Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4′-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging
Abstract
1. Introduction
2. Results and Discussion
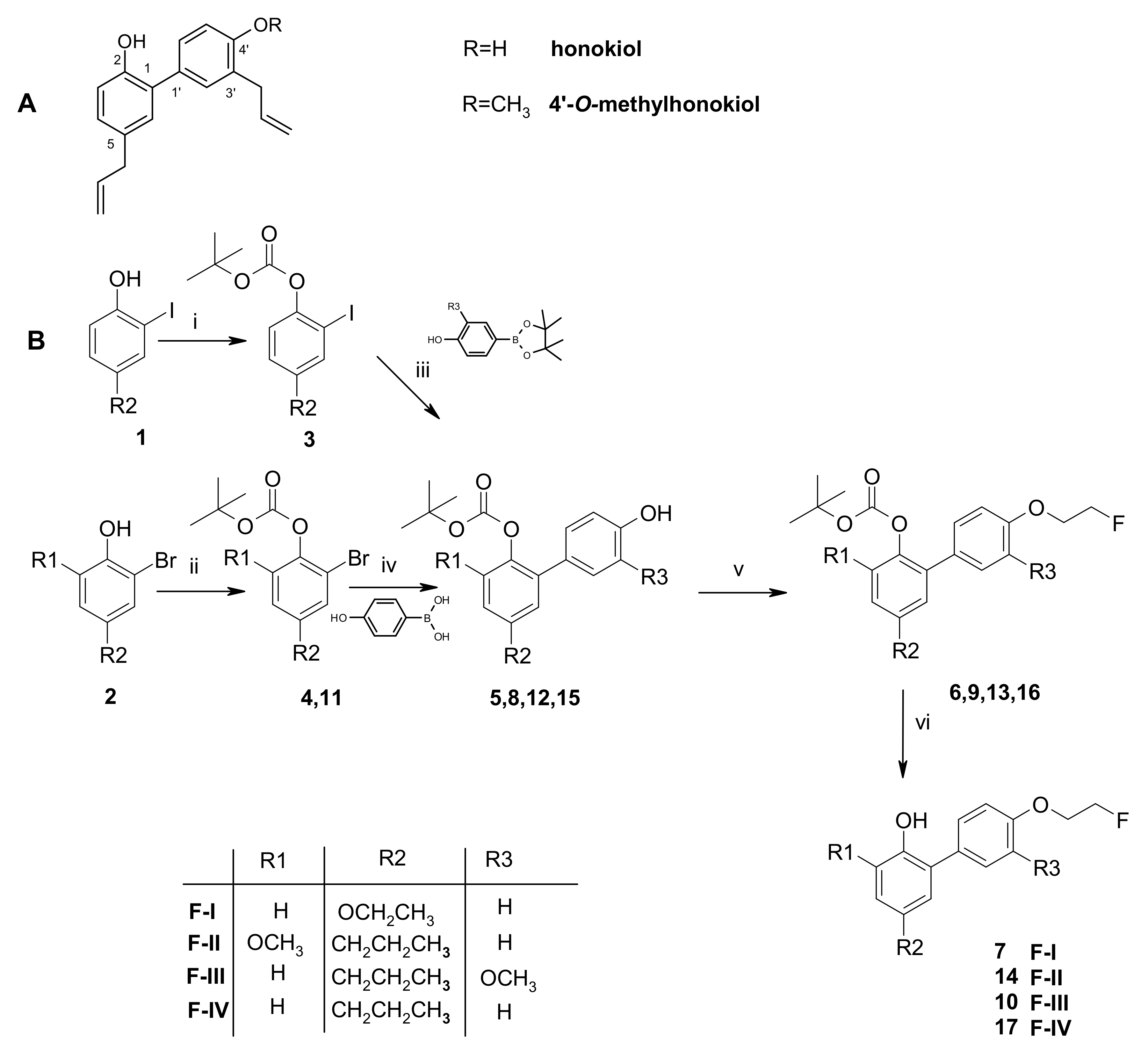
2.1. Chemistry
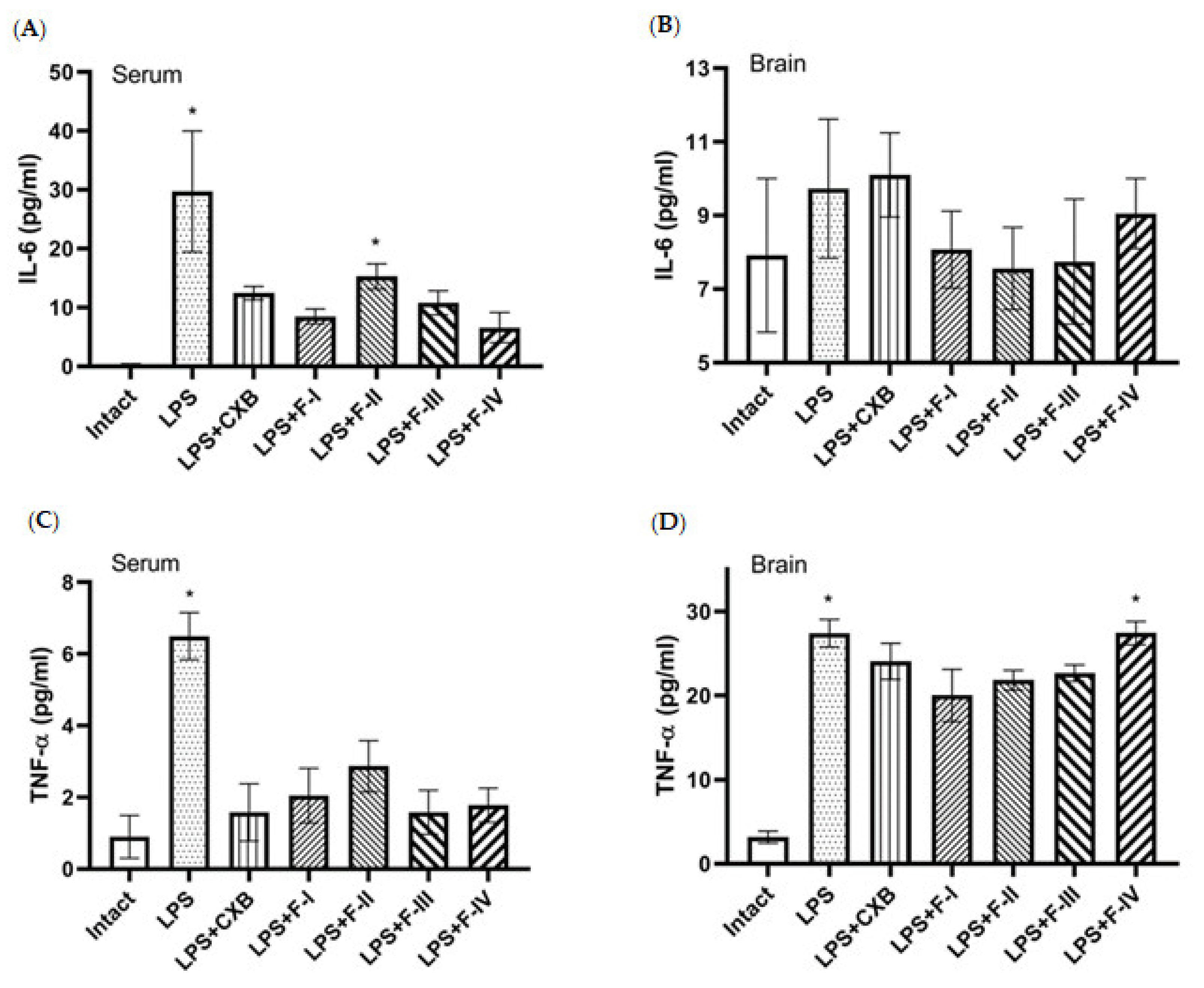
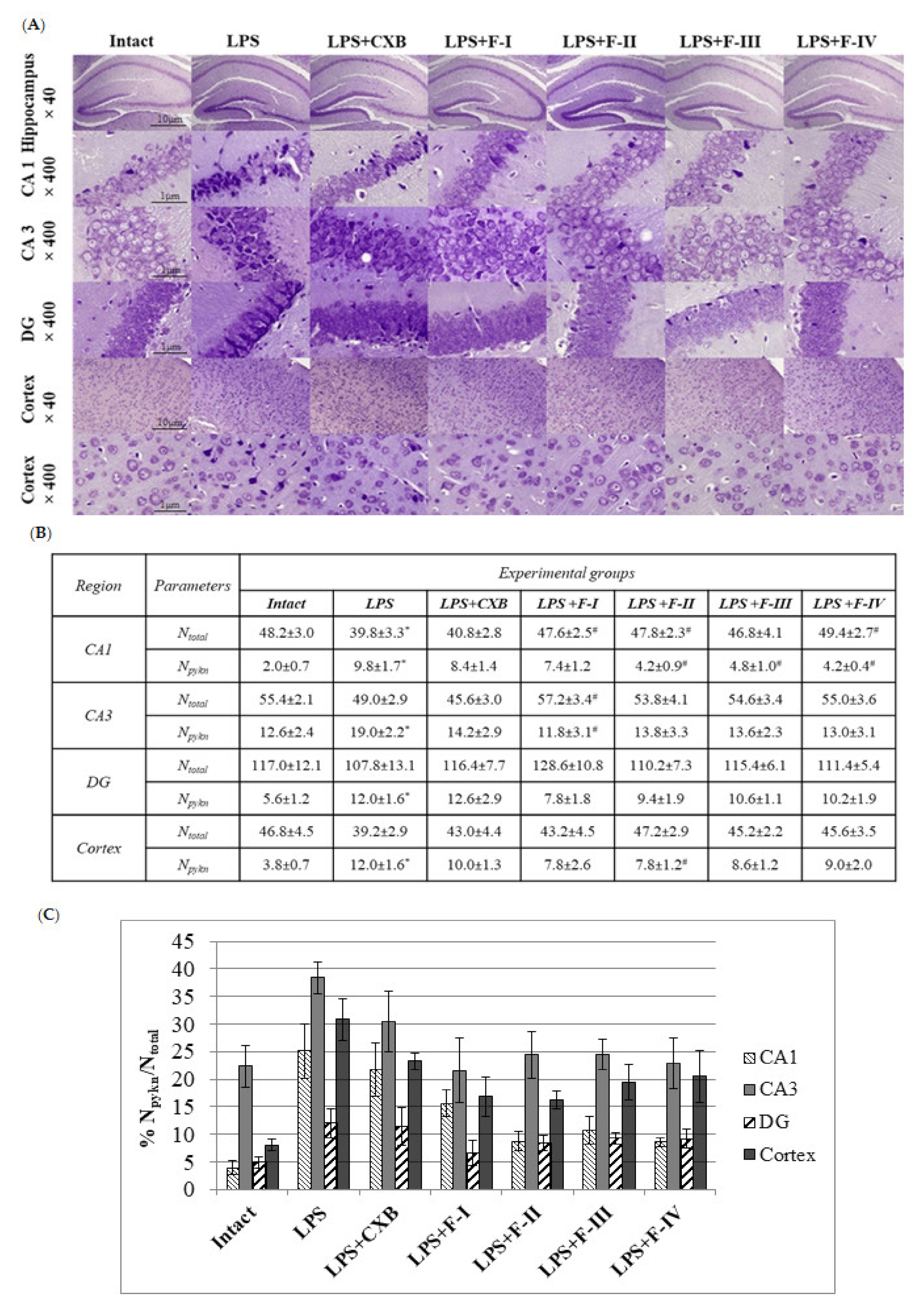
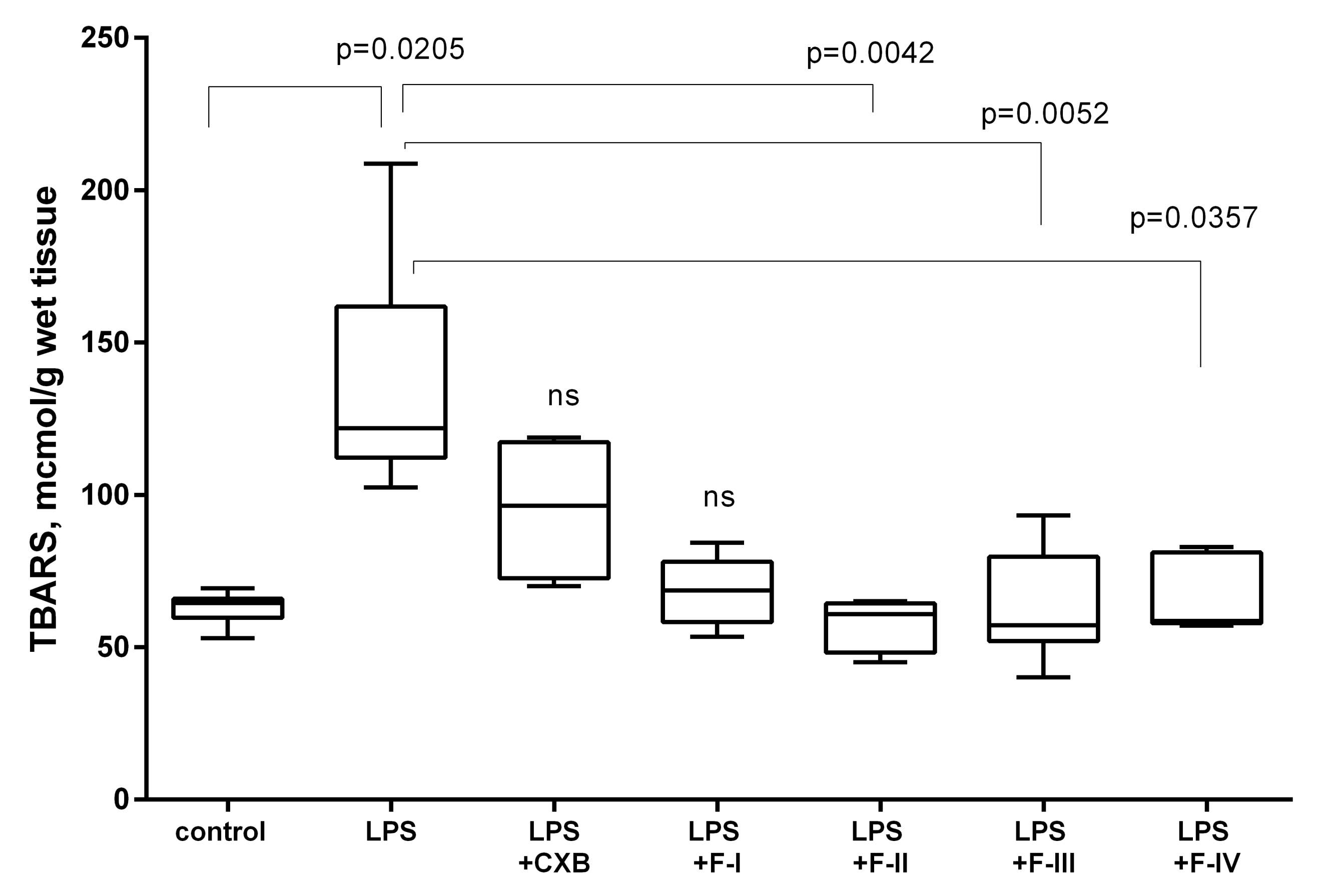
2.2. Anti-Inflammatory Screening
2.3. In Vitro COX-2 Inhibition
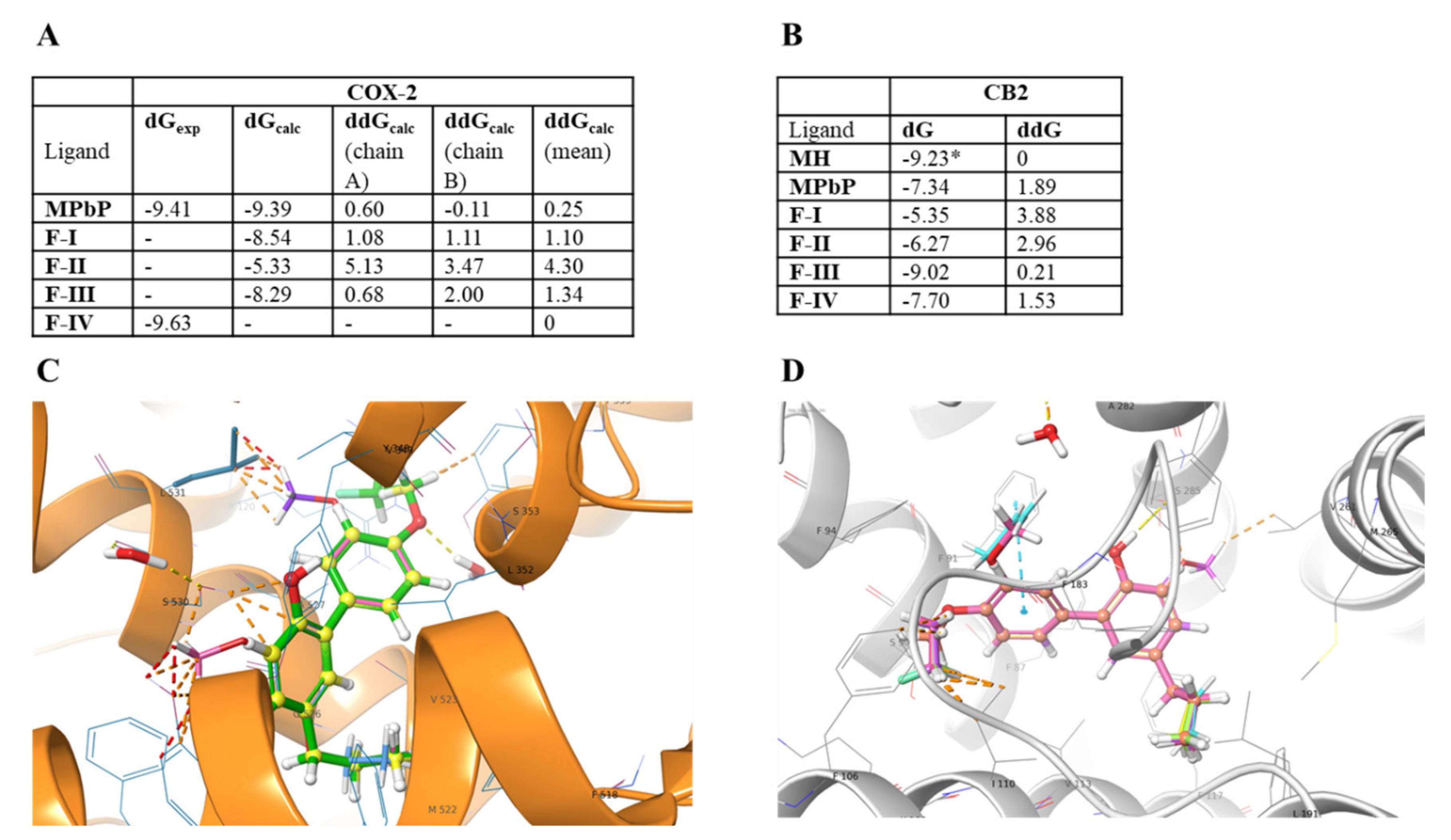
2.4. Computational Analysis
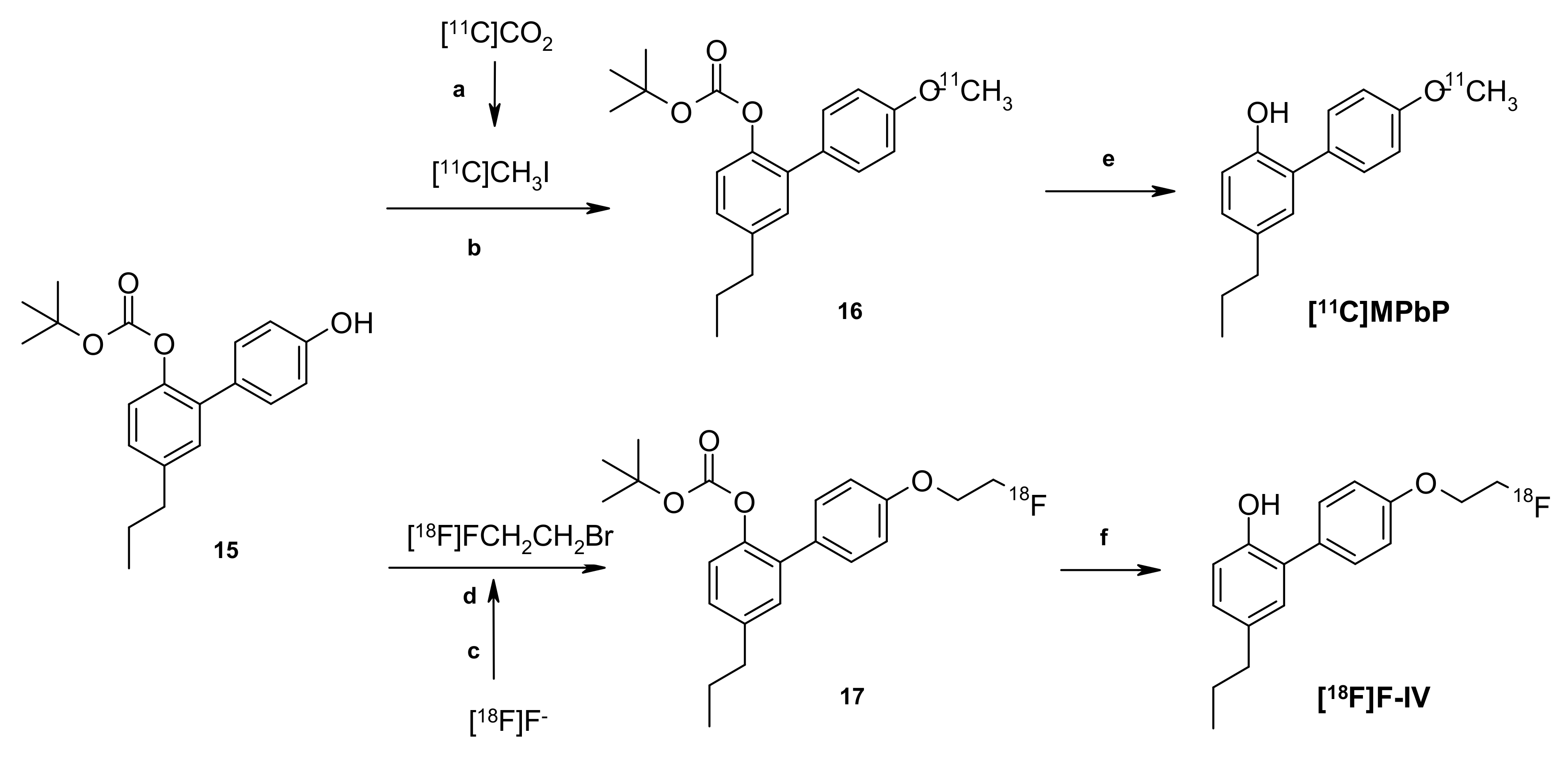
2.5. Radiochemistry
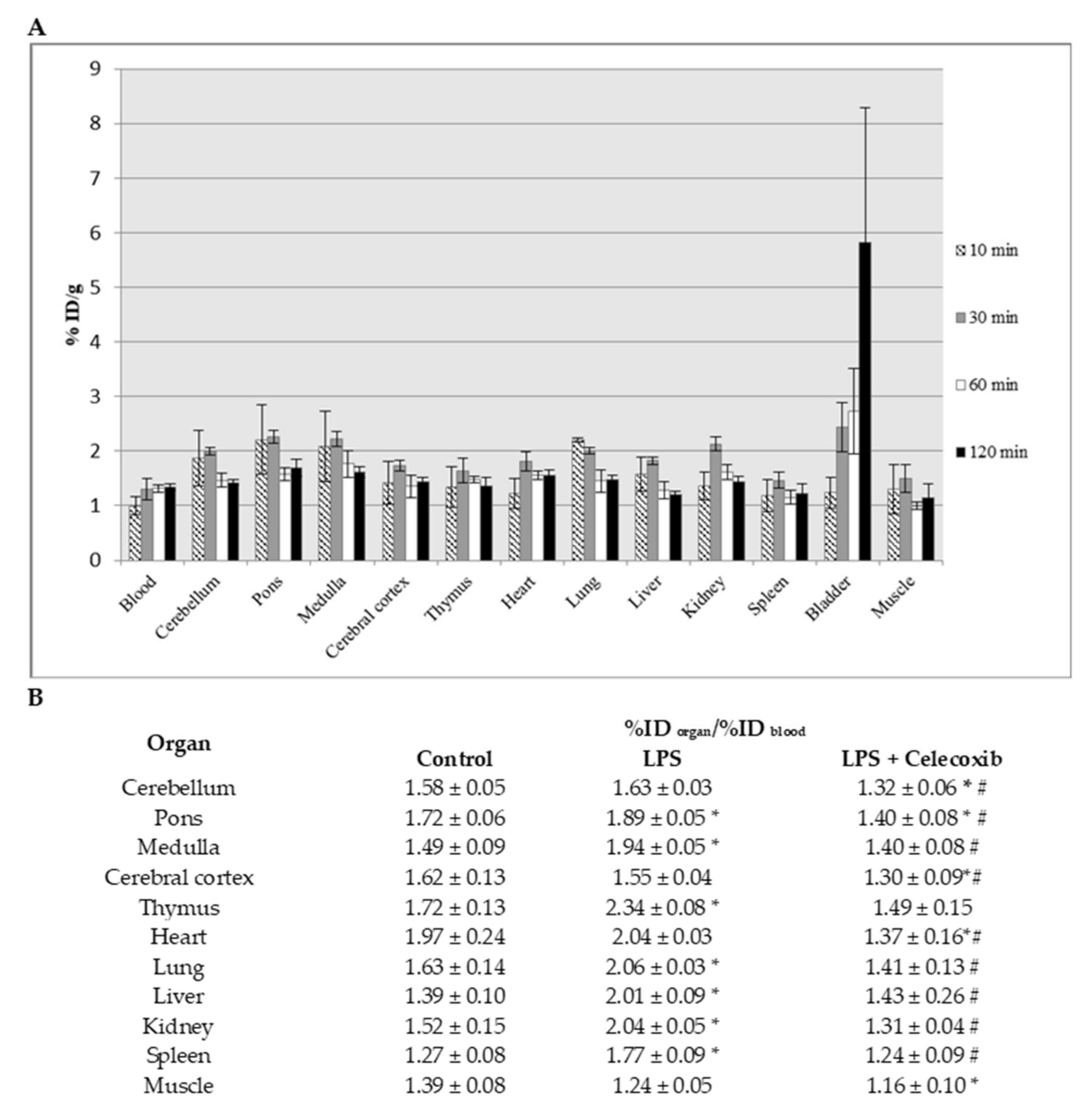
2.6. Ex Vivo Biodistribution Studies of [18F]F-IV
2.7. Plasma Protein Binding (PPB) for [18F]F-IV and [11C]MPbP
3. Experimental Procedure
3.1. General Chemistry
3.2. Synthesis of Structural Analogs of Honokiol with a 4′-O-(2-Fluoroethyl) Moiety (F-I –F-IV)
3.2.1. 5-Ethoxy-4′-(2-fluoroethoxy)-2-hydroxy-1,1′-biphenyl (F-I)
3.2.2. 4′-(2-Fluoroethoxy)-3′-methoxy-5-propyl-2-hydroxy-1,1′-biphenyl (F-III)
3.2.3. 4′-(2-Fluoroethoxy)-3-methoxy-5-propyl-2-hydroxy-1,1′-biphenyl (F-II)
3.2.4. 5-Propyl-1,1′-biphenyl-2,4′-diol (Model Compound)
3.3. Animal Studies
3.4. Anti-Inflammatory Screening
3.4.1. Cytokine Assay
3.4.2. Histology, Cresyl Violet Staining, and Neuronal Morphology
3.4.3. TBARS Assay
3.4.4. Statistical Analysis
3.5. In Vitro COX-1/2 Inhibition Assay
3.6. Relative Binding Free Energies of Ligands F-I–F-IV with CB-2 and COX-2
3.7. Radiochemistry
3.7.1. Radiochemistry Materials and Methods
3.7.2. Radiosynthesis of [18F]FEtPbP and [11C]MPbP
3.7.3. Plasma-Stability Study and logD7.4 Determination for [18F]F-IV
3.8. Ex Vivo Biodistribution
3.9. Plasma Protein Binding (PPB) Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- DiSabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef]
