Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy
Abstract
1. Introduction
2. Pathologies Correlated with Metal Dyshomeostasis
2.1. Genetic Disorders
2.2. Acute and Chronic Metal Intoxication
2.3. Multifactorial Etiology
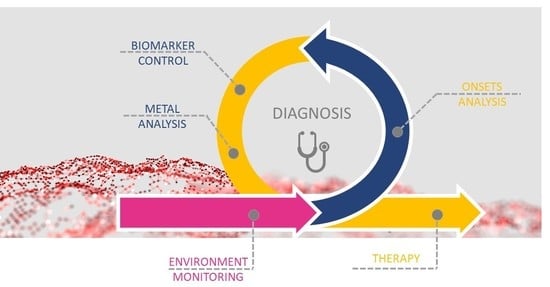
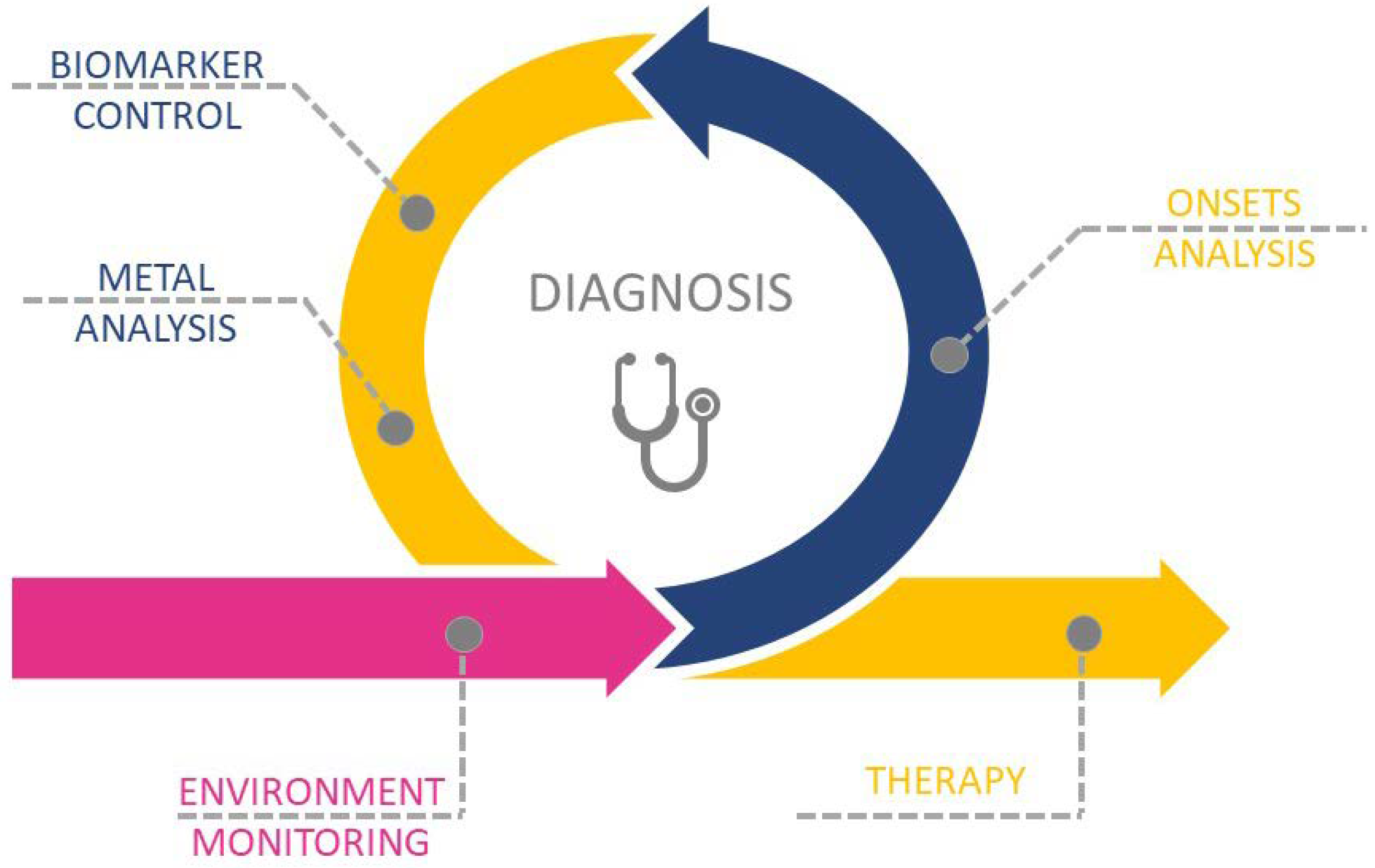
3. Exposure, Diagnosis, and Therapy of Metal-Related Diseases
3.1. Environmental Monitoring
3.2. Metal Accumulation Sites/Tissues
3.3. Drugs Enhancing/Involved in Metal Dyshomeostasis
3.4. Diagnosis
3.5. Therapy for Metal-Related Pathologies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M. Metallodrugs are unique: Opportunities and challenges of discovery and development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef] [PubMed]
- Mohanpuria, P.; Rana, N.K.; Yadav, S.K. Biosynthesis of nanoparticles: Technological concepts and future applications. J. Nanopart. Res. 2008, 10, 507–517. [Google Scholar] [CrossRef]
- Bystrzejewska-Piotrowska, G.; Golimowski, J.; Urban, P.L. Nanoparticles: Their potential toxicity, waste and environmental management. Waste Manag. 2009, 29, 2587–2595. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Medici, S.; Ledda, A.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M. Toxicity of nanoparticles. Curr. Med. Chem. 2014, 21, 3837–3853. [Google Scholar] [CrossRef]
- Bäuerlein, P.S.; Emke, E.; Tromp, P.; Hofman, J.A.; Carboni, A.; Schooneman, F.; de Voogt, P.; van Wezel, A.P. Is there evidence for man-made nanoparticles in the Dutch environment? Sci. Total Environ. 2017, 576, 273–283. [Google Scholar] [CrossRef]
- Keller, A.A.; Lazareva, A. Predicted releases of engineered nanomaterials: From global to regional to local. Environ. Sci. Technol. Lett. 2014, 1, 65–70. [Google Scholar] [CrossRef]
- Crisponi, G.; Nurchi, V.M.; Lachowicz, J.I.; Peana, M.; Medici, S.; Zoroddu, M.A. Toxicity of nanoparticles: Etiology and mechanisms. In Antimicrobial Nanoarchitectonics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 511–546. [Google Scholar]
- Kondaiah, P.; Yaduvanshi, P.S.; Sharp, P.A.; Pullakhandam, R. Iron and zinc homeostasis and interactions: Does enteric zinc excretion cross-talk with intestinal iron absorption? Nutrients 2019, 11, 1885. [Google Scholar] [CrossRef]
- Ugarte, M.; Osborne, N.N.; Brown, L.A.; Bishop, P.N. Iron, zinc, and copper in retinal physiology and disease. Surv. Ophthalmol. 2013, 58, 585–609. [Google Scholar] [CrossRef]
- Park, J.H.; Hogrebe, M.; Fobker, M.; Brackmann, R.; Fiedler, B.; Reunert, J.; Rust, S.; Tsiakas, K.; Santer, R.; Grüneberg, M. SLC39A8 deficiency: Biochemical correction and major clinical improvement by manganese therapy. Genet. Med. 2018, 20, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Piperno, A.; Alessio, M. Aceruloplasminemia: Waiting for an efficient therapy. Front. Neurosci. 2018, 12, 903. [Google Scholar] [CrossRef]
- Lehn, A.; Boyle, R.; Brown, H.; Airey, C.; Mellick, G. Neuroferritinopathy. Parkinsonism Relat. Disord. 2012, 18, 909–915. [Google Scholar] [CrossRef]
- Shribman, S.; Reid, E.; Crosby, A.H.; Houlden, H.; Warner, T.T. Hereditary spastic paraplegia: From diagnosis to emerging therapeutic approaches. Lancet Neurol. 2019, 18, 1136–1146. [Google Scholar] [CrossRef]
- Margetis, K.; Korfias, S.; Boutos, N.; Gatzonis, S.; Themistocleous, M.; Siatouni, A.; Dalivigka, Z.; Flaskas, T.; Stranjalis, G.; Boviatsis, E. Intrathecal baclofen therapy for the symptomatic treatment of hereditary spastic paraplegia. Clin. Neurol. Neurosurg. 2014, 123, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Abusrair, A.H.; Bohlega, S.; Al-Semari, A.; Al-Ajlan, F.S.; Al-Ahmadi, K.; Mohamed, B.; AlDakheel, A. Brain MR Imaging Findings in Woodhouse-Sakati Syndrome. Am. J. Neuroradiol. 2018, 39, 2256–2262. [Google Scholar] [CrossRef]
- Schneider, S.A.; Hardy, J.; Bhatia, K.P. Syndromes of neurodegeneration with brain iron accumulation (NBIA): An update on clinical presentations, histological and genetic underpinnings, and treatment considerations. Mov. Disord. 2012, 27, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Franchini, M.; Veneri, D. Recent advances in hereditary hemochromatosis. Ann. Hematol. 2005, 84, 347–352. [Google Scholar] [CrossRef]
- Brewer, G.J.; Askari, F.K. Wilson’s disease: Clinical management and therapy. J. Hepatol. 2005, 42, S13–S21. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, D.; Travaglini, L.; Drouin, C.A.; Ceballos-Picot, I.; Rizza, T.; Bertini, E.; Carrozzo, R.; Petrini, S.; De Lonlay, P.; El Hachem, M. MEDNIK syndrome: A novel defect of copper metabolism treatable by zinc acetate therapy. Brain 2013, 136, 872–881. [Google Scholar] [CrossRef]
- Sarkar, B.; Lingertat-Walsh, K.; Clarke, J.T. Copper-histidine therapy for Menkes disease. J. Pediatr. 1993, 123, 828–830. [Google Scholar] [CrossRef]
- Ogawa, E.; Kodama, H. Effects of disulfiram treatment in patients with Menkes disease and occipital horn syndrome. J. Trace Elem. Med. Biol. 2012, 26, 102–104. [Google Scholar] [CrossRef]
- Bindu, P.S.; Chiplunkar, S.; Vandana, V.; Nagappa, M.; Govindaraj, P.; Taly, A. Huppke-Brendel Syndrome—GeneReviews®; University of Washington: Seattle, WA, USA, 1993. [Google Scholar] [PubMed]
