Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea
Abstract
1. Introduction
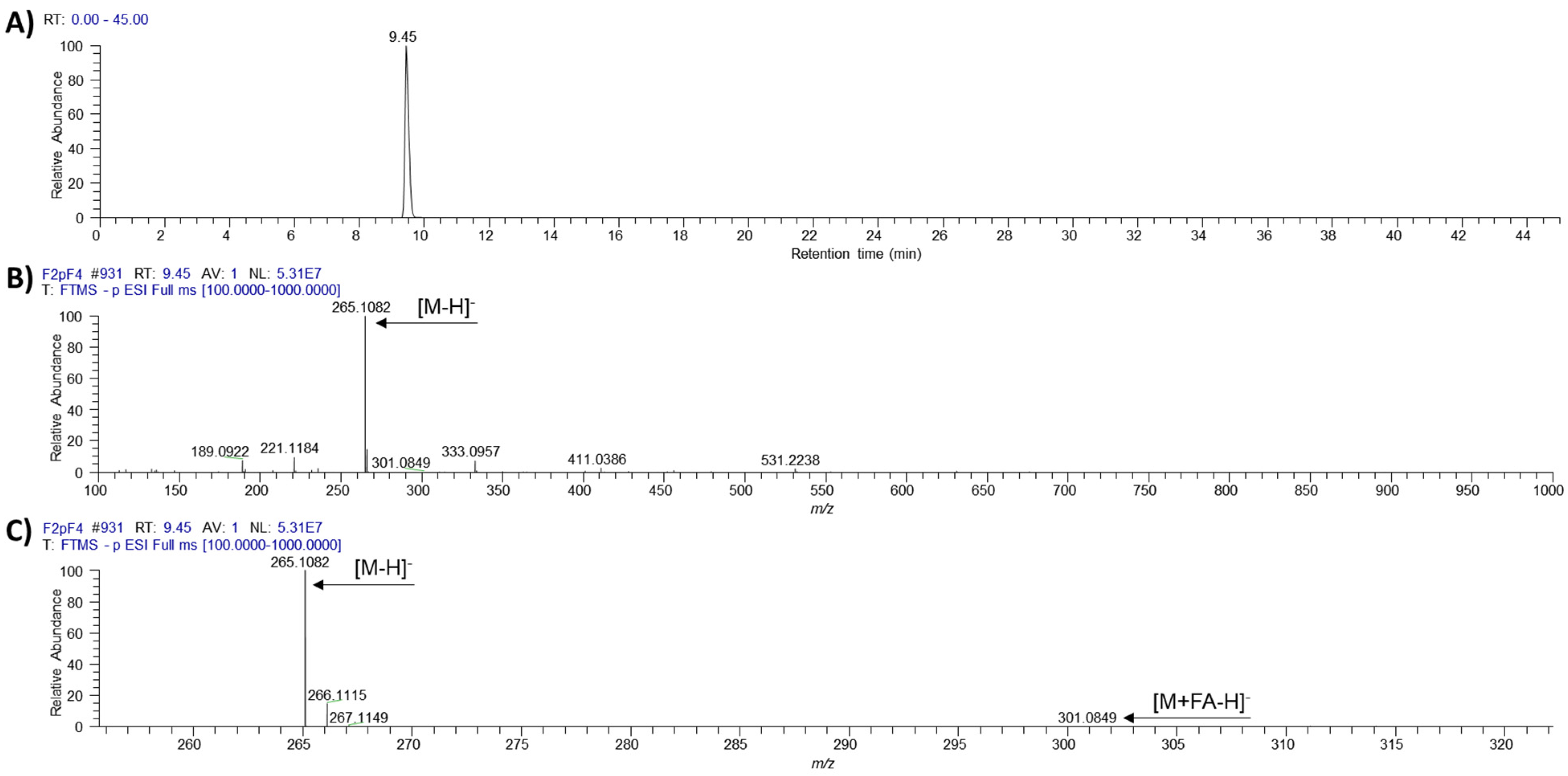
2. Results
3. Discussion
4. Methods
4.1. Fungal Isolation and Taxonomy
4.2. Fermentation and Extraction
4.3. Isolation of Secondary Metabolites
4.4. LC-MS and NMR
4.5. Biological Assays
4.5.1. Antimicrobial Activity
4.5.2. Cytotoxicity Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Labuda, R.; Bernreiter, A.; Schuller, C.; Kubatova, A.; Hellinger, R.; Strauss, J. Metapochonia lutea, a new species isolated from the Danube river in Austria. Nova Hedwig. 2018, 107, 487–500. [Google Scholar] [CrossRef]
- Zare, R.; Gams, W.; Evans, W.H.C. A revision of Verticillium section Prostrata. V. The genus Pochonia, with notes on Rotiferophthora. Nova Hedwig. 2001, 73, 51–86. [Google Scholar] [CrossRef]
- Gams, W.; Zare, R. A taxonomic review of the clavicipitaceous anamorphs parasitizing nematodes and other microinvertebrates. In Clavicipitalean Fungi: Evolutionary Biology, Chemistry, Biocontrol and Cultural Impacts; White, J.F., Bacon, C.W., Hywel-Jones, N.L., Spatafora, J.W., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW-Verlag: Eching, Germany, 2007. [Google Scholar]
- Nonaka, K.; Ōmura, S.; Masuma, R.; Kaifuchi, S.; Masuma, R. Three new Pochonia taxa (Clavicipitaceae) from soils in Japan. Mycologia 2013, 105, 1202–1218. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.; Tichy, H.-V.; Katsiou, E.; Hellwig, V. Chemotaxonomy of Pochonia and other conidial fungi with Verticillium-like anamorphs. Mycol. Prog. 2003, 2, 95–122. [Google Scholar] [CrossRef]
- Degenkolb, T.; Vilcinskas, A. Metabolites from nematophagous fungi and nematicidal natural products from fungi as an alternative for biological control. Part I: Metabolites from nematophagous ascomycetes. Appl. Microbiol. Biotechnol. 2016, 100, 3799–3812. [Google Scholar] [CrossRef] [PubMed]
- Wilk, W.; Waldmann, H.; Kaiser, M. Gamma-pyrone natural products--a privileged compound class provided by nature. Bioorg. Med. Chem. 2009, 17, 2304–2309. [Google Scholar] [CrossRef] [PubMed]
