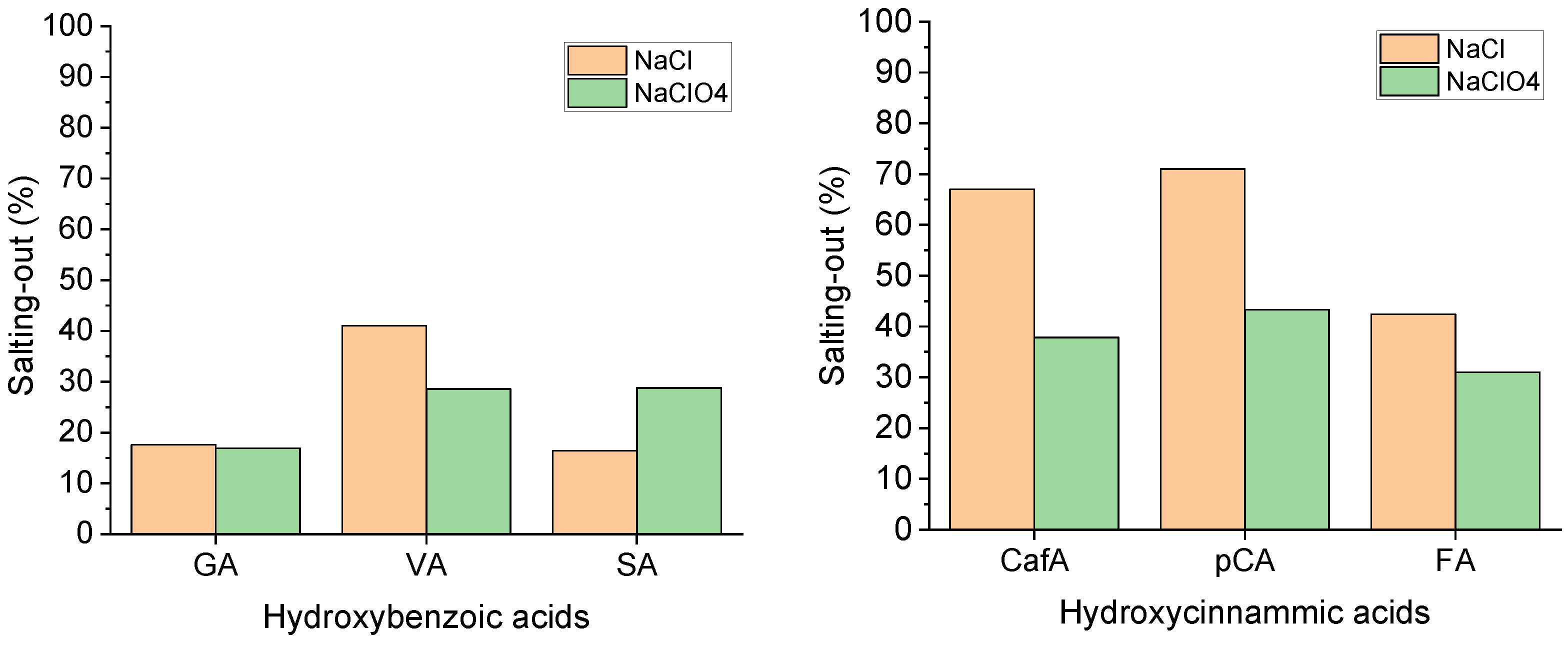Abstract
In this work, the solubility of vanillic, gallic, syringic, p-coumaric, ferulic and caffeic acids was determined at 37 °C under different conditions, namely pure water and two different ionic media, NaCl(aq) and NaClO4(aq), at different ionic strengths (i.e., 0.16, 0.50, 1.0, 2.0 and 3.0 M). The solubility in water of all the acids was found to be higher than that in both of the ionic media. Moreover, the solubility of hydroxycinnamic acids was lower than that of hydroxybenzoic acids. The activity coefficients of neutral species were calculated from these data; this knowledge is necessary when modeling the dependence of equilibrium constants on the ionic strength. Results obtained in this work can be useful for further studies regarding complex formation equilibria between these ligands and bioavailable metal cations.
