Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine
Abstract
1. Introduction
2. Chemical Adulterant in Herbal Medicine
3. Chromatographic Method
3.1. Thin Layer Chromatography
3.2. Liquid Chromatography
3.3. Gas Chromatography
4. Spectroscopic Method
4.1. Infrared Spectroscopy
4.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.3. Mass Spectrometry (MS)
5. Microfluidic Analytical Device
6. Electrochemical Method
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nisar, B.; Sultan, A.; Rubab, S. Comparison of medicinally important natural products versus synthetic drugs-a short commentary. Nat. Prod. Chem. Res. 2018, 6, 308. [Google Scholar] [CrossRef]
- Woerdenbag, H.J.; Kayser, O. Jamu: Indonesian traditional herbal medicine towards rational phytopharmacological use. J. Herbal Med. 2014, 4, 51–73. [Google Scholar]
- Pom, B. Lindungi Masyarakat dari Obat Tradisional, Suplemen Kesehatan, dan Kosmetik yang Berisiko terhadap Kesehatan; Badan POM: Jakarta, Indonesia, 2020. [Google Scholar]
- Minh, D.T.C.; Huyen, N.T.T.; Anh, N.T.K.; Ha, P.T.T. Detection of sildenafil adulterated in herbal products using thin layer chromatography combined with surface enhanced Raman spectroscopy: “Double coffee-ring effect” based enhancement. J. Pharm. Biomed. Anal. 2019, 174, 340–347. [Google Scholar] [CrossRef]
- Wu, N.; Balayssac, S.; Danoun, S.; Malet-Martino, M.; Gilard, V. Chemometric analysis of low-field 1H NMR Spectra for unveiling adulteration of slimming dietary supplements by pharmaceutical compounds. Molecules 2020, 25, 1193. [Google Scholar] [CrossRef]
- Primpray, V.; Chailapakul, O.; Tokeshi, M.; Rojanarata, T.; Laiwattanapaisal, W. A paper-based analytical device coupled with electrochemical detection for the determination of dexamethasone and prednisolone in adulterated traditional medicines. Anal. Chim. Acta 2019, 1078, 16–23. [Google Scholar] [CrossRef]
- Haneef, J.; Shaharyar, M.; Husain, A.; Rashid, M.; Mishra, R.; Siddique, N.A.; Pal, M. Analytical methods for the detection of undeclared synthetic drugs in traditional herbal medicines as adulterants. Drug Test. Anal. 2013, 5, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Calahan, J.; Howard, D.; Almalki, A.J.; Gupta, M.P.; Calderón, A.I. Chemical adulterants in herbal medicinal products: A review. Planta Med. 2016, 82, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-B.; Zheng, J.; Li, J.-J.; Yu, H.-Y.; Li, Q.-Y.; Xu, L.-H.; Liu, M.-J.; Xian, R.-Q.; Sun, Y.-E.; Liu, B.-J. Simultaneous analysis of 23 illegal adulterated aphrodisiac chemical ingredients in health foods and Chinese traditional patent medicines by ultrahigh performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry. J. Food Drug Anal. 2018, 26, 1138–1153. [Google Scholar] [CrossRef]
