In Vitro Liver Metabolism of Six Flavonoid C-Glycosides
Abstract
:1. Introduction
2. Results
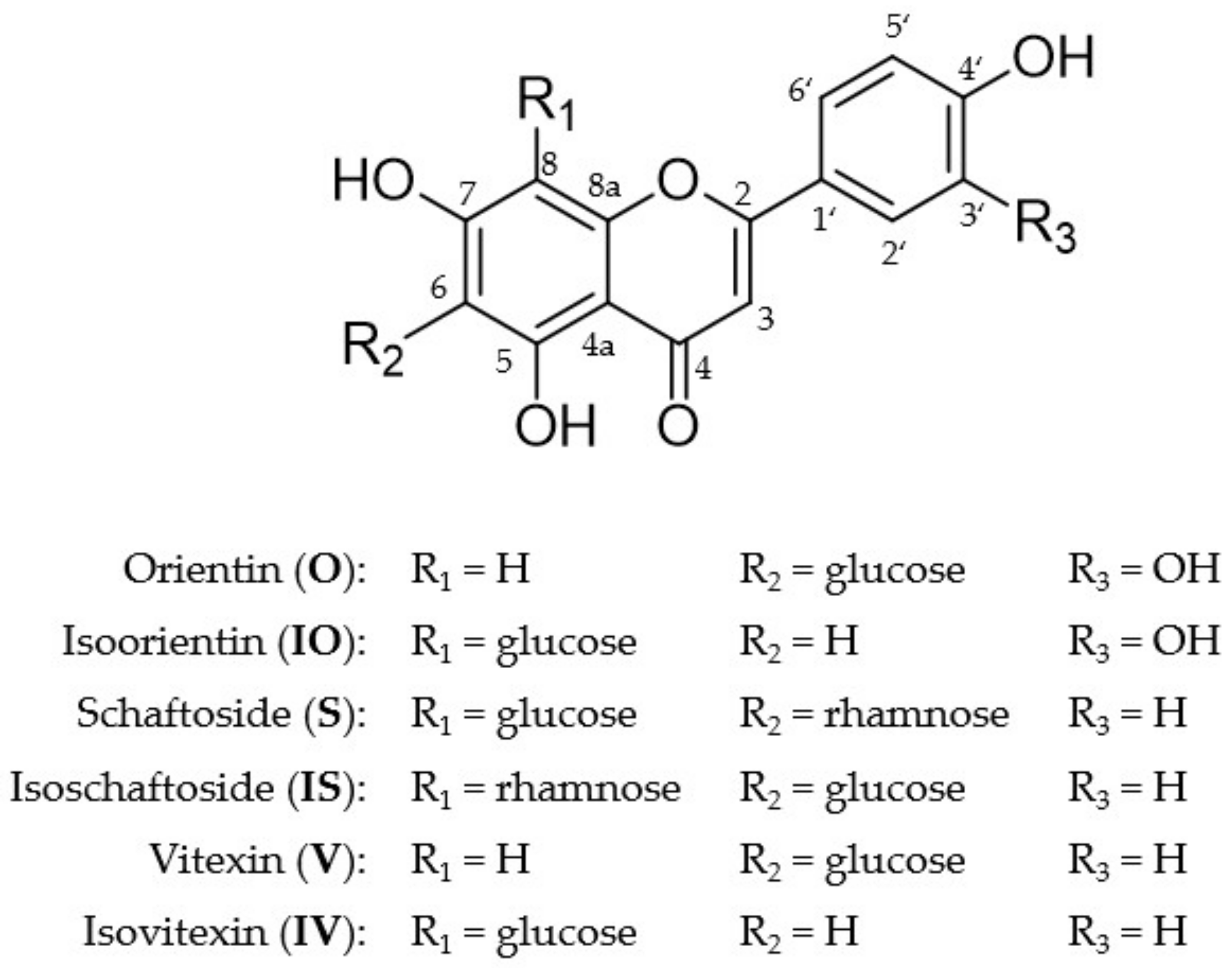
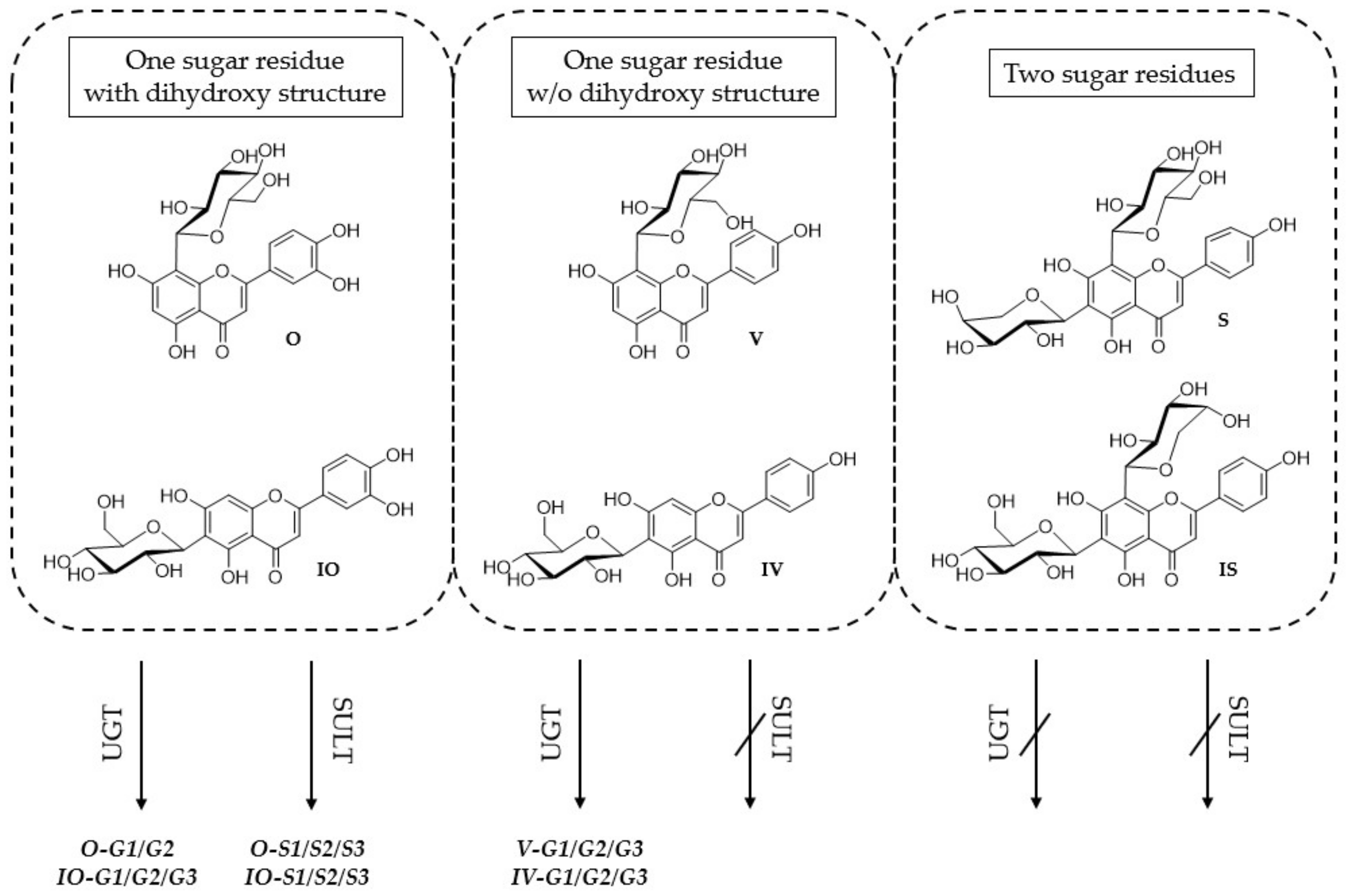
2.1. Metabolite Profiles of the Six C-Glycosides
2.1.1. Metabolites Formed by HLM
2.1.2. Metabolites Formed by Human S9 Fraction
2.2. Up-Scaling and Isolation of Metabolites
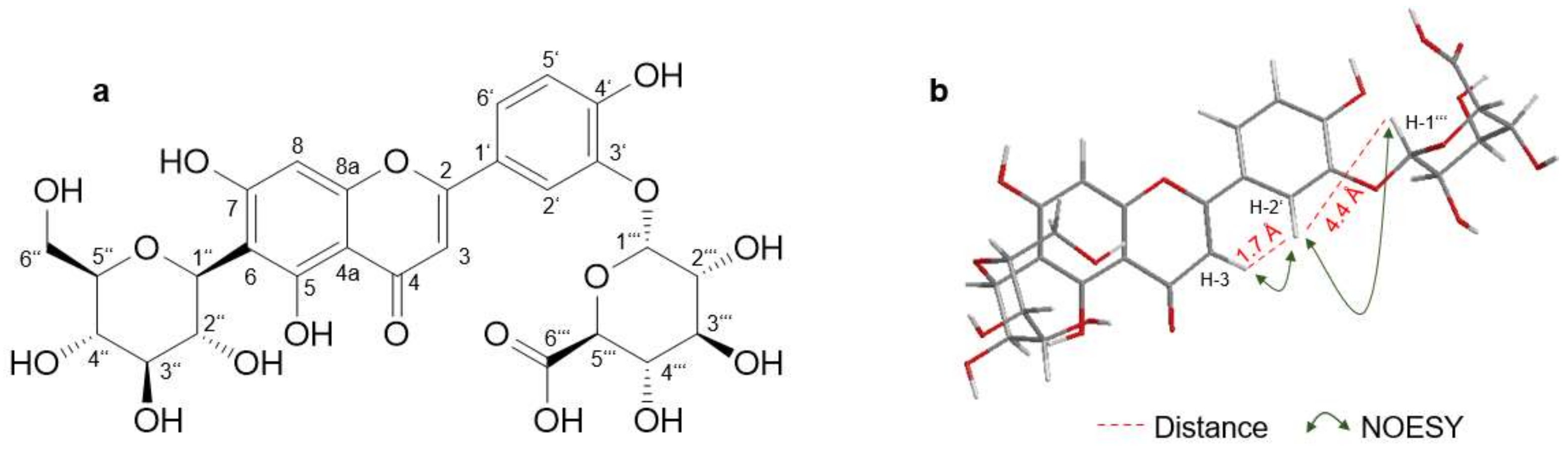
2.2.1. Isoorientin-3′-α-Glucuronide
2.2.2. Isoorientin-4′-O-α-Glucuronide
3. Discussion
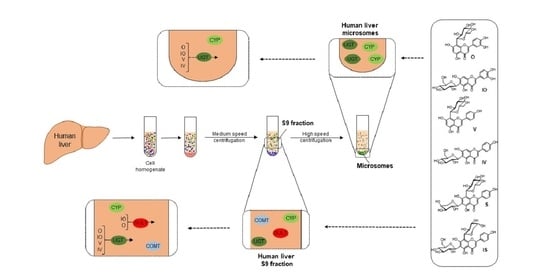
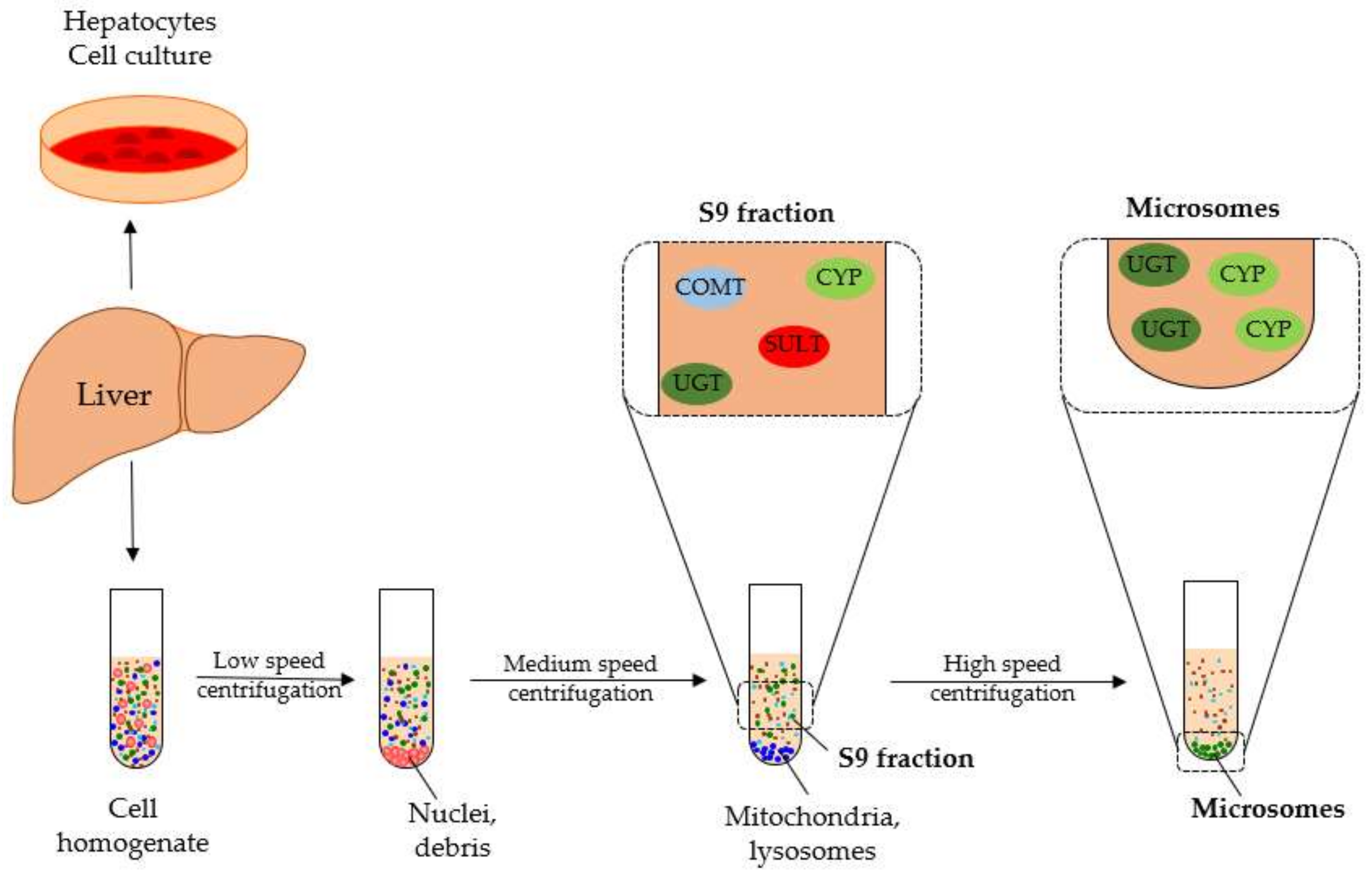
4. Materials and Methods
4.1. Reagents and Chemicals
4.2. Human Liver Microsome Metabolism Assay
4.3. Liver S9 Fraction Metabolism Assay
4.4. HPLC-DAD Analysis of Flavonoids and Their Glucuronides and Sulfates
4.5. LC-MS and LC-MS/MS Analysis and Determination of Main Metabolites
4.6. Isolation of Metabolites
4.6.1. Optimized and Upscaled HLM Metabolism Assay for Metabolite Isolation
4.6.2. Solid Phase Extraction
4.6.3. Isolation via Analytical HPLC
4.6.4. NMR Spectroscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. J. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Teiten, M.-H.; Radogna, F.; Dicato, M.; Diederich, M. From nature to bedside: Pro-survival and cell death mechanisms as therapeutic targets in cancer treatment. Biotechnol. Adv. 2014, 32, 1111–1122. [Google Scholar] [CrossRef]
- Pan, M.-H.; Lai, C.-S.; Ho, C.-T. Anti-inflammatory activity of natural dietary flavonoids. Food Funct. 2010, 1, 15–31. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Lim, H.-S.; Lee, H.-H.; Kim, T.