- Beaino, W.; Janssen, B.; Vugts, D.J.; de Vries, H.E.; Windhorst, A.D. Towards PET imaging of the dynamic phenotypes of microglia. Clin. Exp. Immunol. 2021, 00, 1–19. [Google Scholar] [CrossRef]
- Aïd, S.; Bosetti, F. Targeting cyclooxygenases-1 and -2 in neuroinflammation: Therapeutic implications. Biochimie. 2011, 93, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfari, N.; van Waarde, A.; Dierckx, R.A.J.O.; Doorduin, J.; de Vries, E.F.J. Is cyclooxygenase-1 involved in neuroinflammation? J. Neurosci. Res. 2021, 00, 1–23. [Google Scholar] [CrossRef]
- Ory, D.; Celen, S.; Verbruggen, A.; Bormans, G. PET Radioligands for In Vivo Visualization of Neuroinflammation. Curr. Pharm. Des. 2014, 20, 5897–5913. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, S.S.; Anderson, K.B.; Jenko, K.J.; Luckenbaugh, D.A.; Innis, R.B.; Pike, V.W. On quantitative relationships between drug-like compound lipophilicity and plasma free fraction in monkey and human. J. Pharm. Sci. 2012, 101, 1028–1039. [Google Scholar] [CrossRef]
- Shrestha, S.; Kim, M.J.; Eldridge, M.; Lehmann, M.L.; Frankland, M.; Liow, J.S.; Yu, Z.X.; Cortes-Salva, M.; Telu, S.; Henter, I.D.; et al. PET measurement of cyclooxygenase-2 using a novel radioligand: Upregulation in primate neuroinflammation and first-in-human study. J. Neuroinflammation. 2020, 17, 140. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.S.D.; Zanderigo, F.; Prabhakaran, J.; Rubin-Falcone, H.; Parsey, R.V.; Mann, J.J. In vivo evaluation of [11C]TMI, a COX-2 selective PET tracer, in baboons. Bioorg. Med. Chem. Lett. 2018, 28, 3592–3595. [Google Scholar] [CrossRef]
- Kumar, J.S.D.; Prabhakaran, J.; Molotkov, A.; Sattiraju, A.; Kim, J.; Doubrovin, M.; Mann, J.J.; Mintz, A. Radiosynthesis and evaluation of [18F]FMTP, a COX-2 PET ligand. Pharmacol. Rep. 2020, 72, 1433–1440. [Google Scholar] [CrossRef]