- Nugroho, A.; Ritonga, F.D. Rapid Analysis of Adulterated Dexamethasone in Joint-Pain Killer Traditional Herbal Medicine (THM) Using Infrared Spectroscopy. EKSAKTA J. Sci. Data Anal. 2018, 18, 137–145. [Google Scholar] [CrossRef]
- Nugroho, A.; Febriana, Y.; Septiwi, M.; Pratiwi, D.A. Rapid analysis of adulterated sildenafil citrate in marketed herbal aphrodisiacs using infrared spectroscopy. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2018; p. 020003. [Google Scholar]
- Hayun, H.; Maggadani, B.P.; Amalina, N. Determination of sibutramine adulterated in herbal slimming products using TLC densitometric method. Indones. J. Pharm. 2016, 27, 15. [Google Scholar] [CrossRef][Green Version]
- Pratiwi, R.; Septyani, R.N.; Febriany, R.; Saputri, F.A.; Nuwarda, R.F. Design and Optimization of Colorimetric Paper-Based Analytical Device for Rapid Detection of Allopurinol in Herbal Medicine. Int. J. Anal. Chem. 2019, 2019. [Google Scholar] [CrossRef]
- Hachem, R.; Assemat, G.; Martins, N.; Balayssac, S.; Gilard, V.; Martino, R.; Malet-Martino, M. Proton NMR for detection, identification and quantification of adulterants in 160 herbal food supplements marketed for weight loss. J. Pharm. Biomed. Anal. 2016, 124, 34–47. [Google Scholar] [CrossRef]
- Firozian, F.; Nili-Ahmadabadi, A.; Moradkhani, S.; Moulaei, M.; Fasihi, Z.; Ahmadimoghaddam, D. Adulteration of the Herbal Weight Loss Products by the Illegal Addition of Synthetic Antiobesity Medications: A Pilot Study. J. Obes. 2021, 2021, 9968730. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhuang, Y.; Ma, J.; Wang, J.; Feng, R.; He, R.; Luo, Z.; Wang, H.; Zhan, R. A Rapid Screening Method for Sibutramine Hydrochloride in Natural Herbal Medicines and Dietary Supplements. Int. J. Anal. Chem. 2021, 2021, 8889423. [Google Scholar] [CrossRef]
- Ahmed, N.; Nounou, M.I.; Abouelfetouh, A.; El-Kamel, A. Over-the-counter herbal weight loss supplements in Egypt: Label claim, microbiological and pharmaceutical quality, safety assessments. Med. Princ. Pract. 2019, 28, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Shekari, N.; Vosough, M.; Heidar, K.T. Chromatographic fingerprinting through chemometric techniques for herbal slimming pills: A way of adulterant identification. Forensic Sci. Int. 2018, 286, 213–222. [Google Scholar] [CrossRef]
- Salahshour, B.; Sadeghi, S.; Nazari, H.; Soltaninejad, K. Determining undeclared synthetic pharmaceuticals as adulterants in weight loss herbal medicines. Int. J. Med. Toxicol. Forensic Med. 2020, 10, 26253. [Google Scholar] [CrossRef]
- Dastjerdi, A.G.; Akhgari, M.; Kamali, A.; Mousavi, Z. Principal component analysis of synthetic adulterants in herbal supplements advertised as weight loss drugs. Complement. Ther. Clin. Pract. 2018, 31, 236–241. [Google Scholar] [CrossRef]
- al Lawati, H.A.; al Busaidi, I.; Kadavilpparampu, A.M.; Suliman, F.O. Determination of common adulterants in herbal medicine and food samples using core-shell column coupled to tandem mass spectrometry. J. Chromatogr. Sci. 2017, 55, 232–242. [Google Scholar] [CrossRef]
