Iridal-Type Triterpenoids Displaying Human Neutrophil Elastase Inhibition and Anti-Inflammatory Effects from Belamcanda chinensis
Abstract
1. Introduction
2. Results and Discussion
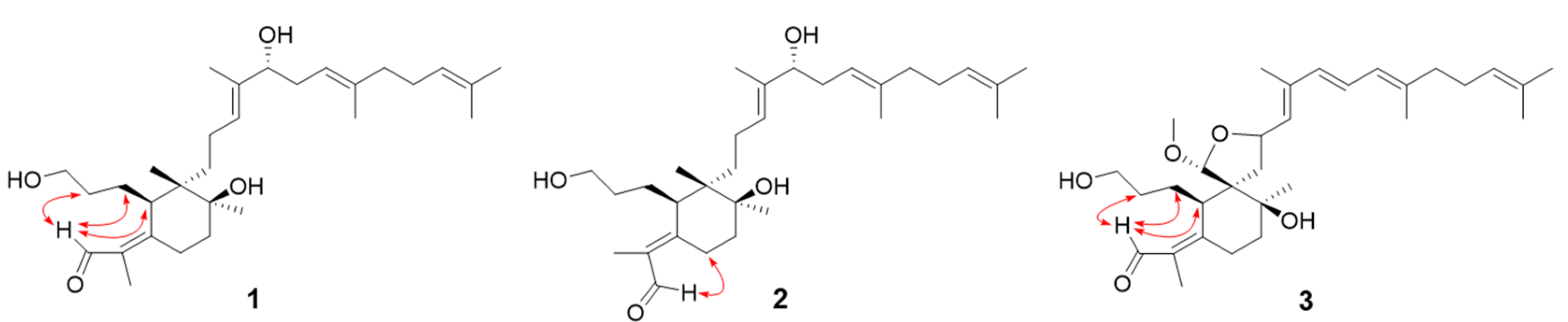
2.1. Isolation and Identification of Iridal-Type Triterpenoids
2.2. Human Neutrophil Elastase Inhibition
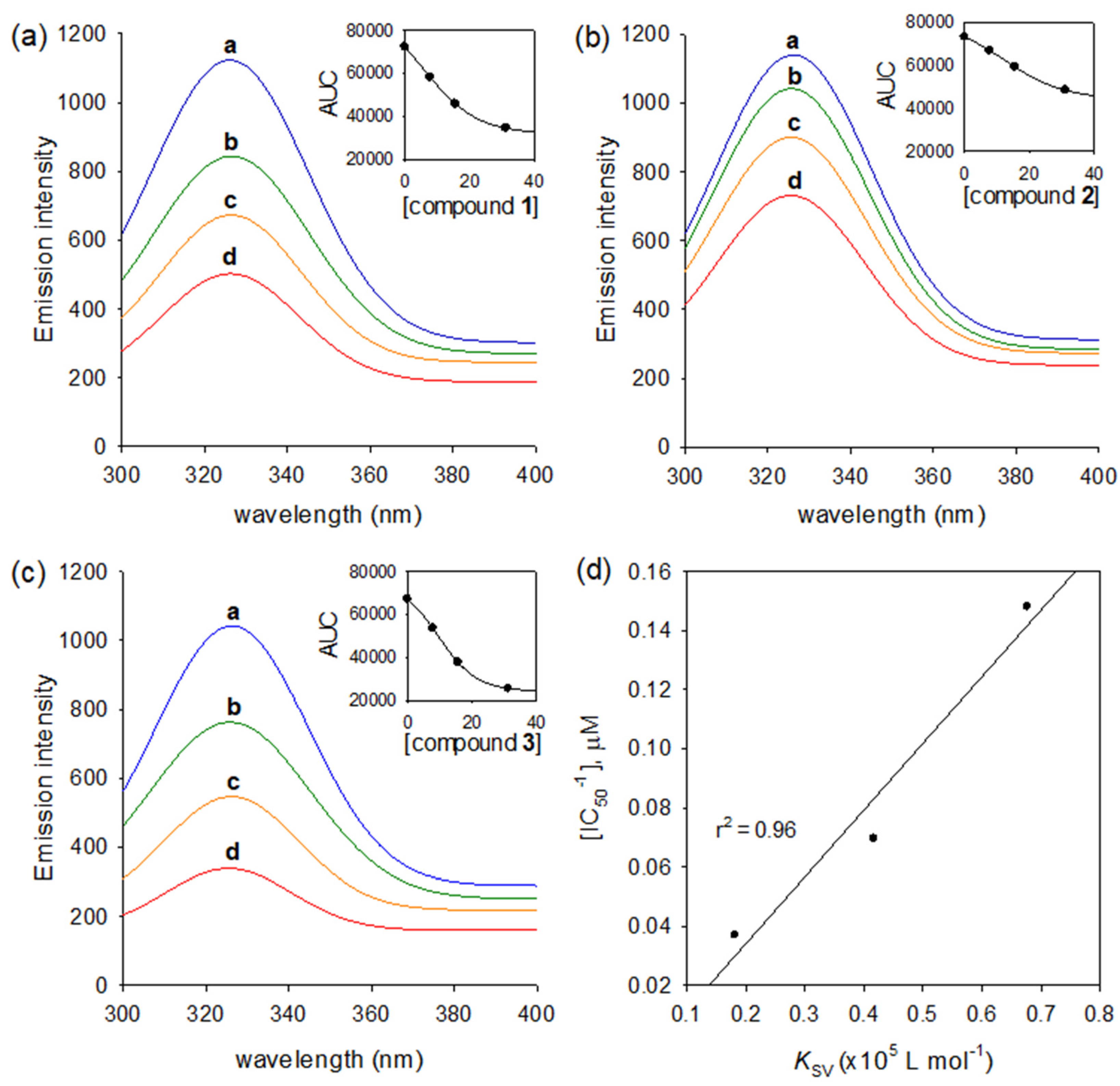
2.3. Binding Affinities to Human Neutrophil Elastase
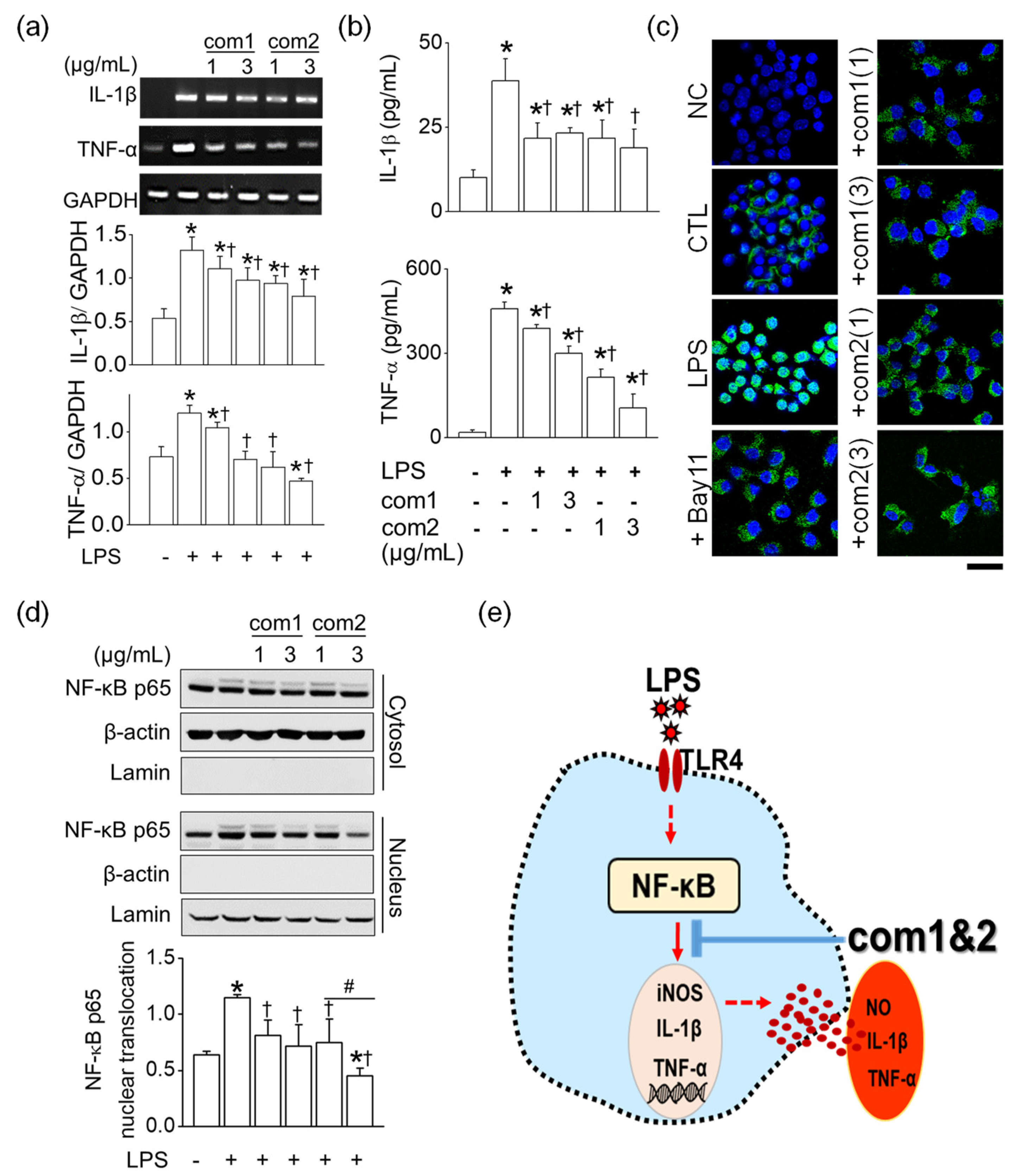
2.4. Anti-Inflammatory Effect of Triterpenoid 1 and 2
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Extraction and Isolation
3.2.1. Isoiridogermanal (1)
3.2.2. Iridobelamal A (2)
3.2.3. Iridobelamal B (3)
3.3. Inhibitory Effects against Human Neutrophil Elastase
3.4. Fluorescence Quenching Measurements
3.5. Cell Culture and Cell Viability Assay
3.6. RNA Isolation and Reverse Transcriptase (RT)-Polymerase Chain Reaction (PCR)
3.7. Measurement of Nitrite Levels
3.8. Measurement of Cytokines Concentration
3.9. Immunocytochemistry
3.10. Western Blot Analysis with Nuclear and Cytoplasmic Fractions
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xin, R.; Zheng, J.; Cheng, L.; Peng, W. Belamcanda chinensis (L.) DC: Ethnopharmacology, Phytochemistry and Pharmacology of An Important Traditional Chinese Medicine. Afr. J. Tradit. Complement. Altern. Med. 2015, 12, 39–70. [Google Scholar] [CrossRef][Green Version]
- Woźniak, D.; Matkowski, A. Belamcandae chinensis rhizoma—A review of phytochemistry and bioactivity. Fitoterapia 2015, 107, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Takahashfi, K.; Hoshino, Y.; Suzuki, S.; Hano, Y.; Nomura, T. Iridals from Iris tectorum and Belamcanda chinensis. Phytochemistry 2000, 53, 925–929. [Google Scholar] [CrossRef]