- Ui, H.; Shiomi, K.; Suzuki, H.; Hatano, H.; Morimoto, H.; Yamaguchi, Y.; Masuma, R.; Sunazuka, T.; Shimamura, H.; Sakamoto, K.; et al. Verticipyrone, a new NADH-fumarate reductase inhibitor, produced by Verticillium sp. FKI-1083. J. Antibiot. 2006, 59, 785–790. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gong, J.; Chen, C.; Mo, S.; Liu, J.; Wang, W.; Zang, Y.; Li, H.; Chai, C.; Zhu, H.; Hu, Z.; et al. Fusaresters A-E, new gamma-pyrone-containing polyketides from fungus Fusarium sp. Hungcl and structure revision of fusariumin D. Org. Biomol. Chem. 2019, 17, 5526–5532. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.G.; Song, J.H.; Park, M.; Kim, S.; Kim, C.E.; Kang, K.S.; Shim, S.H. Neuroprotective gamma-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action. Biomolecules 2020, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Mao, M.J.; Wang, R.Z.; Chang, S.S.; Xiao, T.M.; Wu, Y.X.; Yu, L.Y.; Song, Y.L.; Chen, M.H.; Si, S.Y. Fusapyrone A, a gamma-pyrone derived from a desert Fusarium sp. J. Asian Nat. Prod. Res. 2021, 23, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.P.; Li, G.; Ji, L.X.; Li, Y.X.; Lou, H.X. Acrepyrone A, a new gamma-pyrone derivative from an endophytic fungus, Acremonium citrinum SS-g13. Nat. Prod. Res. 2020, 34, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Yano, K.; Yokoi, K.; Sato, J.; Oono, J.; Kouda, T.; Ogawa, Y. Actinopyrones A, B and C, new physiologically active substances II: Physico-chemical properties and chemical structures. J. Antibiot. 1886, 59, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Kepler, R.M.; Humber, R.A.; Bischoff, J.F.; Rehner, S.A. Clarification of generic and species boundaries for Metarhizium and related fungi through multigene phylogenetics. Mycologia 2014, 106, 811–829. [Google Scholar] [CrossRef] [PubMed]
