Light-Induced Reactions within Poly(4-vinyl pyridine)/Pyridine Gels: The 1,6-Polyazaacetylene Oligomers Formation
Abstract
:1. Introduction
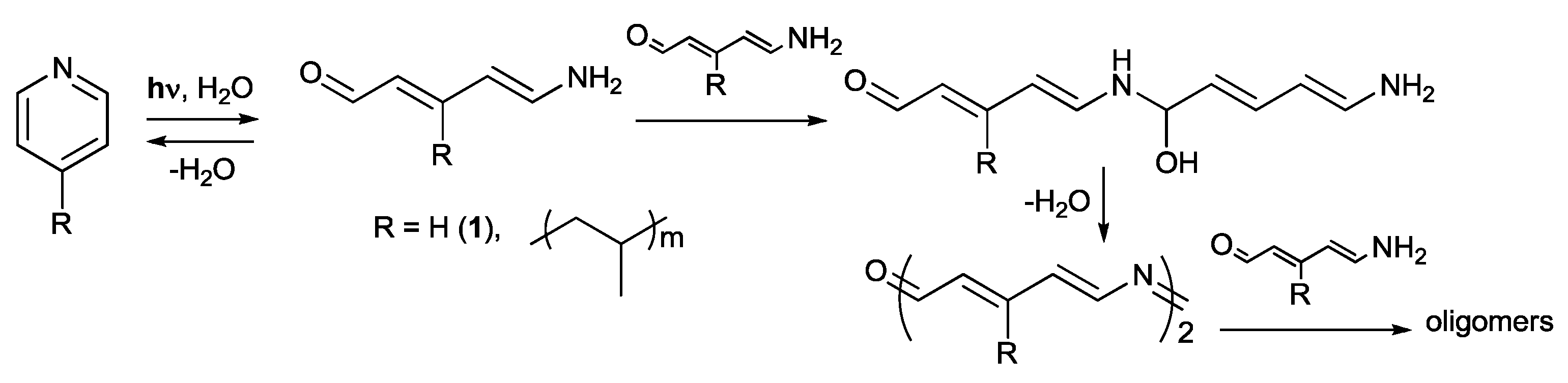
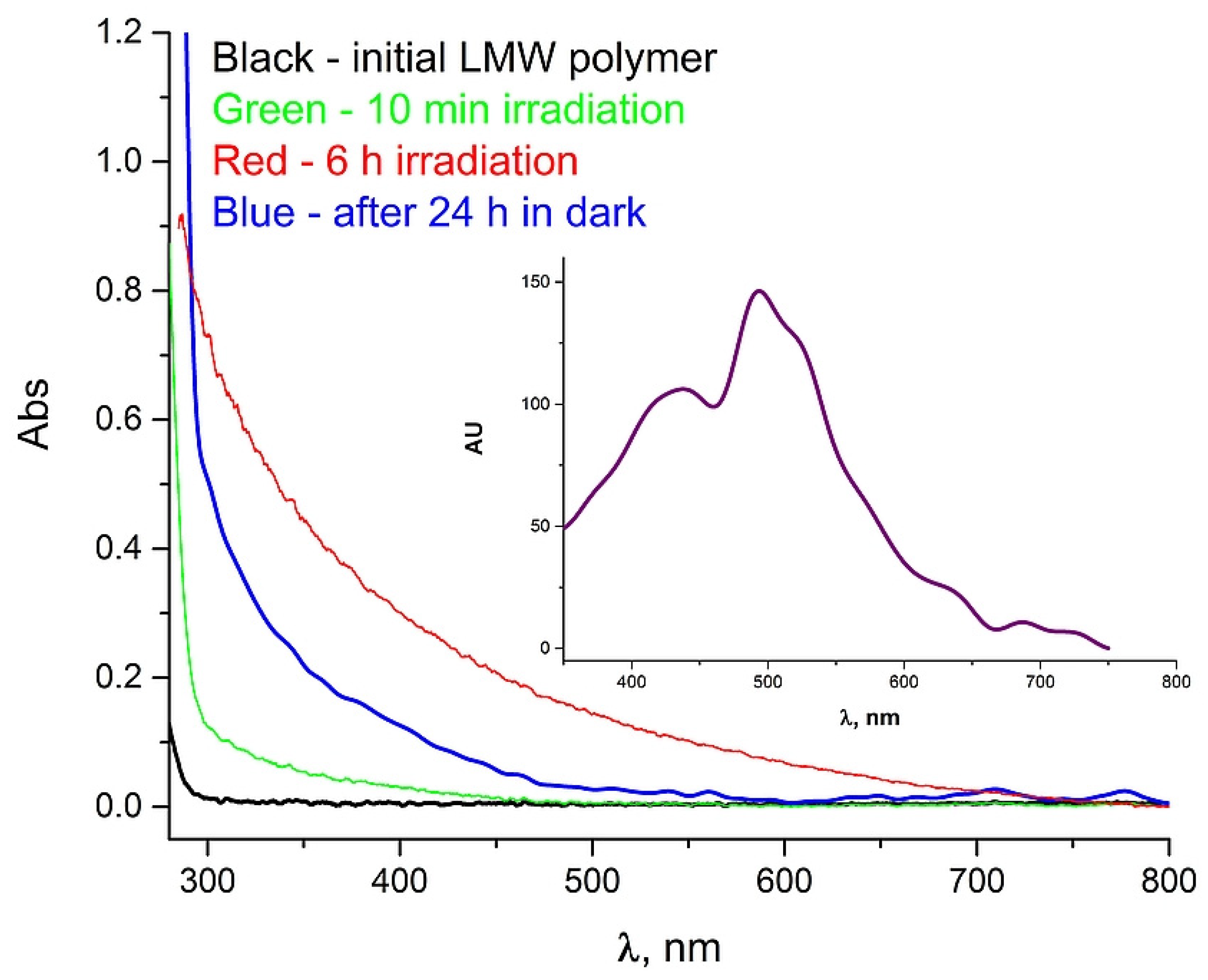
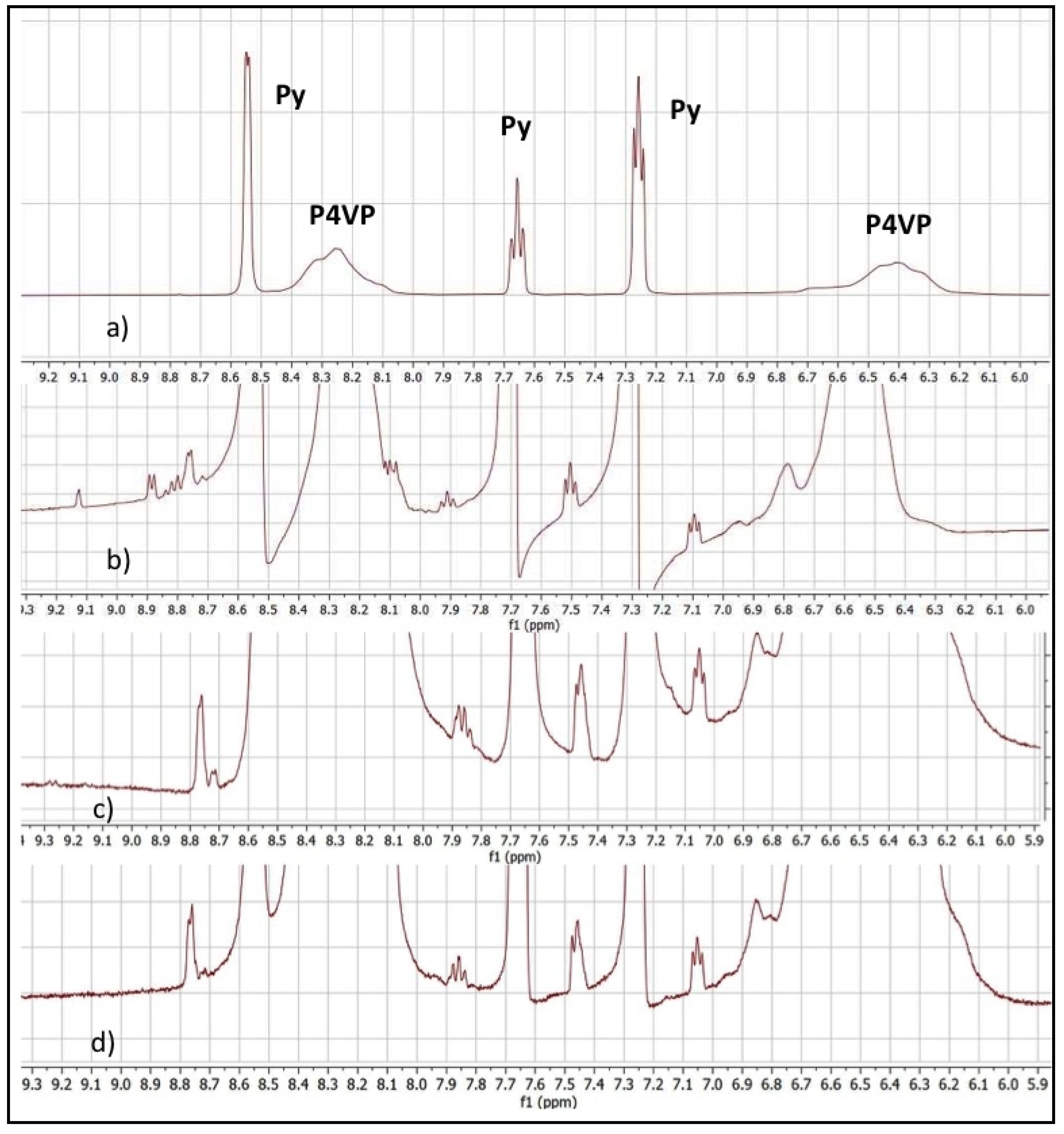
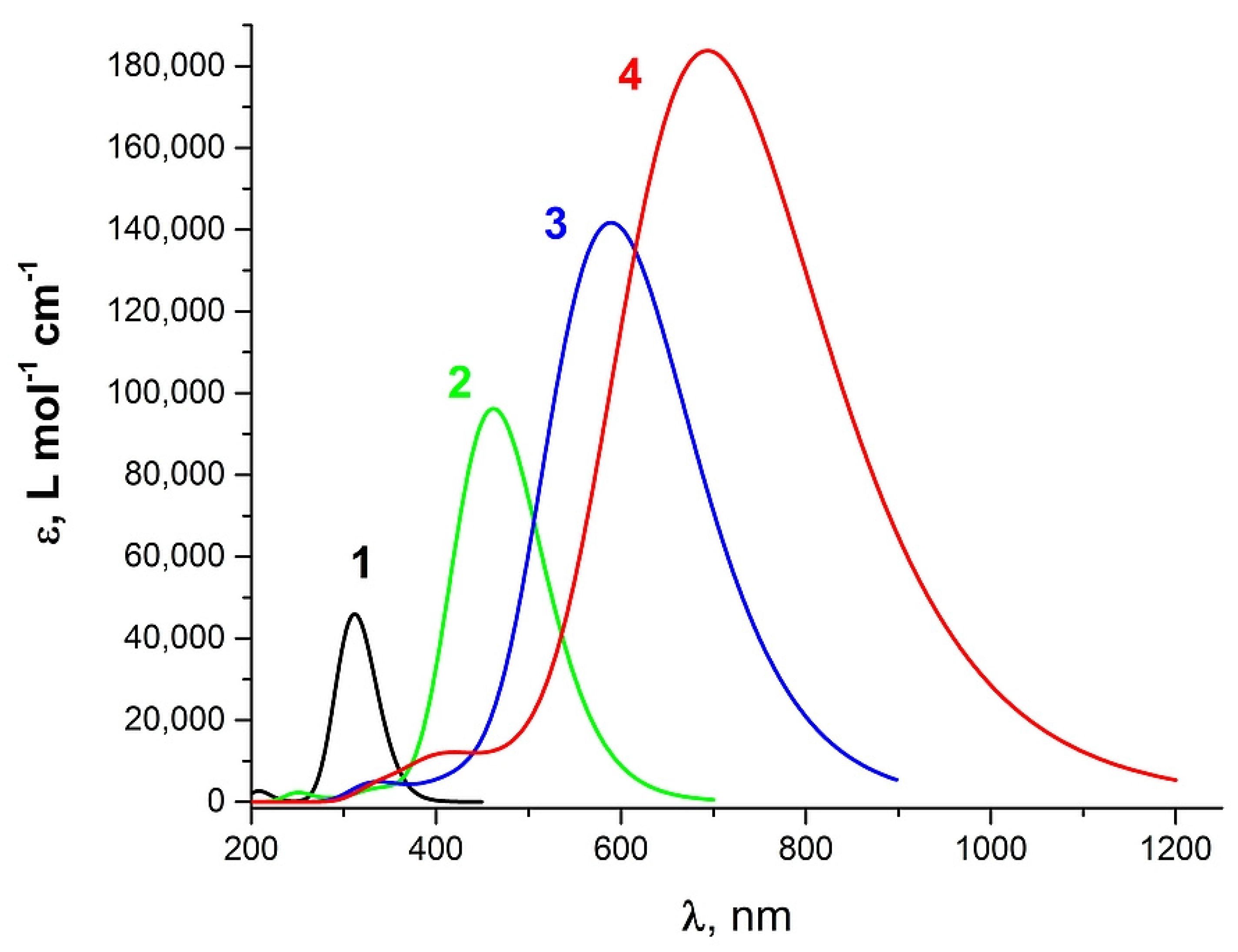
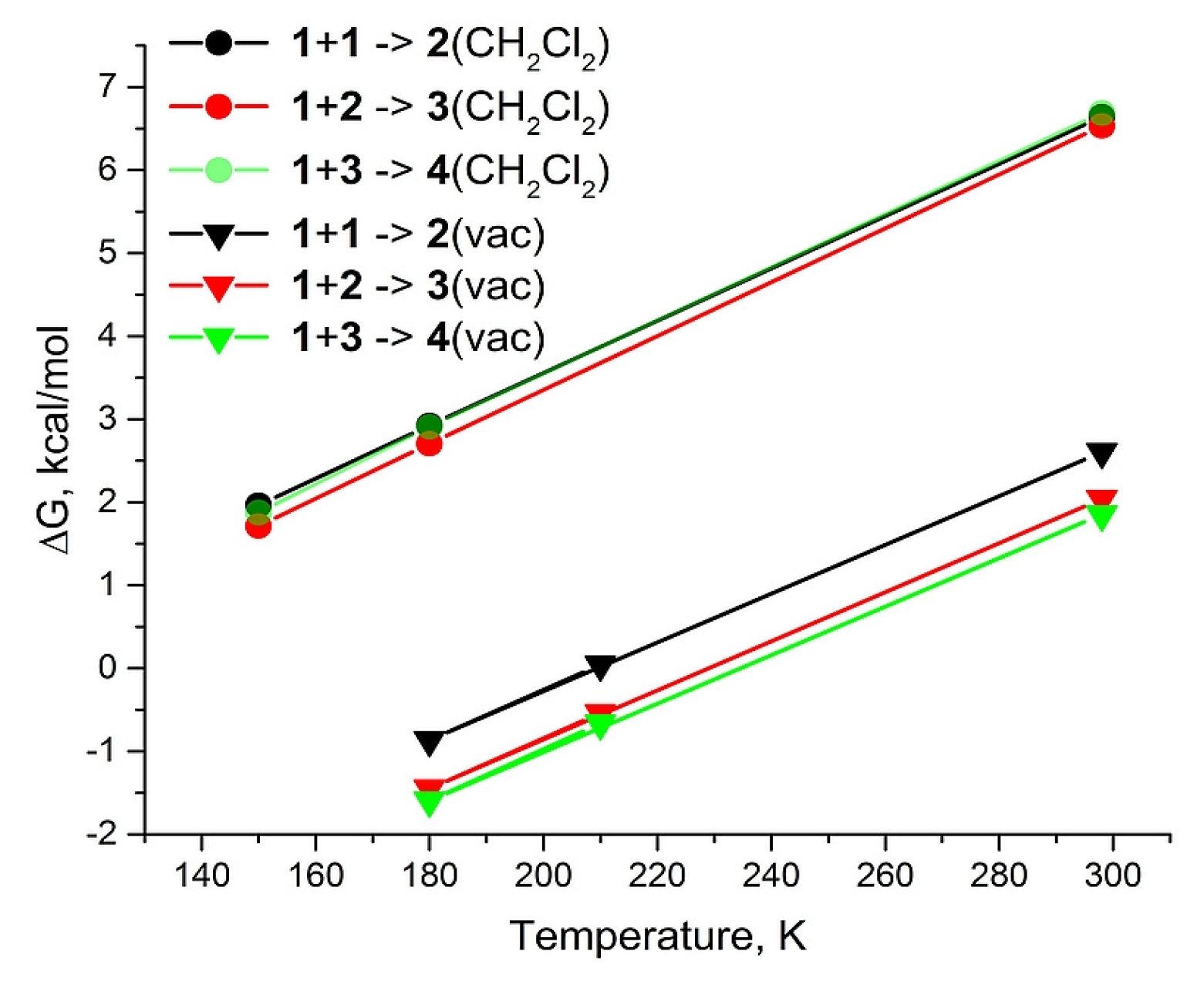
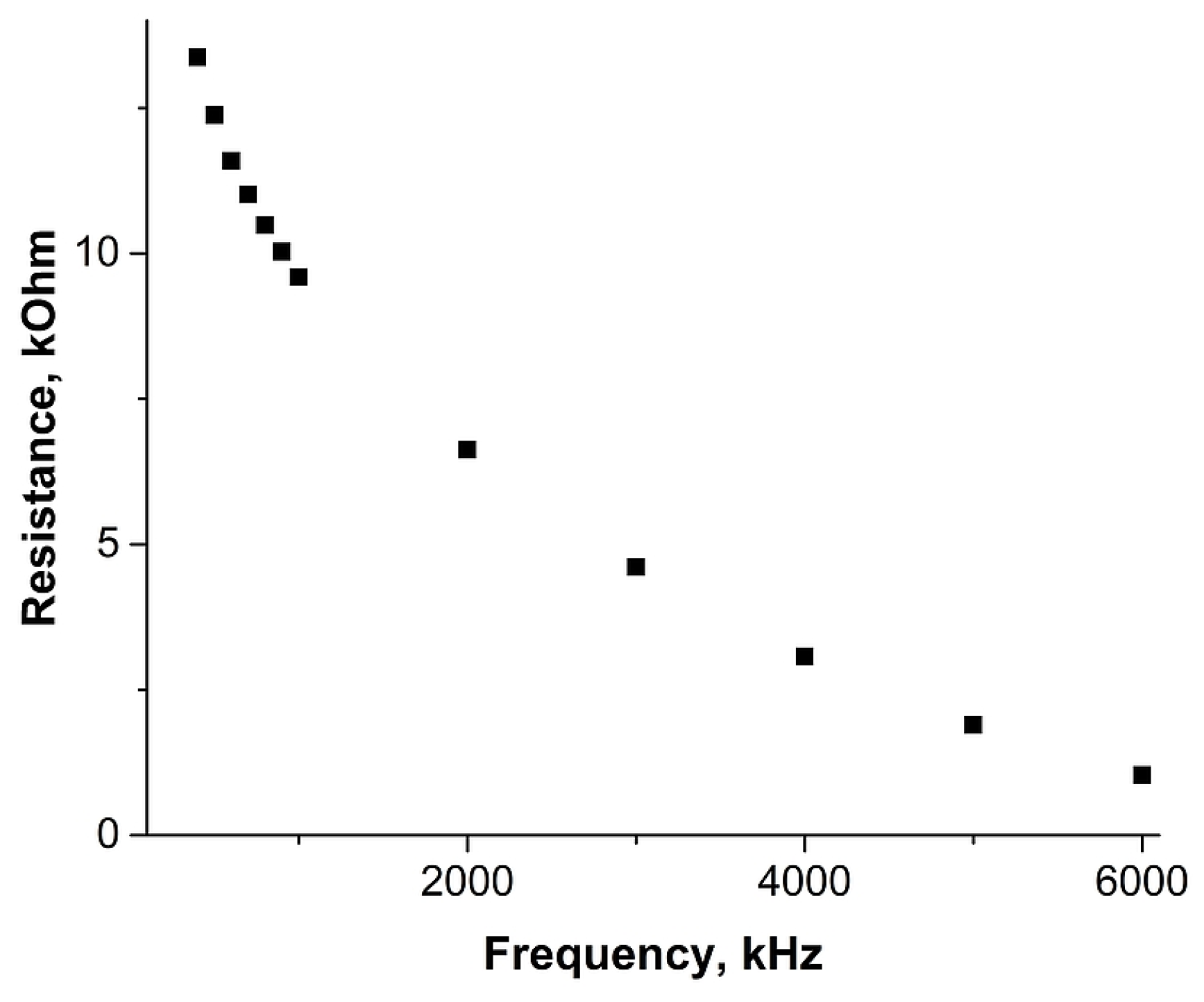
2. Results and Discussion
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bryce-Smith, D.; Gilbert, A. The Organic Photochemistry of Benzene-I. Tetrahedron 1976, 32, 1309–1326. [Google Scholar] [CrossRef]
- Pavlik, J.W. Photoisomerization of Some Nitrogen-Containing Hetero-Aromatic Compounds. In CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; Horspool, W., Lenci, F., Eds.; CRC Press LLC: Boca Raton, FL, USA, 2004; Volume 97, pp. 1–22. [Google Scholar]
