Determination of N-Acetyl-l-cysteine Ethyl Ester (NACET) by Flow Injection Analysis and Spectrophotometric Detection Using Different Thiol-Sensitive Ligands
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Chemical Conditions
2.1.1. Neocuproine (NCN) Ligand
2.1.2. Bicinchoninic Acid (BCA) Ligand
2.1.3. Bathocuproine Disulfonic Acid (BCS) Ligand
2.2. Optimization of the FIA Conditions
2.2.1. Neocuproine (NCN) Ligand
2.2.2. Bicinchoninic Acid (BCA) Ligand
2.2.3. Bathocuproine Disulfonic Acid (BCS) Ligand
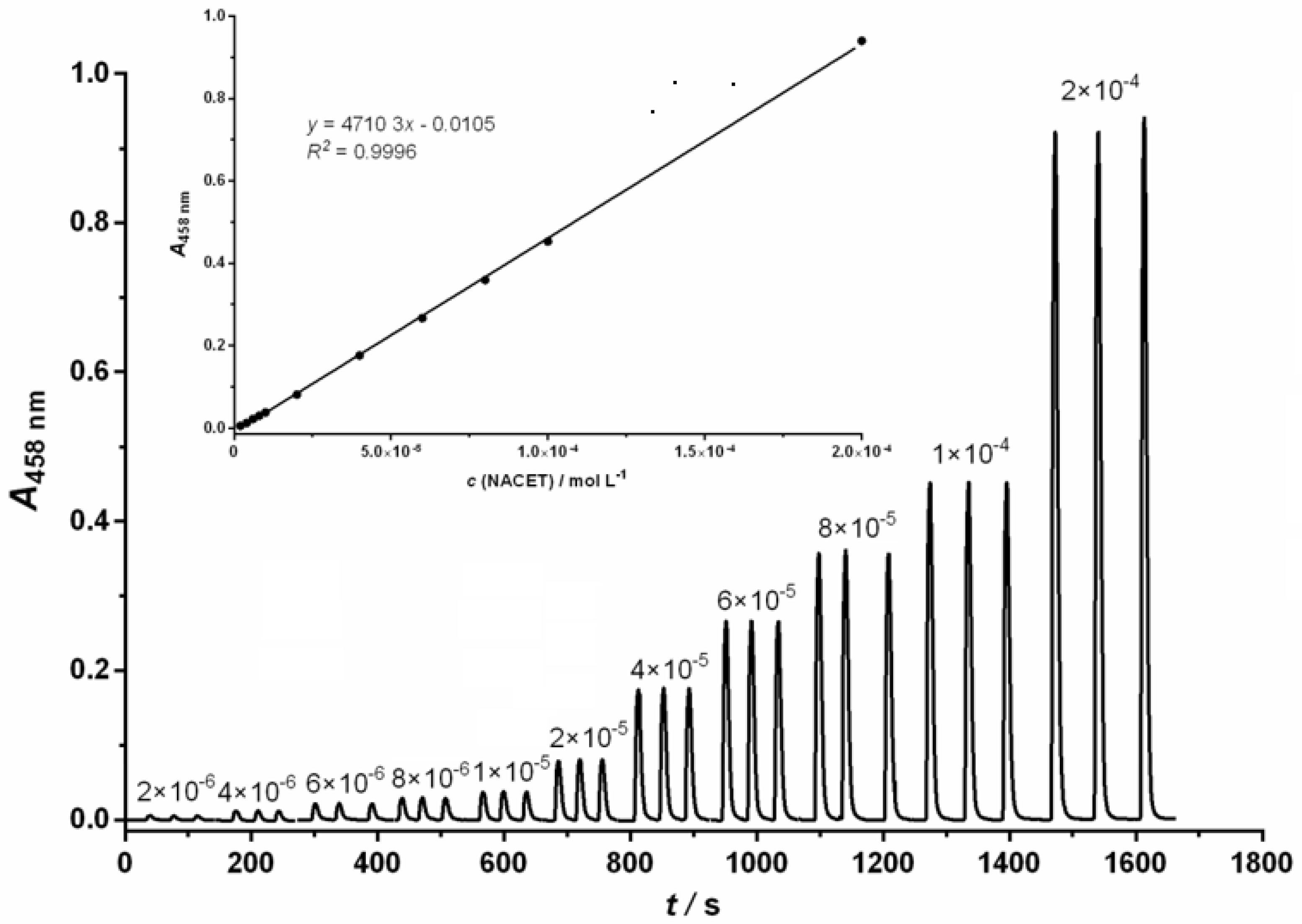
2.3. Analytical Characteristics
2.4. Interference Studies
2.5. Recovery Studies
2.6. Comparison between the Ligands
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Apparatus
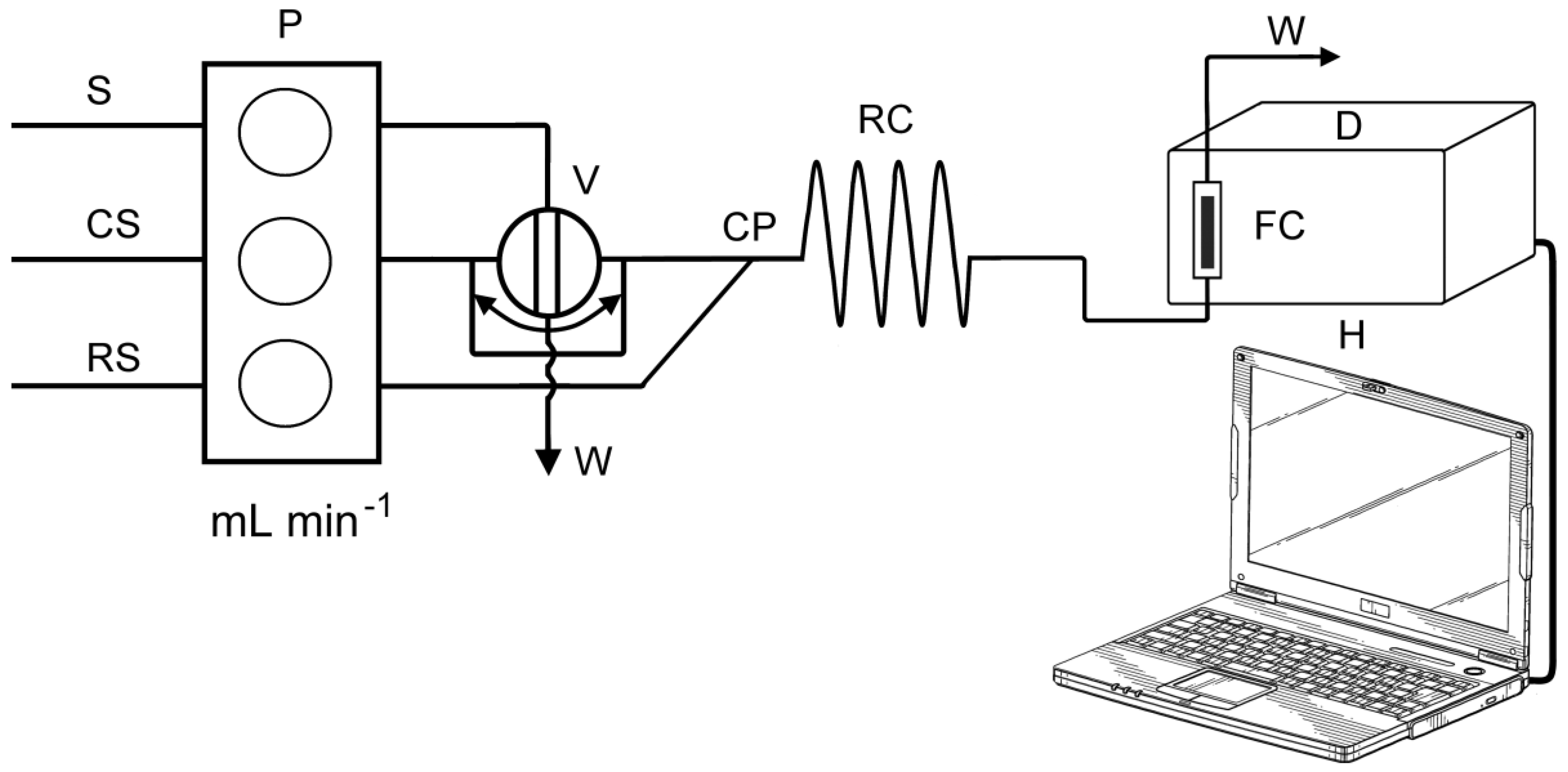
3.3. FIA Manifold and Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Giustarini, D.; Milzani, A.; Dalle-Donne, I.; Tsikas, D.; Rossi, R. N-Acetylcysteine ethyl ester (NACET): A novel lipophilic cell-permeable cysteine derivative with an unusual pharmacokinetic feature and remarkable antioxidant potential. Biochem. Pharmacol. 2012, 84, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D.; Schwedhelm, K.S.; Surdacki, A.; Giustarini, D.; Rossi, R.; Kukoc-Modun, L.; Kedia, G.; Uckert, S. S-Nitroso-N-acetyl-l-cysteine ethyl ester (SNACET) and N-acetyl-l-cysteine ethyl ester (NACET)-cysteine-based drug candidates with unique pharmacological profiles for oral use as NO, H2S and GSH suppliers and as antioxidants: Results and overview. J. Pharm. Anal. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Giustarini, D.; Galvagni, F.; Dalle Donne, I.; Milzani, A.; Severi, F.M.; Santucci, A.; Rossi, R. N-Acetylcysteine ethyl ester as GSH enhancer in human primary endothelial cells: A comparative study with other drugs. Free Radic. Biol. Med. 