- Puri, N.; Puri, A. A study on efficacy of oral zinc therapy for treatment of acrodermatitis enteropathica. Our Dermatol. Online 2013, 4, 162. [Google Scholar] [CrossRef][Green Version]
- Glutsch, V.; Hamm, H.; Goebeler, M. Zinc and skin: An update. JDDG J. Dtsch. Dermatol. Ges. 2019, 17, 589–596. [Google Scholar] [CrossRef]
- Mantle, D.; Wilkins, R.; Preedy, V. A novel therapeutic strategy for Ehlers–Danlos syndrome based on nutritional supplements. Med. Hypotheses 2005, 64, 279–283. [Google Scholar] [CrossRef]
- Rossi, M.; Balint, B.; Millar Vernetti, P.; Bhatia, K.P.; Merello, M. Genetic dystonia-ataxia syndromes: Clinical spectrum, diagnostic approach, and treatment options. Mov. Disord. Clin. Pract. 2018, 5, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.-h.; Wang, X.-f.; Yang, G.; Wei, J.; Tan, W.-h.; Wang, L.-x.; Guo, X.; Lammi, M.J.; Xu, J.-H. Efficacy of long-term selenium supplementation in the treatment of chronic Keshan disease with congestive heart failure. Curr. Med. Sci. 2019, 39, 237–242. [Google Scholar] [CrossRef]
- Maani, N.; Karolczak, S.; Dowling, J.J. Genetic therapy for congenital myopathies. Curr. Opin. Neurol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Umair, M.; Alfadhel, M. Genetic Disorders Associated with Metal Metabolism. Cells 2019, 8, 1598. [Google Scholar] [CrossRef]
- Aaseth, J.; Crisponi, G.; Anderson, O. Chelation Therapy in the Treatment of Metal Intoxication; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Bramanti, E.; Onor, M.; Colombaioni, L. Neurotoxicity induced by low thallium doses in living hippocampal neurons: Evidence of early onset mitochondrial dysfunction and correlation with ethanol production. ACS Chem. Neurosci. 2018, 10, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Kazantzis, G. Thallium in the environment and health effects. Environ. Geochem. Health 2000, 22, 275–280. [Google Scholar] [CrossRef]
- Snipes, G.M.; Hafeez, A.; Marek, G.; Winchester, D.E. Sinus bradycardia with haemodynamic compromise following lithium intoxication. BMJ Case Rep. CP 2021, 14, e242946. [Google Scholar] [CrossRef]
- Diserens, L.; Porretta, A.P.; Trana, C.; Meier, D. Lithium-induced ECG modifications: Navigating from acute coronary syndrome to Brugada syndrome. BMJ Case Rep. CP 2021, 14, e241555. [Google Scholar] [CrossRef]
- Ott, M.; Stegmayr, B.; Salander Renberg, E.; Werneke, U. Lithium intoxication: Incidence, clinical course and renal function—A population-based retrospective cohort study. J. Psychopharmacol. 2016, 30, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.-S.; Song, K.-H.; Chung, J.-Y. Health effects of chronic arsenic exposure. J. Prev. Med. Public Health 2014, 47, 245. [Google Scholar] [CrossRef]
- Neslund-Dudas, C.; Kandegedara, A.; Kryvenko, O.N.; Gupta, N.; Rogers, C.; Rybicki, B.A.; Dou, Q.P.; Mitra, B. Prostate tissue metal levels and prostate cancer recurrence in smokers. Biol. Trace Elem. Res. 2014, 157, 107–112. [Google Scholar] [CrossRef]
- Coudon, T.; Hourani, H.; Nguyen, C.; Faure, E.; Mancini, F.R.; Fervers, B.; Salizzoni, P. Assessment of long-term exposure to airborne dioxin and cadmium concentrations in the Lyon metropolitan area (France). Environ. Int. 2018, 111, 177–190. [Google Scholar] [CrossRef]
- Wallace, D.R. Nanotoxicology and metalloestrogens: Possible involvement in breast cancer. Toxics 2015, 3, 390–413. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 3597. [Google Scholar]
- Zimta, A.-A.; Schitcu, V.; Gurzau, E.; Stavaru, C.; Manda, G.; Szedlacsek, S.; Berindan-Neagoe, I. Biological and molecular modifications induced by cadmium and arsenic during breast and prostate cancer development. Environ. Res. 2019, 178, 108700. [Google Scholar] [CrossRef]
- Fischer, R.S.; Unrine, J.M.; Vangala, C.; Sanderson, W.T.; Mandayam, S.; Murray, K.O. Evidence of nickel and other trace elements and their relationship to clinical findings in acute Mesoamerican Nephropathy: A case-control analysis. PLoS ONE 2020, 15, e0240988. [Google Scholar] [CrossRef]
- Bruehlmeier, M.; Leenders, K.L.; Vontobel, P.; Calonder, C.; Antonini, A.; Weindl, A. Increased cerebral iron uptake in Wilson’s disease: A 52Fe-citrate PET study. J. Nucl. Med. 2000, 41, 781–787. [Google Scholar] [PubMed]
- Tomska, N.; Kosik-Bogacka, D.I.; Łanocha-Arendarczyk, N.; Szylińska, A.; Kotfis, K.; Sipak-Szmigiel, O.; Rotter, I. Relationship between concentrations of elements and geographic location in Poland. Ann. Agric. Environ. Med. 2021, 28, 283–290. [Google Scholar] [CrossRef]
- Brozoska, M.; Moniuszko-Jakoniuk, J. Interactions between cadmium and zink in the organism. Food Chem. Toxicol. 2001, 39, 967–980. [Google Scholar] [CrossRef]
- Exley, C. The toxicity of aluminium in humans. Morphologie 2016, 100, 51–55. [Google Scholar] [CrossRef]
- Sheikh, S.; Haque, E.; Mir, S.S. Neurodegenerative diseases: Multifactorial conformational diseases and their therapeutic interventions. J. Neurodegener. Dis. 2013, 2013, 563481. [Google Scholar] [CrossRef]
- Quintanar, L.; Lim, M.H. Metal Ions and Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Poulson, B.G.; Szczepski, K.; Lachowicz, J.I.; Jaremko, L.; Emwas, A.-H.; Jaremko, M. Aggregation of biologically important peptides and proteins: Inhibition or acceleration depending on protein and metal ion concentrations. RSC Adv. 2020, 10, 215–227. [Google Scholar] [CrossRef]
