The Impact of Selected Essential Oils Applied to Non-Woven Viscose on Bacteria That Cause Lower Urinary Tract Infections—Preliminary Studies
Abstract
1. Introduction
| Microorganisms | Occurrence of Infections |
|---|---|
| Escherichia coli | 73–95% of infections, 53–72% of ambulatory infections and 18–57% of in-hospital infections |
| Staphylococcus epidermidis | 5–10% of all infections |
| Staphylococcus saprophyticus | Up to 2% of ambulatory infections and up to 4% of in-hospital infections |
| Pseudomonas spp. | Up to 4% of ambulatory infections and 1–11% of in-hospital infections |
| Enterococcus spp. | 2–12% of ambulatory infections and 7–16% of in-hospital infections |
| Staphylococcus aureus | Mainly in-hospital infections |
| Enterobacter spp. | 3% of all infections, mostly in-hospital |
| Klebsiella spp. | 3% of all infections, mostly in-hospital, often returns |
| Proteus spp. | 3% of all infections, mostly in-hospital |
| Serratia spp. | Mainly in-hospital infections |
| Mycobacterium spp. | Mainly in-hospital infections; may be spread by blood |
| Neisseria gonorrhoeae | Spread by unprotected sex |
| Chalmydia trachomatis | Spread by unprotected sex |
| Candida albicans, Cryptococcus neoformans, Aspergillus spp. | May be spread by blood |
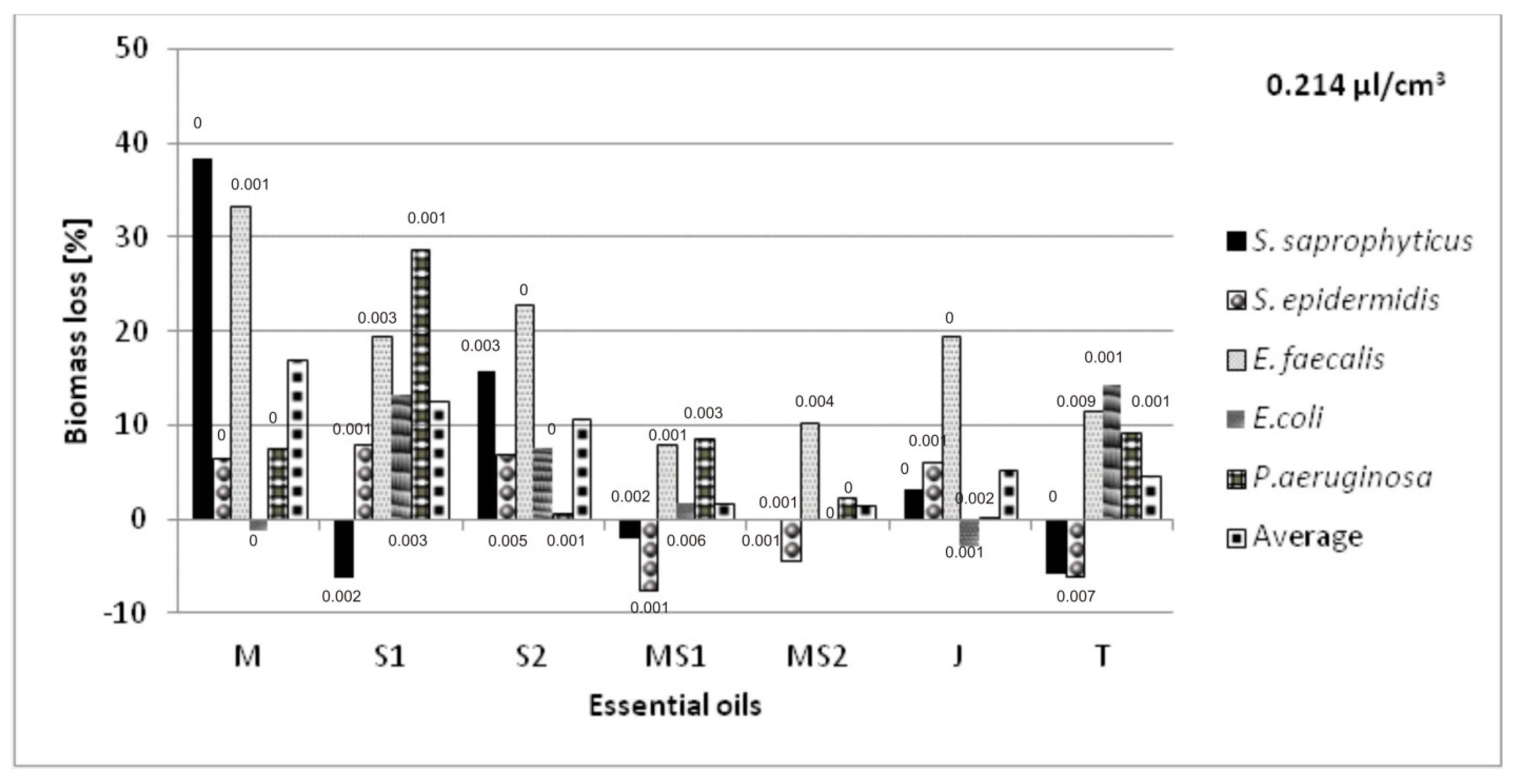
2. Results and Discussion
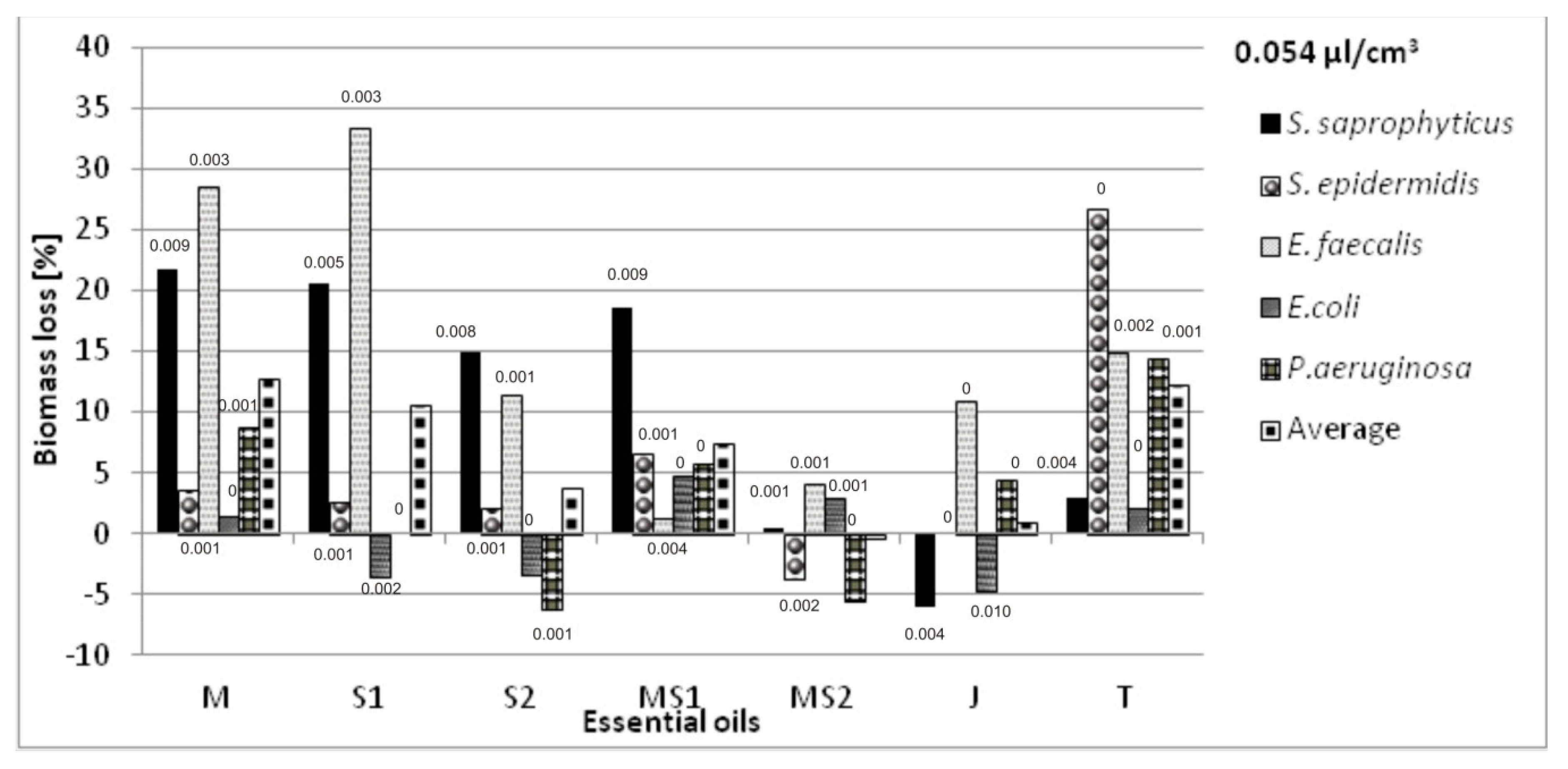
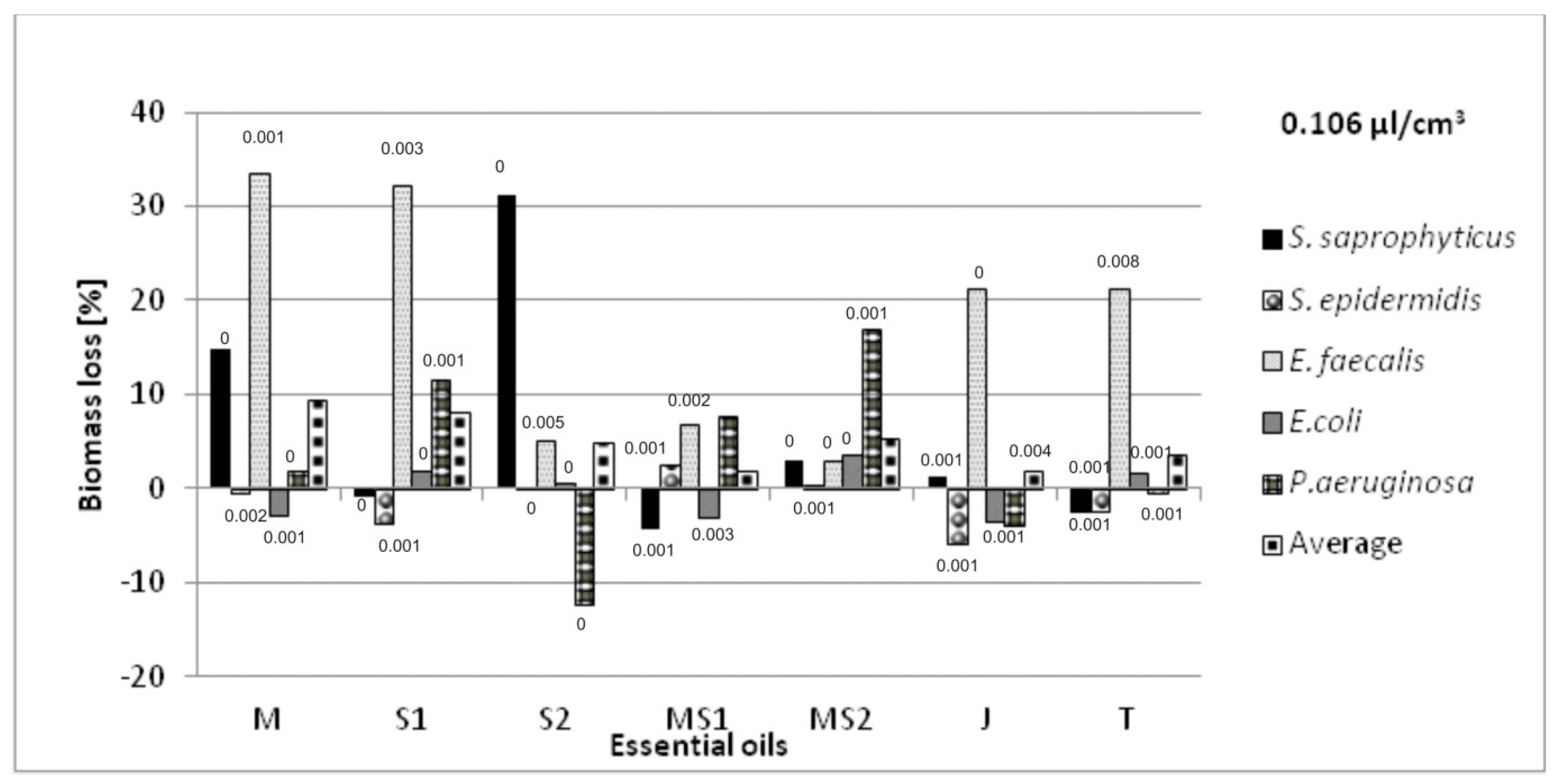
