Iturin A Induces Resistance and Improves the Quality and Safety of Harvested Cherry Tomato
Abstract
:1. Introduction
2. Results
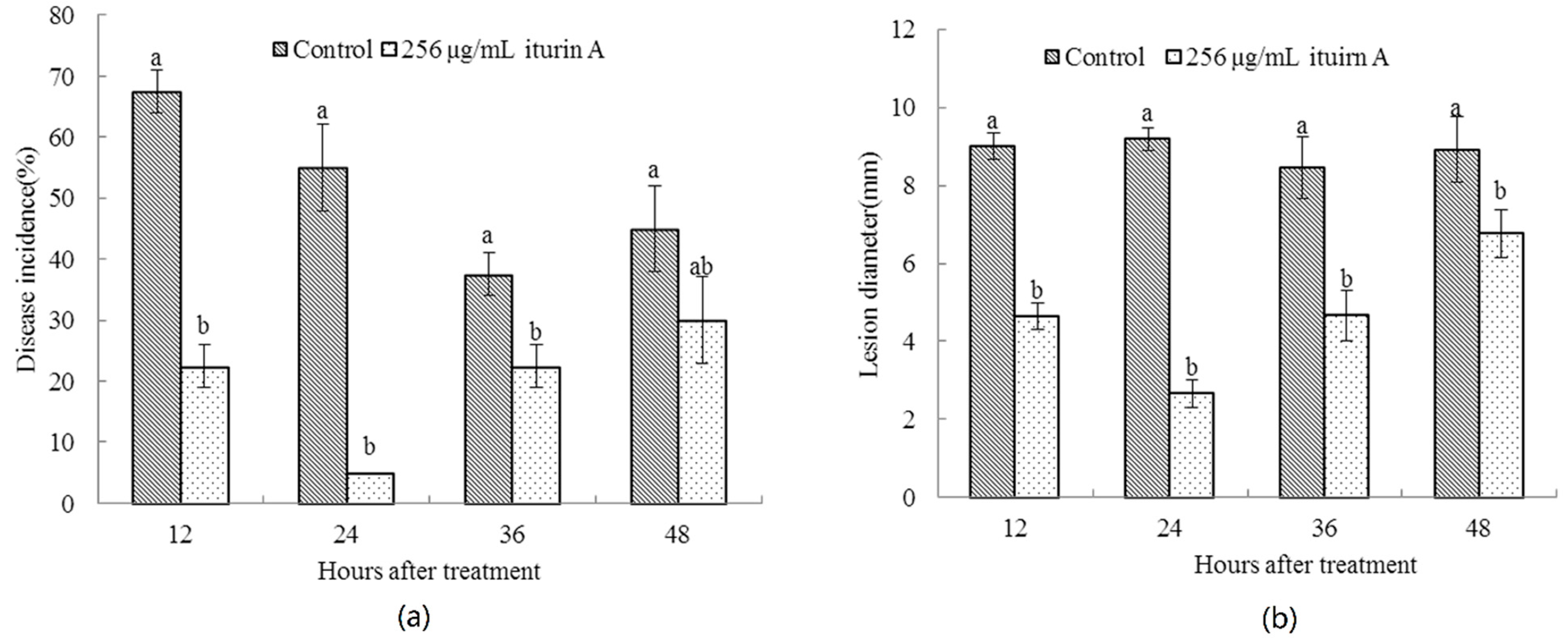
2.1. Effects of Treatment Time of Iturin A on the Disease Incidence of Cherry Tomato Fruit
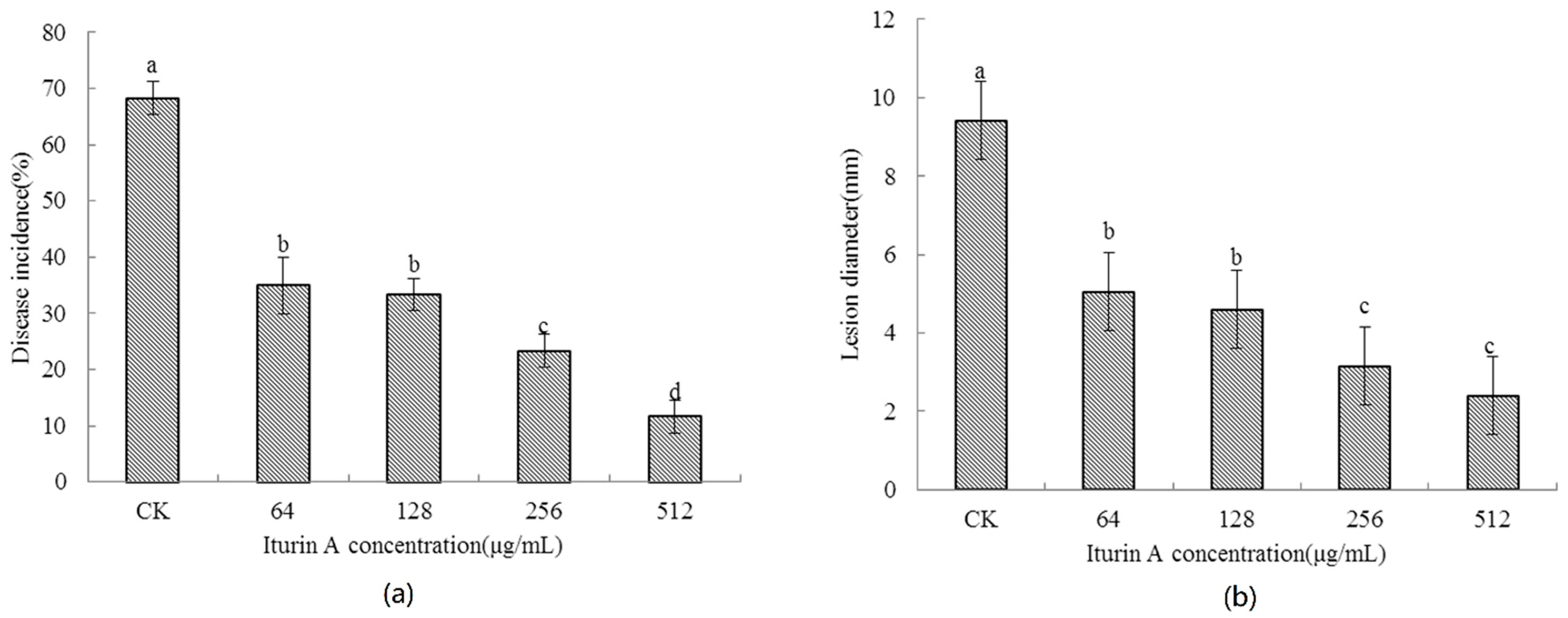
2.2. Effects of Concentration of Iturin A on the Disease Occurrence of Cherry Tomato Fruit
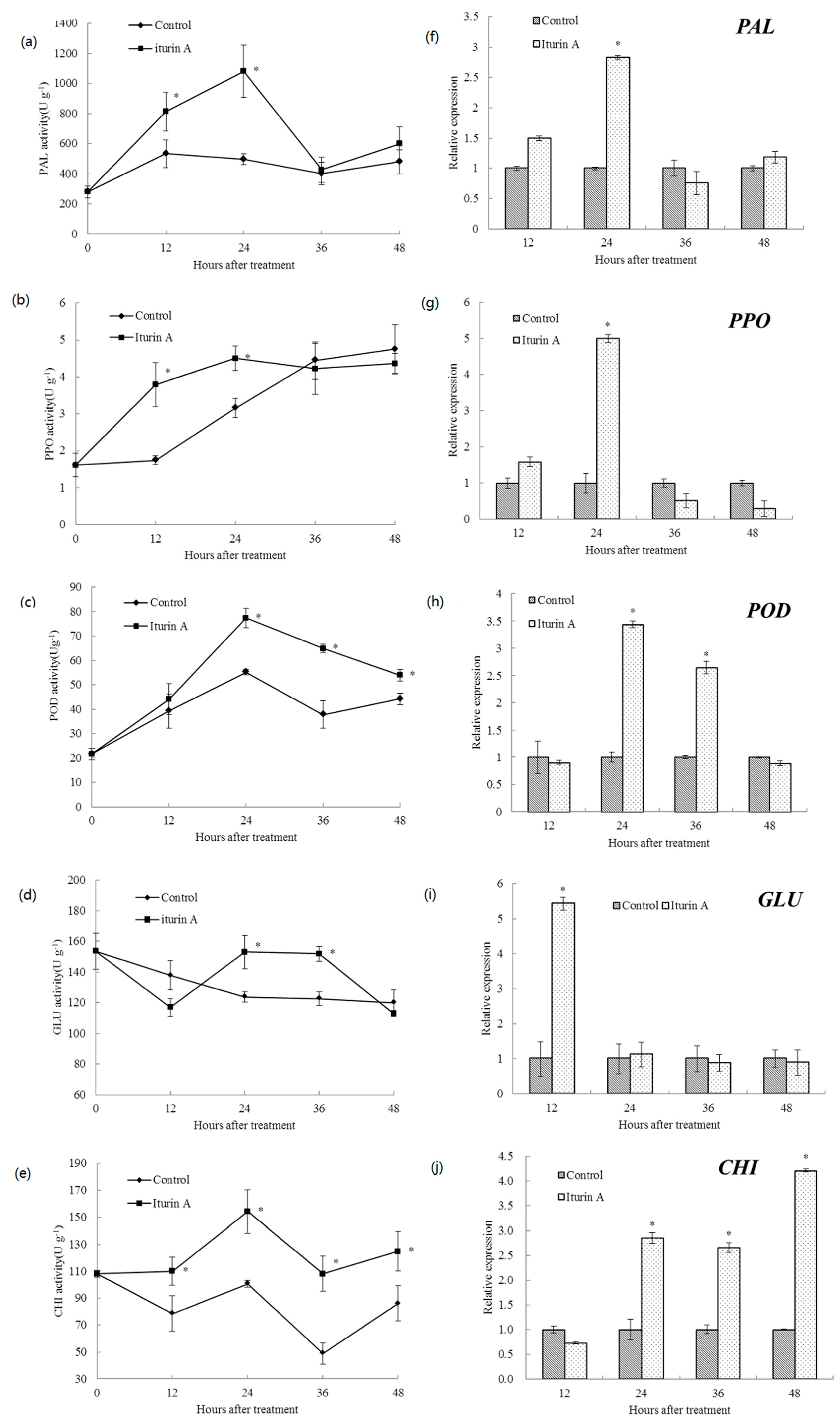
2.3. Effects of Iturin A on Defense-Related Enzyme Activities and Relevant Gene Expression in Cherry Tomato
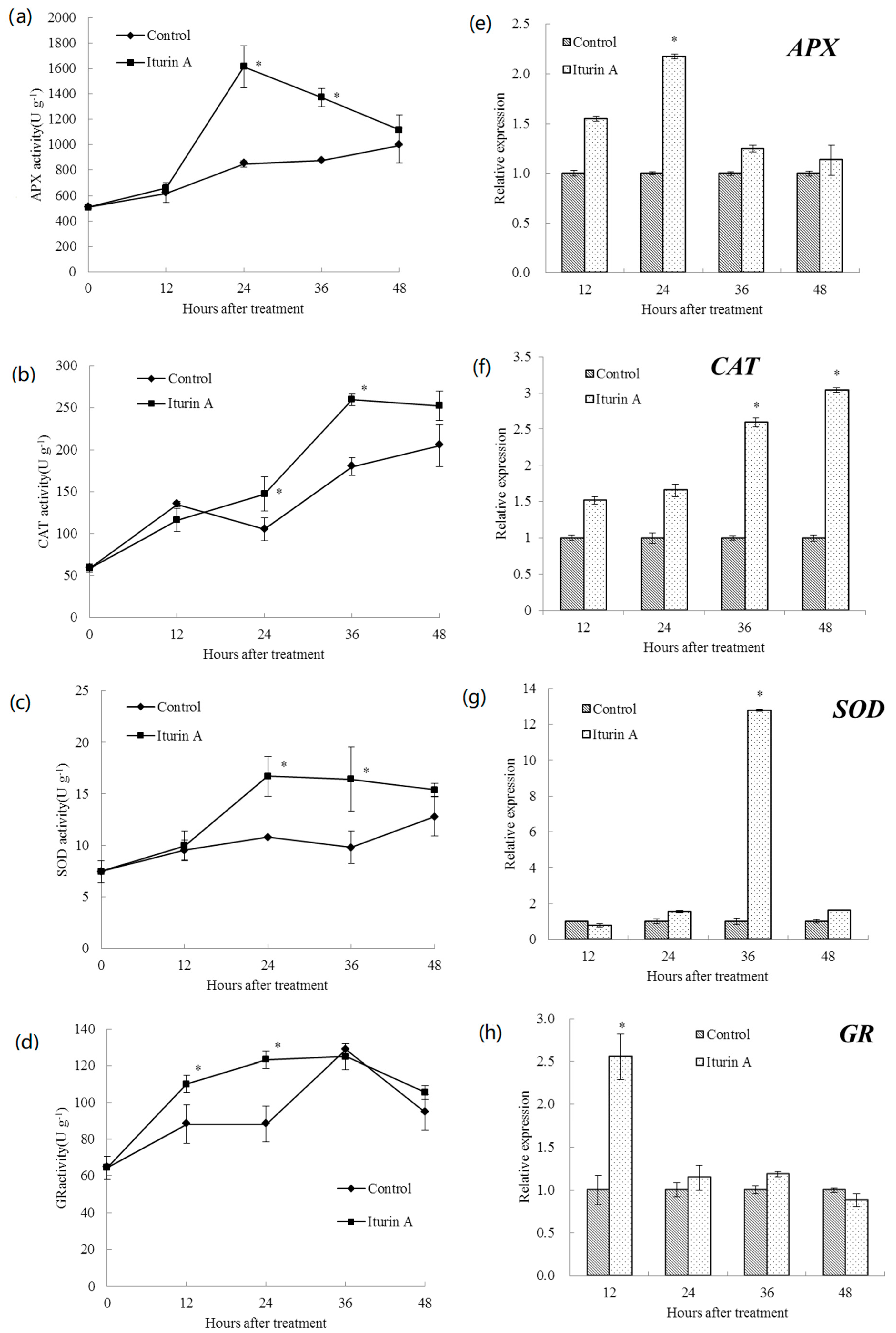
2.4. Effects of Iturin A on Antioxidative Enzyme Activities and Relevant Gene Expression in Cherry Tomato
2.5. Effects of Iturin A on the Quality of Cherry Tomato Fruit
3. Discussion
4. Materials and Methods
4.1. Fruit Material
4.2. Preparation of Spore Suspension
4.3. Production of Iturin A
4.4. Effects of Iturin A Treatment Time on Induction of Resistance against R. stolonifer in Cherry Tomato Fruit
4.5. Effects of Iturin A with Different Concentrations on the Induction of Resistance against R. stolonifer in Cherry Tomato Fruit
4.6. Assay of Enzyme Activity
4.6.1. Treatment
4.6.2. Measurement of Enzyme Activity
4.7. Analysis of Gene Expression by Real-Time Quantitative PCR
4.8. Determination of Quality Index
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dukare, A.S.; Paul, S.; Nambi, V.E.; Gupta, R.K.; Singh, R.; Sharma, K.; Vishwakarma, R.K. Exploitation of microbial antagonists for the control of postharvest diseases of fruits: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1498–1513. [Google Scholar] [CrossRef]
- Hussain, M.; Hamid, M.I.; Ghazanfar, M.U. Salicylic acid induced resistance in fruits to combat against postharvest pathogens: A review. Arch. Phytopathol. Plant Protect. 2015, 48, 34–42. [Google Scholar] [CrossRef]