- Emwas, A.-H.; Alghrably, M.; Dhahri, M.; Sharfalddin, A.; Alsiary, R.; Jaremko, M.; Faa, G.; Campagna, M.; Congiu, T.; Piras, M. Living with the enemy: From protein-misfolding pathologies we know, to those we want to know. Ageing Res. Rev. 2021, 70, 101391. [Google Scholar] [CrossRef] [PubMed]
- Piloni, N.E.; Fermandez, V.; Videla, L.A.; Puntarulo, S. Acute iron overload and oxidative stress in brain. Toxicology 2013, 314, 174–182. [Google Scholar] [CrossRef]
- Stockwell, B.R.; Angeli, J.P.F.; Bayir, H.; Bush, A.I.; Conrad, M.; Dixon, S.J.; Fulda, S.; Gascón, S.; Hatzios, S.K.; Kagan, V.E. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 2017, 171, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Wandt, V.K.; Winkelbeiner, N.; Bornhorst, J.; Witt, B.; Raschke, S.; Simon, L.; Ebert, F.; Kipp, A.P.; Schwerdtle, T. A matter of concern–Trace element dyshomeostasis and genomic stability in neurons. Redox Biol. 2021, 41, 101877. [Google Scholar] [CrossRef] [PubMed]
- Grochowski, C.; Blicharska, E.; Krukow, P.; Jonak, K.; Maciejewski, M.; Szczepanek, D.; Jonak, K.; Flieger, J.; Maciejewski, R. Analysis of trace elements in human brain: Its aim, methods, and concentration levels. Front. Chem. 2019, 7, 115. [Google Scholar] [CrossRef]
- Frederickson, C.J.; Suh, S.W.; Silva, D.; Frederickson, C.J.; Thompson, R.B. Importance of zinc in the central nervous system: The zinc-containing neuron. J. Nutr. 2000, 130, 1471S–1483S. [Google Scholar] [CrossRef] [PubMed]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef]
- Li, D.-D.; Zhang, W.; Wang, Z.-Y.; Zhao, P. Serum copper, zinc, and iron levels in patients with Alzheimer’s disease: A meta-analysis of case-control studies. Front. Aging Neurosci. 2017, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Gromadzka, G.; Tarnacka, B.; Flaga, A.; Adamczyk, A. Copper dyshomeostasis in neurodegenerative diseases—Therapeutic implications. Int. J. Mol. Sci. 2020, 21, 9259. [Google Scholar] [CrossRef]
- Bisaglia, M.; Bubacco, L. Copper ions and Parkinson’s disease: Why is homeostasis so relevant? Biomolecules 2020, 10, 195. [Google Scholar] [CrossRef]
- Dodani, S.C.; Domaille, D.W.; Nam, C.I.; Miller, E.W.; Finney, L.A.; Vogt, S.; Chang, C.J. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and X-ray fluorescence microscopy. Proc. Natl. Acad. Sci. USA 2011, 108, 5980–5985. [Google Scholar] [CrossRef]
- Monzani, E.; Nicolis, S.; Dell’Acqua, S.; Capucciati, A.; Bacchella, C.; Zucca, F.A.; Mosharov, E.V.; Sulzer, D.; Zecca, L.; Casella, L. Dopamine, oxidative stress and protein–quinone modifications in Parkinson’s and other neurodegenerative diseases. Angew. Chem. Int. Ed. 2019, 58, 6512–6527. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Nurchi, V.M.; Crisponi, G.; Cappai, I.; Cappai, R.; Busato, M.; Melchior, A.; Tolazzi, M.; Peana, M.; Garribba, E. para-Aminosalicylic acid in the treatment of manganese toxicity. Complexation of Mn 2+ with 4-amino-2-hydroxybenzoic acid and its N-acetylated metabolite. New J. Chem. 2018, 42, 8035–8049. [Google Scholar] [CrossRef]
- Chen, P.; Bornhorst, J.; Aschner, M.A. Manganese metabolism in humans. Front. Biosci. 2018, 711, 1655–1679. [Google Scholar] [CrossRef]
- Ramos, P.; Santos, A.; Pinto, N.R.; Mendes, R.; Magalhães, T.; Almeida, A. Anatomical region differences and age-related changes in copper, zinc, and manganese levels in the human brain. Biol. Trace Elem. Res. 2014, 161, 190–201. [Google Scholar] [CrossRef]
- Nakayama, A.; Hill, K.E.; Austin, L.M.; Motley, A.K.; Burk, R.F. All regions of mouse brain are dependent on selenoprotein P for maintenance of selenium. J. Nutr. 2007, 137, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Angeli, J.P.F.; Schneider, M.; Proneth, B.; Tyurina, Y.Y.; Tyurin, V.A.; Hammond, V.J.; Herbach, N.; Aichler, M.; Walch, A.; Eggenhofer, E. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 2014, 16, 1180–1191. [Google Scholar] [CrossRef]
- Alim, I.; Caulfield, J.T.; Chen, Y.; Swarup, V.; Geschwind, D.H.; Ivanova, E.; Seravalli, J.; Ai, Y.; Sansing, L.H.; Marie, E.J.S. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 2019, 177, 1262–1279. [Google Scholar] [CrossRef]
- Cascella, R.; Cecchi, C. Calcium Dyshomeostasis in Alzheimer’s Disease Pathogenesis. Int. J. Mol. Sci. 2021, 22, 4914. [Google Scholar] [CrossRef] [PubMed]
- Lynch, G.; Seubert, P. Links between long-term potentiation and neuropathology. An hypothesis involving calcium-activated proteases. Ann. N. Y. Acad. Sci. 1989, 568, 171–180. [Google Scholar] [CrossRef]
- Khachaturian, Z.S. Introduction and overview. Ann. N. Y. Acad. Sci. 1989, 568, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Toescu, E.C. Calcium and neuronal ageing. Trends Neurosci. 1998, 21, 2–7. [Google Scholar] [CrossRef]
- Supnet, C.; Bezprozvanny, I. The dysregulation of intracellular calcium in Alzheimer disease. Cell Calcium 2010, 47, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Europea, C. The Appropriateness of Existing Methodologies to Assess the Potential Risks Associated with Engineered and Adventitious Products of Nanotechnologies; European Commission: Strasbourg, France, 2006.