2018, 126, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Kukoc-Modun, L.; Tsikas, D.; Kraljević, T.; Biocic, M.; Radić, N. Kinetic spectrophotometric determination of N-acetyl-l-cysteine ethyl ester (NACET) generating chromogenic copper(I)Ln complexes with different ligands. Croat. Chem. Acta 2017, 90, 263–271. [Google Scholar] [CrossRef]
- Evgen’ev, M.I.; Garmonov, S.Y.; Shakirova, L.S. Flow-Injection Analysis of Pharmaceuticals. J. Anal. Chem. 2001, 56, 313–323. [Google Scholar] [CrossRef]
- Tzanavaras, P.D.; Themelis, D.G. Review of recent applications of flow injection spectrophotometry to pharmaceutical analysis. Anal. Chim. Acta 2007, 588, 1–9. [Google Scholar] [CrossRef]
- Rocha, F.R.P.; Nóbrega, J.A.; Filho, O.F. Flow analysis strategies to greener analytical chemistry. An overview. Green Chem. 2001, 3, 216–220. [Google Scholar] [CrossRef]
- Rocha, F.R.P.; Reis, B.F.; Zagatto, E.A.G.; Lima, J.L.F.C.; Lapa, R.A.S.; Santos, J.L.M. Multicommutation in flow analysis: Concepts, applications and trends. Anal. Chim. Acta 2002, 468, 119–131. [Google Scholar] [CrossRef]
- Segundo, M.A.; Magalhaes, L.M. Multisyringe Flow Injection Analysis: State-of-the-Art and Perspectives. Anal. Sci. 2006, 22, 3–8. [Google Scholar] [CrossRef][Green Version]
- Itabashi, H.; Kawamoto, H.; Kawashima, T. A Novel Flow Injection Technique: All Injection Analysis. Anal. Sci. 2001, 17, 229–231. [Google Scholar] [CrossRef]
- Lapa, R.A.S.; Lima, J.L.F.C.; Reis, B.F.; Santos, J.L.M.; Zagatto, E.A.G. Multi-pumping in flow analysis: Concepts, instrumentation, potentialities. Anal. Chim. Acta 2002, 466, 125–132. [Google Scholar] [CrossRef]
- Chocholouš, P.; Solich, P.; Šatínský, D. An overview of sequential injection chromatography. Anal. Chim. Acta 2007, 600, 129–135. [Google Scholar] [CrossRef]
