Abstract
Investigation of the n-BuOH extract of the aerial parts of Elsholtzia bodinieri led to the isolation of seven new triterpenoid saponins, Bodiniosides S–Y (1–7, resp.). Their strictures were elucidated on the basis of spectroscopic techniques, including HSQC, HSBC, and HSQC–TOCSY experiments, together with acid hydrolysis and GC analysis. The anti-influenza activities of compounds 1–7 were evaluated against A/WSN/33/2009 (H1N1) virus in MDCK cells. The results showed that compounds 2 and 5 exhibited moderate anti-influenza activities against A/WSN/33/2009 (H1N1), with inhibition rates of 35.33% and 24.08%, respectively.
1. Introduction
Elsholtzia bodinieri Vaniot (Chinese name “Dongzisu”), belonging to the taxonomically diverse group of the family Labiatae, is a medicinal plant that grows in Yunnan and Guizhou Provinces in China. It is commonly known as “yashuacao” and is used as a traditional Chinese medicine for the treatment of cough, headache, pharyngitis, fever and hepatitis [1]. Previous studies on E. bodinieri led to the isolation of triterpenoid saponins [2,3,4,5,6], flavonoid glycosides [7,8], sesquiterpene glycosides [9], clerodane diterpenoid glycosides [10], and phenolic constituents [11] from the aerial parts of this plant. As a continuation of our work, we further systematically investigated the chemical components of the aerial parts of this plant. In our search for secondary metabolites with structural diversity and potential anti-influenza virus activity, seven new triterpenoid saponins, Bodiniosides S–Y (1–7, resp.), were obtained from E. bodinieri. Among them, compounds 2 and 5 exhibited moderate inhibition of influenza virus activities with inhibition rates of 35.33% and 24.08%, respectively. Herein, we report the isolation, structural elucidation and anti-influenza virus activities of the isolated compounds.
2. Results
The n-BuOH soluble fraction of the 75% aqueous acetone extract of the aerial parts of E. bodinieri was subjected to repeated column chromatography over silica gel, Sephadex LH-20, RP-18, and semipreparative reversed-phase HPLC, eluting with various solvent systems, to afford seven new triterpenoid saponins (Figure 1). The spectrums can be found in supplementary materials.
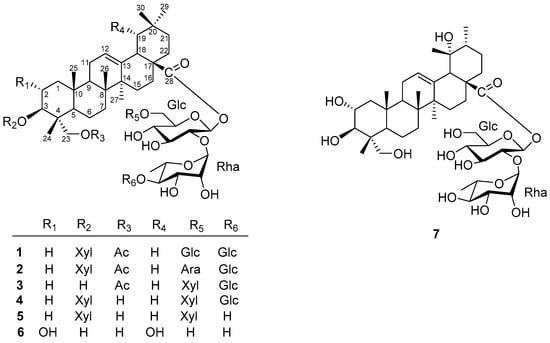
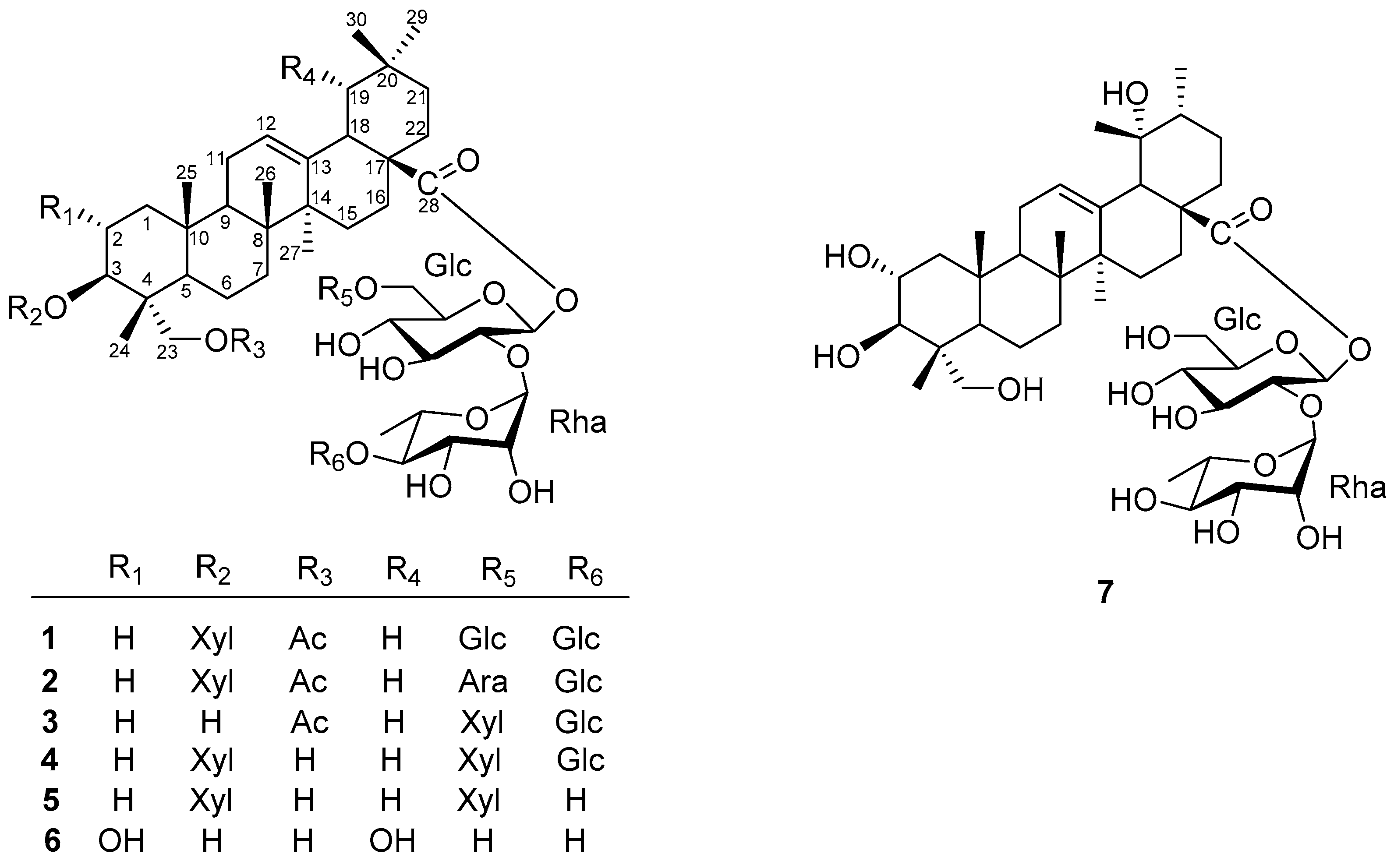
Figure 1.
Chemical structure of compounds 1–7 from Elsholtzia bodinieri.
Compound 1 was obtained as a white amorphous powder. Its molecular formula was determined as C61H98O28, according to the [M − H]− peak at m/z 1277.6148 in the negative HR-ESI-MS, indicating 13 degrees of unsaturation. It exhibited a UV maximum at 204 nm. The IR spectrum showed the presence of hydroxyl (3441 cm−1), carbonyl (1722 cm−1), and olefinic (1635 cm−1) groups. NMR data analysis indicated that 1 was a saponin containing a triterpene sapogenin and five monosaccharides.
