Synthesis, In Silico Studies, and Evaluation of Syn and Anti Isomers of N-Substituted Indole-3-carbaldehyde Oxime Derivatives as Urease Inhibitors against Helicobacter pylori
Abstract
:1. Introduction
2. Results and Discussion
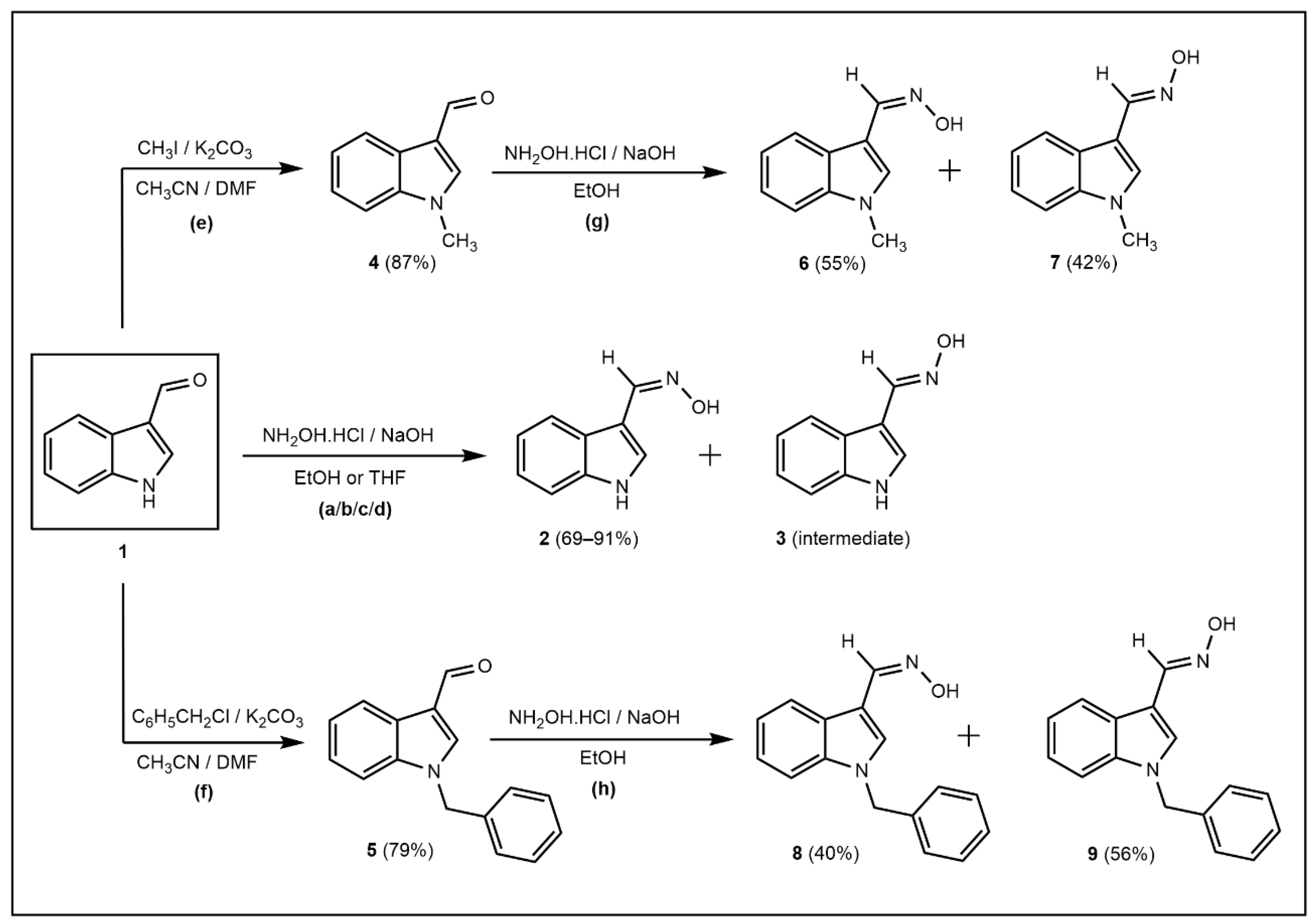
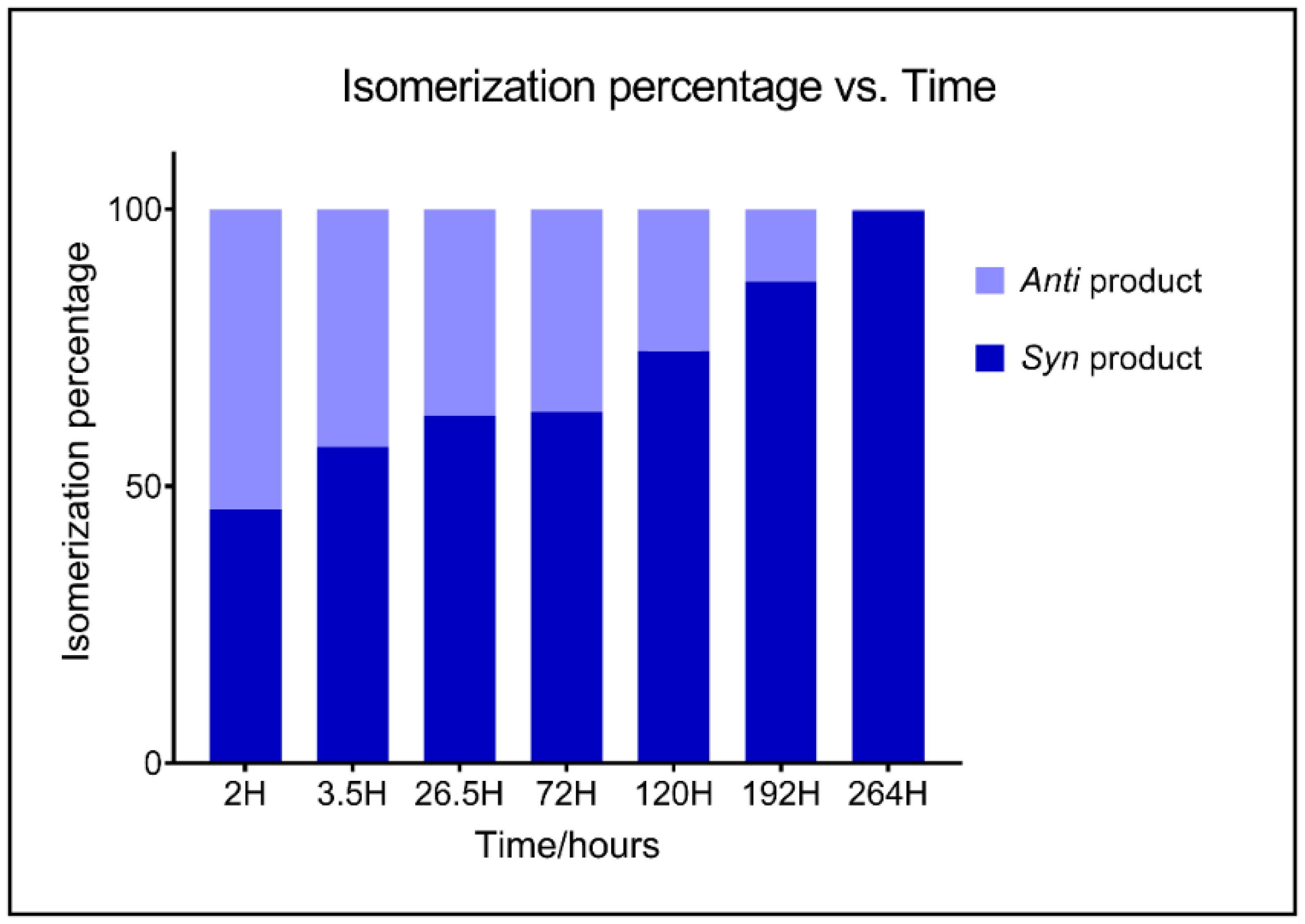
2.1. Chemistry
2.2. Urease Inhibitory Assay
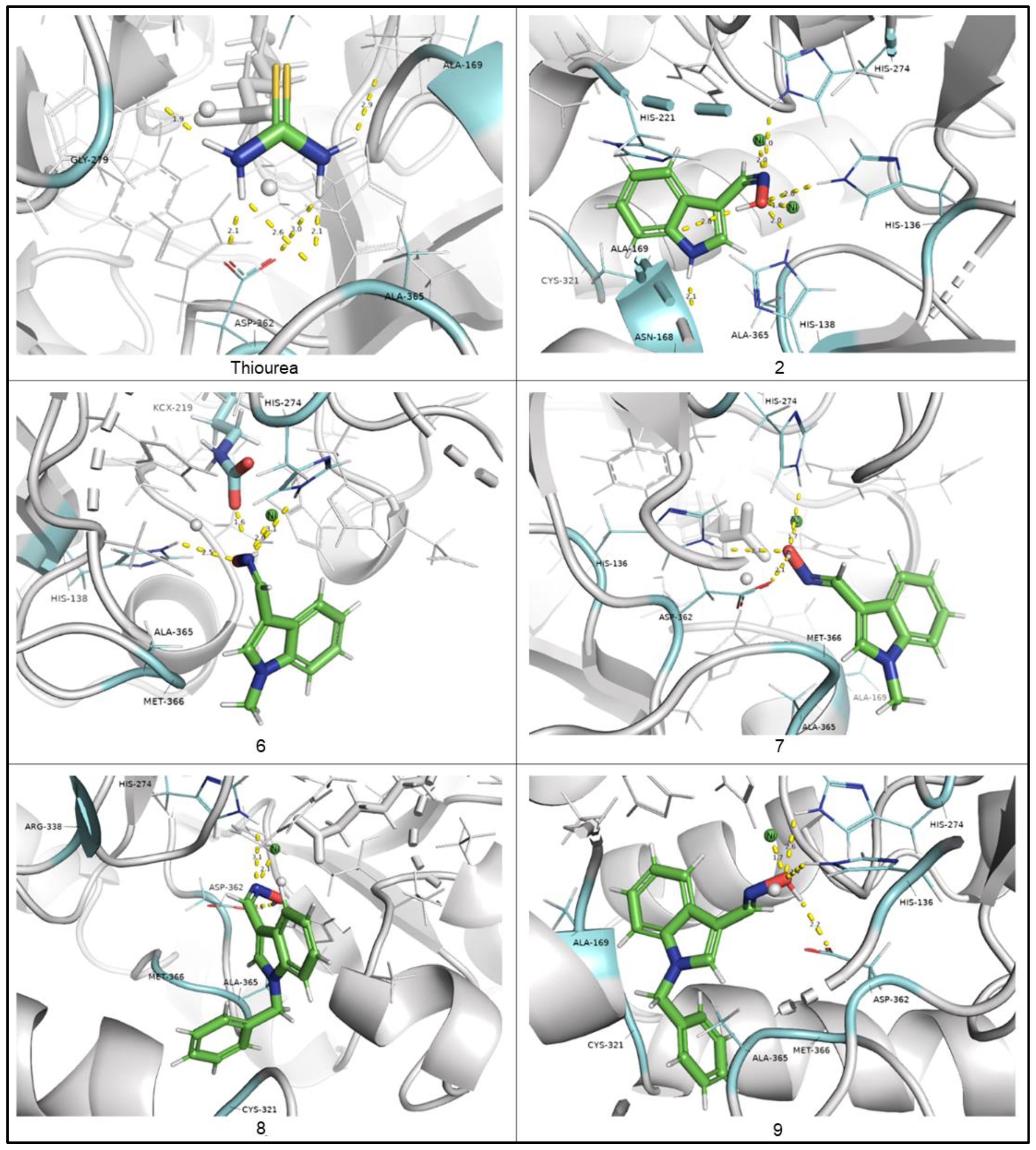
2.3. In Silico Studies of Indole-3-Carbaldehyde Oxime Derivatives
2.4. Drug-Likeness of Indole-3-Carbaldehyde Oxime Derivatives
3. Materials and Methods
3.1. General
3.2. Synthesis of (Z)-N-Hydroxy-1-(1H-indole-3-yl)methanimine (2)
3.2.1. Methods a and b
3.2.2. Methods c and d
3.3. Synthesis of (E)-N-Hydroxy-1-(1H-indole-3-yl)methanimine (3)
3.4. Synthesis of 1-Methyl-1H-indole-3-carbaldehyde (4)
3.5. Synthesis of (Z/E)-N-Hydroxy-1-(1-methyl-1H-indol-3-yl)methanimine (6 and 7)
3.6. Synthesis of 1-Benzyl-1H-indole-3-carbaldehyde (5)
3.7. Synthesis of (Z/E)-1-(1-Benzyl-1H-indol-3-yl)-N-hydroxymethanimine (8 and 9)
3.8. Urease Inhibitory Assay
- = Absorbance of the control sample (C)
- = Absorbance of the negative control sample (N)
- = Absorbance of the test sample (T1 to T7)
- = Absorbance of the negative control of the test sample (N1 to N7)
3.9. Molecular Docking Studies
3.10. Drug-Likeness Prediction of Indole-3-Carbaldehyde Oxime Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xavier, R.J.; Ananthakrishnan, A.N. Gastrointestinal Diseases. In Hunter′s Tropical Medicine and Emerging Infectious Disease, 10th ed.; Ryan, E.T., Hill, D.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 18–27. [Google Scholar]