1. Introduction
A variety of phenolic compounds are currently among the most studied categories of natural antioxidants [1]. Phenolic acids contain one or more hydroxyl groups (polar part) attached to an aromatic ring (non-polar part) and are often found in plants as esters or glycosides [1,2,3,4,5,6]. Due to their ubiquitous presence in plant-based foods (fruit, vegetables, grain, tea, coffee, spices), the intake of phenolic acids is estimated as 25 × 10−3–1 g per day, depending on diet [7]. The interest in phenolic compounds lies mainly in their known health benefits, including their antioxidant activity and ability as free radical scavengers [8,9,10,11]. These properties give them great potential as active principles in the pharmaceutical industry as well as antioxidants in the food industry. For this reason, there is increasing interest in isolating these compounds from their natural matrices [12,13,14]. The efficient design of any extraction process requires the knowledge of the solute’s solubility. Aqueous solubility is a parameter of particular importance for assessing the environmental partitioning of different compounds. It has been reported that the low solubility of some solutes in water can be modified by the presence of co-solutes such as salts or by increasing the temperature [15,16,17,18,19,20,21]. Two phenomena related to solubility changes caused by the presence of co-solutes can be observed: salting-in and salting-out. In general, the solubility of a non-electrolyte can increase or decrease by the addition of an electrolyte, but the effect is dependent on the solvent salt used. Equation (1) relates the solubility of the neutral species, S°, to its activity coefficients, γ:
where S°0 is the solubility at zero ion concentration. The activity coefficient of the neutral species is related to the molality, m, of the solvent electrolyte by Equation (2), also valid for weak electrolytes:
where k is the salting-out (positive) or salting-in (negative) coefficient, also known as the Setschenow coefficient, which may depend on the ionic strength [22]. For a generic non-electrolyte, the effect depends upon the solvent salt; in general, for a given solvent salt, it depends upon the saturating non-electrolyte. According to Debye [23], the salting-out constant generally increases as the polar properties of the non-electrolyte decrease. This theory does not clarify how certain electrolytes salt-out a specified non-electrolyte while others do the opposite. A possible explanation has been proposed by Kruyt and Robinson [24]: in a solution of a non-electrolyte in water, the water dipoles are arranged around a molecule of the non-electrolyte, with their positive or negative end towards the non-electrolyte depending upon the polar properties of the latter. When an electrolyte is added to the solution, due to the hydration of the ions, less water is available to the saturating non-electrolyte and salting-out of the non-electrolyte is expected. There is an opposite effect: water molecules are organized in a manner differing to the arrangement about the non-electrolyte. The result of the approach of the ion to the non-electrolyte is the packing-in of water molecules about the non-electrolyte, i.e., salting-in. The present work represents the continuation of efforts concerning the evaluation of the effect of salt addition on the solubility of organic compounds [25,26,27,28,29]. We report here on the solubility at 37 °C of six phenolic acids of both subclasses, i.e., hydroxybenzoic and hydroxycinnamic acids, generically indicated as HnPh—vanillic (VA), gallic (GA), syringic (SA), p-coumaric (p-CA), ferulic (FA) and caffeic (CafA) acids (Scheme 1)—in pure water as well as in aqueous solutions of different ionic strength in sodium chloride and in sodium perchlorate.
log γ = log (S°0/S°)
log γ = k·m
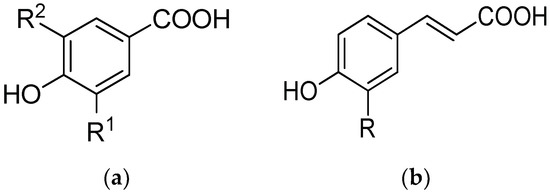
Scheme 1.
Chemical structure of (a) hydroxybenzoic acids (vanillic acid: R1 = OCH3, R2 = H; syringic acid: R1 = R2 = OCH3; gallic acid: R1 = R2 = OH); (b) hydroxycinnamic acids (caffeic acid: R = OH; ferulic acid: R = OCH3; p-coumaric acid: R = H).
Previous literature data report on the solubility of these phenolic acids in pure organic solvents, in mixed organic–aqueous solutions [10,30,31,32,33,34,35,36,37,38,39,40,41,42,43], or in aqueous media but under experimental conditions far from biological. The interaction of these acids with aqueous media is important because such compounds display their antioxidant activity in the biological systems involving water as the natural solvent.