Antibacterial Activity of Essential Oils Applied on Non-Woven Viscose
3. Materials and Methods
3.1. Material for Essentials Oils Application
3.2. Essential Oils
3.3. Microorganisms and Antibacterial Activity Assessment of Essential Oils
Microatmosphere Method
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Kunttu, K. Urinary Tract Infections in Young Women; Ylioppilaiden Terveydenhoitosäätiö: Helsinki, Finland, 2010; pp. 1–12. [Google Scholar]
- Sekulowa, J. Domowy Poradnik Medyczny (eng. Home Medical Guide); Kazimierz, J., Ed.; Państwowy Zakład Wydawnictw Lekarskich: Warsaw, Poland, 1991; pp. 335–338. [Google Scholar]
- Urinary Tract Infection (UTI). A Guide for Women; International Urogynecological Association: Burnsville, MN, USA, 2012; pp. 1–2. [Google Scholar]
- Mashall, K. Interstitial Cystitis: Understanding the Syndrome. Altern. Med. Rev. 2003, 8, 1–12. [Google Scholar]
- Kawecki, D. Infekcje w Układzie Moczowym (eng. Infections in Urinary Tract. In Uroginekologia Schorzenia dna Miednicy (eng. Uroginecology Diseases of Pelvic Floor); Ewa, B., Ed.; Via Medica: Gdańsk, Poland, 2017; pp. 399–406. [Google Scholar]
- Adamkiewicz, K.; Borkowski, A.; Krzeski, T.; Leńko, J.; Marczyńska, A.; Mazurek, L.J.; Stolarczyk, J.; Zieliński, J. UROLOGIA Podręcznik dla Studentów Medycyny (eng. UROLOGY Manual for Medical Students); Leńko, J., Ed.; Państwowy Zakład Wydawnictw Lekarskich: Warsaw, Poland, 1987; pp. 130–133. [Google Scholar]