In the 1H and 13C NMR spectra of aglycone moiety, 6 tertiary methyl groups [δH 0.81, 0.83, 0.84, 0.88, 1.07, and 1.25 (each, 3H, s); δC 12.9, 15.9, 17.5, 23.6, 25.5, and 30.6, (each, q)], 11 methylenes containing an oxygenated one [δH 4.03 (1H, m, Ha-23), 4.12 (1H, m, Hb-23; δC 64.7 (t, C-23)], 5 methines including an oxygenated one[δH 3.73 (1H, br. s, H-3); δC 81.9 (d, C-23)] and 1 unsaturated one [δH 5.27 (1H, m, H-12); δC 123.2 (d, C-12)], as well as 8 quaternary carbons (including a carbonyl carbon (δC 176.4, C-28) and a unsaturated one (δC 144.0, C-13)) were observed (Table 1 and Table 2). This information suggested that the aglycone moiety of compound 1 was 3β, 23-dihydroxyolean-12-en-28-oic acid [12]. Except for the signals for the aglycone, the remaining 31 signals were assigned as five sugar moieties and an acetoxy group, due to signals of δC 172.7 and 20.9. Moreover, comparison of its 1H and 13C NMR spectroscopic data with those of Bodinioside H [4], suggested that they had same 3-hydroxy-23-acetoxy-olean-12-en-28-oic acid as the aglycone, but differed in the sugar moiety.

Table 1.
H NMR data of compounds 1–7 in Pyridine-d5 (600 MHz, δH in ppm, J in Hz).

Table 2.
C NMR data of compounds 1–7 in pyridine-d5 (150 MHz, δC in ppm).
The 1H NMR spectrum showed five signals for anomeric protons at δH 6.45 (1H, br. s), 6.09 (1H, d, J = 7.8 Hz), 5.16 (1H, d, J = 7.8 Hz), 4.97 (1H, d, J = 7.8 Hz) and 4.86 (1H, d, J = 7.8 Hz), which correlated with anomeric carbon signals at δC 101.0, 94.5, 107.2, 107.1 and 105.3 in the HSQC spectrum, respectively, suggesting the presence of five sugar moieties. Acid hydrolysis of 1 with 1 M HCl produced L-rhamnose (Rha), D-glucose (Glc), and D-xylose (Xyl) as sugar residues by GC chromatography with the corresponding trimethylsilylated L-cysteine derivatives. Since NMR signals of five sugar units have undesirable overlapped effects, the HMQC-TOCSY experiment was successfully used to distinguish and assign the 1H and 13C NMR signals of each sugar moiety. The correlations from the anomeric proton signal at δH 4.97 to three carbon signals at δC 75.3, 78.4, and 67.1, as well as from three proton signals at δH 4.22, 4.06, and 3.75 to the anomeric carbon, suggested the presence of D-xylopyranose. In a similar way, the 1H and 13C NMR signals for D-glucopyranosyl and L-rhamnopyranosyl were assigned. In addition, the J H1, H2 coupling constants of four anomeric proton signals at δH 6.09 (1H, d, J = 7.8 Hz), 5.16 (1H, d, J = 7.8 Hz), 4.97 (1H, d, J = 7.8 Hz) and 4.86 (1H, d, J = 7.8 Hz) suggested the β anomeric configuration for the xylopyranosyl and glucopyranosyl units. The inspection of the anomeric proton (6.45, br. s) deduced the α anomeric configuration for the L-rhamnopyranosyl unit [4].