2. Results
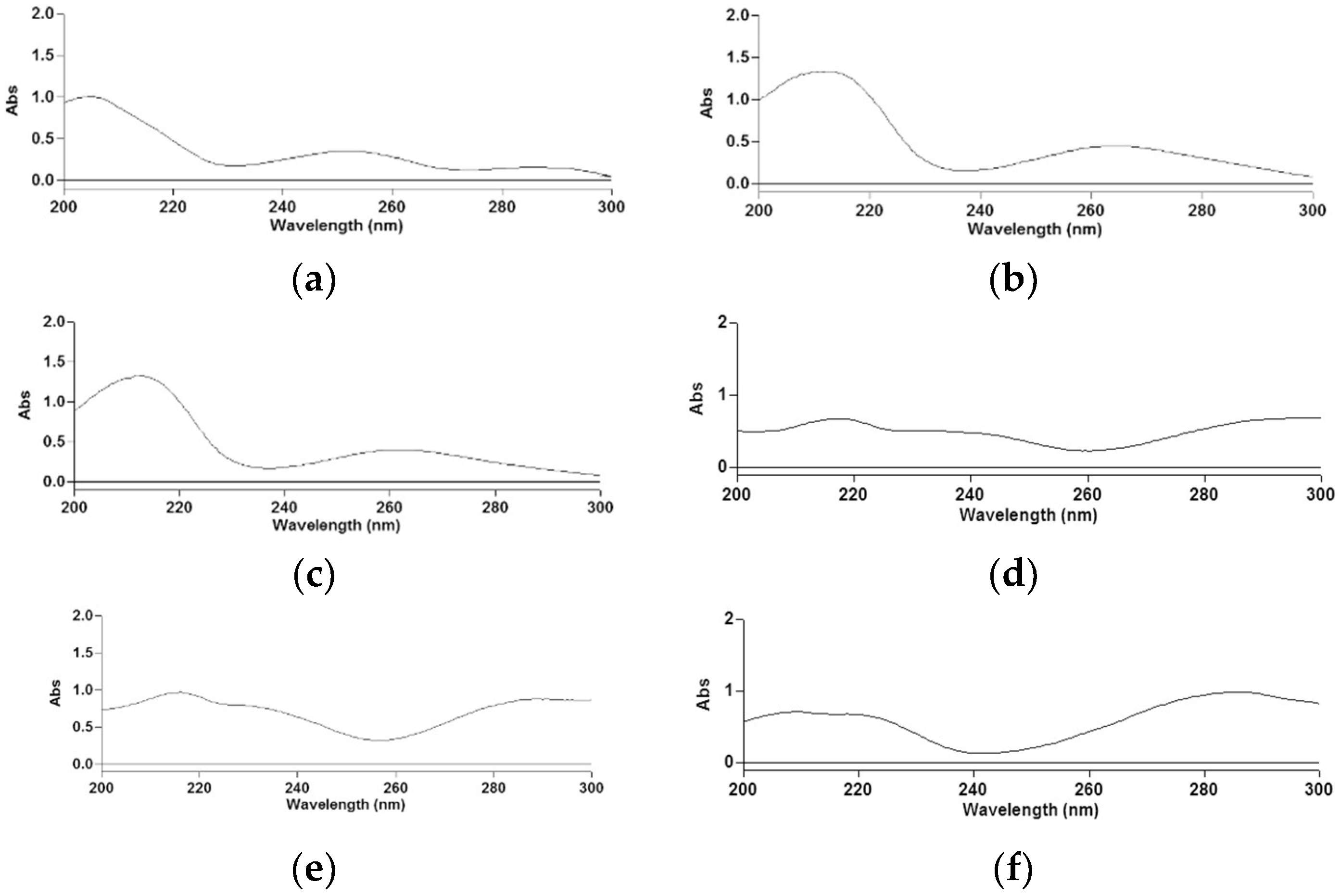
The determination of phenolic acid solubility was achieved by measuring the absorbance values between 200 and 300 nm, every 1 nm, taking the ionic medium as a blank. Three replicates were run for each point. The typical spectra recorded for hydroxybenzoic (Figure 1a–c) and hydroxycinnamic (Figure 1d–f) acids are reported.