- Holecki, M.; Duława, J.; Hryniewicz, W.; Imiela, J.; Klinger, M.; Pawlik, K.; Wanke-Rytt, M. Rekomendacje Diagnostyki, Terapii i Profilaktyki Zakażeń Układu Moczowego u Dorosłych (eng. Recommendations for Diagnostics, Therapy and Prevention of Urinary Tract Infections in Adults); Hryniewicz, W., Holecki, M., Eds.; Narodowy Instytut Leków: Warsaw, Poland, 2015; pp. 7–46. [Google Scholar]
- Welsh, P.C. Campbell’s Urology, 8th ed.; The Curtis Center: Philadelphia, PA, USA, 2002; Volume 1, pp. 572–580. [Google Scholar]
- Boone, T.B.; Coburn, M.; Fishman, I.J.; Gousse, A.E.; Greer, J.A.; Griffith, D.P.; Gurpinar, T.; Harding, J.R.; Hinson, J.L.; Lerner, S.P. Nawracające Zakażenia Dróg Moczowych (eng. Recurrent urinary Tract Infections). In Podstawy Urologii (eng. Basics of Urology); Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 1999; pp. 58–63. [Google Scholar]
- Amalaradjou, M.A.R.; Venkitanarayanan, K. Natural Approaches for Controlling Urinary Tract Infections. Urin. Tract Infect. 2011, 13, 227–244. [Google Scholar] [CrossRef][Green Version]
- Van Vuuren, S.F.; Naidoo, D. An antimicrobial investigation of plants used traditionally in southern Africa to treat sexually transmitted infections. J. Ethnopharmacol. 2010, 130, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Duttke, C.; Hild, J.; Muller, H.J. In vitro activity of essential oils on microorganisms isolated from vaginal infections. Int. J. Aromather. 2006, 16, 169–174. [Google Scholar] [CrossRef]