- Pratiwi, R.; Suherman, S.E.; Poongan, R.A.; Mutakin, M.; Hasanah, A.N. Design of optical sensor membrane based on polymer poly (methyl methacrylate) for paracetamol detection in traditional herbal medicine. Int. J. Anal. Chem. 2018, 2018, 8918329. [Google Scholar] [CrossRef] [PubMed]
- Hemdan, A.; Tawakol, S.M. HPLC–UV chromatographic methods for detection and quantification of undeclared withdrawn synthetic medications in counterfeit herbal medicines with confirmation by HPLC–PDA and mass spectrometry. Chromatographia 2018, 81, 777–783. [Google Scholar] [CrossRef]
- Yu, K.; Powell, M.; Maziarz, M.; Patel, D.N. Analysis of an adulterated herbal medicinal product using ultra-performance liquid chromatography coupled with QTOF mass spectrometry. World J. Tradit. Chin. Med. 2016, 2, 1. [Google Scholar] [CrossRef]
- Jin, R.; Li, L.; Guo, L.; Li, W.; Shen, Q. A graphene tip coupled with liquid chromatography tandem mass spectrometry for the determination of four synthetic adulterants in slimming supplements. Food Chem. 2017, 224, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-P.; Lee, Y.-L.; Hung, C.-Y.; Chang, C.-F.; Chen, Y. Detection of adulterated drugs in traditional Chinese medicine and dietary supplements using hydrogen as a carrier gas. PLoS ONE 2018, 13, e0205371. [Google Scholar] [CrossRef]
- Dasari, R.; Zamborini, F.P. Surface enhanced Raman spectroscopy at electrochemically fabricated silver nanowire junctions. Anal. Chem. 2016, 88, 675–681. [Google Scholar] [CrossRef]
- Langer, J.; Jimenez de Aberasturi, D.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2019, 14, 28–117. [Google Scholar] [CrossRef]
- Parys, W.; Dołowy, M.; Pyka-Pająk, A. Rapid TLC with Densitometry for Evaluation of Naproxen Stability. Processes 2020, 8, 962. [Google Scholar] [CrossRef]
- Ainiyah, E.Q.; Supandi, S.; Anggia, V. Review of Adulterated Herbal Products and Supplements and Methods of Analysis. Pharm. Biomed. Sci. J. (PBSJ) 2021, 2, 5–16. [Google Scholar]
- Gumustas, M.; Kurbanoglu, S.; Uslu, B.; Ozkan, S.A. UPLC versus HPLC on drug analysis: Advantageous, applications and their validation parameters. Chromatographia 2013, 76, 1365–1427. [Google Scholar] [CrossRef]
- Allen, D.R.; McWhinney, B.C. Quadrupole time-of-flight mass spectrometry: A paradigm shift in toxicology screening applications. Clin. Biochem. Rev. 2019, 40, 135. [Google Scholar] [CrossRef] [PubMed]
- Milman, B.L. General principles of identification by mass spectrometry. TrAC Trends Anal. Chem. 2015, 69, 24–33. [Google Scholar] [CrossRef]
- Ng, L.M.; Simmons, R. Infrared spectroscopy. Anal. Chem. 1999, 71, 343–350. [Google Scholar] [CrossRef]