- Taylor, S.; Dirir, O.; Zamanian, R.T.; Rabinovitch, M.; Thompson, A.A.R. The role of neutrophils and neutrophil elastase in pulmonary arterial hypertension. Front. Med. 2018, 5, 217. [Google Scholar] [CrossRef]
- Bardoel, B.W.; Kenny, E.F.; Sollberger, G.; Zychlinsky, A. The balancing act of neutrophils. Cell Host Microbe 2014, 15, 526–536. [Google Scholar] [CrossRef]
- Crocetti, L.; Quinn, M.T.; Schepetkin, I.A.; Giovannoni, M.P. A patenting perspective on human neutrophil elastase (HNE) inhibitors (2014–2018) and their therapeutic applications. Expert Opin. Ther. Pat. 2019, 29, 555–578. [Google Scholar] [CrossRef]
- Lee, W.L.; Downey, G.P. Leukocyte elastase: Physiological functions and role in acute lung injury. Am. J. Respir. Crit. Care Med. 2001, 164, 896–904. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Boutten, A.; Dehoux, M.S.; Seta, N.; Ostinelli, J.; Venembre, P.; Crestani, B.; Dombret, M.C.; Durand, G.; Aubier, M. Compartmentalized IL-8 and elastase release within the human lung in unilateral pneumonia. Am. J. Respir. Crit. Care Med. 1996, 153, 336–342. [Google Scholar] [CrossRef]
- Thulborn, S.J.; Mistry, V.; Brightling, C.E.; Moffitt, K.L.; Ribeiro, D.; Bafadhel, M. Neutrophil elastase as a biomarker for bacterial infection in COPD. Respir. Res. 2019, 20, 170. [Google Scholar] [CrossRef] [PubMed]
- Dollery, C.M.; Owen, C.A.; Sukhova, G.K.; Krettek, A.; Shapiro, S.D.; Libby, P. Neutrophil elastase in human atherosclerotic plaques production by macrophages. Circulation 2003, 107, 2829–2836. [Google Scholar] [CrossRef]
- Muley, M.M.; Reid, A.R.; Botz, B.; Bölcskei, K.; Helyes, Z.; McDougall, J.J. Neutrophil elastase induces inflammation and pain in mouse knee joints via activation of proteinase-activated receptor-2. Br. J. Pharmacol. 2016, 173, 766–777. [Google Scholar] [CrossRef]
- Xu, R.; Huang, H.; Zhang, Z.; Wang, F.S. The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 2014, 11, 224–231. [Google Scholar] [CrossRef]