-H. Role identification of Passiflora incarnata Linnaeus: A Mini Review. J. Menopausal Med. 2017, 23, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Blaschek, W.; Hänsel, R.; Keller, K.; Reichling, J.; Rimpler, H.; Schneider, G. Hagers Handbuch der Pharmazeutischen Praxis: Folgeband 2; Drogen A-K, 5., vollständig neubearbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 1998; ISBN 978-3-642-58928-7. [Google Scholar]
- Marchart, E.; Krenn, L.; Kopp, B. Quantification of the flavonoid glycosides in Passiflora incarnata by capillary electrophoresis. Planta Med. 2003, 69, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Androutsopoulos, V.P.; Papakyriakou, A.; Vourloumis, D.; Tsatsakis, A.M.; Spandidos, D.A. Dietary flavonoids in cancer therapy and prevention: Substrates and inhibitors of cytochrome P450 CYP1 enzymes. Pharmacol. Ther. 2010, 126, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Paine, M.F.; Hart, H.L.; Ludington, S.S.; Haining, R.L.; Rettie, A.E.; Zeldin, D.C. The human intestinal cytochrome P450 “pie”. Drug Metab. Dispos. 2006, 34, 880–886. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Pan, H.; Sun, S.; Li, L.; Zeng, S.; Jiang, H. Role of catechol-O-methyltransferase in the disposition of luteolin in rats. Drug Metab. Dispos. 2011, 39, 667–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Männistö, P.T.; Kaakkola, S. Catechol-O-methyltransferase (COMT): Biochemistry, Molecular Biology, Pharmacology, and Clinical Efficacy of the New Selective COMT Inhibitors. Pharm. Rev. 1999, 51, 593–628. [Google Scholar]
- Rowland, A.; Miners, J.O.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Maul, R.; Siegl, D.; Kulling, S.E. Glucuronidation of the red clover isoflavone irilone by liver microsomes from different species and human UDP-glucuronosyltransferases. Drug Metab. Dispos. 