- Smith, B.C. Fundamentals of Fourier Transform Infrared Spectroscop; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Walkowiak, A.; Ledziński, Ł.; Zapadka, M.; Kupcewicz, B. Detection of adulterants in dietary supplements with Ginkgo biloba extract by attenuated total reflectance Fourier transform infrared spectroscopy and multivariate methods PLS-DA and PCA. Spectrochimica Acta Part A Mol. Biomol. Spectrosc. 2019, 208, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Liu, Y.; Li, H.; Qi, Y.; Lu, F. Two-dimensional correlation infrared spectroscopy applied to the identification of ephedrine and pseudoephedrine in illegally adulterated slimming herbal products. Drug Test. Anal. 2017, 9, 221–229. [Google Scholar] [CrossRef]
- Günther, H. NMR Spectroscopy: Basic Principles, Concepts and Applications in Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hu, B.; Huang, Y.; Yin, G.; Zhang, G.; Zhang, L.; Wang, T.; Yao, Z.-P. Rapid detection of adulterated drugs in herbal dietary supplements by wooden-tip electrospray ionization mass spectrometry. Anal. Methods 2016, 8, 6840–6846. [Google Scholar] [CrossRef]
- Yao, Y.N.; Wu, L.; Sun, W.Y.; Luo, Z.H.; Di, D.; Yuan, Z.C.; Huang, Z.; Hu, B. Fast-switching high-voltage porous-tip electrospray ionization mass spectrometry for rapid detection of antirheumatic drugs in adulterated herbal dietary supplements. Rapid Commun. Mass Spectrom. 2019, 33, 1877–1883. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, Y.; Lu, Y.; Zhang, F.; Su, Y.; Guo, Y. Ultrasonic extraction and nebulization in real-time coupled with carbon fiber ionization mass spectrometry for rapid screening of the synthetic drugs adulterated into herbal products. Anal. Chim. Acta 2020, 1136, 62–71. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2014, 87, 19–41. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. Int. Ed. 2007, 46, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Noviana, E.; Nguyen, M.P.; Geiss, B.J.; Dandy, D.S.; Henry, C.S. Paper-based microfluidic devices: Emerging themes and applications. Anal. Chem. 2017, 89, 71–91. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Carrão, D.B.; Pratiwi, R.; Henry, C.S. Emerging applications of paper-based analytical devices for drug analysis: A review. Anal. Chim. Acta 2020, 1116, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Murphy, A.; Gorey, B.; de Guzman, K.; Kelly, N.; Nesterenko, E.; Morrin, A. Microfluidic paper analytical device for the chromatographic separation of ascorbic acid and dopamine. RSC Adv. 2015, 5, 93162–93169. [Google Scholar] [CrossRef]
- Da Silva, G.O.; de Araujo, W.R.; Paixão, T.R. Portable and low-cost colorimetric office paper-based device for phenacetin detection in seized cocaine samples. Talanta 2018, 176, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Tran, V.; Ko, E.; Park, C.H.; Chung, W.S.; Seong, G.H. Determination of acetaminophen using functional paper-based electrochemical devices. Sens. Actuators B Chem. 2016, 232, 514–522. [Google Scholar] [CrossRef]