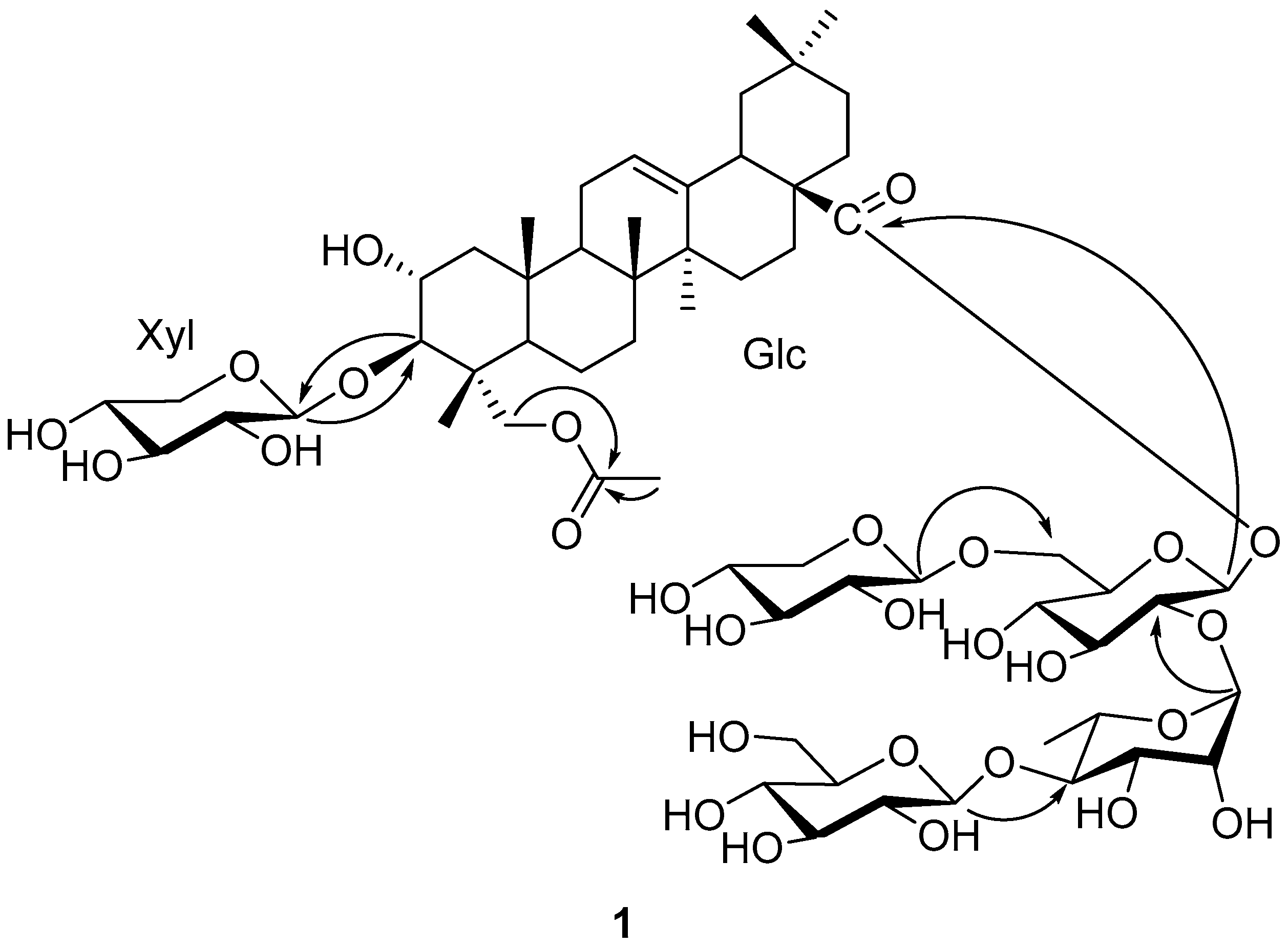
The sequence of the glycoside chains connected to C-3 and C-28 was established by analysis of the HMBC correlations (Figure 2). The absence of any glycosidation shift for Xyl suggested that Xyl was the singlet sugar unit attached at C-3 of the aglycone, which was further confirmed by HMBC correlation of HXyl-1 (δH 4.97) of C-3. A series of HMBC correlations from HGlc-1 (δH 6.09) to C-28, from HRha-1 (δH 6.45) to CGlc-2 (δc 76.3), from HGlc′-1 (δH 4.86) to CGlc-6 (δc 68.2), and from HGlc′′-1 (δH 5.16) to CRha-4 (δc 85.7) enabled the sugar chain of C-28 to be assigned as β-D-glucopyranosyl(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl(1→2)]-β-D-glucopyranoside. Thus, the structure of compound 1 was elucidated to be 3-O-β-D-xylopyranosyl-23-acetyloxy-olean-12-en-28-oic acid 28-O-β-D-glucopyranosyl(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl(1→2)]-β-D-glucopyranoside, a new oleanane triterpenoid saponin, named Bodinioside S.