2011, 39, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Y.; Zhou, T.; Chen, G. Sulfation of dietary flavonoids by human sulfotransferases. Xenobiotica 2009, 39, 312–322. [Google Scholar] [CrossRef] [Green Version]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W.H. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: The SULT “pie”. Drug Metab. Dispos. 2009, 37, 2255–2261. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-G.; Li, P.; Li, P.; Yan, R.; Zhang, X.-Q.; Wang, Y.; Zhang, X.-T.; Ye, W.-C.; Zhang, Q.-W. α-Glucosidase inhibitory effect and simultaneous quantification of three major flavonoid glycosides in Microctis folium. Molecules 2013, 18, 4221–4232. [Google Scholar] [CrossRef]
- Hawas, U.W.; Soliman, G.M.; El-Kassem, L.T.A.; Farrag, A.R.H.; Mahmoud, K.; León, F. A new flavonoid C-glycoside from Solanum elaeagnifolium with hepatoprotective and curative activities against paracetamol- induced liver injury in mice. Z. Nat. C 2013, 68, 19–28. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Xiao, H.; Xiao, C.; Wang, Y.; Liu, X. Isoorientin induces apoptosis through mitochondrial dysfunction and inhibition of PI3K/Akt signaling pathway in HepG2 cancer cells. Toxicol. Appl. Pharmacol. 2012, 265, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Miroddi, M.; Calapai, G.; Navarra, M.; Minciullo, P.L.; Gangemi, S. Passiflora incarnata L.: Ethnopharmacology, clinical application, safety and evaluation of clinical trials. J. Ethnopharmacol. 2013, 150, 791–804. [Google Scholar] [CrossRef]