- Luo, G.; Du, L.; Wang, Y.; Wang, K. Recent developments in microfluidic device-based preparation, functionalization, manipulation of nano-and micro-materials. Particuology 2019, 45, 1–19. [Google Scholar] [CrossRef]
- Tsao, C.-W.; De Voe, D.L. Bonding of thermoplastic polymer microfluidics. Microfluid. Nanofluid. 2009, 6, 1–16. [Google Scholar] [CrossRef]
- Nery, E.W.; Kubota, L.T. Sensing approaches on paper-based devices: A review. Anal. Bioanal. Chem. 2013, 405, 7573–7595. [Google Scholar] [CrossRef]
- Sharma, N.; Barstis, T.; Giri, B. Advances in paper-analytical methods for pharmaceutical analysis. Eur. J. Pharm. Sci. 2018, 111, 46–56. [Google Scholar] [CrossRef]
- Costa-Rama, E.; Nouws, H.; Delerue-Matos, C.; Blanco-López, M.d.C.; Fernández-Abedul, M.T. Preconcentration and sensitive determination of the anti-inflammatory drug diclofenac on a paper-based electroanalytical platform. Anal. Chim. Acta 2019, 1074, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, R.; Nguyen, M.P.; Ibrahim, S.; Yoshioka, N.; Henry, C.S.; Tjahjono, D.H. A selective distance-based paper analytical device for copper (II) determination using a porphyrin derivative. Talanta 2017, 174, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Cate, D.M.; Noblitt, S.D.; Volckens, J.; Henry, C.S. Multiplexed paper analytical device for quantification of metals using distance-based detection. Lab. Chip 2015, 15, 2808–2818. [Google Scholar] [CrossRef]
- Cate, D.M.; Dungchai, W.; Cunningham, J.C.; Volckens, J.; Henry, C.S. Simple, distance-based measurement for paper analytical devices. Lab. Chip 2013, 13, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Karamahito, P.; Sitanurak, J.; Nacapricha, D.; Wilairat, P.; Chaisiwamongkhol, K.; Phonchai, A. Paper device for distance-based visual quantification of sibutramine adulteration in slimming products. Microchem. J. 2021, 162, 105784. [Google Scholar] [CrossRef]
- Kuswandi, B.; Kartika, A.S.; Kristiningrum, N.; Pratoko, D.K.; Sary, I.P. Simple and rapid dipstick test for detection of dexamethasone adulteration in traditional herbal medicines. Pharm. Sci. Asia 2021, 48, 115–121. [Google Scholar] [CrossRef]
- Freitas, J.M.; Ramos, D.L.O.; Sousa, R.M.F.; Paixão, T.R.L.C.; Santana, M.H.P.; Muñoz, R.A.A.; Richter, E.M. A portable electrochemical method for cocaine quantification and rapid screening of common adulterants in seized samples. Sens. Actuators B Chem. 2017, 243, 557–565. [Google Scholar] [CrossRef]
- Freitas, J.M.; Oliveira, T.C.; Santana, M.H.; Banks, C.E.; Munoz, R.A.; Richter, E.M. A simple and fast-portable method for the screening of the appetite-suppressant drug sibutramine in natural products and multivitamins supplements. Sens. Actuators B Chem. 2019, 282, 449–456. [Google Scholar] [CrossRef]