- Prabhakaran, J.; Molotkov, A.; Mintz, A.; Mann, J.J. Progress in PET Imaging of Neuroinflammation Targeting COX-2 Enzyme. Molecules 2021, 26, 3208. [Google Scholar] [CrossRef] [PubMed]
- Attiq, A.; Jalil, J.; Husain, K.; Ahmad, W. Raging the War Against Inflammation with Natural Products. Front. Pharmacol. 2018, 9, 976. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Poenca, C.; Varela, C.; Janela, J.; da Silva, E.J.T.; Fernandes, E.; Roleira, F.M.F. New phenolic cinnamic acid derivatives as selective COX-2 inhibitors. Design, synthesis, biological activity and structure-activity relationships. Bioorg. Chem. 2019, 91, 103179. [Google Scholar] [CrossRef] [PubMed]
- Uzuegbunam, B.C.; Librizzi, D.; Yousefi, B.H. PET Radiopharmaceuticals for Alzheimer’s Disease and Parkinson’s Disease Diagnosis, the Current and Future Landscape Molecules. Molecules 2020, 25, 977. [Google Scholar] [CrossRef]
- Ono, M.; Watanabe, R.; Kawashima, H.; Kawai, T.; Watanabe, H.; Haratake, M.; Saji, H.; Nakayama, M. 18F-labeled flavones for in vivo imaging of b-amyloid plaques in Alzheimer’s brains. Bioorg. Med. Chem. 2009, 17, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preuße-Prange, A.; Wilms, H.; Lucius, R. Anti-inflammatory properties of Honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef]
- Chen, H.H.; Chang, P.C.; Chen, C.; Chan, M.H. Protective and therapeutic activity of honokiol in reversing motor deficits and neuronal degeneration in the mouse model of Parkinson’s disease. Pharmacol. Rep. 2018, 70, 668–676. [Google Scholar] [CrossRef]