- Gadioli, I.L.; da Cunha, M.D.S.B.; de Carvalho, M.V.O.; Costa, A.M.; Pineli, L.D.L.D.O. A systematic review on phenolic compounds in Passiflora plants: Exploring biodiversity for food, nutrition, and popular medicine. Crit. Rev. Food Sci. Nutr. 2018, 58, 785–807. [Google Scholar] [CrossRef]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 4104595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-L.; Ma, S.-C.; Yang, Y.-T.; Ye, S.-M.; But, P.P.-H. Antiviral activities of flavonoids and organic acid from Trollius chinensis Bunge. J. Ethnopharmacol. 2002, 79, 365–368. [Google Scholar] [CrossRef]
- Liu, Y.; Lan, N.; Ren, J.; Wu, Y.; Wang, S.-t.; Huang, X.-F.; Yu, Y. Orientin improves depression-like behavior and BDNF in chronic stressed mice. Mol. Nutr. Food Res. 2015, 59, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Ku, S.-K.; Kwak, S.; Bae, J.-S. Orientin inhibits high glucose-induced vascular inflammation in vitro and in vivo. Inflammation 2014, 37, 2164–2173. [Google Scholar] [CrossRef]
- Lee, W.; Ku, S.-K.; Bae, J.-S. Vascular barrier protective effects of orientin and isoorientin in LPS-induced inflammation in vitro and in vivo. Vascul. Pharmacol. 2014, 62, 3–14. [Google Scholar] [CrossRef]
- An, F.; Yang, G.; Tian, J.; Wang, S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen. Res. 2012, 7, 2565–2575. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, B.C.; Kim, J.H.; Sim, G.S.; Lee, D.H.; Lee, K.E.; Yun, Y.P.; Pyo, H.B. The isolation and antioxidative effects of vitexin from Acer palmatum. Arch. Pharm Res. 2005, 28, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, D.; Yang, L.; Zhou, D.; Zhang, J. Purification and characterization of flavonoids from the leaves of Zanthoxylum bungeanum and correlation between their structure and antioxidant activity. PLoS ONE 2014, 9, e105725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dos