- D’Auria, M. Photochemical Isomerization of Hexatomic Heterocyclic Compounds. Curr. Org. Chem. 2021, 25, 1659–1685. [Google Scholar] [CrossRef]
- Freytag, H.; Neudert, W. Einwirkung ultravioletter Strahlen auf Pyridin. (I. Mitteilung). Ein neuer Nachweis einiger primärer aromatischer Amine und des Pyridins. J. Prakt. Chem. 1932, 135, 15–35. [Google Scholar] [CrossRef]
- Joussot-Dubien, J.; Houdard, J. Reversible photolysis of pyridine in aqueous solution. Tetrahedron Lett. 1967, 8, 4389–4391. [Google Scholar] [CrossRef]
- Joussot-Dubien, J.; Houdard-Pereyre, J. Photolyse de la pyridine en solution aqueuse. Bull. Soc. Chim. Fr. 1969, 8, 2619–2623. [Google Scholar]
- Wilzbach, K.E.; Rausch, D.J. Photochemistry of nitrogen heterocycles. Dewar pyridine and its intermediacy in photoreduction and photohydration of pyridine. J. Am. Chem. Soc. 1970, 92, 2178–2179. [Google Scholar] [CrossRef]
- Caplain, S.; Castellano, A.; Catteau, J.-P.; Lablache-Combier, A. Etudes Photochimiques-VI. Mecanisme de la photosubstitution de la pyridine en solution. Tetrahedron 1971, 27, 3541–3553. [Google Scholar] [CrossRef]
- Andre, J.C.; Niclause, M.; Joussot-Dubien, J.; Deglise, X.J. Photohydration of pyridine in aqueous solution. An undergraduate experiment in photochemical kinetics. J. Chem. Educ. 1977, 54, 387–388. [Google Scholar] [CrossRef]
- Reinehr, D.; Winkler, T. (E,E)-5-Amino-2,4-pentadienal: The first preparative synthesis of hydrolyzed pyridine. Angew. Chem. Int. Ed. Engl. 1981, 20, 881–882. [Google Scholar] [CrossRef]
- Vaganova, E.; Damm, C.; Israel, G.; Yitzchaik, S. Photoinduced pyridine cleavage-closure in viscous polymer solutions. J. Fluoresc. 2002, 12, 219–224. [Google Scholar] [CrossRef]