- Raufa, A.; Olatundeb, A.; Imranc, M.; Alhumaydhi, F.A.; Aljohani, A.S.M.; Shahid, A.K.; Uddin, M.S.; Mitra, S.; Emran, T.B.; Khayrullin, M.; et al. Honokiol: A review of its pharmacological potential and therapeutic insights. Phytomedicine 2021, 90, 153647. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, M.M.; Vaulina, D.D.; Sivak, K.V.; Alexandrov, A.G.; Kuzmich, N.N.; Viktorov, N.B.; Kuznetsova, O.F.; Gomzina, N.A. Radiosynthesis of a Novel 11C-Labeled Derivative of 4’-O-Methylhonokiol and Its Preliminary Evaluation in an LPS Rat Model of Neuroinflammation. Chem. Sel. 2020, 5, 2685–2689. [Google Scholar] [CrossRef]
- Pike, V.W. Considerations in the Development of Reversibly Binding PET Radioligands for Brain Imaging. Curr. Med. Chem. 2016, 23, 1818–1869. [Google Scholar] [CrossRef]
- Hoogland, I.C.M.; Houbolt, C.; van Westerloo, D.J.; van Gool, W.A.; van de Beek, D. Systemic inflammation and microglial activation: Systematic review of animal experiments. J. Neuroinflamm. 2015, 12, 114. [Google Scholar] [CrossRef]
- Banks, W.A.; Gray, A.M.; Erickson, M.A.; Salameh, T.S.; Damodarasamy, M.; Sheibani, N.; Meabon, J.S.; Wing, E.E.; Morofuji, Y.; Cook, D.G.; et al. Lipopolysaccharide-induced blood-brain barrier disruption: Roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflamm. 2015, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Ann. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Dawn-Linsley, M.; Ekinci, F.J.; Ortiz, D.; Rogers, E.; Shea, T.B. Monitoring thiobarbituric acid-reactive substances (TBARs) as an assay for oxidative damage in neuronal cultures and central nervous system. J. Neurosci. Methods 2005, 141, 219–222. [Google Scholar] [CrossRef]
- Kim, H.S.; Ryu, H.S.; Kim, J.S.; Kim, Y.G.; Lee, H.K.; Jung, J.K.; Kwak, Y.S.; Lee, K.; Seo, S.Y.; Yun, J.; et al. Validation of cyclooxygenase-2 as a direct anti-inflammatory target of 4-O-methylhonokiol in zymosan-induced animal models. Arch. Pharmacal. Res. 2015, 38, 813–825. [Google Scholar] [CrossRef]