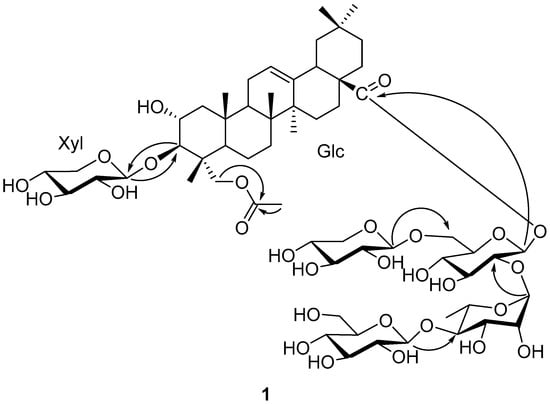
Figure 2.
Key HMBC correlation of compound 1.
Compound 2 was obtained as white amorphous powder. Its positive HR-ESI-MS spectrum indicated the molecular formula to be C60H96O27 by the observation quasi-molecular ion peak [M − H]− at m/z 1247.6070 and with the help of the NMR spectroscopic date, indicating 13 degrees of unsaturation. Detailed comparison of the 1H and 13C NMR spectral data (Table 1 and Table 2) of 2 with those of 1 revealed that they were highly structural similar, except for the replacement of signals for the Glc’ at C-28 in 1 by the Ara in 2. Acid hydrolysis of 2 yielded L-rhamnose, D-glucose, L-arabinose and D-xylose as sugar residues as determined by GC analysis. In the 1H NMR spectrum of 2, five anomeric H-atom at δH 6.47 (br. s), 6.14 (d, J = 7.8 Hz), 5.62 (br. s), 5.12 (d, J = 7.8 Hz) and 4.86 (d, J = 7.5 Hz) correlated with anomeric carbon signals at δC 101.2, 94.4, 109.7, 107.0, and 107.2 in the HSQC spectrum, respectively, suggesting the presence of five sugar residues: one rhamnopyranosyl (Rha), one arabinopyranosyl (Ara), one xylopyranosyl (Xyl) and two glucopyranosyl (Glc and Glc’) units. The Xyl unit was still linked to C-3 (δC 82.8) of the aglycone based on the HMBC correlation between HXyl-1 (δH 4.86) of Xyl and C-3 (δc 82.8). A series of HMBC correlations from HGlc-1 (δH 6.14) to C-28, from HRha-1 (δH 6.47) to CGlc-2 (δc 76.3), from HAra-1 (δH 5.62) to CGlc-6 (δc 68.3), and from HGlc′-1 (δH 5.12) to CRha-4 (δc 85.7) enabled the sugar chain of C-28 to be assigned as α-L -arabinopyranosyl(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl(1→2)]-β-D-glucopyranoside. From the foregoing evidence, the structure of 1 was unequivocally determined to be 3-O-β-D-xylopyranosyl-23-acetyloxy-olean-12-en-28-oic acid 28-O-α-L-arabinopyranosyl(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside, and named Bodinioside T.
Compound 3 was isolated as white amorphous powder. The molecular formula was established as C55H88O23 by positive HR-ESI-MS (m/z 1139.5614, [M + Na]+) and NMR spectral data, indicating 12 degrees of unsaturation. In the 1H NMR spectrum of 3, four anomeric H-atom at δH 6.45 (br. s), 6.15 (d, J = 7.8 Hz), 5.13 (d, J = 7.8 Hz) and 4.89 (d, J = 7.8 Hz) correlated with anomeric carbon signals at δC 101.2, 94.5, 105.5 and 107.1 in the HSQC spectrum, respectively, suggesting the presence of four sugar residues: one rhamnopyranosyl (Rha), one xylopyranosyl (Xyl), and two glucopyranosyl (Glc and Glc’) units. Detailed comparison of the 1H and 13C NMR spectra of 3 (Table 1 and Table 2) with those of Bodinioside H [4] revealed that they were identical, except for the absence of signals for Xyl moiety on C-3. GC analysis after acid hydrolysis of 3 as the same manner with 1 gave D-glucose, D-xylose, and L-rhamnose in a ratio of 2:1:1. Moreover, a series of HMBC correlations from HGlc-1 (δH 6.15) to C-28, from HRha-1 (δH 6.45) to CGlc-2 (δc 76.3), from HXyl-1 (δH 5.13) to CGlc-6 (δc 68.3), and from HGlc’-1 (δH 4.89) to CRha-4 (δc 85.7), adequately illustrated the structure of 3 as 3β-hydroxy-23-acetyloxy-olean-12-en-28-oic acid 28-O-β-D-xylopyranosyl(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside, named Bodinioside U.