- Tsai, Y.F.; Yu, H.P.; Chang, W.Y.; Liu, F.C.; Huang, Z.C.; Hwang, T.L. Sirtinol Inhibits Neutrophil Elastase Activity and Attenuates Lipopolysaccharide-Mediated Acute Lung Injury in Mice. Sci. Rep. 2015, 5, 8347. [Google Scholar] [CrossRef]
- Ryu, H.W.; Kim, K.O.; Yuk, H.J.; Kwon, O.K.; Kim, J.H.; Kim, D.Y.; Na, M.K.; Ahn, K.S.; Oh, S.R. The constituent, anti-inflammation, and human neutrophil elastase inhibitory activity of Gnaphalium affine. J. Funct. Foods 2016, 27, 674–684. [Google Scholar] [CrossRef]
- Henriksen, P.A. The potential of neutrophil elastase inhibitors as anti-inflammatory therapies. Curr. Opin. Hematol. 2014, 21, 23–28. [Google Scholar] [CrossRef]
- Fürst, R.; Zündorf, I. Plant-derived anti-inflammatory compounds: Hopes and disappointments regarding the translation of preclinical knowledge into clinical progress. Mediat. Inflamm. 2014, 2014, 146832. [Google Scholar] [CrossRef] [PubMed]
- Checker, R.; Sandur, S.K.; Sharma, D.; Patwardhan, R.S.; Jayakumar, S.; Kohli, V.; Sethi, G.; Aggarwal, B.B.; Sainis, K.B. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-κB, AP-1 and NF-AT. PLoS ONE 2012, 7, e31318. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, Y.; Liu, Y.F.; Liang, D.; Hao, Z.Y.; Luo, H.; Zhang, Q.J.; Shi, G.R.; Chen, R.Y.; Cao, Z.Y.; et al. Cytotoxic iridal-type triterpenoids from Iris tectorum. Tetrahedron 2015, 71, 5579–5583. [Google Scholar] [CrossRef]
- Takahashi, K.; Suzuki, S.; Hano, Y.; Nomura, T. Protein kinase C activation by iridal type triterpenoids. Biol. Pharm. Bull. 2002, 25, 432–436. [Google Scholar] [CrossRef]