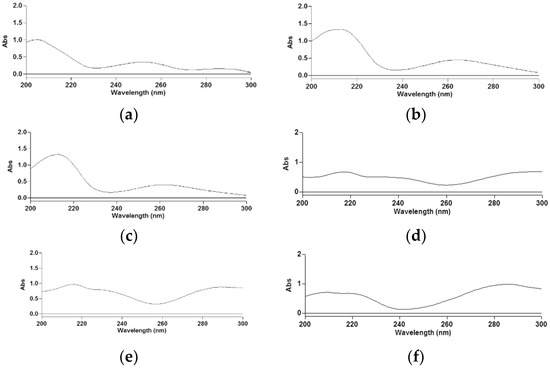
Figure 1.
Absorption spectra of vanillic (a), syringic (b), gallic (c), caffeic (d), ferulic (e) and p-coumaric (f) acids.
For the solubility determination of vanillic, syringic and gallic acids, peaks at 251, at 262 and at 258 nm, respectively, were considered. Concerning hydroxycinnamic acids, peaks at 287 for the quantitative determination of caffeic and ferulic acids and at 288 nm for p-coumaric acid were considered. The absorbance, Aλ, of phenolic acids, generically HnPh, was expressed according to Equation (3):
where l is the optical path and ελ is the molar absorptivity. The total solubility in pure water, ST°, as well as in aqueous solutions of different ionic strength in sodium chloride and in sodium perchlorate, generically ST, was deduced by interpolation on a calibration curve, based on standard solutions prepared in the range 2.0 × 10−3 and 25.0 × 10−3 molal for hydroxybenzoic acids and in the range 1.0 × 10−3 and 10.0 × 10−3 molal for hydroxycinnamic acids. In all cases, the correlation coefficient, R2, was ≥0.999 and the limit of detection was 0.50 × 10−3 molal. The total solubility values obtained at 37 °C are reported in Table 1, along with the outcomes of the statistical analysis.
Aλ = l ελ [HnPh]

Table 1.
Total solubility at 37 °C of phenolic acids in water and in aqueous solutions of NaCl and NaClO4 at different ionic strengths.
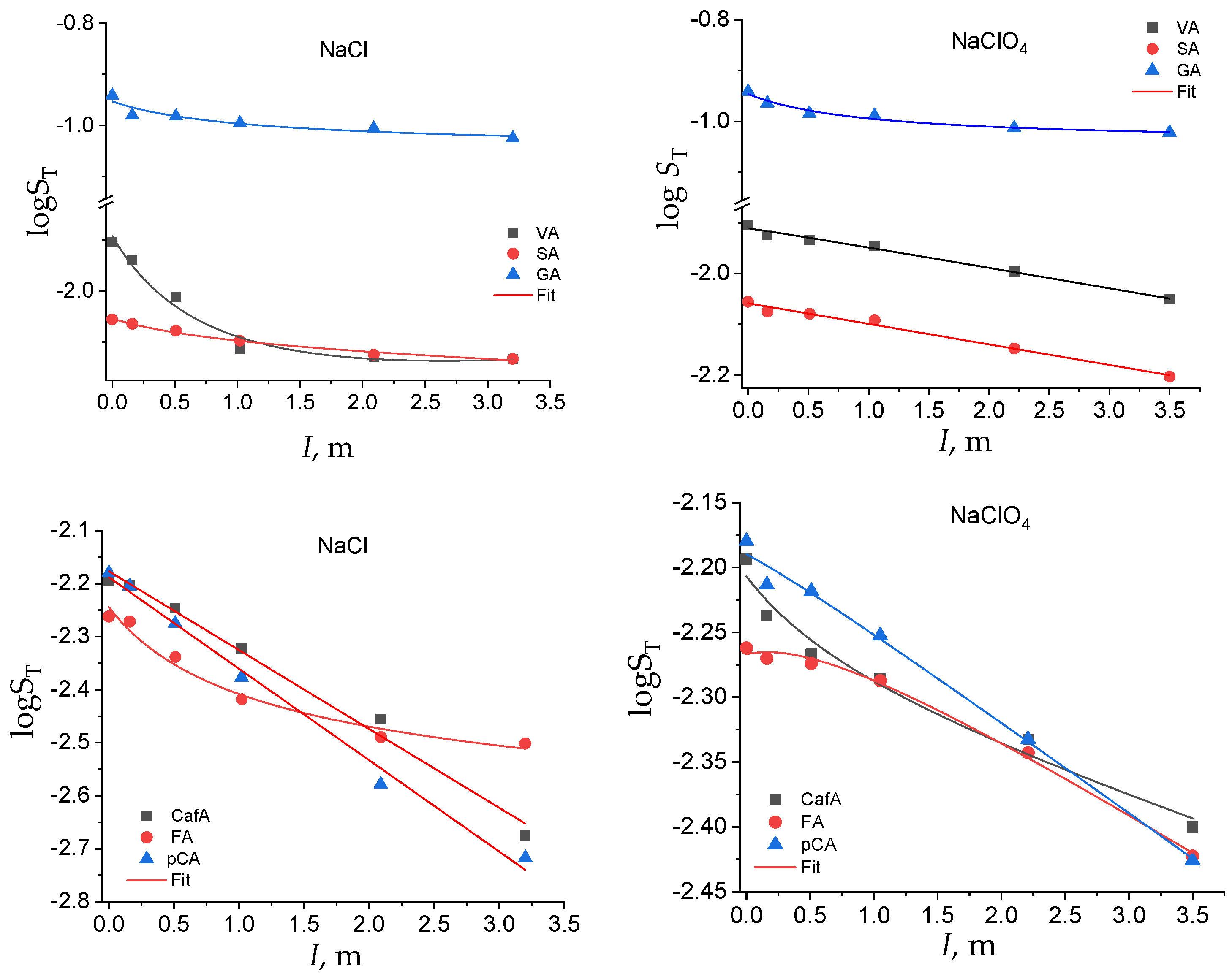
As a general trend, the solubility in water of all the acids was higher than that in both ionic media at each of the electrolyte concentrations. The hydroxybenzoic acids were more soluble than the hydroxycinnamic ones and, among them, gallic acid showed the highest solubility, more than one order of magnitude higher. As expected, among the hydroxybenzoic acids, syringic acid was the least soluble, due to the presence of two methoxy groups, and the solubility of the hydroxycinnamic acids was generally lower than that of