- Chicca, A.; Gachet, M.S.; Petrucci, V.; Schuehly, W.; Charles, R.-P.; Gertsch, J. 4′-O-methylhonokiol increases levels of 2-arachidonoyl glycerol in mouse brain via selective inhibition of its COX-2-mediated oxygenation. J. Neuroinflamm. 2015, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Gomzina, N.A.; Vasil’ev, D.A.; Krasikova, R.N. Use of 2-[18F] Fluoroethyl Bromide in Synthesis of O-(2-[18F] Fluoroethyl)-L-tyrosine, a Radiotracer for PET Diagnostics of Brain Tumors. Radiochemistry 2007, 49, 299. [Google Scholar] [CrossRef]
- Nakamura, K.; Nakajima, T.; Kayahara, H.; Nomurab, E.; Taniguchi, H. Base-labile tert-butoxycarbonyl (Boc) group on phenols. Tetrahedron Lett. 2004, 45, 495–499. [Google Scholar] [CrossRef]
- Cheraiet, Z.; Hessainia, S.; Ouarna, S.; Berredjem, M.; Aouf, N.-E. A simple and eco-sustainable method for the O-Boc protection/deprotection of various phenolic structures under water-mediated/catalyst-free conditions. Green Chem. Lett. Rev. 2013, 6, 211–216. [Google Scholar] [CrossRef][Green Version]
- Kim, M.-J.; Shrestha, S.S.; Cortes, M.; Singh, P.; Morse, C.; Liow, J.-S.; Gladding, R.L.; Brouwer, C.; Henry, K.; Gallagher, E.; et al. Evaluation of Two Potent and Selective PET Radioligands to Image COX-1 and COX-2 in Rhesus Monkeys. J. Nucl. Med. 2018, 59, 1907–1912. [Google Scholar] [CrossRef]
- Horti, A.G.; Naik, R.; Foss, C.A.; Minn, I.; Misheneva, V.; Du, Y.; Wang, Y.; Mathews, W.B.; Wu, Y.; Hall, A.; et al. PET imaging of microglia by targeting macrophage colony-stimulating factor 1 receptor (CSF1R). Proc. Natl. Acad. Sci. USA 2019, 116, 1686–1691. [Google Scholar] [CrossRef]