- Shinomiya, S.; Iwasaki, A.; Ohno, O.; Suenaga, K. Total synthesis and stereochemical determination of yoshinone A. Phytochemistry 2016, 132, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Kawazoe, Y.; Iwasaki, A.; Ohno, O.; Suenaga, K.; Uemura, D. Anti-obesity activities of the yoshinone A and the related marine gamma-pyrone compounds. J. Antibiot. 2016, 69, 348–351. [Google Scholar] [CrossRef] [PubMed]

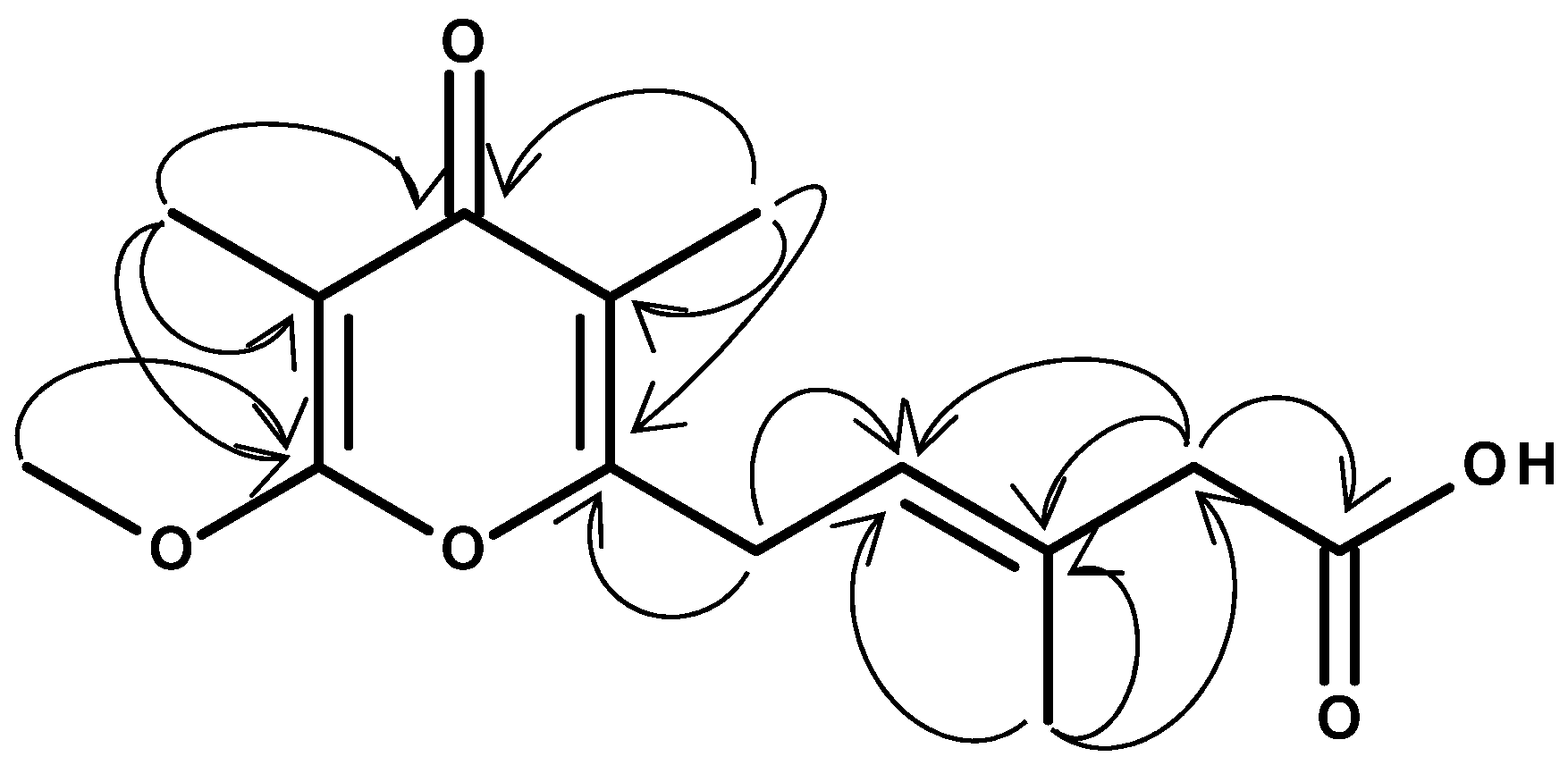


| 1H | 13C | |
|---|---|---|
| 2 | - | 164.7 |
| 3 | - | 100.0 |
| 4 | - | 183.2 |
| 5 | - | 119.0 |
| 6 | - | 159.5 |
| 7 | 3.48 (d, 2H, J = 7.4) | 30.7 |
| 8 | 5.44 (tq, 3H, J = 7.4, 1.3) | 122.5 |
| 9 | - | 134.5 |
| 10 | 3.05 (br.s, 2H) | 45.7 |
| 11 | - | 175.7 |
| 12 | 1.81 (s, 3H) | 7.1 |
| 13 | 1.95 (s, 3H) | 10.0 |
| 14 | 1.86 (br.s, 3H) | 16.8 |
| OMe | 4.02 (s, 3H) | 56.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labuda, R.; Bacher, M.; Gratzl, H.; Doppler, M.; Parich, A.; Aufy, M.; Lemmens-Gruber, R.; Schuhmacher, R.; Rychli, K.; Wagner, M.; et al. Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea. Molecules 2021, 26, 6589. https://doi.org/10.3390/molecules26216589
Labuda R, Bacher M, Gratzl H, Doppler M, Parich A, Aufy M, Lemmens-Gruber R, Schuhmacher R, Rychli K, Wagner M, et al. Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea. Molecules. 2021; 26(21):6589. https://doi.org/10.3390/molecules26216589
Chicago/Turabian StyleLabuda, Roman, Markus Bacher, Hannes Gratzl, Maria Doppler, Alexandra Parich, Mohammed Aufy, Rosa Lemmens-Gruber, Rainer Schuhmacher, Kathrin Rychli, Martin Wagner, and et al. 2021. "Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea" Molecules 26, no. 21: 6589. https://doi.org/10.3390/molecules26216589
APA StyleLabuda, R., Bacher, M., Gratzl, H., Doppler, M., Parich, A., Aufy, M., Lemmens-Gruber, R., Schuhmacher, R., Rychli, K., Wagner, M., Rosenau, T., Strauss, J., & Schüller, C. (2021). Luteapyrone, a Novel ƴ-Pyrone Isolated from the Filamentous Fungus Metapochonia lutea. Molecules, 26(21), 6589. https://doi.org/10.3390/molecules26216589