Compound 4 was obtained as white amorphous powder. It exhibited a quasi-molecular ion peak at m/z 1205.5950 [M − H]− in the negative HR-ESI-MS spectrum, suggesting the molecular formula C58H94O26, indicating 12 degrees of unsaturation. Besides one hydroxyl taking the place of an acetoxy group substituent on C-23, most NMR signals (1 and 2) of 4 were nearly identical to those of Bodinioside H [4]. Five anomeric H-atom at δH 6.38 (br. s), 6.11 (d, J = 7.9 Hz), 5.15 (d, J = 7.6 Hz), 5.02 (d, J = 7.4 Hz) and 4.85 (d, J = 7.4 Hz) in the 1H NMR spectrum were ascribed to D-xylose, D-glucose, and L-rhamnose, respectively, in combination with acid hydrolysis and GC analysis. The Xyl unit was assigned to C-3 (δc 82.1) of the aglycone on the basis of the long-range correlation between H-1 (δH 5.02) of Xyl and C-3. Meanwhile, a series of HMBC correlations from HGlc-1 (δH 6.11) to C-28 (δc 176.7), from HRha-1 (δH 6.38) to CGlc-2 (δc 77.6), from HXyl′-1 (δH 4.85) to CGlc-6 (δc 68.6), and from HGlc′-1 (δH 5.15) to CRha-4 (δc 85.6) clarified the linkage of Glc, Xyl and Rha units at C-28 as shown. The structure of compound 4 was, therefore, concluded to be 3-O-β-D-xylopyranosyl-23-hydroxy-olean-12-en-28-oic acid 28-O-β-D- xylopyranosyl-(1→6)-[β-D-glucopyranosyl-(1→4)-α-L-rhamnopyranosyl-(1→2)]-β-D- glucopyranoside, and named Bodinioside V.
The molecular formula of Bodinioside W (5) was established as C52H84O21 on the basis of the negative HR-ESI-MS from the quasi molecular ion peak at m/z 1089.5454 [M + COOH]−, indicating 11 degrees of unsaturation. In the 1H NMR spectrum of 5, four anomeric H-atom at δH 6.51 (br. s), 6.11 (d, J = 8.0 Hz), 5.01 (d, J = 7.8 Hz) and 4.85 (d, J = 7.4 Hz) correlated with anomeric carbon signals at δC 101.3, 94.6, 106.7 and 105.4 in the HSQC spectrum, respectively. Acid hydrolysis of 5 yielded two D-xylose (Xyl), L-rhamnose (Rha) and D-glucose (Glc) as sugar residues by GC chromatography. Interpretation of its NMR data (Table 1 and Table 2) revealed that the structure of compound 5 was closely related to compound 4, except for the presence of an additional Glc at C-28 of 4. Thus, the structure of compound 5 was 3-O-β-D-xylopyranosyl-23-hydroxy-olean-12-en-28-oic acid 28-O-β-D-xylopyranosyl- (1→6)-[α-L-rhamnopyranosyl-(1→2)]-β-D-glucopyranoside, and named Bodinioside W.
Compound 6 was obtained as white amorphous powder. It exhibited a quasi-molecular ion peak at m/z 835.4459 [M + Na]+ in the positive HR-ESI-MS spectrum, suggesting the molecular formula of C42H68O15, indicating nine degrees of unsaturation. The 1H NMR spectrum (Table 1 and Table 2) of compound 6 showed two signals for anomeric protons at δH 6.60 (1H, br. s) and 6.23 (1H, d, J = 8.1 Hz), which correlated with anomeric carbon signals at δC 101.6 and 95.1 in the HSQC spectrum, respectively, suggesting the presence of two sugar moieties. Acid hydrolysis of 6 yielded L-rhamnose (Rha) and D-glucose (Glc) as sugar residues by GC chromatography. The NMR data of 6 were highly analogous to the sericoside [13], suggested that they had same 2, 3, 19, 23-tetrahydroxy-olean-12-en-28-oic acid as the aglycone, except for the presence of an additional Rha at C-28 of 6. The HMBC correlations from HGlc-1 (δH 6.23) to C-28, and from HRha-1 (δH 6.60) to CGlc-2 (δc 75.7), adequately illustrated the structure of 6 as 2α, 3β, 19α, 23- tetrahydroxy-olean-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl-(1→2)-β-D- glucopyranoside, named Bodinioside X.