- Salabert, A.-S.; Mora-Ramirez, E.; Beaurain, M.; Alonso, M.; Fontan, C.; Tahar, H.B.; Boizeau, M.L.; Tafani, M.; Bardiès, M.; Payoux, P. Evaluation of [18F]FNM biodistribution and dosimetry based on whole-body PET imaging of rats. Nucl. Med. Biol. 2018, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Nakao, R.; Amini, N.; Abdel-Rehim, M.; Schou, M.; Halldin, C. Sample preparation techniques for radiometabolite analysis of positron emission tomography radioligands; trends, progress, limitations and future prospects. Trends Anal. Chem. 2019, 110, 1–7. [Google Scholar] [CrossRef]
- Gobbi, L.; Mercier, J.; Bang-Andersen, B.; Nicolas, J.-M.; Reilly, J.; Wagner, B.; Whitehead, D.; Briard, E.; Maguire, R.P.; Borroni, E.; et al. A comparative study of in vitro assays for predicting the non-specific binding of PET imaging agents in vivo. ChemMedChem. 2020, 15, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Sivak, K.V.; Stosman, K.I.; Muzhikyan, A.A.; Alexandrov, A.G.; Viktorov, N.B.; Vaulina, D.D.; Gomzina, N.A. Evaluation of Antiinflammatory Activity of 4′-O-Methylhonokiol Derivatives in a Neuroinflammation Model. Russ. J. Bioorg. Chem. 2019, 45, 425–429. [Google Scholar] [CrossRef]
- Gu, Y.G.; Clark, R.F.; Li, Q.; Weitzberg, M.; Sham, H. Novel acetyl-CoA carboxylase (acc) inhibitors and their use in diabetes, obesity and metabolic syndrome. WO2008079610A2, 3 July 2008. [Google Scholar]
- Ruffin, B.; Grelier, S.; Nourmamode, A.; Castellan, A. Attempt to approach the role of phenolic phenylpropenol structures in the photoyellowing of softwood mechanical pulps. Can. J. Chem. 2002, 80, 1223–1231. [Google Scholar] [CrossRef]
- Matalka, K.Z.; Tutunji, M.F.; Abu-Baker, M.; Abu Baker, Y. Measurement of protein cytokines in tissue extracts by enzyme-linked immunosorbent assays: Application to lipopolysaccharide-induced differential milieu of cytokines. Neuro. Endocrinol. Lett. 2005, 26, 231–236. [Google Scholar]
