0740 | 0.9955 |
| 333.15 | 0.6506 | −0.1629 | −0.0040 | 0.0139 | −0.0043 | 0.9995 |
| 343.15 | 0.5228 | −0.1292 | 0.0577 | −0.0096 | −0.0021 | 0.9969 |
| Chemical Names | CAS | Level | Purity c | Final Water Mass Fraction | Source |
|---|---|---|---|---|---|
| TEA | 102-71-6 | Analytical reagent | ≥0.99 | 0.0001 a | Tianjin Hedong District Hongyan reagent plant |
| [EMIM] [TFA] | 174899-65-1 | - | ≥0.99 | 0.00167 b | Homemade |
| 1-Methylimidazole | 616-47-7 | Analytical reagent | ≥0.98 | Shijiazhuang Sydano Fine Chemical Co. Ltd | |
| Silver trifluoroacetate | 2966-50-9 | Analytical reagent | ≥0.99 | Tianjin Hines Biochemical Technology Co. Ltd | |
| Bromoethane | 74-96-4 | Analytical reagent | ≥0.99 | Tianjin Damao chemical reagent plant | |
| NO2 | 10102-44-0 | Analytical reagent | >0.9999 | Qingdao Antaike Gas Co. Ltd | |
| Glycerol | 56-81-5 | Analytical reagent | ≥0.99 | 0.0005 b | Tianjin Yongda Chemical Reagent Co. Ltd |
| Water | 7732-18-5 | - | UPH-1V-10T, Chengdu ultra pure water technology Co. Ltd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Wang, X.; Tian, J.; Zhang, P.; Yang, H.; Jin, N. Experimental Research on NO2 Viscosity and Absorption for (1-Ethyl-3-methylimidazolium Trifluoroacetate + Triethanolamine) Binary Mixtures. Molecules 2021, 26, 6953. https://doi.org/10.3390/molecules26226953
Liu B, Wang X, Tian J, Zhang P, Yang H, Jin N. Experimental Research on NO2 Viscosity and Absorption for (1-Ethyl-3-methylimidazolium Trifluoroacetate + Triethanolamine) Binary Mixtures. Molecules. 2021; 26(22):6953. https://doi.org/10.3390/molecules26226953
Chicago/Turabian StyleLiu, Baoyou, Xinyu Wang, Jie Tian, Peiwen Zhang, Huilong Yang, and Nanxi Jin. 2021. "Experimental Research on NO2 Viscosity and Absorption for (1-Ethyl-3-methylimidazolium Trifluoroacetate + Triethanolamine) Binary Mixtures" Molecules 26, no. 22: 6953. https://doi.org/10.3390/molecules26226953
APA StyleLiu, B., Wang, X., Tian, J., Zhang, P., Yang, H., & Jin, N. (2021). Experimental Research on NO2 Viscosity and Absorption for (1-Ethyl-3-methylimidazolium Trifluoroacetate + Triethanolamine) Binary Mixtures. Molecules, 26(22), 6953. https://doi.org/10.3390/molecules26226953