- Vaganova, E.; Dubnikov, F.; Kesselman, E.; Lokshin, V.; Khodorkovsky, V. What can be learned from the polymerization of a pyridine-based two-component system. Macromol. Symp. 2019, 385, 1800157. [Google Scholar] [CrossRef]
- Avadanei, M.I.; Barboiu, V.; Luca, C. Contributions to the photochemistry of poly(4-vinylpyridine) and its ionic derivatives in the presence of water. J. Photochem. Photobiol. A Chem. 2009, 207, 260–267. [Google Scholar] [CrossRef]
- Berestetsky, N.; Vaganova, E.; Wachtel, E.; Leitus, G.; Goldberg, A.; Yitzchaik, S. Photoactive proton conductor: Poly(4-vinyl pyridine) gel. J. Phys. Chem. B 2008, 112, 3662–3667. [Google Scholar] [CrossRef] [PubMed]
- Vaganova, E.; Yitzchaik, S.; Shapiro, L.; Sigalov, M.; Khodorkhovsky, V. Photo-induced reversible emission switching in pyridine/Europium(III) chloride/organic fluorophore system. Adv. Mater. 2000, 12, 1669–1671. [Google Scholar] [CrossRef]
- Vaganova, E.; Wachtel, E.; Goldberg, A.; Yitzchaik, S. White Light and Heat Sensitivity in a Pyridine-Based Polymer Blend. J. Phys. Chem. C 2012, 116, 25028–25033. [Google Scholar] [CrossRef]
- Vaganova, E.; Yitzchaik, S. Tunable emission in poly(4-vinylpyridine)-based gel. Acta Polym. 1998, 49, 636–641. [Google Scholar] [CrossRef]
- Kuhn, R.; Teller, E. Azapolyenaldehyde. Ann. Chem. 1968, 715, 106–121. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Petersson, G.; Nakatsuji, H. Gaussian 16, Revision A03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Khodorkovsky, V.; Becker, J.Y. Molecular Design of Organic Conductors. In Organic Conductors: Fundamentals and Applications; Farges, J., Ed.; Marcel Dekker: New York, NY, USA, 1994; Chapter III; pp. 75–114. [Google Scholar]