- Wang, F.; Deng, J.; Jiao, J.; Lu, Y.; Yang, L.; Shi, Z. The combined effects of Carboxymethyl chitosan and Cryptococcus laurentii treatment on postharvest blue mold caused by Penicillium italicum in grapefruit fruit. Sci. Hortic. 2019, 253, 35–41. [Google Scholar] [CrossRef]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-based resistance inducers for sustainable plant protection against pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Enebe, M.C.; Babalola, O.O. The impact of microbes in the orchestration of plants’s resistance to biotic stress: A disease management approach. Appl. Microbiol. Biotechnol. 2019, 103, 9–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, P.N.; Swapna, T.H.; Khan, M.Y.; Reddy, G.; Hameeda, B. Statistical optimization of antifungal iturin A production from Bacillus amyloliquefaciens RHNK22 using agro-industrial wastes. Saudi J. Biol. Sci. 2017, 24, 1722–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meena, K.R.; Kanwar, S.S. Lipopeptides as the Antifungal and Antibacterial Agents: Applications in Food Safety and Therapeutics. Biomed. Res. Int. 2015, 473050. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.S.; Ano, T.; Shoda, M. Biofilm fermentation of iturin A by a recombinant strain of Bacillus subtilis 168. J. Biotechnol. 2007, 127, 503–507. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, T.S. Toxicity and characteristics of antifungal substances produced by Bacillus amyloliquefaciens IUB158-03. J. Life Sci. 2009, 19, 1672–1678. [Google Scholar]
- Zhang, S.M.; Wang, Y.X.; Meng, L.Q.; Li, J.; Zhao, X.Y.; Cao, X.; Chen, X.L.; Wang, A.X.; Li, F. Isolation and characterization of antifungal lipopeptides produced by ndophytic Bacillus amyloliquefaciens TF28. Afr. J. Microbiol. Res. 2012, 6, 1747–1755. [Google Scholar]