- Keller, A.A.; McFerran, S.; Lazareva, A.; Suh, S. Global life cycle releases of engineered nanomaterials. J. Nanopart. Res. 2013, 15, 1692. [Google Scholar] [CrossRef]
- Kim, Y.; Smith, J.G.; Jain, P.K. Harvesting multiple electron–hole pairs generated through plasmonic excitation of Au nanoparticles. Nat. Chem. 2018, 10, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Laux, P.; Riebeling, C.; Booth, A.M.; Brain, J.D.; Brunner, J.; Cerrillo, C.; Creutzenberg, O.; Estrela-Lopis, I.; Gebel, T.; Johanson, G. Challenges in characterizing the environmental fate and effects of carbon nanotubes and inorganic nanomaterials in aquatic systems. Environ. Sci. Nano 2018, 5, 48–63. [Google Scholar] [CrossRef]
- Pati, S.S.; Singh, L.H.; Guimarães, E.; Mantilla, J.; Coaquira, J.; Oliveira, A.; Sharma, V.K.; Garg, V.K. Magnetic chitosan-functionalized Fe3O4@ Au nanoparticles: Synthesis and characterization. J. Alloys Compd. 2016, 684, 68–74. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Birla, S.; Yadav, A.; Santos, C.A.D. Strategic role of selected noble metal nanoparticles in medicine. Crit. Rev. Microbiol. 2016, 42, 696–719. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sayes, C.M.; Guo, B.; Pillai, S.; Parsons, J.G.; Wang, C.; Yan, B.; Ma, X. Interactions between silver nanoparticles and other metal nanoparticles under environmentally relevant conditions: A review. Sci. Total Environ. 2019, 653, 1042–1051. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, D.W.; Lee, Y.H.; Oh, J.H.; Yoon, S.; Choi, M.S.; Lee, S.K.; Kim, J.W.; Lee, K.; Song, C.-W. Silver nanoparticles induce apoptosis and G2/M arrest via PKCζ-dependent signaling in A549 lung cells. Arch. Toxicol. 2011, 85, 1529–1540. [Google Scholar] [CrossRef]
- Pietruska, J.R.; Liu, X.; Smith, A.; McNeil, K.; Weston, P.; Zhitkovich, A.; Hurt, R.; Kane, A.B. Bioavailability, intracellular mobilization of nickel, and HIF-1α activation in human lung epithelial cells exposed to metallic nickel and nickel oxide nanoparticles. Toxicol. Sci. 2011, 124, 138–148. [Google Scholar] [CrossRef]
- Morawska, L.; Wang, H.; Ristovski, Z.; Jayaratne, E.; Johnson, G.; Cheung, H.; Ling, X.; He, C. JEM spotlight: Environmental monitoring of airborne nanoparticles. J. Environ. Monit. 2009, 11, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Aiken, G.R.; Hsu-Kim, H.; Ryan, J.N. Influence of Dissolved Organic Matter on the Environmental Fate of Metals, Nanoparticles, and Colloids. Environ. Sci. Technol. 2011, 45, 3196–3201. [Google Scholar] [CrossRef]
- Baalousha, M.; Yang, Y.; Vance, M.E.; Colman, B.P.; McNeal, S.; Xu, J.; Blaszczak, J.; Steele, M.; Bernhardt, E.; Hochella, M.F., Jr. Outdoor urban nanomaterials: The emergence of a new, integrated, and critical field of study. Sci. Total Environ. 2016, 557, 740–753. [Google Scholar] [CrossRef]
- Jiang, C.; Castellon, B.T.; Matson, C.W.; Aiken, G.R.; Hsu-Kim, H. Relative contributions of copper oxide nanoparticles and dissolved copper to Cu uptake kinetics of Gulf killifish (Fundulus grandis) embryos. Environ. Sci. Technol. 2017, 51, 1395–1404. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Hu, X.; Zhou, Q.; Li, X.; Miao, X.; Zhou, R. Nanocolloids in natural water: Isolation, characterization, and toxicity. Environ. Sci. Technol. 2018, 52, 4850–4860. [Google Scholar] [CrossRef] [PubMed]
- Rajput, V.; Minkina, T.; Fedorenko, A.; Sushkova, S.; Mandzhieva, S.; Lysenko, V.; Duplii, N.; Fedorenko, G.; Dvadnenko, K.; Ghazaryan, K. Toxicity of copper oxide nanoparticles on spring barley (Hordeum sativum distichum). Sci. Total Environ. 2018, 645, 1103–1113. [Google Scholar] [CrossRef]
- Wimmer, A.; Kalinnik, A.; Schuster, M. New insights into the formation of silver-based nanoparticles under natural and semi-natural conditions. Water Res. 2018, 141, 227–234. [Google Scholar] [CrossRef]
- Bakshi, S.; He, Z.L.; Harris, W.G. Natural nanoparticles: Implications for environment and human health. Crit. Rev. Environ. Sci. Technol. 2015, 45, 861–904. [Google Scholar] [CrossRef]
- Dong, S.; Qu, M.; Rui, Q.; Wang, D. Combinational effect of titanium dioxide nanoparticles and nanopolystyrene particles at environmentally relevant concentrations on nematode Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2018, 161, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Garner, K.L.; Keller, A.A. Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies. J. Nanopart. Res. 2014, 16, 1–28. [Google Scholar] [CrossRef]
- Manfra, L.; Rotini, A.; Bergami, E.; Grassi, G.; Faleri, C.; Corsi, I. Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis. Ecotoxicol. Environ. Saf. 2017, 145, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles–formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef]
- Wilke, C.M.; Tong, T.; Gaillard, J.-F.; Gray, K.A. Attenuation of microbial stress due to nano-Ag and nano-TiO2 interactions under dark conditions. Environ. Sci. Technol. 2016, 50, 11302–11310. [Google Scholar] [CrossRef] [PubMed]
- Schulte, P.; Murashov, V.; Zumwalde, R.; Kuempel, E.; Geraci, C. Occupational exposure limits for nanomaterials: State of the art. J. Nanopart. Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Seaton, A.; Tran, L.; Aitken, R.; Donaldson, K. Nanoparticles, human health hazard and regulation. J. R. Soc. Interface 2010, 7 (Suppl. 1), S119–S129. [Google Scholar] [CrossRef]
- Elder, A.; Oberdörster, G. Translocation and effects of ultrafine particles outside of the lung. Clin. Occup. Environ. Med. 2006, 5, 785–796. [Google Scholar]
- Medina, C.; Santos-Martinez, M.; Radomski, A.; Corrigan, O.; Radomski, M. Nanoparticles: Pharmacological and toxicological significance. Br. J. Pharmacol. 2007, 150, 552–558. [Google Scholar] [CrossRef]
- Remelli, M.; Peana, M.; Medici, S.; Delogu, L.G.; Zoroddu, M.A. Interaction of divalent cations with peptide fragments from Parkinson’s disease genes. Dalton Trans. 2013, 42, 5964–5974. [Google Scholar] [CrossRef]
- Oberdörster, G.; Oberdörster, E.; Oberdörster, J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005, 113, 823–839. [Google Scholar] [CrossRef]
- Montanari, S.; Gatti, A.M. Nanopathology: The Health Impact of Nanoparticles; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Gatti, A.M.; Bosco, P.; Rivasi, F.; Bianca, S.; Ettore, G.; Gaetti, L.; Montanari, S.; Bartoloni, G.; Gazzolo, D. Heavy metals nanoparticles in fetal kidney and liver tissues. Front. Biosci. 2011, 3, 221–226. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iannitti, T.; Capone, S.; Gatti, A.; Capitani, F.; Cetta, F.; Palmieri, B. Intracellular heavy metal nanoparticle storage: Progressive accumulation within lymph nodes with transformation from chronic inflammation to malignancy. Int. J. Nanomed. 2010, 5, 955. [Google Scholar] [CrossRef]
- Elder, A.; Gelein, R.; Silva, V.; Feikert, T.; Opanashuk, L.; Carter, J.; Potter, R.; Maynard, A.; Ito, Y.; Finkelstein, J. Translocation of inhaled ultrafine manganese oxide particles to the central nervous system. Environ. Health Perspect. 2006, 114, 1172–1178. [Google Scholar] [CrossRef]
- Oberdörster, G.; Sharp, Z.; Atudorei, V.; Elder, A.; Gelein, R.; Kreyling, W.; Cox, C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004, 16, 437–445. [Google Scholar] [CrossRef]
- Gatti, A.M.; Montanari, S. The side effects of drugs: Nanopathological hazards and risks. In Particles and Nanoparticles in Pharmaceutical Products; Springer: Berlin/Heidelberg, Germany, 2018; pp. 429–443. [Google Scholar]
- Snyder, W.; Cook, M.; Nasset, E.; Karhausen, L.; Howells, G.P.; Tipton, I. Report of the Task Group on Reference Man; ICRP Publication: Oxford, UK, 1975; Volume 23. [Google Scholar]