- Gniewosz, M.; Kraśniewska, K.; Węglarz, Z.; Przybył, J.L. Porównanie przeciwdrobnoustrojowej aktywności etanolowego i wodnego ekstraktu z szałwii lekarskiej (Salvia officinalis L.) (eng. Comparison of the antimicrobial activity of ethanolic and aqueous sage extracts from sage (Salvia officinalis L.)). Bromatologia i Chemia Toksykologiczna–XLV 2012, 3, 743–748. [Google Scholar]
- Darwish, M.A.; Al-Ramamneh, E.A.; Abu-Dieyeh, I.S.Z.; Al-Nawaiseh, M.; Albdour, T. Determination of essential oil bioactive components and rosmarinic acid of Salvia officinalis cultivated under different intra-row spacing. Not. Sci. Biol. 2013, 5, 198–203. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef]
- Kałużna, O. Badanie Wpływu Rożnych Stężeń Olejku Eterycznego i Naparu z Szałwii Lekarskiej Salvia officinalis L. na Tempo Wzrostu Hodowli Płynnej Bakterii Escherichia coli Szczepów MC4100 i MC4100ΔClpB (eng. Study of the Effect of Various Concentrations of Essential Oil and Infusion of Salvia officinalis L. on the Growth Rate of the Liquid Culture of Escherichia coli Strains MC4100 and MC4100ΔClpB). Available online: https://docplayer.pl/26459575-Badanie-wplywu-roznych-stezen-olejku-eterycznego-i-naparu-z-szalwii-lekarskiej-salvia-officinalis.html (accessed on 10 September 2021).