- Dey, G.; Bharti, R.; Banerjee, I.; Das, A.K.; Das, C.K.; Das, S.; Jena, B.C.; Misra, M.; Sen, R.; Mandal, M. Pre-clinical risk assessment and therapeutic potential of antitumor ipopeptide ‘Iturin A’ in an in vivo and in vitro model. RSC Adv. 2016, 6, 71612–71623. [Google Scholar] [CrossRef]
- Cao, Y.; Cheng, W.; Huang, D.; Zheng, L.; Cai, M.; Lin, D.; Yu, Z.; Zhang, J. 2017 reparation characterization of iturin a microcapsules in sodium alginate/Poly(γglutamic acid) by spray drying. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 479–484. [Google Scholar]
- Kawagoe, Y.; Shiraishi, S.; Kondo, H.; Yamamoto, S.; Aoki, Y.; Suzuki, S. Cyclic lipopeptide iturin A structure-dependently induces defense response in Arabidopsis plants by activating SA and JA signaling pathways. Biochem. Biophy. Res. Commun. 2015, 460, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Shiraishi, S.; Suzuki, S. Are cyclic lipopeptides produced by Bacillus amyloliquefaciens S13-3 responsible for the plant defence response in strawberry against Colletotrichum gloeosporioides? Lett. Appl. Microbiol. 2015, 60, 79–386. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Zhang, Y.; Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Quek, S.Y.; Yao, W. Antifungal effects of thymol and salicylic acid on cell membrane and mitochondria of Rhizopus stolonifer and their application in postharvest preservation of tomatoes. Food Chem. 2019, 285, 380–388. [Google Scholar] [CrossRef]
- Alfaro-Sifuentes, L.; Juan, M.; Troncoso-Rojas, R.; Meca, D.E.; Elorrieta, M.A.; Valenzuela, J.L. Effectiveness of chemical and thermal treatments on control Rhizopus stolonifer fruit infection comparing tomato cultivars with different sensitivities to cracking. Int. J. Environ. Res. Public Health 2019, 16, 2754. [Google Scholar] [CrossRef] [Green Version]
- Kwon, J.H.; Kang, S.W.; Kim, J.S.; Park, C.S. Rhizopus Soft Rot on Cherry Tomato Caused by Rhizopus stolonifer in Korea. Mycobiology 2001, 29, 176–178. [Google Scholar] [CrossRef]