- Locci, E.; Pilia, I.; Piras, R.; Pili, S.; Marcias, G.; Cocco, P.; De Giorgio, F.; Bernabei, M.; Brusadin, V.; Allegrucci, L. Particle Background Levels in Human Tissues—PABALIHT project. Part I: A nanometallomic study of metal-based micro-and nanoparticles in liver and kidney in an Italian population group. J. Nanopart. Res. 2019, 21, 45. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.; Elisaf, M. Blood pressure drug therapy and electrolyte disturbances. Int. J. Clin. Pract. 2008, 62, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Braun, L.A.; Rosenfeldt, F. Pharmaco-nutrient Interactions—A systematic review of zinc and antihypertensive therapy. Int. J. Clin. Pract. 2013, 67, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, J.I.; Nurchi, V.M.; Crisponi, G.; de Guadalupe Jaraquemada-Pelaez, M.; Caltagirone, C.; Peana, M.; Zoroddu, M.A.; Szewczuk, Z.; Cooper, G.J. Complex formation equilibria of Cu2+ and Zn2+ with Irbesartan and Losartan. Eur. J. Pharm. Sci. 2017, 97, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Karges, W.; Rink, L. Zinc and Diabetes—Clinical links and molecular mechanisms. J. Nutr. Biochem. 2009, 20, 399–417. [Google Scholar] [CrossRef]
- Yetley, E.A. Multivitamin and multimineral dietary supplements: Definitions, characterization, bioavailability, and drug interactions. Am. J. Clin. Nutr. 2007, 85, 269S–276S. [Google Scholar] [CrossRef] [PubMed]
- Pitt, C.G.; Martell, A.E. The Design of Chelating Agents for the Treatment of Iron Overload; ACS Publications: Washington, DC, USA, 1980. [Google Scholar] [CrossRef]
- Saran, B.M.; Russell, G.F. The effects of administering lithium carbonate on the balance of Na, K and water in manic-depressive patients. Psychol. Med. 1976, 6, 381–392. [Google Scholar] [CrossRef]
- Schrauzer, G.N.; Shrestha, K.P. Lithium in drinking water and the incidences of crimes, suicides, and arrests related to drug addictions. Biol. Trace Elem. Res. 1990, 25, 105–113. [Google Scholar] [CrossRef]
- Ohgami, H.; Terao, T.; Shiotsuki, I.; Ishii, N.; Iwata, N. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry 2009, 194, 464–465. [Google Scholar] [CrossRef] [PubMed]
- Fels, A. Should we all take a bit of lithium. New York Times, 14 September 2014. [Google Scholar]
- Finley, P.R. Drug interactions with lithium: An update. Clin. Pharmacokinet. 2016, 55, 925–941. [Google Scholar] [CrossRef]
- Vasantha, P.; Shekhar, B.; PV, A.L. Copper-metformin ternary complexes: Thermal, photochemosensitivity and molecular docking studies. Mater. Sci. Eng. C 2018, 90, 621–633. [Google Scholar]
- Zhu, B.-Z.; Mao, L.; Fan, R.-M.; Zhu, J.-G.; Zhang, Y.-N.; Wang, J.; Kalyanaraman, B.; Frei, B. Ergothioneine prevents copper-induced oxidative damage to DNA and protein by forming a redox-inactive Ergothioneine—Copper complex. Chem. Res. Toxicol. 2011, 24, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, S.; Alghrably, M.; Campagna, M.; Hauser, C.A.E.; Jaremko, M.; Lachowicz, J.I. Metal Complex Formation and Anticancer Activity of Cu (I) and Cu (II) Complexes with Metformin. Molecules 2021, 26, 4730. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Hudecek, R.; Stejskal, J.; Sterzl, I. Diagnosis and treatment of metal-induced side-effects. Neuro Endocrinol. Lett. 2006, 27 (Suppl. 1), 7–16. [Google Scholar] [PubMed]
- Weiss, N.S.; LIFF, J.M. Accounting for the multicausal nature of disease in the design and analysis of epidemiologic studies. Am. J. Epidemiol. 1983, 117, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Sirlin, C.B.; Reeder, S.B. Magnetic resonance imaging quantification of liver iron. Magn. Reson. Imaging Clin. 2010, 18, 359–381. [Google Scholar] [CrossRef] [PubMed]
- Biglia, A.; Morandi, V.; Monti, S.; Delvino, P.; Cavagna, L.; Montecucco, C. Cobalt hip prosthesis intoxication mimicking an autoimmune disease. Jt. Bone Spine 2020, 87, 652–654. [Google Scholar] [CrossRef]
- Pornwilard, M.; Weiskirchen, R.; Gassler, N.; Bosserhoff, A.K.; Becker, J.S. Novel bioimaging techniques of metals by laser ablation inductively coupled plasma mass spectrometry for diagnosis of fibrotic and cirrhotic liver disorders. PLoS ONE 2013, 8, e58702. [Google Scholar]
- Susnea, I.; Weiskirchen, R. Trace metal imaging in diagnostic of hepatic metal disease. Mass Spectrom. Rev. 2016, 35, 666–686. [Google Scholar] [CrossRef]
- Henderson, R.; Hobbie, J.; Landrigan, P.; Mattisoti, D.; Perera, F.; Pfttaer, E.; Silbergeld, E.; Wogan, G. Biological markers in environmental health research. Environ. Health Perspect. 1987, 7, 3–9. [Google Scholar]
- Westphal, G.; Schnuch, A.; Schulz, T.; Reich, K.; Aberer, W.; Brasch, J.; Koch, P.; Wessbecher, R.; Szliska, C.; Bauer, A. Homozygous gene deletions of the glutathione S-transferases M1 and T1 are associated with thimerosal sensitization. Int. Arch. Occup. Environ. Health 2000, 73, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Lindstedt, G.; Gottberg, I.; Holmgren, B.; Jonsson, T.; Karlsson, G. Individual mercury exposure of chloralkali workers and its relation to blood and urinary mercury levels. Scand. J. Work Environ. Health 1979, 5, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.R.; Shannon, M.W.; Committee on Environmental Health. Technical report: Mercury in the environment: Implications for pediatricians. Pediatrics 2001, 108, 197–205. [Google Scholar] [CrossRef]
- Hegde, A.; Shetty, G.; Jayasheelan, N. The Perturbation Encompassing Dental Amalgam Toxicity: A Review. Indian J. Forensic Med. Toxicol. 2020, 14, 147–152. [Google Scholar]
- Bernhoft, R.A. Mercury toxicity and treatment: A review of the literature. J. Environ. Public Health 2012, 2012, 460508. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.-J.; Kim, B.-G.; Jeon, M.-J.; Kim, S.-Y.; Kim, H.-C.; Jang, T.-W.; Chae, H.-J.; Choi, W.-J.; Ha, M.-N.; Hong, Y.-S. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann. Occup. Environ. Med. 2016, 28, 5. [Google Scholar] [CrossRef] [PubMed]
- Bonenfant, J.; Miller, G.; Roy, P. Quebec beer-drinkers’ cardiomyopathy: Pathological studies. Can. Med. Assoc. J. 1967, 97, 910. [Google Scholar]
- Linna, A.; Oksa, P.; Groundstroem, K.; Halkosaari, M.; Palmroos, P.; Huikko, S.; Uitti, J. Exposure to cobalt in the production of cobalt and cobalt compounds and its effect on the heart. Occup. Environ. Med. 2004, 61, 877–885. [Google Scholar] [CrossRef]
- Peters, R.M.; Willemse, P.; Rijk, P.C.; Hoogendoorn, M.; Zijlstra, W.P. Fatal cobalt toxicity after a non-metal-on-metal total hip arthroplasty. Case Rep. Orthop. 2017, 2017, 9123684. [Google Scholar] [CrossRef] [PubMed]
- Paustenbach, D.J.; Galbraith, D.A.; Finley, B.L. Interpreting cobalt blood concentrations in hip implant patients. Clin. Toxicol. 2014, 52, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Allen, L.A.; Ambardekar, A.V.; Devaraj, K.M.; Maleszewski, J.J.; Wolfel, E.E. Missing elements of the history. N. Engl. J. Med. 2014, 370, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, I.; Nikolopoulos, D.; Michos, I.; Patsouris, E.; Theocharis, S. Local and systemic toxicity of nanoscale debris particles in total hip arthroplasty. J. Appl. Toxicol. 2012, 32, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.; Holland, J.; Quigley, L.; Sprague, S.; Bhandari, M. Pseudotumors associated with total hip arthroplasty. JBJS 2012, 94, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Aaseth, J.; Skaug, M.A.; Cao, Y.; Andersen, O. Chelation in metal intoxication—principles and paradigms. J. Trace Elem. Med. Biol. 2015, 31, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Foreman, H.; Hamilton, J.G. The Use of Chelating Agents for Accelerating Excretion of Radioelements; US Atomic Energy Commission, Technical Information Service: San Francisco, CA, USA, 1951; Volume 3247.