- Kędzia, A.; Dera-Tomaszewska, B.; Ziółkowska-Klinkosz, M.; Wojtaszek-Słomińska, A.; Czernecka, B. Działanie olejku szałwiowego (Oleum Salviae lavandulaefoliae) na bakterie tlenowe izolowane z jamy ustnej, dróg oddechowych i przewodu pokarmowego (eng. Activity of Oleum salviae (Spanish sage essential oil) against aerobic bacteria isolated from oral cavity, respiratory and gasrtointestinal tract). Postępy Fitoterapii 2011, 4, 238–242. [Google Scholar]
- Porres-Martínez, M.; Carretero Accame, E.; Gómez-Serranillos, P. Pharmacological activity of Salvia lavandulifolia and chemical components of its essential oil. A review. Lazaroa 2013, 34, 237–254. [Google Scholar] [CrossRef]
- Kazemi, M. Chemical composition and antimicrobial activity of essential oil of Matricaria chamomilla. Bulletin of Environment. Pharmacol. Life Sci. 2014, 3, 148–153. [Google Scholar]
- The Longwood Herbal Task Force. Gardiner P. Chamomile (Matricaria recutita, Anthemis nobilis). 1999, pp. 1–21. Available online: https://tratamientocelular.com/papers/cmran.pdf (accessed on 15 September 2021).
- Hosseinpour, M.; Mobini-Dehkordi, M.; Saffar, B.; Teimori, H. Antiproliferative effects of Matricaria chamomilla on Saccharomyces cerevisiae. J. HerbMed Pharmacol. 2013, 2, 49–51. [Google Scholar]
- Kędzia, A.; Dera-Tomaszewska, B.; Ziółkowska-Klinkosz, M.; Kędzia, A.W.; Kochańska, B.; Gębska, A. Aktywność olejku tymiankowego (Oleum Thymi) wobec bakterii tlenowych (eng. Activity of thyme essentials oil (Oleum Thymi) against aerobic bacteria). Postępy Fitoterapii 2012, 2, 67–71. [Google Scholar]
- Sienkiewicz, M.; Wasiela, M. Aktywność olejków tymiankowego i lawendowego wobec opornych na antybiotyki szczepów klinicznych Pseudomonas aeruginosa (eng. Activity of thyme and lavender essential oils against antibiotic resistant clinical strains of Pseudomonas aeruginosa). Postępy Fitoterapii 2012, 3, 139–145. [Google Scholar]
- Shabnum, S.; Muzafar, G.W. Essential oil composition of Thymus Vulgaris L. and their uses. J. Res. Dev. 2011, 11, 83–94. [Google Scholar]
- Moghtader, M. Antifungal effects of the essential oil from Thymus vulgaris L. and comparison with synthetic thymol on Aspergillus niger. J. Yeast Fungal Res. 2012, 3, 83–88. [Google Scholar]
- Filipowicz, N.H.; Kamiński, M.M.; Kurlenda, J.; Asztemborska, J. Antibacterial and antifungal activity of juniper berry oil and its selected components. Phytoth. Res. 2003, 17, 227–231. [Google Scholar] [CrossRef]