- Leclère, V.; Béchet, M.; Adam, A.; Guez, J.S.; Wathelet, B.; Ongena, M.; Thonart, P.; Gancel, F.; Chollet-Imbert, M.; Jacques, P. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl. Environ. Microbiol. 2005, 71, 4577–4584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanazzi, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martínez, P.G.; Alkan, N. Induced resistance to control postharvest decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Farzand, A.; Moosa, A.; Zubair, M.; Khan, A.R.; Massawe, V.C.; Tahir HA, S.; Sheikh, T.M.M.; Ayaz, M.; Gao, X. Suppression of Sclerotinia sclerotiorum by the Induction of Systemic Resistance and Regulation of Antioxidant Pathways in Tomato Using Fengycin Produced by Bacillus amyloliquefaciens FZB42. Biomolecules 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Arrebola, E.; Jacobs, R.; Korsten, L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 2010, 108, 386–395. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, K.; Wang, G. Combination of the biocontrol yeast Cryptococcus laurentii with UV-C treatment for control of postharvest diseases of tomato fruit. BioControl 2013, 58, 269–281. [Google Scholar] [CrossRef]
- Ballester, A.R.; Izquierdo, A.; Lafuente, M.T.; González-Candelas, L. Biochemical and molecular characterization of induced resistance against Penicillium digitatum in citrus fruit. Postharvest Biol. Technol. 2010, 56, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Constabel, C.P.; Bergey, D.R.; Ryan, C.A. Systemin activates synthesis of wound-inducible tomato leaf polyphenol oxidase via the octadecanoid defense signaling pathway. Proc. Natl. Acad. Sci. USA 1995, 92, 407–411. [Google Scholar] [CrossRef] [Green Version]