- Soares, F.A.; Farina, M.; Santos, F.W.; Souza, D.; Rocha, J.B.T.; Nogueira, C.W. Interaction between metals and chelating agents affects glutamate binding on brain synaptic membranes. Neurochem. Res. 2003, 28, 1859–1865. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O. Principles and recent developments in chelation treatment of metal intoxication. Chem. Rev. 1999, 99, 2683–2710. [Google Scholar] [CrossRef] [PubMed]
- Modell, W.; Gold, H.; Cattell, M. Clinical uses of 2,3-dimercaptopropanol (BAL). IV. Pharmacologic observations on BAL by intramuscular injection in man. J. Clin. Investig. 1946, 25, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Vilensky, J.A.; Redman, K. British anti-Lewisite (dimercaprol): An amazing history. Ann. Emerg. Med. 2003, 41, 378–383. [Google Scholar] [CrossRef]
- Genoud, S.; Roberts, B.R.; Gunn, A.P.; Halliday, G.M.; Lewis, S.J.; Ball, H.J.; Hare, D.J.; Double, K.L. Subcellular compartmentalisation of copper, iron, manganese, and zinc in the Parkinson’s disease brain. Metallomics 2017, 9, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- McLeary, F.A.; Rcom-H’cheo-Gauthier, A.N.; Goulding, M.; Radford, R.A.; Okita, Y.; Faller, P.; Chung, R.S.; Pountney, D.L. Switching on Endogenous metal binding proteins in Parkinson’s Disease. Cells 2019, 8, 179. [Google Scholar] [CrossRef]
- Davies, K.M.; Bohic, S.; Carmona, A.; Ortega, R.; Cottam, V.; Hare, D.J.; Finberg, J.P.; Reyes, S.; Halliday, G.M.; Mercer, J.F. Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol. Aging 2014, 35, 858–866. [Google Scholar] [CrossRef]
- Tórsdóttir, G.; Kristinsson, J.; Sveinbjörnsdóttir, S.; Snaedal, J.; Jóhannesson, T. Copper, ceruloplasmin, superoxide dismutase and iron parameters in Parkinson’s disease. Pharmacol. Toxicol. 1999, 85, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Bjorklund, G.; Stejskal, V.; Urbina, M.A.; Dadar, M.; Chirumbolo, S.; Mutter, J. Metals and Parkinson’s disease: Mechanisms and biochemical processes. Curr. Med. Chem. 2018, 25, 2198–2214. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.H.; Karri, V.; Tay, N.W.R.; Chang, K.H.; Ah, H.Y.; Ng, P.Q.; San Ho, H.; Keh, H.W.; Candasamy, M. Emerging pathways to neurodegeneration: Dissecting the critical molecular mechanisms in Alzheimer’s disease, Parkinson’s disease. Biomed. Pharmacother. 2019, 111, 765–777. [Google Scholar] [CrossRef] [PubMed]
- Tosato, M.; Di Marco, V. Metal chelation therapy and Parkinson’s disease: A critical review on the thermodynamics of complex formation between relevant metal ions and promising or established drugs. Biomolecules 2019, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Prachayasittikul, V.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V. 8-Hydroxyquinolines: A review of their metal chelating properties and medicinal applications. Drug Des. Dev. Ther. 2013, 7, 1157. [Google Scholar] [CrossRef] [PubMed]
- Grasso, G.; Santoro, A.M.; Lanza, V.; Sbardella, D.; Tundo, G.R.; Ciaccio, C.; Marini, S.; Coletta, M.; Milardi, D. The double faced role of copper in Aβ homeostasis: A survey on the interrelationship between metal dyshomeostasis, UPS functioning and autophagy in neurodegeneration. Coord. Chem. Rev. 2017, 347, 1–22. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, H.; Watanabe, R.; Haratake, M.; Nakayama, M.; Saji, H. Diphenylpropynone derivatives as probes for imaging β-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. Lett. 2011, 21, 117–120. [Google Scholar] [CrossRef]
- Jones, M.R.; Mathieu, E.; Dyrager, C.; Faissner, S.; Vaillancourt, Z.; Korshavn, K.J.; Lim, M.H.; Ramamoorthy, A.; Yong, V.W.; Tsutsui, S. Multi-target-directed phenol–triazole ligands as therapeutic agents for Alzheimer’s disease. Chem. Sci. 2017, 8, 5636–5643. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gomes, L.M.; Zhang, T.; Storr, T. A small bifunctional chelator that modulates Aβ42 aggregation. Can. J. Chem. 2018, 96, 78–82. [Google Scholar] [CrossRef]
- Liu, Y.; Kochi, A.; Pithadia, A.S.; Lee, S.; Nam, Y.; Beck, M.W.; He, X.; Lee, D.; Lim, M.H. Tuning reactivity of diphenylpropynone derivatives with metal-associated amyloid-β species via structural modifications. Inorg. Chem. 2013, 52, 8121–8130. [Google Scholar] [CrossRef]
- Cao, Z.; Yang, J.; Xu, R.; Song, Q.; Zhang, X.; Liu, H.; Qiang, X.; Li, Y.; Tan, Z.; Deng, Y. Design, synthesis and evaluation of 4′-OH-flurbiprofen-chalcone hybrids as potential multifunctional agents for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2018, 26, 1102–1115. [Google Scholar] [CrossRef] [PubMed]
- Fosso, M.Y.; LeVine, H., 3rd; Green, K.D.; Tsodikov, O.V.; Garneau-Tsodikova, S. Effects of structural modifications on the metal binding, anti-amyloid activity, and cholinesterase inhibitory activity of chalcones. Org. Biomol. Chem. 2015, 13, 9418–9426. [Google Scholar] [CrossRef]
- Schugar, H.; Green, D.E.; Bowen, M.L.; Scott, L.E.; Storr, T.; Böhmerle, K.; Thomas, F.; Allen, D.D.; Lockman, P.R.; Merkel, M. Combating Alzheimer’s disease with multifunctional molecules designed for metal passivation. Angew. Chem. 2007, 119, 1746–1748. [Google Scholar] [CrossRef]
- Telpoukhovskaia, M.A.; Cawthray, J.F.; Rodríguez-Rodríguez, C.; Scott, L.E.; Page, B.D.; Patrick, B.O.; Orvig, C. 3-Hydroxy-4-pyridinone derivatives designed for fluorescence studies to determine interaction with amyloid protein as well as cell permeability. Bioorg. Med. Chem. Lett. 2015, 25, 3654–3657. [Google Scholar] [CrossRef]
- Green, D.E.; Bowen, M.L.; Scott, L.E.; Storr, T.; Merkel, M.; Böhmerle, K.; Thompson, K.H.; Patrick, B.O.; Schugar, H.J.; Orvig, C. In vitro studies of 3-hydroxy-4-pyridinones and their glycosylated derivatives as potential agents for Alzheimer’s disease. Dalton Trans. 2010, 39, 1604–1615. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Cai, P.; Liu, Q.; Wu, J.; Yin, Y.; Wang, X.; Kong, L. Novel 8-hydroxyquinoline derivatives targeting β-amyloid aggregation, metal chelation and oxidative stress against Alzheimer’s disease. Bioorg. Med. Chem. 2018, 26, 3191–3201. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.; Vieira, R.P.; Jones, M.R.; Wang, M.C.; Dyrager, C.; Souza-Fagundes, E.M.; Da Silva, J.G.; Storr, T.; Beraldo, H. 8-Hydroxyquinoline Schiff-base compounds as antioxidants and modulators of copper-mediated Aβ peptide aggregation. J. Inorg. Biochem. 2014, 139, 106–116. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Yang, X.; Feng, X.; Li, X.; Huang, L.; Chan, A.S. Design, synthesis, and evaluation of orally bioavailable quinoline–indole derivatives as innovative multitarget-directed ligands: Promotion of cell proliferation in the adult murine Hippocampus for the treatment of alzheimer’s disease. J. Med. Chem. 2018, 61, 1871–1894. [Google Scholar] [CrossRef]