- Pepeljnjak, S.; Kosalec, I.; Kalodera, Z.; Blazevic, N. Antimicrobial activity of juniper berry essential oil (Juniperus communis L., Cupressaceae). Acta Pharm. 2005, 55, 417–422. [Google Scholar] [PubMed]
- Das, S.; Horvath, B.; Safranko, S.; Jokić, S.; Szechenyi, A.; Koszegi, T. Antimicrobial activity of chamomile essential oil: Effect of different formulations. Molecules 2019, 24, 4321. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential oils as antimicrobial agents—myth or real alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Reyes-Jurado, F.; Navarro-Cruz, A.R.; Ochoa-Velasco, C.E.; Lopez-Malo, A.; Avila-Sosa, R. Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. 2020, 60, 1641–1650. [Google Scholar] [CrossRef]
- Pierozan, M.K.; Pauletti, G.F.; Rota, L.; Santos, A.C.A.; Lerin, L.A.; Di Luccio, M.; Mossi, A.J.; Atti-Serafini, L.; Cansian, R.L.; de Oliveira, V.J. Chemical characterization and antimicrobial activity of essential oils of salvia L. species. Ciência e Tecnologia de Alimentos 2009, 29, 764–770. [Google Scholar] [CrossRef]
- Martin, E.; Lingbeck, J.; Adams, J.; O’Bryan, C.; Crandall, P. Sweetgum: An ancient source of beneficial compounds with modern benefits. Pharm. Rev. 2015, 9, 1–11. [Google Scholar]
- Longaray Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Cutillas, A.-B.; Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Salvia officinalis L. essential oil from Spain: Determination of composition, antioxidant capacity, antienzymatic and antimicrobial bioactivitie. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef]
- Salehi, B.; Abu-Darwish, M.S.; Tarawneh, A.H.; Cabral, C.; Gadetskaya, A.V.; Salgueiro, L.; Hosseinabadii, T.; Rajabi, S.; Chandak, W.; Sharifi-Rad, M.; et al. Thymus spp. plants -food applications and phytopharmacy properties. Trends Food Sci. Technol. 2019, 85, 286–306. [Google Scholar] [CrossRef]
- Rajkowska, K.; Nowak, A.; Kunicka-Styczyńska, A.; Siadura, A. Biological effects of various chemically characterized essential oils: Investigation of the mode of action against Candida albicans and HeLa cells. RSC Adv. 2016, 6, 97199–97207. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharm. Pharmacol. 2014, 67, 473–485. [Google Scholar] [CrossRef]
- Jäger, W.; Buchbauer, G.; Jirovetz, L.; Fritzer, M. Percutaneous absorption of lavender oil from a massage oil. J. Soc. Cosmet. Chem. 1992, 43, 49–54. [Google Scholar]