Compound 7 was obtained as white amorphous powder. It exhibited a quasi-molecular ion peak at m/z 835.4456 [M + Na]+ in the positive HR-ESI-MS spectrum, suggesting a molecular formula C42H68O15, indicating nine degrees of unsaturation. The 1H NMR spectrum (Table 1 and Table 2) of 7 revealed six methyl signals at δH 1.13 (3H, s), 1.39 (3H, s), 1.03 (3H, s), 1.19 (3H, s), 1.62 (3H, s), and 1.06 (3H, s) in correlation with carbons at δC 14.2 (C-24), 17.5 (C-25), 17.4 (C-26), 24.1 (C-27), 26.9 (C-29), and 16.6 (C-30) in the HSQC spectrum, respectively. The signal at δH 5.55 (1H, br. s), corresponding to the carbon at δC 128.2 (C-12), coupled with δC 139.2 (C-13) in the 13C NMR spectrum. On the basis of the above spectroscopic data, compound 7 was suggested to possess an ursane-12-ene skeleton [6]. Comparison of its NMR spectroscopic data with those of niga-ichigoside F1 [14], suggested that they had the same 2, 3, 19, 23-tetrahydroxy-urs-12-en-28-oic acid as the aglycone. Detailed comparison of 1H and 13C NMR spectral data of 7 with those of niga-ichigoside F1 indicated that they are highly structurally similar, except for the presence of an additional Rha at C-28 of 7. Acid hydrolysis of 7 yielded D-glucose (Glc) and L-rhamnose (Rha) as sugar residues by GC chromatography. The structure of compound 7 was, therefore, concluded to be 2α, 3β, 19α, 23-tetrahydroxy-urs-12-en-28-oic acid 28-O-α-L-rhamnopyranosyl- (1→2)-β-D-glucopyranoside, and named Bodinioside Y.
The anti-influenza A virus activity of compounds 1–7 against strain A/WSN/33/2009 was evaluated in MDCK cells, and their cytotoxicity was measured in parallel with the determination of antiviral activity, using oseltamivir as a positive control. It was found that compounds 1–7 displayed no significant cytotoxicity at 50 µM concentration. Then, under this concentration, the in vitro potential anti-influenza A virus effects of all isolates were investigated. The results showed that compounds 2 and 5 exhibited moderate inhibition of influenza virus activities with inhibition rates of 35.33% and 24.08%, while the inhibition rate of the positive control (oseltamivir) was 71.20%.
3. Discussion
In summary, seven new triterpenoid saponins, including six oleanane triterpenoid saponins and a ursane one, named Bodiniosides S–Y, were isolated from the aerial parts of Elsholtzia bodinieri. Elucidation of their structures was performed based on extensive spectroscopic analyses. The anti-influenza activities of the isolates against A/WSN/33/2009 (H1N1) virus were investigated. The results demonstrated that compounds 2 and 5 exhibited potent inhibition of influenza virus activities, with inhibition rates of 35.33% and 24.08%; meanwhile, compounds 1, 3, 4, 6 and 7 were found to be inactive, with an inhibition rate lower than 10%. A previous study revealed that pentacyclic triterpenoids, including ursane, oleanane, and lupane types, have anti-influenza virus activity [15]. Our results further confirmed that the pentacyclic triterpenoids were active against influenza virus. This investigation should provide valuable information for further understanding of E. bodinieri.
4. Materials and Methods
4.1. General Experimental Procedures
Optical rotations were recorded using a Jasco DIP-370 digital polarimeter (Jasco, Tokyo, Japan). UV spectra were performed on a UV-210A spectrophotometer (Shimadzu, Kyoto, Japan). IR spectra were obtained on a Bio-Rad FtS-135 spectrophotometer (Bio-Rad Laboratories, California, CA, USA) with KBr pellets. The 1D- and 2D NMR spectra were run on Bruker DRX-600 instruments (Bruker BioSpin Group, Rheinstetten, Germany) with TMS as an internal standard. ESI-MS and HR-ESI-MS were measured with an API-Qstar-TOF instrument (Applied Biosystem/MSD Sciex, Concord, ON, Canada). GC analysis was taken on Agilent Technologies HP5890 gas chromatograph (Agilent Technologies Inc., Massy, France) with flame ionization detector. Semi-preparative HPLC was run on an Agilent 1200 liquid chromatograph (Agilent Technologies Inc., Palo Alto, CA, USA) with a ZORBAX SB-C18 (5 Ao, 9.4 × 250 mm) column. Column chromatography (CC) was carried out on silica gel (200–300 mesh, 80–100 mesh, Qingdao Marine Chemical Factory, Qingdao, China), Diaion HP-20SS (63–150 mm, Mitsubishi Fine Chemical Industries Co., Ltd., Tokyo, Japan), ODS-C18 (75 μm, YMC Co., Ltd., Tokyo, Japan), and Sephadex LH-20 (Amersham Biosciences AB, Uppsala, Sweden); thin-layer chromatography (TLC) was monitored by TLC plates (Si gel GF254, Qingdao Marine Chemical Factory, Qingdao, China), and spots were visualized by spraying with 5% H2SO4–EtOH, followed by heating on a hot plate. The purity (>95%) of compounds 1–7 was determined by HPLC.
4.2. Materials
The aerial parts of E. bodinieri were collected in Yuxi city, Yunnan Province, P. R. China, in May 2016, and identified by Dr. Jindong Zhong. A voucher specimen (KMUST 20160005) was deposited at the Laboratory of Phytochemistry, Faculty of Life Science and Technology, Kunming University of Science and Technology.
4.3. Extraction and Isolation
The powered air-dried aerial parts of E. bodinieri (15 kg) were extracted with 75% aq. Me2CO (3 × 35 L, 24 h, each) at room temperature and filtered. The filtrate was concentrated in vacuo, and the resulting residue was extracted successively with CHCl3, AcOEt and n-BuOH, respectively.