the hydroxybenzoic acids in all the media investigated. The salting-out effect is related to the strong tendency of ionic solutes to form hydration shells. In fact, as the concentration of the ionic salt increased, more and more water molecules were bound up in the hydration shells, and therefore the solubility decreased. The solubility trend of hydroxybenzoic and hydroxycinnamic acids as a function of the ionic strength can be better appreciated in Figure 2, in the two electrolyte media.
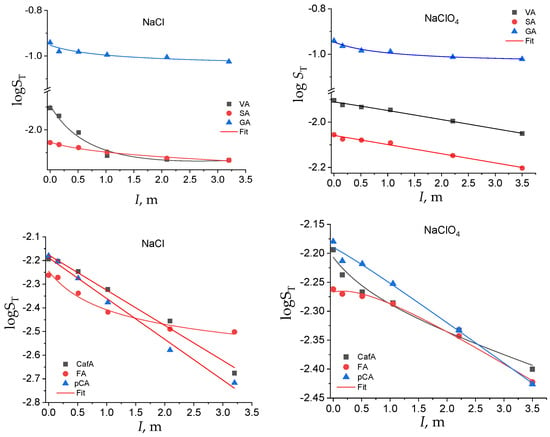
Figure 2.
Total solubility in the logarithmic scale of the hydroxybenzoic and hydroxycinnamic acids as a function of the solvent salts and of the ionic strength.
In both sets of acids, the solubility was affected by the -OH and -OCH3 substituents on the phenyl moiety, with the hydroxy and methoxy groups contributing to an increase and a decrease in the solubility, respectively, especially at lower ionic strengths. The following order of solubility, that holds up to approximately I = 1 m, was found: GA > VA > SA and CafA > p-CA > FA. The effect of the substituents on the total solubility was more evident on the benzoic acid series. If we look at the dependence of ST on the ionic strength for the benzoic acid series, the salting-out effect of the electrolyte was different for the two salts. In sodium chloride, the solubility dropped to low values in a smoothed way, at low ionic strength, and then it tended towards a plateau value. In perchlorate, instead, the solubility decreased almost linearly with I (except for GA).
In the hydroxycinnamic acid series the solubility decreased quite linearly with the ionic strength, independently of the ionic medium used, except for the least soluble ferulic acid, which showed a nonlinear dependence of its ST vs. I (Table S1 in Supplementary Material). The solid lines are the best fitting curves obtained by Equation (5) or by a line. The related fitting parameters are reported in Table S1.