- Diliberto, J.J.; Usha, G.; Birnbaum, L.S. Disposition of citral in male Fischer rats. Drug Metab. Dispos. 1988, 16, 721–727. [Google Scholar]
- Srivastava, J.K.; Gupta, S. Antiproliferative and apoptotic effects of chamomile extract in various human cancer cells. J. Agric. Food Chem. 2007, 55, 9470–9478. [Google Scholar] [CrossRef] [PubMed]
- Mahdavia, B.; Ghorat, F.; Nasrollahzadeh, M.S.; Hosseyni-Tabara, M.; Rezaei-Seresht, H. Chemical composition, antioxidant, antibacterial, cytotoxicity, and hemolyses activity of essential oils from flower of Matricaria chamomilla var. chamomilla. Anti-Infect. Agents 2020, 18, 224–232. [Google Scholar] [CrossRef]
- Russo, R.; Corasaniti, M.T.; Bagetta, G.; Morrone, L.A. Exploitation of cytotoxicity of some essential oils for translation in cancer therapy. Evid.-Based Compl. Alt. Med. 2015, 397821, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, A.; Przychodna, M.; Sopata, S.; Bodalska, A.; Fecka, I. Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules 2020, 25, 4125. [Google Scholar] [CrossRef] [PubMed]
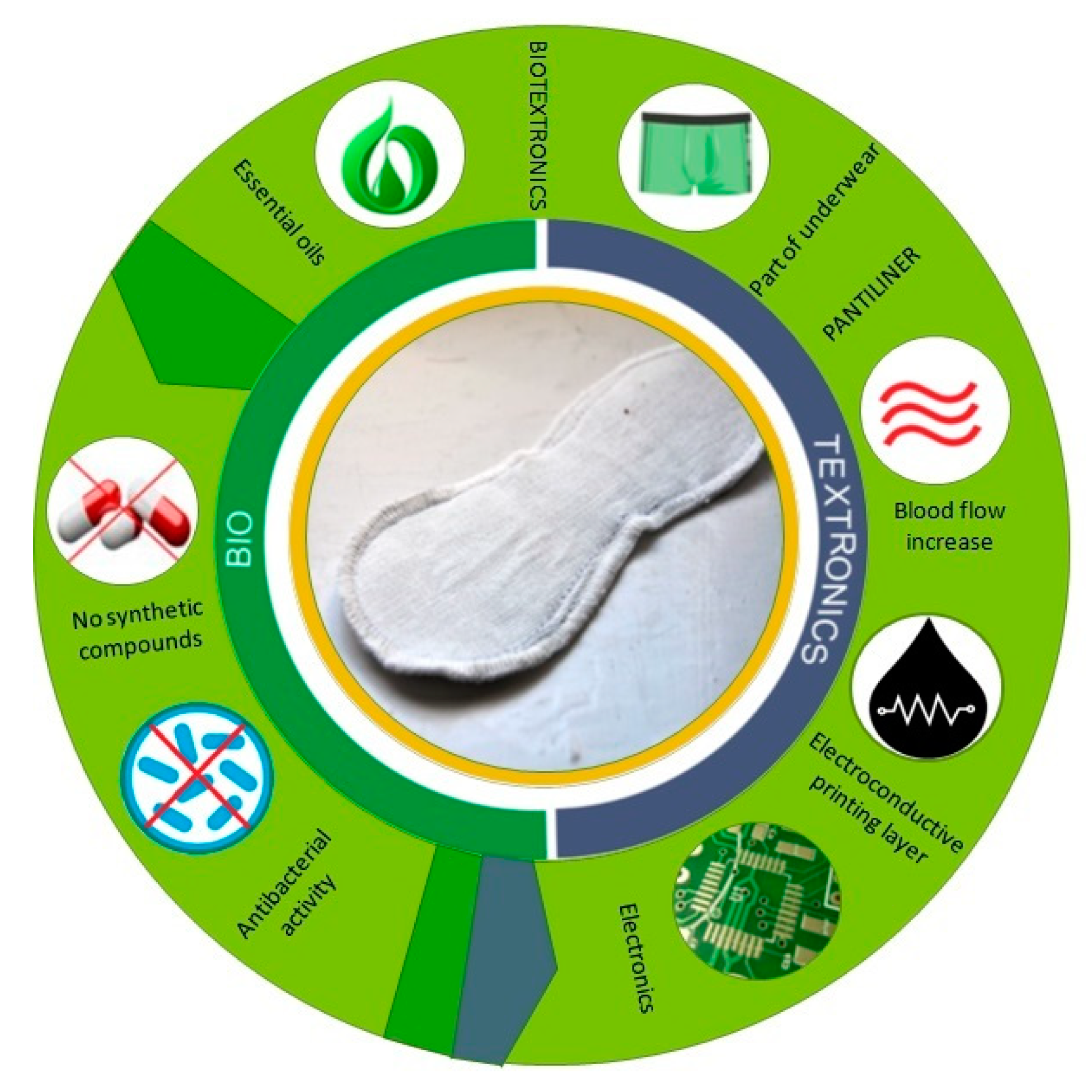
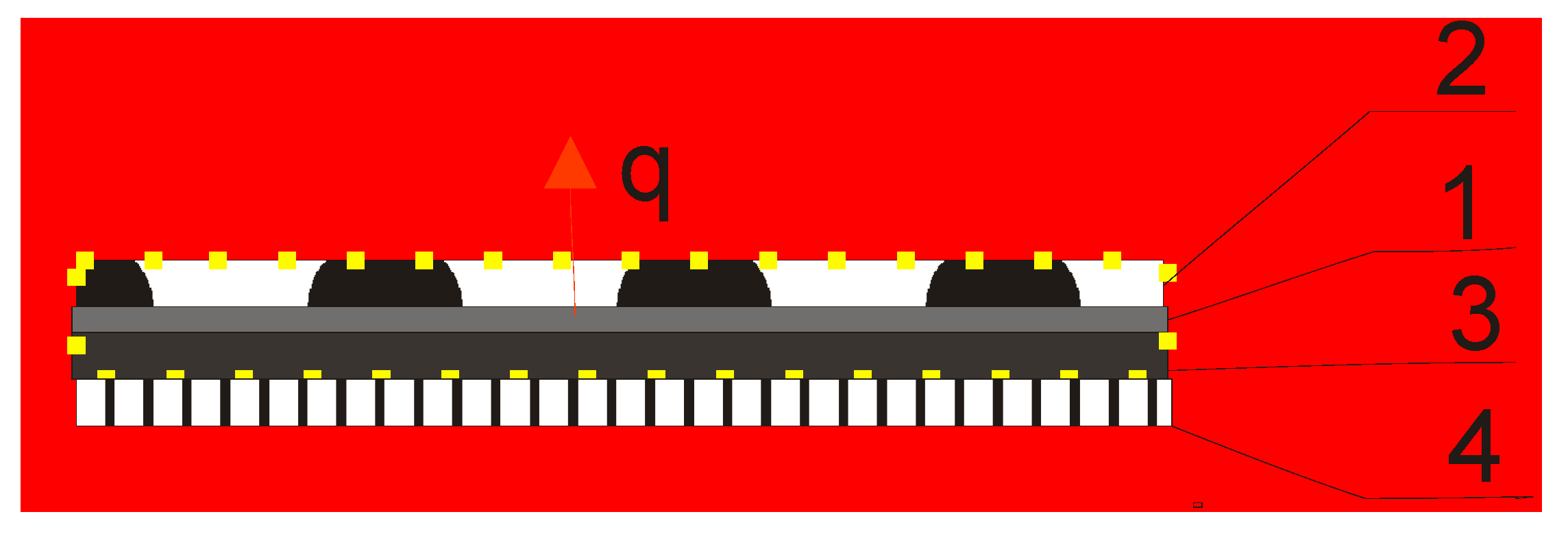
| Material | Function | Surface Mass [g/m2] | Thickness [mm] |
|---|---|---|---|
| Non-woven viscose | External, removable insert | 30 | 0.2 |
| Matricaria chamomilla L. Essential Oil | |
|---|---|
| Organoleptic Description | clear, viscous liquid, dark blue, with a characteristic odor |
| Analytical Data | density (at 20 °C): 0.946 to 0.969 g/cm3 refractive index (at 20 °C): 1.496–1.516 |
| Chromatographic Profile | (−)-α-bisabolol: 10–65% chamazulene: ≥1.0% bisabolol oxide and (−)-α-bisabolol: ≥20% |
| Salvia officinalis L. Essential Oil | |
| Organoleptic Description | clear liquid, slightly yellow or slightly green, with a characteristic odor |
| Analytical Data | density (at 20 °C): 0.905 to 0.925 g/cm3 refractive index (at 20 °C): 1.457 to 1.473 optical rotation (at 20 °C): −3.0° to +15.0° |
| Chromatographic Profile | 1,8-cineole: 6.0–16.0% α- and β-thujone: 20–40% camphor: 14.0–37.0% bornyl acetate: max. 5.0% borneol: max 5.0% |
| Salvia lavandulaefolia Vahl. Essential Oil | |
| Organoleptic Description | clear liquid, light yellow, with a characteristic odor |
| Analytical Data | density (at 20 °C): 0.907 to 0.927 g/cm3 refractive index (at 20 °C): 1.458 to 1.478 optical rotation (at 20 °C): +9° to +19° flash point: 54 °C |