- Kosikowska, P.; Berlicki, Ł. Urease inhibitors as potential drugs for gastric and urinary tract infections: A patent review. Expert Opin. Ther. Pat. 2011, 21, 945–957. [Google Scholar] [CrossRef] [PubMed]
- Kusters, J.G.; Van Vliet, A.H.M.; Kuipers, E.J. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006, 19, 449–490. [Google Scholar] [CrossRef] [Green Version]
- Owen, R.J. Helicobacter—species classification and identification. Br. Med. Bull. 1998, 54, 17–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mobley, H.L.T.; Hu, L.T.; Foxall, P.A. Helicobacter pylori urease: Properties and role in pathogenesis. Scand. J. Gastroenterol. 1991, 26, 39–46. [Google Scholar] [CrossRef]
- Maroney, M.J.; Ciurli, S. Nonredox nickel enzymes. Chem. Rev. 2014, 114, 4206–4228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menteşe, E.; Emirik, M.; Sökmen, B.B. Design, molecular docking and synthesis of novel 5,6-dichloro-2-methyl-1H- benzimidazole derivatives as potential urease enzyme inhibitors. Bioorg. Chem. 2019, 86, 151–158. [Google Scholar] [CrossRef]
- Konieczna, I.; Zarnowiec, P.; Kwinkowski, M.; Kolesinska, B.; Fraczyk, J.; Kaminski, Z.; Kaca, W. Bacterial urease and its role in long-lasting human diseases. Curr. Protein Pept. Sci. 2012, 13, 789–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estiu, G.; Merz, K.M. The hydrolysis of urea and the proficiency of urease. J. Am. Chem. Soc. 2004, 126, 6932–6944. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Turner, B.S.; Afdhal, N.H.; Keates, S.; Ghiran, I.; Kelly, C.P.; Ewoldt, R.H.; McKinley, G.H.; So, P.; Erramilli, S.; et al. Helicobacter pylori moves through mucus by reducing mucin viscoelasticity. Proc. Natl. Acad. Sci. USA 2009, 106, 14321–14326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Follmer, C. Ureases as a target for the treatment of gastric and urinary infections. J. Clin. Pathol. 2010, 63, 424–430. [Google Scholar] [CrossRef]
- Fagoonee, S.; Pellicano, R. Helicobacter pylori: Molecular basis for colonization and survival in gastric environment and resistance to antibiotics. A short review. Infect. Dis. 2019, 51, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, K.; Hase, J.; Uehara, K. Specific inhibition of urease by hydroxamic acids. BBA—Biochim. Biophys. Acta 1962, 65, 380–383. [Google Scholar] [CrossRef]
- Bayless, A.V.; Millner, O.E. N-[Diaminophosphinyl]arylcarboxamides. U.S. Patent US-4182881-A, 8 January 1980. [Google Scholar]
- Sivapriya, K.; Suguna, P.; Banerjee, A.; Saravanan, V.; Rao, D.N.; Chandrasekaran, S. Facile one-pot synthesis of thio and selenourea derivatives: A new class of potent urease inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 6387–6391. [Google Scholar] [CrossRef] [PubMed]