- Song, L.F.; Merz, K.M. Evolution of Alchemical Free Energy Methods in Drug Discovery. J. Chem. Inform. Model 2020, 60, 5308–5318. [Google Scholar] [CrossRef]
- Schindler, C.E.M.; Baumann, H.; Blum, A.; Böse, D.; Buchstaller, H.-P.; Burgdorf, L.; Cappel, D.; Chekler, E.; Czodrowski, P.; Dorsch, D.; et al. Large-Scale Assessment of Binding Free Energy Calculations in Active Drug Discovery Projects. J. Chem. Inform. Model. 2020, 60, 5457–5474. [Google Scholar] [CrossRef]
- Schrödinger Release 2020-1: FEP+; Schrödinger, LLC: New York, NY, USA, 2020.
- Schrödinger Release 2020-2: Prime; Schrödinger, LLC: New York, NY, USA, 2020.
- Gomzina, N.A.; Zaitsev, V.V.; Krasikova, R.N. Optimization of nucleophilic fluorination step in the synthesis of various compounds labelled with fluorine-18 for their use as PET radiotracers. J. Label. Comp. Radiopharm. 2001, 44, S515–S517. [Google Scholar] [CrossRef]
- Slavik, R.; Müller Herde, A.; Haider, A.; Krämer, S.D.; Weber, M.; Schibli, R.; Ametamey, S.M.; Mu, L. Discovery of a fluorinated 4-oxo-quinoline derivative as a potential positron emission tomography radiotracer for imaging cannabinoid receptor type 2. J. Neurochem. 2016, 138, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.A.; Jin, L.; Garcia, A.; DaSilva, J.N.; Houle, S. An admonition when measuring the lipophilicity of radiotracers using counting techniques. Appl. Radiat. Isot. 2001, 54, 203–208. [Google Scholar] [CrossRef]

| Compound | IC50 (µM) | SI (Selectivity Index) a | Ref | |
|---|---|---|---|---|
| COX-1 | COX-2 | |||
| Celecoxib | 12.73 >10.00 | 0.05 0.05 | 254 | [25] b |
| >200 | [18] c | |||
| MH | 2.40 | 0.06 | 40 | [25] b |