- Cai, Z.-L.; Reimers, J.R. The first singlet (n,π*) and (π,π*) excited states of the hydrogen-bonded complex between water and pyridine. J. Phys. Chem. A 2002, 106, 8769–8778. [Google Scholar] [CrossRef]
- Sawada, H. Thermodynamics of Polymerization; Marcel Dekker Inc.: New York, NY, USA, 1976; p. 403. [Google Scholar]
- Odian, J. Principles of Polymerization; Wiley-Interscience: Hoboken, NJ, USA, 2004; p. 812. [Google Scholar]



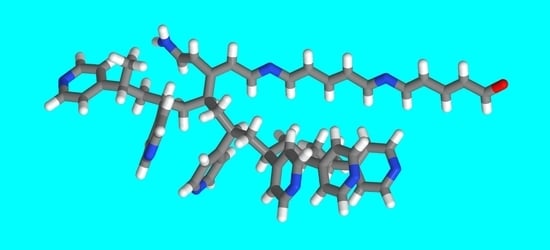
| Structure | IP (eV) | EA (eV) |
|---|---|---|
| 1 | 7.39 | 0.26 |
| 2 | 6.60 | 1.21 |
| 3 | 6.23 | 1.84 |
| 4 | 6.01 | 2.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaganova, E.; Eliaz, D.; Shimanovich, U.; Leitus, G.; Aqad, E.; Lokshin, V.; Khodorkovsky, V. Light-Induced Reactions within Poly(4-vinyl pyridine)/Pyridine Gels: The 1,6-Polyazaacetylene Oligomers Formation. Molecules 2021, 26, 6925. https://doi.org/10.3390/molecules26226925
Vaganova E, Eliaz D, Shimanovich U, Leitus G, Aqad E, Lokshin V, Khodorkovsky V. Light-Induced Reactions within Poly(4-vinyl pyridine)/Pyridine Gels: The 1,6-Polyazaacetylene Oligomers Formation. Molecules. 2021; 26(22):6925. https://doi.org/10.3390/molecules26226925
Chicago/Turabian StyleVaganova, Evgenia, Dror Eliaz, Ulyana Shimanovich, Gregory Leitus, Emad Aqad, Vladimir Lokshin, and Vladimir Khodorkovsky. 2021. "Light-Induced Reactions within Poly(4-vinyl pyridine)/Pyridine Gels: The 1,6-Polyazaacetylene Oligomers Formation" Molecules 26, no. 22: 6925. https://doi.org/10.3390/molecules26226925
APA StyleVaganova, E., Eliaz, D., Shimanovich, U., Leitus, G., Aqad, E., Lokshin, V., & Khodorkovsky, V. (2021). Light-Induced Reactions within Poly(4-vinyl pyridine)/Pyridine Gels: The 1,6-Polyazaacetylene Oligomers Formation. Molecules, 26(22), 6925. https://doi.org/10.3390/molecules26226925