- Saichanapan, J.; Promsuwan, K.; Limbut, W. Adsorption and determination of sibutramine in illegal slimming product using porous graphene ink-modified electrode. Talanta 2020, 212, 120788. [Google Scholar] [CrossRef]
| Analyte | Matrix | Method | Elution Type and Mobile Phase/Flow Rate | Column/Temperature | Detector | LOD/LOQ | % RSD/ Recovery | Ref. |
|---|---|---|---|---|---|---|---|---|
| Sildenafil | Capsule, granule, herbal extract claimed for treatment of erectile dysfunction | TLC-SERS | Mobile phase: ethyl acetate-isopropanol-25% ammonia (45:5:2.6, v/v/v) | Stationary phase: 20 × 10 cm aluminium TLC plate | Surface-enhanced Raman Spectroscopy (SERS) | LOD = 2 ng/spot | - | [4] |
| Sibutramine | Herbal slimming product | TLC-Densitometric | Mobile phase: toluene-diethylamine (10:0.3, v/v) | Stationary phase: aluminum TLC plates coated with silica gel 60 F254 with 250 μm thickness | Densitometric scanning at 227 nm with Camag® TLC Scanner III | LOD = 217.5 ng LOQ = 724.9 ng/spot | Average recovery (%): 99.70 ± 1.22 RSD: <2% | [12] |
| Sibutramine phenolphthalein, sildenafil | Capsules (Sliming Bomb, Zotreem Plus, and Enjoy) | HPLC-UV | Mobile phase SIB and PPH: potassium dihydrogen orthophosphate buffer (adjusted to pH 3 using o-phosphoric acid)/acetonitrile (40/60 v/v) Mobile phase SLD: acetonitrile–potassium hydrogen phosphate buffer (pH 3.2) adjusted using o-phosphoric acid (50/50 v/v) Flow rate: 1 mL/min | Inertsil C18 Column (4.6 × 100 mm with 5 μm particle size) | UV at 223 nm for SIB dan PPH and UV at 230 for SLD | - | RSD = 1.926% (SIB), 1.779% (PPH), 1.709% (SLD) Recovery: 98.64 ± 1.151 (SIB), 98.78 ± 1.537 (PPH), 99.11 ± 1.814 (SLD) | [23] |
| Sildenafil, tadalafil, vardenafil hydrochloride | Capsule, cream, gel, etc. from 33 screened samples supplied by Public Authority of Customer Protection, Ministry of Health. | HPLC-MS-MS | Gradient elution. 0–2 min 5% B, 2–4 min 5% B, 4–7 min 40% B, 7–14 min 65% B, 14–18 min 90% B, 18–23 min 90% B, 23–23.5 min 5% B, and 23.5–27 min 5% B to equilibrate for the next injection (the total run time was 27 min). Mobile phase: A = 0.1% formic acid in water; B = 1% formic acid in 15% acetonitrile and 85% methanol flow rate: 0.3 mL min−1 | Stationary phase: Poroshell 120 EC C18 column (3.0 ID × 100 mm length, 2.7 μm) (PC18) Temperature: 40 °C | diode array | - | Coefficient variance CV = < 4% Relative error RE = < 3% | [21] |
| Caffeine, chlorpheniramine, piroxicam, betamethasone, oxethazaine | Capsule * | UPLC-QTOF-MS | Mobile phase: water with 0.1% formic acid (A) and acetonitrile (B) Elution type: gradient elution from 1% B to 70% B in 26 min with additional 2 min re-equilibration Flow Rate: 0.6 mL/min | ACQUITY UPLC HSS T3 column (1.8 μm, 2.1 × 100 mm) Temperature: 45 °C | QTOF-MS with DIA as data acquisition | - | - | [24] |