The n-BuOH extract (300.0 g) was separated over macroporous resin CC (Diaion HP-20SS) eluting with MeOH/H2O (gradient30, 60, 90, and 100%, each 15 L) to obtain four fractions (Fr. A–D). Fr. C (eluted with 60% MeOH/H2O, 86.5 g) was chromatographed successively over Sephadex LH-20 gel column (20%, 30%, 40%, 50%, 60%, and 100% MeOH/H2O, each 8 L) to obtain subfractions Fr. C-1–C-6. Fr. C-1 (31 g) was isolated by ODS CC (eluted with 10%, 30%, 60%, and 100% MeOH/H2O) to obtain subfractions Fr. C-1-1–C-1-4. Fr. C-1-3 (13 g) was chromatographed over silica gel CC (eluted with CHCl3/MeOH 15: 1 to 0: 1) to yield Fr. C-1-3-1–C-1-3-5. Compounds 1 (tR = 15.0 min, 5.6 mg) and 2 (tR = 20.1 min, 6.2 mg) were purified from Fr. C-1-3-4 (167 mg) via semi-preparative HPLC (58% MeOH, 3 mL/min). Compounds 3 (tR = 17.4 min, 4.3 mg) and 6 (tR = 22.1 min, 4.8 mg) were purified from Fr. C-1-3-3 (103 mg) via semi-preparative HPLC (56% MeOH, 3 mL/min). Compound 4 (tR = 14.6 min, 5.6 mg) was purified from Fr. C-1-3-2 (84 mg) via semi-preparative HPLC (52% MeOH, 3 mL/min). Fr. C-1-4 (2.5 g) was chromatographed over silica gel CC (eluted with CHCl3/MeOH 10: 1 to 0: 1) to yield Fr. C-1-4-1–C-1-4-3. Compounds 5 (tR = 15.6 min, 4.8 mg) and 7 (tR = 17.6 min, 7.3 mg) were purified from Fr. C-1-4-2 (163 mg) via semi-preparative HPLC (55% MeOH, 3 mL/min).
4.3.1. Bodinioside S (1)
4.3.2. Bodinioside T (2)
4.3.3. Bodinioside U (3)
4.3.4. Bodinioside V (4)
4.3.5. Bodinioside W (5)
4.3.6. Bodinioside X (6)
4.3.7. Bodinioside Y (7)
4.4. Acid Hydrolysis for Sugar Analysis
Compounds 1–7 (1.0 mg for each compound) were hydrolyzed with 1 M HCl (0.4 mL) and heated at 90–100 °C for 5 h. The mixture was neutralized by the addition of Amberlite IRA400 (OH− form) and then filtered. The filtrate was dried in vacuo, dissolved in 0.2 mL of pyridine containing L-cysteine methyl ester (10 mg/mL) and reacted at 60 °C for 1 h. To this mixture, a solution (0.2 mL) of trimethylsilyl imidazole in pyridine (10 mg/mL) was added, and then heated at 60 °C for 1 h. The final mixture was directly analyzed by GC [30QC2/AC-5 quartz capillary column (30 m × 0.32 mm) with the following conditions: column temperature: 180/280 °C; programmed increase 3 °C/min; carrier gas: N2 (1 mL/min); injection and detector temperature: 250 °C; injection volume: 4 μL; split ratio: 1/50]. The authentic samples D- and L-glucose, D- and L-xylose, L-arabinose, and L-rhamnose were treated in the same manner. Under these conditions, the retention times of authentic samples D- and L-glucose, D- and L-xylose, L-arabinose and L-rhamnose were 18.29, 18.87, 13.35, 14.01, 14.30 and 14.97 min, respectively. During our studies, identical retention times observed between the different hydrolysates and authentic standards.
4.5. Anti-Influenza Virus Activity
The anti-influenza virus activities of compounds 1–7 were evaluated, using influenza strain A/WSN/33/2009 (H1N1). For the inhibitory activity assays, compounds 1–7 were dissolved and then diluted with DMSO, using Oseltamivir as a positive control. MDCK cells were seeded into 96-well plates, incubated overnight and infected with influenza virus (MOI ¼ 0.1). The cells were suspended in DMEM supplemented with 1% fetal bovine serum (FBS), containing test compound and 2 mg/mL TPCK-treated trypsin, and a final DMSO concentration of 1% was added in each well. After 40 h of incubation, Cell Titer-Glo reagent was added, and the plates were read, using a plate reader [15]. The inhibition rate was calculated by the following formula: inhibition rate (%) = [1 − (luminescence with compounds − luminescence with compounds and virus)/(luminescence with DMSO − luminescence with DMSO and virus)] × 100%. Assessment of anti-influenza virus activity was performed as described previously [16].
Supplementary Materials
The following are available online. The IR, HR-ESI-MS, and 1D and 2D NMR spectra of the seven compounds are available in the supplementary materials.