- Fishbein, N.; Carbone, P.P. Urease Catalysis: II. Inhibition of the enzyme by hydroxyurea, hydroxylamine, and acetohydroxamic acid. J. Biol. Chem. 1965, 240, 2407–2414. [Google Scholar] [CrossRef]
- Hassan, S.; Šudomová, M. The Development of urease inhibitors: What opportunities exist for better treatment of Helicobacter pylori infection in children? Children 2017, 4, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahyoun, T.; Arrault, A.; Schneider, R. Amidoximes and oximes: Synthesis, structure, and their key role as NO donors. Molecules 2019, 24, 2470. [Google Scholar] [CrossRef] [Green Version]
- Baláž, M.; Kudličková, Z.; Vilková, M.; Imrich, J.; Balážová, L.; Daneu, N. Mechanochemical synthesis and isomerization of N- substituted indole-3-carboxaldehyde oximes. Molecules 2019, 24, 3347. [Google Scholar] [CrossRef] [Green Version]
- Chakrabarty, M.; Sarkar, S.; Khasnobis, S.; Harigaya, Y.; Sato, N.; Arima, S. Study of the reactions of four indolic 1-azadienes with a few enoic, ynoic, and azo dienophiles. Synth. Commun. 2002, 32, 2295–2306. [Google Scholar] [CrossRef]
- Budovská, M.; Pilátová, M.B.; Tischlerová, V.; Mojžiš, J. Spirocyclization reactions and antiproliferative activity of indole phytoalexins 1-methoxybrassinin and its 1-substituted derivatives. Arkivoc 2016, 2016, 198–234. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, N.; Xu, J. A concise synthesis of cyclobrassinin and its analogues via a thiyl radical aromatic substitution. New J. Chem. 2018, 42, 13549–13557. [Google Scholar] [CrossRef]