| Sildenafil, tadalafil, aildenafil, sulfoaildenafil | Pellets, capsules, tablets, or oral liquid, claimed functions of aphrodisiac, enhancement of sexual performance, physical fatigue relief or immunity enhancement | UHPLCQ-TOF HRMS | Gradient elution: began at 25% B for 2 min, then linearly ramped to 55% B within 11 min, and then ramped to 90% B in 1 min, and held at 90% B for 2.0 min mobile phase: A = 5 mmol/L ammonium acetate (adjusted pH to 3.4 with acetic acid) B = acetonitrile. Flow rate: 0.4 mL/min | Column: Agilent SB-C18 RRHD column (100 mm × 3.0 mm, 1.8 mm) Temperature: 40 ℃. | QTOF-MS/MS | LOD = 0.002–0.1 µg/g LLOQ = 0.005–0.25 mcg/g | Recovery: 82.5–103.6%. RSD (Intra and inter-day): 0.4 to 13.6%. | [9] |
| Fenfluramine, phenolphthalein, bumetanide, and sibutramine | Slimming supplement: tea powder, capsule, tablets | Gtip SPE- UPLC–MS/MS | Gradient elution: initial 10% A, 4 min 50% A, 6 min 60% A, and 10 min 100% A. Total run time of 12 min. Mobile phase: Acetonitrile (A) and ultrapure water with 0.1% formic acid (B). flow rate: 0.40 mL/min | Stationary phase: HaloTM fused-core C18 column (100 mm × 2.1 mm, 2.7 mcm) Temperature column 30 °C | MS/MS | LOD = 1.8 ng mL−1) LOQ = 5.6 ng mL−1 | Recovery = 82.9–95.2% RSD = <7.3% | [25] |
| Amitriptyline acetaminophen, Ibuprofen, chlorzoxazone, sulfamethoxazole, tadalafil, and sildenafil | 83 Traditional Chinese medicines and 40 food supplements samples | GC-MS | Hydrogen as a carrier gas; 1.0 mL/min | A silica capillary column, Agilent HP-5 MS (30 m × 0.25 mm i.d. 0.25 μm film thickness); temperature programming | MS electron ionization | LOD = 10 to 1000 μg/g. | - | [26] |
| Analyte | Matrix | Method | Max Wavelength or Frequency | LOD/LOQ | %RSD/Recovery | Reference |
|---|---|---|---|---|---|---|
| Dexamethasone | Herb claimed for joint painkiller * | Infrared spectroscopy combined with partial least square (PLS) | Wavenumbers 3646, 3642, 2461, 2453, 2432, 2406, 2229, 2209, 2197, 2097, 2092, 2064, 2059, 2047, 2026, 2009, 1969, and 1513 cm−1 | - | Validation: R2 = −0.9988 RMSEC = 0.009455 PRESS = 0.0022721 RMSECV = 0.02902 | [10] |
| Sildenafil | An herbal product claimed to enhance sexual activity * | FTIR-SMLR Stepwise multiple linear regression | wavenumbers 1791, 1692, 1691 and 971 cm−1 | - | Validation: R2 = 1.00 RMSEC = 0.000310913 PRESS = 0.0009191 | [11] |
| Sibutramine, phenolphtalein | Capsule, tablet, powder for weight-loss | Low-field 1H NMR spectroscopy | Frequency = 59.7 mHz for 1H | Lowes limit 3 mg/100 mg | - | [5] |
| Rutin, quercetin, kaempferol | Capsule and tablet containing Ginkgo biloba | FTIR-PLS DA | Wavelength = 900–1800 cm−1 | - | Validation RMSEC = 0.393 RMSECV = 0.570 | [36] |
| Ephedrin, pseudoephedrine | Slimming herbal product | 2DCOS | Wavelength: 4000–400 cm−1 | LOD = <1% | - | [37] |
| Melatonin, doxepin, diazepam, chlorpheniramine, zopiclone, nitrazepam, zaleplon, alprazolam, clonazepam and chlordiazepoxide | Herbal dietary supplements (pills, tablets, capsules, or soft-gel capsules) | WT-ESI-MS | Tranquilizer and aphrodisiac samples = 230 nm Weight loss samples = 225 nm or 265 nm. | LOD = 0.1 mg/g | - | [39] |