Author Contributions
L.Y., J.D., R.L. and J.Z. conceived and designed the experiments; L.Y. and J.D. isolated the compounds; L.Y., J.D. and J.Z. elucidated the structures; F.Y. carried out the biological assay and helped with the preparation of the manuscript; L.Y., J.D. and J.Z. wrote the paper; and J.Z. managed the research project. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the grants of National Natural Science Foundation of China (grant number 31660100, U1602222), Yunnan Province applied Basic research project (grant number 2017BF034).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of compounds 1–7 are available from the authors.
References
- Jiangsu New Medical College. Dictionary of Traditional Chinese Medicine; Shanghai Science and Technology Publishing House: Shanghai, China, 1985; 489p. [Google Scholar]
- Li, H.Z.; Fu, L.Z.; Li, H.M.; Li, R.T.; Deng, X.L. Two new oleanane triterpenoid saponins from Elsholtzia bodinieri. Phytochem. Lett. 2012, 5, 572–575. [Google Scholar] [CrossRef]
- Zhao, X.W.; Zhong, J.D.; Li, H.M.; Li, R.T. Three new 18, 19-seco-ursane glycosides from Elsholtzia bodinieri. Phytochem. Lett. 2015, 12, 308–312. [Google Scholar] [CrossRef]
- Zhong, J.D.; Zhao, X.W.; Li, H.M.; Gao, L.H.; Li, R.T. Five new oleanane triterpenoid saponins from the aerial parts of Elsholtzia bodinieri. Helv. Chim. Acta 2016, 99, 204–209. [Google Scholar] [CrossRef]
- Zhong, J.D.; Zhao, X.W.; Chen, X.Q.; Li, H.M.; Chen, C.H.; Xia, X.S.; Li, R.T. Two new ursane-type triterpenoid saponins from Elsholtzia bodinieri. Arch. Pharm. Res. 2016, 39, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.L.; Zhang, L.; Chen, X.Q.; Xia, X.S.; Li, R.T.; Zhong, J.D. Ursane-type triterpenoid saponins from Elsholtzia bodinieri. Nat. Prod. Res. 2018, 33, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Li, R.T.; Li, J.T.; Wang, J.K.; Han, Q.B.; Zhu, Z.Y.; Sun, H.D. Three new flavonoid glycosides isolated from Elsholtzia bodinieri. Chem. Pharm. Bull. 2008, 56, 592–594. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhong, J.D.; Feng, Y.; Li, H.M.; Li, H.Z.; Xia, X.S.; Li, R.T. A new flavonoid glycoside from Elsholtzia bodinieri. Nat. Prod. Res. 2016, 30, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.B.; Jian, Y.F.; Zheng, X.D.; Cao, H. Three sesquiterpene glycosides from Elsholtzia bodinieri. Bull. Korean Chem. Soc. 2007, 28, 467–470. [Google Scholar] [CrossRef]
- Hu, H.B.; Cao, H.; Jian, Y.F.; Zheng, X.D.; Liu, J.X. Two new clerodane diterpenoid glucosides and other constituents from the roots of Elsholtzia bodinieri Van’t. Indian J. Chem. B. 2008, 47B, 166–170. [Google Scholar]
- Hu, H.B.; Jian, Y.F.; Cao, H.; Zheng, X.D. Phenolic compounds from Elsholtzia bodinieri Van’t. J. Chin. Chem. Soc. 2007, 54, 1189–1194. [Google Scholar] [CrossRef]
- Mohammad, F.V.; Noorwala, M.; Ahmad, V.U.; Sener, B. A bidesmosidic hederagenin hexasaccharide from the roots of Symphytum officinale. Phytochemistry 1995, 40, 213–218. [Google Scholar] [CrossRef]
- Ponou, B.K.; Teponno, R.B.; Ricciutelli, M.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Barboni, L.; Tapondjou, L.A. Dimeric antioxidant and cytotoxic triterpenoid saponins from Terminalia ivorensis A. Chev. Phytochemistry 2010, 71, 2108–2115. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Jung, H.J.; Tapondjou, L.A.; Lee, K.T.; Choi, J.H.; Kim, W.B.; Park, H.J. The anti-hyperlipidemic effects and constituents of the 19α- hydroxyursane-type triterpenoid fraction obtained from the leaves of Rubus crataegifolius. Nat. Prod. Sci. 2007, 13, 152–159. [Google Scholar]
- Wang, H.; Xu, R.Y.; Shi, Y.Y.; Si, L.L.; Jiao, P.X.; Fan, Z.B.; Han, X.; Wu, X.Y.; Zhou, X.S.; Yu, F.; et al. Design, synthesis and biological evaluation of novel L-ascorbic acid-conjugated pentacyclic triterpene derivatives as potential influenza virus entry inhibitors. Eur. J. Med. Chem. 2016, 110, 376–388. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Si, L.L.; Ji, J.S.; Wang, H.; Fang, X.M.; Yu, L.Y.; Li, R.Y.; Liang, L.N.; Zhou, D.; Ye, M. Uralsaponins M-Y, antiviral triterpenoid saponins from the roots of Glycyrrhiza uralensis. J. Nat. Prod. 2014, 77, 1632–1643. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).