| MPbP | 2.53 | 0.14 | 18 | [18] c |
| F-IV | 4.61 | 0.09 | 48 | This study c |
| Radioligand | fu in Rat Plasma, %(n = 3) | fu in Human Plasma, %(n = 6) |
|---|---|---|
| [18F]F-IV | 2.01 ± 0.26 | 0.19 ± 0.05 |
| [11C]MPbP | 8.02 ± 0.37 | 1.88 ± 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vaulina, D.D.; Stosman, K.I.; Sivak, K.V.; Aleksandrov, A.G.; Viktorov, N.B.; Kuzmich, N.N.; Kiseleva, M.M.; Kuznetsova, O.F.; Gomzina, N.A. Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4′-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging. Molecules 2021, 26, 6630. https://doi.org/10.3390/molecules26216630
Vaulina DD, Stosman KI, Sivak KV, Aleksandrov AG, Viktorov NB, Kuzmich NN, Kiseleva MM, Kuznetsova OF, Gomzina NA. Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4′-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging. Molecules. 2021; 26(21):6630. https://doi.org/10.3390/molecules26216630
Chicago/Turabian StyleVaulina, Daria D., Kira I. Stosman, Konstantin V. Sivak, Andrey G. Aleksandrov, Nikolai B. Viktorov, Nikolay N. Kuzmich, Mariia M. Kiseleva, Olga F. Kuznetsova, and Natalia A. Gomzina. 2021. "Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4′-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging" Molecules 26, no. 21: 6630. https://doi.org/10.3390/molecules26216630
APA StyleVaulina, D. D., Stosman, K. I., Sivak, K. V., Aleksandrov, A. G., Viktorov, N. B., Kuzmich, N. N., Kiseleva, M. M., Kuznetsova, O. F., & Gomzina, N. A. (2021). Preliminary Assessment of the Anti-inflammatory Activity of New Structural Honokiol Analogs with a 4′-O-(2-Fluoroethyl) Moiety and the Potential of Their 18F-Labeled Derivatives for Neuroinflammation Imaging. Molecules, 26(21), 6630. https://doi.org/10.3390/molecules26216630