- Grudpan, K. Some recent developments on cost-effective flow-based analysis. Talanta 2004, 64, 1084–1090. [Google Scholar] [CrossRef]
- Teshima, N.; Ohno, S.; Sakai, T. Stopped-in-Loop Flow Analysis of Trace Vanadium in Water. Anal. Sci. 2007, 23, 1–2. [Google Scholar] [CrossRef][Green Version]
- Teshima, N.; Kuno, M.; Ueda, M.; Ueda, H.; Ohno, S.; Sakai, T. Automated stopped-in-dual-loop flow analysis system for catalytic determination of vanadium in drinking water. Talanta 2009, 79, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Teshima, N.; Noguchi, D.; Joichi, Y.; Lenghor, N.; Ohno, N.; Sakai, T.; Motomizu, S. Simultaneous Injection-Effective Mixing Analysis of Palladium. Anal. Sci. 2010, 26, 143–144. [Google Scholar] [CrossRef]
- Ponhong, K.; Teshima, N.; Grudpan, K.; Vichapong, J.; Motomizu, S.; Sakai, T. Successive determination of urinary bilirubin and creatinine employing simultaneous injection effective mixing flow analysis. Talanta 2015, 133, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, J. Redesigning flow injection after 40 years of development: Flow programming. Talanta 2018, 176, 437–443. [Google Scholar] [CrossRef]
- Besada, A.; Tadros, N.B.; Gawargious, Y.A. Copper(II)-neocuproine as colour reagent for some biologically active thiols: Spectrophotometric determination of cysteine, penicillamine, glutathione, and 6-mercaptopurine. Mikrochim. Acta 1989, 99, 143–146. [Google Scholar] [CrossRef]
- Brenner, A.J.; Harris, E.D. A Quantitative Test for Copper Using Bicinchoninic Acid. Anal. Biochem. 1995, 226, 80–84. [Google Scholar] [CrossRef]
- Blair, D.; Diehl, H. Bathophenanthrolinedisulphonic acid and bathocuproinedisulphonic acid, water soluble reagents for iron and copper. Talanta 1961, 7, 163–174. [Google Scholar] [CrossRef]