Reis, G.O.; Vicente, G.; Kanumfre de Carvalho, F.; Heller, M.; Micke, G.A.; Pizzolatti, M.G.; Fröde, T.S. Croton antisyphiliticus Mart. attenuates the inflammatory response to carrageenan-induced pleurisy in mice. Inflammopharmacol 2014, 22, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Das, M.C.; Sandhu, P.; Gupta, P.; Rudrapaul, P.; De, U.C.; Tribedi, P.; Akhter, Y.; Bhattacharjee, S. Attenuation of Pseudomonas aeruginosa biofilm formation by vitexin: A combinatorial study with azithromycin and gentamicin. Sci. Rep. 2016, 6, 23347. [Google Scholar] [CrossRef] [Green Version]
- Knipping, K.; Garssen, J.; Van’t Land, B. An evaluation of the inhibitory effects against rotavirus infection of edible plant extracts. Virol J. 2012, 9, 137. [Google Scholar] [CrossRef] [Green Version]
- He, M.; Min, J.-W.; Kong, W.-L.; He, X.-H.; Li, J.-X.; Peng, B.-W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia 2016, 115, 74–85. [Google Scholar] [CrossRef]
- Tremmel, M.; Kiermaier, J.; Heilmann, J. In vitro metabolism of six C-Glycosidic flavonoids from Passiflora incarnata L. Int. J. Mol. Sci. 2021, 22, 6566. [Google Scholar] [CrossRef]
- Brandon, E.F.A.; Raap, C.D.; Meijerman, I.; Beijnen, J.H.; Schellens, J.H.M. An update on in vitro test methods in human hepatic drug biotransformation research: Pros and cons. Toxicol. Appl. Pharmacol. 2003, 189, 233–246. [Google Scholar] [CrossRef]
- Serralta, A.; Donato, M.T.; Orbis, F.; Castell, J.V.; Mir, J.; Gómez-Lechón, M.J. Functionality of cultured human hepatocytes from elective samples, cadaveric grafts and hepatectomies. Toxicology In Vitro 2003, 17, 769–774. [Google Scholar] [CrossRef]