- Fang, R.; Houghton, P.J.; Hylands, P.J. Cytotoxic effects of compounds from Iris tectorum on human cancer cell lines. J. Ethnopharmacol. 2008, 118, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Topic, A.; Milovanovic, V.; Lazic, Z.; Ivosevic, A.; Radojkovic, D. Oxidized Alpha-1-Antitrypsin as a Potential Biomarker Associated with Onset and Severity of Chronic Obstructive Pulmonary Disease in Adult Population. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Watorek, W.; Karr, S.; Giles, J.; Bode, W.; Travis, J. Primary structure of human neutrophil elastase. Proc. Natl. Acad. Sci. USA 1987, 84, 2228–2232. [Google Scholar] [CrossRef]
- Van Amersfoort, E.S.; Van Berkel, T.J.C.; Kuiper, J. Receptors, mediators, and mechanisms involved in bacterial sepsis and septic shock. Clin. Microbiol. Rev. 2003, 16, 379–414. [Google Scholar] [CrossRef]
- Ryu, J.H.; Sung, J.; Xie, C.; Shin, M.K.; Kim, C.W.; Kim, N.G.; Choi, Y.J.; Choi, B.D.; Kang, S.S.; Kang, D. Aplysia kurodai-derived glycosaminoglycans increase the phagocytic ability of macrophages via the activation of AMP-activated protein kinase and cytoskeletal reorganization in RAW264.7 cells. J. Funct. Foods 2016, 27, 122–130. [Google Scholar] [CrossRef]
- Kim, J.Y.; Wang, Y.; Uddin, Z.; Song, Y.H.; Li, Z.P.; Jenis, J.; Park, K.H. Competitive neutrophil elastase inhibitory isoflavones from the roots of Flemingia philippinensis. Bioorg. Chem. 2018, 78, 249–257. [Google Scholar] [CrossRef]
- Li, Z.P.; Kim, J.Y.; Ban, Y.J.; Park, K.H. Human neutrophil elastase (HNE) inhibitory polyprenylated acylphloroglucinols from the flowers of Hypericum ascyron. Bioorg. Chem. 2019, 90, 103075. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, D.H.; Lee, K.W.; Kim, K.D.; Shah, A.B.; Zhumanova, K.; Park, K.H. Tyrosinase Inhibition and Kinetic Details of Puerol A Having But-2-Enolide Structure from Amorpha fruticosa. Molecules 2020, 25, 2344. [Google Scholar] [CrossRef]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of anti-oxidant and anti-inflammatory effects between fresh and aged black garlic extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]