- Zheng, H.; Youdim, M.B.; Fridkin, M. Site-activated multifunctional chelator with acetylcholinesterase and neuroprotective− neurorestorative moieties for Alzheimer’s therapy. J. Med. Chem. 2009, 52, 4095–4098. [Google Scholar] [CrossRef]
- Oliveri, V.; Grasso, G.I.; Bellia, F.; Attanasio, F.; Viale, M.; Vecchio, G. Soluble sugar-based quinoline derivatives as new antioxidant modulators of metal-induced amyloid aggregation. Inorg. Chem. 2015, 54, 2591–2602. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, T.; Zhu, S.; Gu, X.; Jia, X.; Lu, Y.; Zhu, L. Two macrocyclic polyamines as modulators of metal-mediated Aβ40 aggregation. Integr. Biol. 2015, 7, 655–662. [Google Scholar] [CrossRef]
- Lanza, V.; D’Agata, R.; Iacono, G.; Bellia, F.; Spoto, G.; Vecchio, G. Cyclam glycoconjugates as lectin ligands and protective agents of metal-induced amyloid aggregation. J. Inorg. Biochem. 2015, 153, 377–382. [Google Scholar] [CrossRef]
- Lincoln, K.M.; Gonzalez, P.; Richardson, T.E.; Julovich, D.A.; Saunders, R.; Simpkins, J.W.; Green, K.N. A potent antioxidant small molecule aimed at targeting metal-based oxidative stress in neurodegenerative disorders. Chem. Commun. 2013, 49, 2712–2714. [Google Scholar] [CrossRef]
- Lincoln, K.M.; Richardson, T.E.; Rutter, L.; Gonzalez, P.; Simpkins, J.W.; Green, K.N. An N-heterocyclic amine chelate capable of antioxidant capacity and amyloid disaggregation. ACS Chem. Neurosci. 2012, 3, 919–927. [Google Scholar] [CrossRef]
- Gonzalez, P.; Da Costa, V.C.; Hyde, K.; Wu, Q.; Annunziata, O.; Rizo, J.; Akkaraju, G.; Green, K.N. Bimodal-hybrid heterocyclic amine targeting oxidative pathways and copper mis-regulation in Alzheimer’s disease. Metallomics 2014, 6, 2072–2082. [Google Scholar] [CrossRef]
- Sharma, A.K.; Pavlova, S.T.; Kim, J.; Finkelstein, D.; Hawco, N.J.; Rath, N.P.; Kim, J.; Mirica, L.M. Bifunctional compounds for controlling metal-mediated aggregation of the Aβ42 peptide. J. Am. Chem. Soc. 2012, 134, 6625–6636. [Google Scholar] [CrossRef]
- Jones, M.R.; Mu, C.; Wang, M.C.; Webb, M.I.; Walsby, C.J.; Storr, T. Modulation of the Aβ peptide aggregation pathway by KP1019 limits Aβ-associated neurotoxicity. Metallomics 2015, 7, 129–135. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.-Y.; Yin, W.-X.; Yin, J.; Zhang, S.-B.; Liu, C.-L. The chelation targeting metal–Aβ40 aggregates may lead to formation of Aβ40 oligomers. Dalton Trans. 2011, 40, 4830–4833. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Telpoukhovskaia, M.; Alí-Torres, J.; Rodríguez-Santiago, L.; Manso, Y.; Bailey, G.; Hidalgo, J.; Sodupe, M.; Orvig, C. Thioflavin-based molecular probes for application in Alzheimer’s disease: From in silico to in vitro models. Metallomics 2015, 7, 83–92. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.; Sanchez de Groot, N.; Rimola, A.; Alvarez-Larena, A.; Lloveras, V.; Vidal-Gancedo, J.; Ventura, S.; Vendrell, J.; Sodupe, M.; González-Duarte, P. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J. Am. Chem. Soc. 2009, 131, 1436–1451. [Google Scholar] [CrossRef]
- Viveiros, R.; Karim, K.; Piletsky, S.; Heggie, W.; Casimiro, T. Development of a molecularly imprinted polymer for a pharmaceutical impurity in supercritical CO2: Rational design using computational approach. J. Clean. Prod. 2017, 168, 1025–1031. [Google Scholar] [CrossRef]
- Beck, M.W.; Derrick, J.S.; Kerr, R.A.; Oh, S.B.; Cho, W.J.; Lee, S.J.C.; Ji, Y.; Han, J.; Tehrani, Z.A.; Suh, N. Structure-mechanism-based engineering of chemical regulators targeting distinct pathological factors in Alzheimer’s disease. Nat. Commun. 2016, 7, 13115. [Google Scholar] [CrossRef]
- Xu, P.; Zhang, M.; Sheng, R.; Ma, Y. Synthesis and biological evaluation of deferiprone-resveratrol hybrids as antioxidants, Aβ1–42 aggregation inhibitors and metal-chelating agents for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 127, 174–186. [Google Scholar] [CrossRef]
- Li, S.-Y.; Wang, X.-B.; Kong, L.-Y. Design, synthesis and biological evaluation of imine resveratrol derivatives as multi-targeted agents against Alzheimer’s disease. Eur. J. Med. Chem. 2014, 71, 36–45. [Google Scholar] [CrossRef]
- DeToma, A.S.; Krishnamoorthy, J.; Nam, Y.; Lee, H.J.; Brender, J.R.; Kochi, A.; Lee, D.; Onnis, V.; Congiu, C.; Manfredini, S. Interaction and reactivity of synthetic aminoisoflavones with metal-free and metal-associated amyloid-β. Chem. Sci. 2014, 5, 4851–4862. [Google Scholar] [CrossRef]
- He, X.; Park, H.M.; Hyung, S.-J.; DeToma, A.S.; Kim, C.; Ruotolo, B.T.; Lim, M.H. Exploring the reactivity of flavonoid compounds with metal-associated amyloid-β species. Dalton Trans. 2012, 41, 6558–6566. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Cai, P.; Liu, Q.-H.; Xu, D.-Q.; Yang, X.-L.; Wu, J.-J.; Kong, L.-Y.; Wang, X.-B. Rational modification of donepezil as multifunctional acetylcholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2016, 123, 282–297. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.-M.; Wu, J.-J.; Wang, J.; Xie, S.-S.; Lan, J.-S.; Xu, W.; Kong, L.-Y.; Wang, X.-B. Synthesis and pharmacological evaluation of donepezil-based agents as new cholinesterase/monoamine oxidase inhibitors for the potential application against Alzheimer’s disease. J. Enzym. Inhib. Med. Chem. 2016, 31 (Suppl. 3), 41–53. [Google Scholar] [CrossRef]
- Benchekroun, M.; Romero, A.; Egea, J.; Leon, R.; Michalska, P.; Buendía, I.; Jimeno, M.L.; Jun, D.; Janockova, J.; Sepsova, V. The antioxidant additive approach for Alzheimer’s disease therapy: New ferulic (lipoic) acid plus melatonin modified tacrines as cholinesterases inhibitors, direct antioxidants, and nuclear factor (erythroid-derived 2)-like 2 activators. J. Med. Chem. 2016, 59, 9967–9973. [Google Scholar] [CrossRef]