- Goyard, D.; Kónya, B.; Chajistamatiou, A.S.; Chrysina, E.D.; Leroy, J.; Balzarin, S.; Tournier, M.; Tousch, D.; Petit, P.; Duret, C.; et al. Glucose-derived spiro-isoxazolines are anti-hyperglycemic agents against type 2 diabetes through glycogen phosphorylase inhibition. Eur. J. Med. Chem. 2016, 108, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Shaw, K.N.F.; Mcmillan, A.; Gudmundson, A.G.; Armstrong, M.D. Preparation and properties of β-3-indolyl compounds related to tryptophan metabolism. J. Org. Chem. 1958, 17, 1171–1178. [Google Scholar] [CrossRef]
- Ramón, R.S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S.P. Au/Ag-cocatalyzed aldoximes to amides rearrangement under solvent- and acid-free conditions. J. Org. Chem. 2010, 75, 1197–1202. [Google Scholar] [CrossRef]
- Vasil′Tsov, A.M.; Zhang, K.; Ivanov, A.V.; Ushakov, I.A.; Afonin, A.V.; Petrushenko, K.B.; Li, S.; Ma, J.S.; Mikhaleva, A.I.; Trofimov, B.A.; et al. 1-Vinylpyrrole-2-carbaldehyde oximes: Synthesis, isomerization, and spectral properties. Monatsh. Chem. 2009, 140, 1475–1480. [Google Scholar] [CrossRef]
- O′Ferrall, R.A.M.; O’Brien, D. Rate and equilibrium constants for hydrolysis and isomerization of (E)- and (Z)-p-methoxybenzaldehyde oximes. J. Phys. Org. Chem. 2004, 17, 631–640. [Google Scholar] [CrossRef]
- ImageJ; Version 1.53e; Software for Image Process; U. S. National Institutes of Health: Bethesda, MD, USA, 2020.