- Tsikas, D.; Dehnert, S.; Urban, K.; Surdacki, A.; Meyer, H.H. GC–MS analysis of S-nitrosothiols after conversion to S-nitroso-N-acetyl cysteine ethyl ester and in-injector nitrosation of ethyl acetate. J. Chromatogr. B 2009, 877, 3442–3455. [Google Scholar] [CrossRef] [PubMed]
- Kukoc-Modun, L.; Tsikas, D.; Biocic, M.; Radić, N. Flow injection analysis of N-acetyl-l-cysteine based on the reduction of copper(II)-neocuproine reagent. Anal. Lett. 2016, 49, 607–617. [Google Scholar] [CrossRef]
| Variable | Studied Range | Optimum Conditions | ||
|---|---|---|---|---|
| NCN | BCA | BCS | ||
| Wavelength (nm) | 400–800 | 458 | 562 | 483 |
| pH | 2.0–8.0 | 5.0 | 7.0 | 5.0 |
| Temperature (°C) | 20–40 | 25 | 25 | 25 |
| Molar ratio Cu(II)/ligand | 1/1.0–1/3.5 | 1/2.4 | 1/2 | 1/2 |
| CS 1 flow rate (mL min−1) | 0.5–6.0 | 6.0 | 5.0 | 5.0 |
| RS 2 flow rate (mL min−1) | 1.0–4.0 | 2.0 | 1.0 | 1.0 |
| Injection sample volume (µL) | 100–1000 | 500 | 500 | 500 |
| Reaction coil length (cm) | 30–525 | 40 | 40 | 30 |
| NCN | BCA | BCS | |
|---|---|---|---|
| Linear range (mol L−1) | 2.0 × 10−6 −2.0 × 10−4 | 2.0 × 10−6 −1.0 × 10−4 | 6.0 × 10−7 −1.2 × 10−4 |
| Regression equation | y = 4710x −0.0105 | y = 3850x −0.0108 | y = 8437x −0.003 |
| LOD (mol L−1) 1 | 6.0 × 10−7 | 8.3 × 10−7 | 2.2 × 10−7 |
| Correlation coefficient, R2 | 0.9996 | 0.9993 | 0.9995 |
| Relative standard deviation 2, RSD (%) | 0.41 | 0.70 | 0.36 |
| Analytical frequency (h−1) | 90 | 90 | 90 |
| Substance | Optimum Conditions 1 | ||
|---|---|---|---|
| NCN | BCA | BCS | |
| Glucose | 1:500 | 1:500 | 1:500 |
| Fructose | 1:500 | 1:500 | 1:500 |
| KNO3 | 1:500 | 1:500 | 1:500 |
| Lactose | 1:5 | 1:500 | 1:500 |
| Sucrose | 1:5 | 1:50 | 1:100 |
| Citric acid | 1:10 | 1:1 | 1:250 |
| Tartaric acid | 1:50 | 1:5 | 1:500 |
| Na2SO4 | 1:500 | 1:500 | 1:500 |
| Na-citrate | 1:500 | 1:1 | 1:250 |
| H3BO3 | 1:500 | 1:500 | 1:100 |