| Paracetamol, naproxen, sulfamethoxazole, diclofenac, and phenylbutazone | Tablet and capsules (anti rheumatism health care products) | Fast-switching +/− HV tip-ESI--MS | - | LOD: (<0.1 ng/g) PCM = 0.05 ng/g NPX 0.1 ng/g for NPX, 0.01 ng/g for SMZ, 0.01 ng/g for DCM, and 0.1 ng/g for PBZ | - | [40] |
| Nifedipine, nisoldipine, nicardipine, lercanidipine, felodipine, clevidipine, metformin hydrochloride, lovastatin, Simvastatin, gemfibrozil, and fenofibrate | Tablets, pills, granules, and capsules | UEN/CFI-MS | - | LOD = 2 mcg/g to 50 mcg/g | RSD = less than 15% | [41] |
| Analyte | Matrix | Method | Media | LOD/LOQ | %RSD/ Recovery | Reference |
|---|---|---|---|---|---|---|
| Paracetamol | Herbal medicine powder | Optical sensor membrane | Polymer poly(methyl methacrylate) (PMMA) | Lowest measurable: 2.55–4.01 mg/mL | - | [22] |
| Allopurinol | Herbal medicine powder | A paper-based analytical device using colorimetry | Whatman filter paper No. 1, 4, 6 and Whatman 1 chromatography | Lowest measurable: 75 mg/mL | - | [13] |
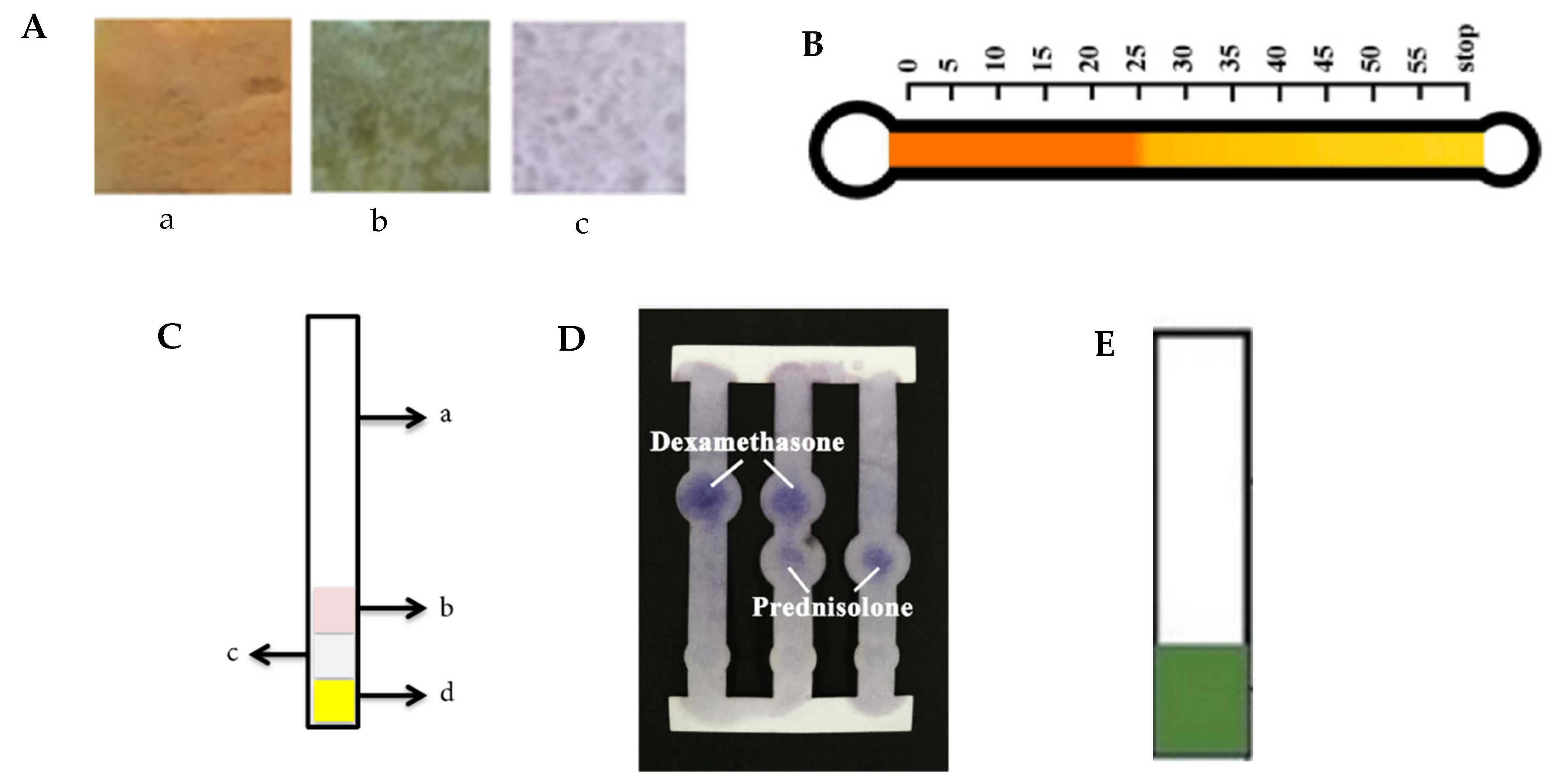
| Dexamethasone and Prednisolone | Herbal medicine pellets | Electrochemical paper-based analytical device | Whatman SG81 paper | LOD dexamethasone: 3.59 µg/mL prednisolone: 11.988 µg/mL LOQ Dexamethasone: 6.00 µg/mL prednisolone: 20.02 µg/mL | Dexamethasone: 83 to 108% (recovery) and 5.6 to 10.1% (Coefficient of variation) prednisolone: 88 to 134% (recovery) and 4.4 to 13.8% (coefficient of variation) | [6] |
| Sibutramine | Powder (weight loss product) | A distance-based paper device with colorimetry | Filter paper | LOQ 0.22 mmol/L | Less than 4.4%RSD | [58] |
| Dexamethasone | Powder (joint-paint killer product) | Dipstick test using sensing film | Cellulose acetate film | LOD: 0.422 μg/mL LOQ: 1.406 μg/mL | Recovery: 99.978–101.144% RSD: <0.196% | [59] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratiwi, R.; Dipadharma, R.H.F.; Prayugo, I.J.; Layandro, O.A. Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine. Molecules 2021, 26, 6606. https://doi.org/10.3390/molecules26216606
Pratiwi R, Dipadharma RHF, Prayugo IJ, Layandro OA. Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine. Molecules. 2021; 26(21):6606. https://doi.org/10.3390/molecules26216606
Chicago/Turabian StylePratiwi, Rimadani, Ratu Hanifa Fayza Dipadharma, Ishmat Jati Prayugo, and Olivia Angelina Layandro. 2021. "Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine" Molecules 26, no. 21: 6606. https://doi.org/10.3390/molecules26216606
APA StylePratiwi, R., Dipadharma, R. H. F., Prayugo, I. J., & Layandro, O. A. (2021). Recent Analytical Method for Detection of Chemical Adulterants in Herbal Medicine. Molecules, 26(21), 6606. https://doi.org/10.3390/molecules26216606