- Tie-xin, T.; Hong, W. An image analysis system for thin-layer chromatography quantification and its validation. J. Chromatogr. Sci. 2008, 46, 560–564. [Google Scholar] [CrossRef]
- Gunaratna, M.J.; Hua, D.H.; Zou, B.; Pascual, C.; Cao, W.; Zhang, M.; Weerasekara, S.; Nguyen, T.D.T.; Xiao, K.; Xie, X.S. Synthesis of 1,4- and 1,4,4-substituted piperidines for the inhibition of neuronal T-type Ca2+ channels and mitigation of neuropathic pain in mice. Arkivoc 2019, 2019, 22–39. [Google Scholar] [CrossRef]
- Mondal, R.; Mallik, A.K. Recent applications of potassium carbonate in organic synthesis. Org. Prep. Proced. Int. 2014, 46, 391–434. [Google Scholar] [CrossRef]
- Gordon, S.A.; Fleck, A.; Bell, J. Optimal conditions for the estimation of ammonium by the Berthelot reaction. Ann. Clin. Biochem. 1978, 15, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Searle, P.L. The Berthelot or indophenol reaction and its use in the analytical chemistry of nitrogen: A review. Analyst 1984, 109, 549–568. [Google Scholar] [CrossRef]
- Blakeley, R.L.; Webb, E.C.; Zerner, B. Jack bean urease (EC 3.5.1.5). A new purification and reliable rate assay. Biochemistry 1969, 8, 1984–1990. [Google Scholar] [CrossRef] [PubMed]
- Li, W.Y.; Ni, W.W.; Ye, Y.X.; Fang, H.L.; Pan, X.M.; He, J.L.; Zhou, T.L.; Yi, J.; Liu, S.S.; Zhou, M.; et al. N-monoarylacetothioureas as potent urease inhibitors: Synthesis, SAR, and biological evaluation. J. Enzyme Inhib. Med. Chem. 2020, 35, 404–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanif, M.; Shoaib, K.; Saleem, M.; Hasan Rama, N.; Zaib, S.; Iqbal, J. Synthesis, urease inhibition, antioxidant, antibacterial, and molecular docking studies of 1,3,4-oxadiazole derivatives. ISRN Pharmacol. 2012, 2012, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amtul, Z.; Atta-ur-Rahman, B.S.P.; Siddiqui, R.; Choudhary, M. Chemistry and mechanism of urease inhibition. Curr. Med. Chem. 2012, 9, 1323–1348. [Google Scholar] [CrossRef] [PubMed]
- Minkara, M.S.; Ucisik, M.N.; Weaver, M.N.; Merz, K.M. Molecular dynamics study of Helicobacter pylori urease. J. Chem. Theory Comput. 2014, 10, 1852–1862. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Issar, U.; Kakkar, R. In Silico study of the active site of Helicobacter pylori urease and its inhibition by hydroxamic acids. J. Mol. Graph. Model. 2018, 83, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3717. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]
- Barret, R. Importance and evaluation of the Polar Surface Area (PSA and TPSA). In Therapeutical Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 89–95. [Google Scholar]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [Green Version]
- Pervez, H.; Iqbal, M.S.; Tahir, M.Y.; Nasim, F.U.H.; Choudhary, M.I.; Khan, K.M. In vitro cytotoxic, antibacterial, antifungal and urease inhibitory activities of some N4—substituted isatin-3-thiosemicarbazones. J. Enzyme Inhib. Med. Chem. 2008, 23, 848–854. [Google Scholar] [CrossRef]
- RCSB PDB. Available online: https://www.rcsb.org/structure/1E9Y (accessed on 26 June 2021).