- Brisson, L.F.; Tenhaken, R.; Lamb, C. Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 1994, 6, 1703–1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, T.P.; Dias, R.O.; Silva-Filho, M.C. Defense-related proteins involved in sugarcane responses to biotic stress. Genet. Mol. Biol. 2017, 40, 360–372. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Xue, Y.; Huang, Z.; Jiang, M.; Lu, F.; Bie, X.; Song, M.; Lu, Z. Bacillomycin D inhibits growth of Rhizopus stolonifer and induces defense-related mechanism in cherry tomato. Appl. Microbiol. Biotechnol. 2019, 103, 7663–7674. [Google Scholar] [CrossRef]
- Waewthongrak, W.; Pisuchpen, S.; Leelasuphakul, W. Effect of Bacillus subtilis and chitosan applications on green mold (Penicilium digitatum Sacc.) decay in citrus fruit. Postharvest Biol. Technol. 2015, 99, 44–49. [Google Scholar] [CrossRef]
- Han, Q.; Wu, F.; Wang, X.; Qi, H.; Shi, L.; Ren, A.; Liu, Q.; Zhao, M.; Tang, C. The bacterial lipopeptide iturins induce Verticillium dahliae cell death by affecting fungal signalling pathways and mediate plant defence responses involved in pathogen-associated molecular pattern-triggered immunity. Environ. Microbiol. 2015, 17, 1166–1188. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Duan, X.; Yang, J.; Beeching, J.R.; Zhang, P. Enhanced reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol. 2013, 161, 1517–1528. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Yousuf, P.Y.; Hakeem KU, R.; Chandna, R.; Ahmad, P. Role of glutathione reductase in plant abiotic stress. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 149–158. [Google Scholar]