| NCN | BCA | BCS | |||||
|---|---|---|---|---|---|---|---|
| Sample | Added (µg mL−1) | Found (µg mL−1) | Recovery (%) | Found (µg mL−1) | Recovery (%) | Found (µg mL−1) | Recovery (%) |
| Placebo | 0 | 0 | - | 0 | - | 0 | - |
| 50 | 50.6 ± 0.7 | 101.2 | 49.4 ± 0.5 | 98.8 | 50.3 ± 0.6 | 100.6 | |
| 100 | 102.6 ± 1.2 | 102.6 | 101.1 ± 1.0 | 101.1 | 102.3 ± 1.4 | 102.3 | |
| 150 | 153.8 ± 2.0 | 102.5 | 148.9 ± 2.2 | 99.3 | 152.9 ± 2.5 | 101.9 | |
| 200 | 204.1 ± 2.4 | 102.1 | 203.4 ± 3.1 | 101.7 | 203.9 ± 3.0 | 102.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukoc-Modun, L.; Kraljević, T.; Tsikas, D.; Radić, N.; Modun, D. Determination of N-Acetyl-l-cysteine Ethyl Ester (NACET) by Flow Injection Analysis and Spectrophotometric Detection Using Different Thiol-Sensitive Ligands. Molecules 2021, 26, 6826. https://doi.org/10.3390/molecules26226826
Kukoc-Modun L, Kraljević T, Tsikas D, Radić N, Modun D. Determination of N-Acetyl-l-cysteine Ethyl Ester (NACET) by Flow Injection Analysis and Spectrophotometric Detection Using Different Thiol-Sensitive Ligands. Molecules. 2021; 26(22):6826. https://doi.org/10.3390/molecules26226826
Chicago/Turabian StyleKukoc-Modun, Lea, Tomislav Kraljević, Dimitrios Tsikas, Njegomir Radić, and Darko Modun. 2021. "Determination of N-Acetyl-l-cysteine Ethyl Ester (NACET) by Flow Injection Analysis and Spectrophotometric Detection Using Different Thiol-Sensitive Ligands" Molecules 26, no. 22: 6826. https://doi.org/10.3390/molecules26226826
APA StyleKukoc-Modun, L., Kraljević, T., Tsikas, D., Radić, N., & Modun, D. (2021). Determination of N-Acetyl-l-cysteine Ethyl Ester (NACET) by Flow Injection Analysis and Spectrophotometric Detection Using Different Thiol-Sensitive Ligands. Molecules, 26(22), 6826. https://doi.org/10.3390/molecules26226826