- Richardson, S.J.; Bai, A.; Kulkarni, A.A.; Moghaddam, M.F. Efficiency in drug discovery: Liver S9 fraction assay as a screen for metabolic stability. Drug Metab. Lett. 2016, 10, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Zhu, L.; Li, Y.; Zheng, H.; Yu, J.; Lu, L.; Liu, Z. In vitro study of UGT metabolism and permeability of orientin and isoorientin, two active flavonoid C-glycosides. Drug Metab. Lett. 2016, 10, 101–110. [Google Scholar] [CrossRef]
- Wang, Q.; Kuang, Y.; Song, W.; Qian, Y.; Qiao, X.; Guo, D.-A.; Ye, M. Permeability through the Caco-2 cell monolayer of 42 bioactive compounds in the TCM formula Gegen-Qinlian Decoction by liquid chromatography tandem mass spectrometry analysis. J. Pharm. Biomed. Anal. 2017, 146, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Caldwell, G.W. Metabolic assessment in liver microsomes by co-activating cytochrome P450s and UDP-glycosyltransferases. Eur. J. Drug Metab. Pharmacokinet. 2003, 28, 223–232. [Google Scholar] [CrossRef]

| Compound | Liver Fraction | RT [min] | m/z [M-H]− Found | m/z [M-H]− Calculated | M Formula |
|---|---|---|---|---|---|
| Isoorientin | HLM, S9F | 9.625 | 447.0939 | 447.0933 | C21H20O11 |
| Isoorientin-G1 | HLM, S9F | 6.584 | 623.1265 | 623.1254 | C27H28O17 |
| Isoorientin-G2 | HLM, S9F | 8.441 | 623.1258 | 623.1254 | C27H28O17 |
| Isoorientin-G3 | HLM, S9F | 9.108 | 623.1263 | 623.1254 | C27H28O17 |
| Isoorientin-OH1 | HLM, S9F | 7.498 | 463.0877 | 463.0882 | C21H20O12 |
| Isoorientin-S1 | S9F | 6.377 | 527.0501 | 527.0501 | C21H20O14S |
| Isoorientin-S2 | S9F | 7.852 | 527.0503 | 527.0501 | C21H20O14S |
| Isoorientin-S3 | S9F | 8.566 | 527.0509 | 527.0501 | C21H20O14S |
| Orientin | HLM, S9F | 10.229 | 447.0934 | 447.0933 | C21H20O11 |