- Verdonk, M.L.; Cole, J.C.; Hartshorn, M.J.; Murray, C.W.; Taylor, R.D. Improved protein-ligand docking using GOLD. Proteins Struct. Funct. Genet. 2003, 52, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C.; Leach, A.R.; Taylor, R. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997, 267, 727–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wan, H.; Shi, Y.; Ouyang, P. Personal experience with four kinds of chemical structure drawing software: Review on Chemdraw, Chemwindow, ISIS/Draw, and Chemsketch. J. Chem. Inf. Comput. Sci. 2004, 44, 1886–1890. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Compound | IC50 Value (mM) | Maximum Percentage of Inhibition |
|---|---|---|
| Thiourea | 0.2387 ± 0.0048 | 85.14 |
| 2 | 0.1412 ± 0.0075 | 67.81 |
| 6 | 0.1405 ± 0.0045 | 77.05 |
| 7 | 0.1530 ± 0.0016 | 57.17 |
| 8 | 0.0516 ± 0.0035 | 83.78 |
| 9 | 0.0345 ± 0.0008 | 86.09 |
| Compound | ChemPLP Score |
|---|---|
| Thiourea | 23.27 |
| 2 | 60.50 |
| 6 | 62.06 |
| 7 | 64.92 |
| 8 | 80.47 |
| 9 | 82.17 |
| Compound | MW a/(g/mol) | TPSA b (Å) | Consensus Log Po/w c | Log S d (mol/L) | n-HA e | n-HD f | n-Violations g | GI Absorption h |
|---|---|---|---|---|---|---|---|---|
| Thiourea | 76.12 | 84.13 | −0.43 | 0.29 | 2 | 4 | 0 | high |
| 2 | 160.17 | 48.38 | 1.72 | −2.49 | 3 | 2 | 0 | high |
| 6 | 174.20 | 37.52 | 1.71 | −2.78 | 3 | 1 | 0 | high |
| 7 | 174.20 | 37.52 | 1.74 | −2.78 | 3 | 1 | 0 | high |
| 8 | 250.30 | 37.52 | 2.99 | −4.65 | 3 | 1 | 0 | high |
| 9 | 250.30 | 37.52 | 3.01 | −4.65 | 3 | 1 | 0 | high |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalatuwawege, I.P.; Gunaratna, M.J.; Udukala, D.N. Synthesis, In Silico Studies, and Evaluation of Syn and Anti Isomers of N-Substituted Indole-3-carbaldehyde Oxime Derivatives as Urease Inhibitors against Helicobacter pylori. Molecules 2021, 26, 6658. https://doi.org/10.3390/molecules26216658
Kalatuwawege IP, Gunaratna MJ, Udukala DN. Synthesis, In Silico Studies, and Evaluation of Syn and Anti Isomers of N-Substituted Indole-3-carbaldehyde Oxime Derivatives as Urease Inhibitors against Helicobacter pylori. Molecules. 2021; 26(21):6658. https://doi.org/10.3390/molecules26216658
Chicago/Turabian StyleKalatuwawege, Ishani P., Medha J. Gunaratna, and Dinusha N. Udukala. 2021. "Synthesis, In Silico Studies, and Evaluation of Syn and Anti Isomers of N-Substituted Indole-3-carbaldehyde Oxime Derivatives as Urease Inhibitors against Helicobacter pylori" Molecules 26, no. 21: 6658. https://doi.org/10.3390/molecules26216658
APA StyleKalatuwawege, I. P., Gunaratna, M. J., & Udukala, D. N. (2021). Synthesis, In Silico Studies, and Evaluation of Syn and Anti Isomers of N-Substituted Indole-3-carbaldehyde Oxime Derivatives as Urease Inhibitors against Helicobacter pylori. Molecules, 26(21), 6658. https://doi.org/10.3390/molecules26216658