- Skibiński, R.; Czarnecka, K.; Girek, M.; Bilichowski, I.; Chufarova, N.; Mikiciuk-Olasik, E.; Szymański, P. Novel tetrahydroacridine derivatives with iodobenzoic acid moiety as multifunctional acetylcholinesterase inhibitors. Chem. Biol. Drug Des. 2018, 91, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jin, B.; Shi, Z.; Wang, X.; Lei, S.; Tang, X.; Liang, H.; Liu, Q.; Gong, M.; Peng, R. New tris (dopamine) derivative as an iron chelator. Synthesis, solution thermodynamic stability, and antioxidant research. J. Inorg. Biochem. 2017, 171, 29–36. [Google Scholar] [CrossRef]
- Bolognin, S.; Drago, D.; Messori, L.; Zatta, P. Chelation therapy for neurodegenerative diseases. Med. Res. Rev. 2009, 29, 547–570. [Google Scholar] [CrossRef]
- Chianella, C.; Gragnaniello, D.; Delser, P.M.; Visentini, M.F.; Sette, E.; Tola, M.R.; Barbujani, G.; Fuselli, S. BCHE and CYP2D6 genetic variation in Alzheimer’s disease patients treated with cholinesterase inhibitors. Eur. J. Clin. Pharmacol. 2011, 67, 1147–1157. [Google Scholar] [CrossRef]
- Członkowska, A.; Litwin, T.; Karliński, M.; Dziezyc, K.; Chabik, G.; Czerska, M. D-penicillamine versus zinc sulfate as first-line therapy for Wilson’s disease. Eur. J. Neurol. 2014, 21, 599–606. [Google Scholar] [CrossRef]
- Borowska, S.; Brzóska, M.M.; Gałażyn-Sidorczuk, M.; Rogalska, J. Effect of an extract from Aronia melanocarpa L. berries on the body status of zinc and copper under chronic exposure to cadmium: An in vivo experimental study. Nutrients 2017, 9, 1374. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, C.W.; Bush, A.I.; Masters, C.L. Metal-protein attenuating compounds and Alzheimer’s disease. Expert Opin. Investig. Drugs 2004, 13, 1585–1592. [Google Scholar] [CrossRef]
- Matlack, K.E.; Tardiff, D.F.; Narayan, P.; Hamamichi, S.; Caldwell, K.A.; Caldwell, G.A.; Lindquist, S. Clioquinol promotes the degradation of metal-dependent amyloid-β (Aβ) oligomers to restore endocytosis and ameliorate Aβ toxicity. Proc. Natl. Acad. Sci. USA 2014, 111, 4013–4018. [Google Scholar] [CrossRef]
| Metal | Disease | Gene | Affected Tissue | Therapy |
|---|---|---|---|---|
| Mn | Manganese transporter deficiency | SLC30A10 SLC39A14 (manganese transporter) | Liver, Nervous system | Manganese(II)-sulfate monohydrate [10] |
| Fe | Aceruloplasminemia | CPL (Ceruloplasmin) CP (Ferroxidase) | Liver, pancreas, nervous system | Iron chelation (Deferoxamine, Deferasirox) + Vitamin E and C/+ Fresh Frozen Plasma, Zinc administration, Minocycline administration, Enzyme Replacement Therapy, Gene Therapy [11] |
| Neuroferritinopathy, Hyperferritinemia-cataract syndrome, L-ferritin deficiency | FTL (iron storage) | Nervous system | Iron chelation (Deferoxamine, Deferasirox), dopamine-related drugs [12] | |
| Spastic paraplegia type 35 | FA2H (fatty acid 2-hydroxylase (Synthesis of sphingolipids)) | Botulinum toxin injections, microtubule destabilizing drugs (e.g., vinblastine) [13] | ||
| HARP syndrome (hypoprebetalipoproteinemia, acanthocytosis, retinitis pigmentosa, and pallidal degeneration) | PANK2 (Pantothenate kinase (CoA synthesis)) | No therapy | ||
| Pontocerebellar hypoplasia type 12 | COASY (CoA synthesis) | |||
| Infantile neuroaxonal dystrophy 1, Neurodegeneration with brain iron accumulation 2B, Parkinson’s disease type 14 | PLA2G6 (Phospholipase) | |||
| Spastic paraplegia 43, Neurodegeneration with brain iron accumulation 4 | C19orf12 (Mitochondrial magnesium homeostasis) | Intrathecal baclofen [14] | ||
| Woodhouse–Sakati syndrome | DCAF17 (Ubiquitinylation) | Treatment is symptomatic (e.g., hormone replacement therapy for hypogonadism) [15] | ||
| Neurodegeneration with brain iron accumulation type 5 | WDR45 (Autophagy) | Treatment is symptomatic [16] | ||
| Kufor–Rakeb syndrome, Spastic paraplegia type 78 | ATP13A2 (Lysosomal divalent cation (transition metal) transporter) | Treatment is symptomatic [16] | ||
| Hereditary hemochromatosis | HFE1 (HFE protein), HJV (Hemojuvelin), TrR2 (Trasferrin receptor-2), SLC40A1 (Ferroportin), HAMP (Hepcidin) | Liver, pancreas, heart | Therapeutic phlebotomy, iron chelating therapy, erythropoietin [17] | |
| Cu | Wilson’s disease | ATP7B (beta polypeptide, ATPase, CuII transporting) | Liver, brain, kidneys, cornea | Copper chelation (e.g., Penicillamine, Trientine), zinc supplementation, Tetrathiomolybdate [18] |
| MEDNIK syndrome | AP1S1 (adaptor protein complex 1 subunit β1) | liver, nervous system | Zinc supplementation (e.g., zinc acetate) [19] | |
| Menkes Disease | ATP7A (ATPase Copper Transporting Alpha) | Nervous system, skeletal, skin | Copper supplementation (e.g., copper histidine) [20] | |
| Occipital Horn Syndrome (OHS) | ATP7A (P-type ATPase) | Nervous system, skeletal, skin | Copper supplementation, disulfiram [21] | |
| Huppke-Brendel Syndrome (HBS) | SLC33A1 | Nervous system | Treatment is symptomatic [22] | |
| Zn | Acrodermatitis Enteropathica | SLC39A4 (Solute Carrier Family 39 Member 4) | Liver | Zinc supplementation [23] |
| Transient Neonatal Zinc Deficiency | SLC30A2 (Solute carrier family 30 member 2) | Skin | Zinc replacement therapy [24] | |
| Ehlers-Danlos Syndrome | SLC39A13 (zinc transporter ZIP13) | Nervous system, Muscle, skeletal | Nutritional supplements [25] | |
| Birk-Landau-Perez Syndrome | SLC30A9 (Zinc transporter 9) | Nervous system, kidneys, | Symptomatic Therapy [26] | |
| Se | Keshan Disease | Under investigation, genes related to selenoproteins and thioredoxin reductase | Heart | Selenium supplementation [27] |
| Rigid spine muscular dystrophy 1 (RSMD1) and congenital myopathy with fiber-type disproportion | SEPN1 (Selenoprotein N) | Muscle nervous system | No approved drug therapies [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639. https://doi.org/10.3390/molecules26216639
Lachowicz JI, Lecca LI, Meloni F, Campagna M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules. 2021; 26(21):6639. https://doi.org/10.3390/molecules26216639
Chicago/Turabian StyleLachowicz, Joanna Izabela, Luigi Isaia Lecca, Federico Meloni, and Marcello Campagna. 2021. "Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy" Molecules 26, no. 21: 6639. https://doi.org/10.3390/molecules26216639
APA StyleLachowicz, J. I., Lecca, L. I., Meloni, F., & Campagna, M. (2021). Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules, 26(21), 6639. https://doi.org/10.3390/molecules26216639