- Toral, L.; Rodríguez, M.; Bejar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Vilcinskas, A.; Rahnamaeian, M. Cooperative interaction of antimicrobial peptides with the interrelated immune pathways in plants. Mol. Plant Pathol. 2016, 17, 464–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, M.S.; Sivakumar, D.; Korsten, L. Effect of biocontrol agent Bacillus amyloliquefaciens and 1-methyl cyclopropene on the control of postharvest diseases and maintenance of fruit quality. Crop. Prot. 2011, 30, 173–178. [Google Scholar] [CrossRef]
- Wang, L.; Ning, T.; Chen, X. Postharvest storage quality of citrus fruit treated with a liquid ferment of Chinese herbs and probiotics. Sci. Hortic. 2019, 5, 169–174. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Zhu, X.R.; Li, Y.B. Postharvest control of litchi fruit rot by Bacillus subtilis. LWT 2001, 34, 430–436. [Google Scholar] [CrossRef]
- Wan, C.; Fan, X.; Lou, Z.; Wang, H.; Olatunde, A.; Rengasamy, K.R. Iturin: Cyclic lipopeptide with multifunction biological potential. Crit. Rev. Food Sci. Nutr. 2021, 1–13. [Google Scholar]
- Aghdam, M.S.; Asghari, M.; Farmani, B.; Mohayeji, M.; Moradbeygi, H. Impact of postharvest brassinosteroids treatment on PAL activity in tomato fruit in response to chilling stress. Sci. Hortic. 2012, 144, 116–120. [Google Scholar] [CrossRef]
- Liu, J.; Tian, S.; Meng, X.; Xu, Y. Effects of chitosan on control of postharvest diseases and physiological responses of tomato fruit. Postharvest Biol. Technol. 2007, 44, 300–306. [Google Scholar] [CrossRef]
- Zheng, Y.; Sheng, J.; Zhao, R.; Zhang, J.; Lv, S.; Liu, L.; Shen, L. Preharvest l-Arginine Treatment Induced Postharvest Disease Resistance to Botrysis cinerea in Tomato Fruits. J. Agric. Food Chem. 2011, 59, 6543–6549. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.; Schofield, A.; Paliyath, G. Changes in antioxidant enzyme activities during tomato fruit development. Physiol. Mol. Biol. Plants 2002, 8, 241–249. [Google Scholar]
- Imahori, Y.; Bai, J.; Baldwin, E. Antioxidative responses of ripe tomato fruit to postharvest chilling and heating treatments. Sci. Hortic. 2016, 198, 398–406. [Google Scholar] [CrossRef]