| Compounds | IC50 a (µM) | Type of Inhibition (Ki b, µM) |
|---|---|---|
| 1 | 14.4 ± 0.3 | Noncompetitive (12.7 ± 0.3) |
| 2 | 27.0 ± 0.6 | Noncompetitive (24.9 ± 0.5) |
| 3 | 6.8 ± 0.3 | Noncompetitive (6.2 ± 0.3) |
| Oleanolic acid d | 38.8 ± 0.8 | NT c |
| Compounds | KSVa (×105 L mol−1) | KAb (×106 L mol−1) | n c |
|---|---|---|---|
| 1 | 0.4166 | 0.0411 | 1.0039 |
| 2 | 0.1816 | 0.0199 | 0.9734 |
| 3 | 0.6764 | 0.0743 | 0.9727 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Ban, Y.J.; Baiseitova, A.; Nyiramana, M.M.; Kang, S.S.; Kang, D.; Park, K.H. Iridal-Type Triterpenoids Displaying Human Neutrophil Elastase Inhibition and Anti-Inflammatory Effects from Belamcanda chinensis. Molecules 2021, 26, 6602. https://doi.org/10.3390/molecules26216602
Kim JH, Ban YJ, Baiseitova A, Nyiramana MM, Kang SS, Kang D, Park KH. Iridal-Type Triterpenoids Displaying Human Neutrophil Elastase Inhibition and Anti-Inflammatory Effects from Belamcanda chinensis. Molecules. 2021; 26(21):6602. https://doi.org/10.3390/molecules26216602
Chicago/Turabian StyleKim, Jeong Ho, Yeong Jun Ban, Aizhamal Baiseitova, Marie Merci Nyiramana, Sang Soo Kang, Dawon Kang, and Ki Hun Park. 2021. "Iridal-Type Triterpenoids Displaying Human Neutrophil Elastase Inhibition and Anti-Inflammatory Effects from Belamcanda chinensis" Molecules 26, no. 21: 6602. https://doi.org/10.3390/molecules26216602
APA StyleKim, J. H., Ban, Y. J., Baiseitova, A., Nyiramana, M. M., Kang, S. S., Kang, D., & Park, K. H. (2021). Iridal-Type Triterpenoids Displaying Human Neutrophil Elastase Inhibition and Anti-Inflammatory Effects from Belamcanda chinensis. Molecules, 26(21), 6602. https://doi.org/10.3390/molecules26216602