| Orientin-G1 | HLM, S9F | 8.007 | 623.1247 | 623.1254 | C27H28O17 |
| Orientin-G2 | HLM, S9F | 9.495 | 623.1249 | 623.1254 | C27H28O17 |
| Orientin-S1 | S9F | 7.692 | 527.0498 | 527.0501 | C21H20O14S |
| Orientin-S2 | S9F | 8.593 | 527.0504 | 527.0501 | C21H20O14S |
| Orientin-S3 | S9F | 9.364 | 527.0510 | 527.0501 | C21H20O14S |
| Vitexin | HLM, S9F | 11.525 | 431.0989 | 431.0984 | C21H20O10 |
| Vitexin-G1 | HLM, S9F | 4.590 | 607.1304 | 607.1305 | C27H28O16 |
| Vitexin-G2 | HLM, S9F | 8.072 | 607.1312 | 607.1305 | C27H28O16 |
| Vitexin-G3 | HLM, S9F | 10.899 | 607.1307 | 607.1305 | C27H28O16 |
| Isovitexin | HLM, S9F | 11.509 | 431.0992 | 431.0984 | C21H20O10 |
| Isovitexin-G1 | HLM, S9F | 6.813 | 607.1317 | 607.1305 | C27H28O16 |
| Isovitexin-G2 | HLM, S9F | 9.416 | 607.1306 | 607.1305 | C27H28O16 |
| Isovitexin-G3 | HLM, S9F | 10.295 | 607.1313 | 607.1305 | C27H28O16 |
| No. | Isoorientin | Isoorientin-3′-O-α-glucuronide | ||
|---|---|---|---|---|
| δH | δC | δH | δC | |
| 2 | 163.7 | 162.9 | ||
| 3 | 6.67 (1H, s) | 102.5 | 6.85 (1H, s) | 102.9 |
| 4 | 181.8 | 181.8 | ||
| 4a | 103.4 | 103.1 | ||
| 5 | 159.9 | 160.4 | ||
| 6 | 108.5 | 108.5 | ||
| 7 | 163.2 | 163.1 | ||
| 8 | 6.48 (1H, s) | 93.3 | 6.55 (1H, s) | 93.6 |
| 8a | 156.0 | 156.0 | ||
| 1′ | 121.3 | 121.3 | ||
| 2′ | 7.40 (1H, s) | 113.2 | 7.71 (1H, s) | 113.8 |
| 3′ | 145.3 | 145.0 | ||
| 4′ | 149.3 | 150.6 | ||
| 5′ | 6.89 (1H, d, 8.1) | 115.9 | 6.99 (1H, d, 8.2) | 116.4 |
| 6′ | 7.42 (1H, dd, 2.0, 8.1) | 118.8 | 7.65 (1H, dd, 2.0, 8.2) | 121.6 |
| 1″ | 4.58 (1H, d, 9.8) | 72.9 | 4.59 (1H, d, 9.5) | 72.8 |
| 2″ | 4.04 1 (1H, m) | 70.0 | 4.021 (1H, m) | 70.1 |
| 3″ | 3.19 (1H, dd, 9.0, 9.0) | 78.8 | 3.20 (1H, m) | 78.6 |