- Yao, W.; Xu, T.; Farooq, S.U.; Jin, P.; Zheng, Y. Glycine betaine treatment alleviates chilling injury in zucchini fruit (Cucurbita pepo L.) by modulating antioxidant enzymes and membrane fatty acid metabolism. Postharvest Biol. Technol. 2018, 144, 20–28. [Google Scholar] [CrossRef]
- Asadi Karam, E.; Keramat, B.; Asrar, Z.; Mozafari, H. Study of interaction effect between triacontanol and nitric oxide on alleviating of oxidative stress arsenic toxicity in coriander seedlings. J. Plant Interact. 2017, 12, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.; Zhou, T.; Zheng, X.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 9–37. [Google Scholar] [CrossRef]
| Treatment | Weight Loss Rate (%) | Firmness (N) | TA (%) | TSS (%) |
|---|---|---|---|---|
| 0 day | 0 | 1.46 ± 0.10 | 2.47 ± 0.22 | 6.23 ± 0.28 |
| 3 day control | 0.18 ± 0.01 | 1.06 ± 0.05 | 1.77 ± 0.19 | 6.03 ± 0.21 |
| 512 μg/mL iturin A | 0.21 ± 0.03 | 1.29 ± 0.09 * | 1.84 ± 0.02 | 5.38 ± 0.54 |
| 6 day control | 1.19 ± 0.17 | 0.80 ± 0.09 | 1.77 ± 0.05 | 4.80 ± 0.42 |
| 512 μg/mL iturin A | 0.43 ± 0.01 * | 1.02 ± 0.10 * | 1.79 ± 0.22 | 4.67 ± 0.23 |
| 9 day control | 4.29 ± 0.50 | 0.73 ± 0.09 | 1.85 ± 0.24 | 5.43 ± 0.35 |
| 512 μg/mL iturin A | 0.49 ± 0.10 * | 0.97 ± 0.08 * | 1.64 ± 0.17 | 4.90 ± 0.10 |
| 12 day control | 6.50 ± 0.93 | 0.68 ± 0.06 | 1.99 ± 0.07 | 5.48 ± 0.26 |
| 512 μg/mL iturin A | 0.69 ± 0.12 * | 0.89 ± 0.07 * | 1.66 ± 0.07 * | 4.63 ± 0.26 * |
| 15 day control | 8.91 ± 0.35 | 0.58 ± 0.07 | 1.94 ± 0.02 | 5.37 ± 0.15 |
| 512 μg/mL iturin A | 0.81 ± 0.12 * | 0.77 ± 0.07 * | 1.48 ± 0.09 * | 4.80 ± 0.10 * |
| Gene | GeneBankNumber | Primer Sequence (5′→3′) | Product Size |
|---|---|---|---|
| Actin | AB199316.1 | Forward: acaccctgttctcctgactg Reverse: agagaaagcacagcctggat | 126 |