| Chromatographic Profile | α-thujone: 30.3% camphor: 20.3% 1,8-cineole: 12.5% α- and β-pinene: 0.6–5.9% borneol: 2.7% α- and β-caryophyllene: 1.6–2.7% bornyl acetate 1.9% α-terpineol: 1.2% |
| Juniperus communis L. Essential Oil | |
| Organoleptic description | Clear liquid, colorless or slightly yellow, with a characteristic odor |
| Analytical Data | density (at 20 °C): 0.857 to 0.876 g/cm3 refractive index (at 20 °C): 1.471–1.483 optical rotation (at 20 °C): −15° to −0.5° |
| Chromatographic Profile | α-pinene: 20.0–50.0% sabinen: ≤20.0% β-pinene: 1.0–12.0% β-myrcene: 1.0–35.0% α- phellandrene: ≤1.0% limonene: 2.0–12.0% terpinen-4-ol: 0.5–10.0% bornyl acetate: ≤2.0% β-caryophyllene: ≤ 7.0% |
| Thymus vulgaris L. Essential Oil | |
| Organoleptic Description | clear, yellow to dark red-brown liquid, with a strong odor of thymol |
| Analytical Data | density (at 20 °C): 0.915 to 0.935 g/cm3 refractive index (at 20 °C): 1.490 to 1.505 optical rotation: −7° to +3° flash point: 58 °C |
| Chromatographic Profile | α-thujen: 0.2–1.5% β-myrcene: 1.0–3.0% α-terpinene: 0.9–2.6% ρ-cymene: 14.0–28.0% γ-terpinene: 4.0–12.0% linalool: 1.5–6.5% terpinen-4-ol: 0.1–2.5% methyl carvacrol ether: 0.05–1.5% thymol: 37.0–55.0% carvacrol: 0.5–5.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frydrysiak, E.; Kunicka-Styczyńska, A.; Śmigielski, K.; Frydrysiak, M. The Impact of Selected Essential Oils Applied to Non-Woven Viscose on Bacteria That Cause Lower Urinary Tract Infections—Preliminary Studies. Molecules 2021, 26, 6854. https://doi.org/10.3390/molecules26226854
Frydrysiak E, Kunicka-Styczyńska A, Śmigielski K, Frydrysiak M. The Impact of Selected Essential Oils Applied to Non-Woven Viscose on Bacteria That Cause Lower Urinary Tract Infections—Preliminary Studies. Molecules. 2021; 26(22):6854. https://doi.org/10.3390/molecules26226854
Chicago/Turabian StyleFrydrysiak, Emilia, Alina Kunicka-Styczyńska, Krzysztof Śmigielski, and Michał Frydrysiak. 2021. "The Impact of Selected Essential Oils Applied to Non-Woven Viscose on Bacteria That Cause Lower Urinary Tract Infections—Preliminary Studies" Molecules 26, no. 22: 6854. https://doi.org/10.3390/molecules26226854
APA StyleFrydrysiak, E., Kunicka-Styczyńska, A., Śmigielski, K., & Frydrysiak, M. (2021). The Impact of Selected Essential Oils Applied to Non-Woven Viscose on Bacteria That Cause Lower Urinary Tract Infections—Preliminary Studies. Molecules, 26(22), 6854. https://doi.org/10.3390/molecules26226854