| 4″ | 3.12 (1H, dd, 9.0, 9.0) | 70.5 | 3.13 (1H, m) | 70.3 |
| 5″ | 3.16 (1H, m) | 81.4 | 3.17 (1H, m) | 81.4 |
| 6″ | 3.40 (1H, m) 3.68 (1H, bd, 11.0) | 61.3 | 3.41 (1H, m) 3.68 (1H, bd, 11.0) | 61.3 |
| 1‴ | 5.17 (1H, d, 4.0) | 100.4 | ||
| 2‴ | 3.36 (1H, m) | 72.8 | ||
| 3‴ | 3.35 (1H, m) | 75.2 | ||
| 4‴ | 3.42 (1H, m) | 71.2 | ||
| 5‴ | 3.98 (1H, m) | 74.9 | ||
| 6‴ | 169.9 |
| Reagent | HLM | S9F | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Matrix | Stab | w/o cof | w/o HLM | Ph I + II | Matrix | Stab | w/o cof | w/o S9F | Ph I + II | |
| PBS | X | X | X | X | X | X | X | X | X | X |
| Glc-6-P | X | X | X | X | X | X | X | X | ||
| Glc-6-PDH | X | X | X | X | X | X | X | X | ||
| alamethicin | X | X | X | X | X | X | X | X | ||
| [substance] | X | X | X | X | X | X | X | X | ||
| HLM | X | X | X | |||||||
| S9F | X | X | X | |||||||
| NADP | X | X | X | X | X | X | ||||
| UDPGA | X | X | X | X | X | X | ||||
| PAPS | X | X | X | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tremmel, M.; Paetz, C.; Heilmann, J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules 2021, 26, 6632. https://doi.org/10.3390/molecules26216632
Tremmel M, Paetz C, Heilmann J. In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules. 2021; 26(21):6632. https://doi.org/10.3390/molecules26216632
Chicago/Turabian StyleTremmel, Martina, Christian Paetz, and Jörg Heilmann. 2021. "In Vitro Liver Metabolism of Six Flavonoid C-Glycosides" Molecules 26, no. 21: 6632. https://doi.org/10.3390/molecules26216632
APA StyleTremmel, M., Paetz, C., & Heilmann, J. (2021). In Vitro Liver Metabolism of Six Flavonoid C-Glycosides. Molecules, 26(21), 6632. https://doi.org/10.3390/molecules26216632