| PAL5 | NM_001320040.1 | Forward: attgctggtttgctcactgg Reverse: tccttaggctgcaactcgaa | 128 |
| CHI | FJ849060.1 | Forward: tggtggtagtgcaggaacat Reverse: tgtccagctcgttcgtagtt | 126 |
| SOD | LC203075.1 | Forward: atgcccaccccttactgttt Reverse: taccgtagttggaccagcag | 118 |
| CAT1 | NM_001247898.1 | Forward: gcagctcccagttaatgctc Reverse: agcaggacgacaaggatcaa | 127 |
| GR | NM_001247314.2 | Forward: cctgacagaagaagaggcca Reverse: catgtgcaagcccagaactt | 157 |
| APX | LC203076.1 | Forward: gaggcccgaaaattcccatg Reverse: caaatgagcagcaggggaag | 113 |
| GLU | NM_001247483.2 | Forward: gcacaatcggtaactctggc Reverse: gcaggctcaaaccaatgtga | 154 |
| POD | NM_001247041.2 | Forward: acagctcctccgaattccaa Reverse: ggaatcacgagcagcaagag | 126 |
| PPO | NM_001309397.1 | Forward: ttgccacatgttcacagagc Reverse: gtaccagagtcaccgcgata | 127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, M.; Pang, X.; Liu, H.; Lin, F.; Lu, F.; Bie, X.; Lu, Z.; Lu, Y. Iturin A Induces Resistance and Improves the Quality and Safety of Harvested Cherry Tomato. Molecules 2021, 26, 6905. https://doi.org/10.3390/molecules26226905
Jiang M, Pang X, Liu H, Lin F, Lu F, Bie X, Lu Z, Lu Y. Iturin A Induces Resistance and Improves the Quality and Safety of Harvested Cherry Tomato. Molecules. 2021; 26(22):6905. https://doi.org/10.3390/molecules26226905
Chicago/Turabian StyleJiang, Mengxi, Xinyi Pang, Huawei Liu, Fuxing Lin, Fengxia Lu, Xiaomei Bie, Zhaoxin Lu, and Yingjian Lu. 2021. "Iturin A Induces Resistance and Improves the Quality and Safety of Harvested Cherry Tomato" Molecules 26, no. 22: 6905. https://doi.org/10.3390/molecules26226905
APA StyleJiang, M., Pang, X., Liu, H., Lin, F., Lu, F., Bie, X., Lu, Z., & Lu, Y. (2021). Iturin A Induces Resistance and Improves the Quality and Safety of Harvested Cherry Tomato. Molecules, 26(22), 6905. https://doi.org/10.3390/molecules26226905