The order of solubility traced above seems to be related to a polarity decrease in the acids as the hydroxyl groups are replaced by either a hydrogen or a methoxy group, and to the decrease in the capability of the molecule to make hydrogen bonds with water. This argument may explain why gallic acid has the highest solubility, almost one order of magnitude higher than the other acids of both series, at all ionic strengths, and why its solubility is slightly affected by the increase in the molality of the solvent salt.
However, this consideration is mostly important for the benzoic acids, while, in the hydroxycinnamic ones, the unsaturated alkyl chain plays a major role in determining the overall solubility behavior.
In a recent computational study, it was shown that the free energy of solvation in water is negative for gallic and caffeic acids and positive for ferulic acid, with gallic acid having the most negative solvation free energy [44], indicating that the highest solubility of gallic acid is due to the high polar interaction density formed between the phenolic and carboxylic hydroxyl groups with water. In the same study, the authors showed also that the phenolic hydroxyl and the carboxyl functions tend to form hydrogen bonds with water, giving rise to a twist in the molecule structure close to the carboxyl group in the case of ferulic and caffeic acids. The effects of the phenyl hydroxy and methoxy substituents on the solid–liquid equilibrium of syringic and vanillic acids in water was recently investigated by the Abraham solvation model [45]. It was found that vanillic acid, with two hydroxyl substituents, had the highest value for the H-bond acidity (solute acidity descriptor), which described the tendency of the solute to form hydrogen bonds with its acid hydrogens, followed by syringic acid, which had one hydroxyl and two methoxy substituents. Moreover, the H-bond acidity of the carboxyl group increased by the electron-donating resonance effect of the methoxy substituents in the aromatic ring. In contrast, the H-bond acidity decreased due to the intramolecular hydrogen bond between two adjacent OH-OR substituents [46].
These acids may also form hydrogen bonds by sharing an oxygen lone electron pair of one of their substituents, i.e., they have hydrogen bond basicity. This is the highest for syringic acid due to the higher number of available lone electron pairs, even though intramolecular H-bonds may lead to a decrease in this parameter for vanillic acid [46]. Analogous results were obtained for ferulic and p-coumaric acids, the latter showing the highest value for the H-bond acidity parameter, which is partially reduced in ferulic acid due to the intramolecular hydrogen bond between the hydroxyl group and the methoxy group in the meta position. In contrast, the H-bond basicity character decreases from ferulic acid to p-coumaric acid, according to the increase in the number of hydrogen acceptors in the molecules [47].
Thus, the presence of hydroxyl groups increases the H-bond acidity, i.e., the strength of the hydrogen bonds formed by the donor hydroxyl groups with water, whereas the H-bond basicity descriptor, related to the strength of the hydrogen bonds formed by the acceptor groups in the molecules with water, increases with the number of methoxy substituents on the ring. On the other hand, when the hydroxy and/or the methoxy groups are absent, the above solute descriptors significantly decrease in value, such as in the case of veratric and cinnamic acids [46,47].
The largest salting-out effect calculated at the highest ionic strength is reported in Figure 3 in terms of the percentage decrease in the total solubility with respect to the value in pure water (Equation (4)).
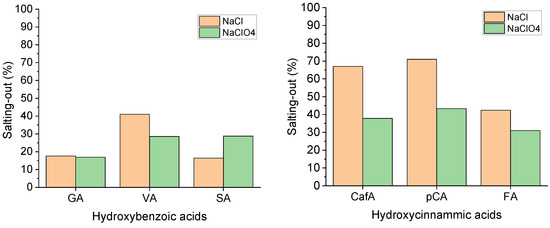
Figure 3.
Salting-out effect calculated at the highest ionic strength for the two series of acids and in both electrolytes.
The salting-out effect is remarkable especially for the hydroxycinnamic acids, with values ranging from 40 up to 70% and mostly in NaCl. The strong decrease in the solubility with the ionic strength determines this result and highlights the low tendency of the hydroxycinnamic acids to interact with water, which becomes worse in the presence of highly polar salts such as sodium chloride. On the other hand, the salting-out effect seems to depend less on the type of electrolyte in the hydroxybenzoic acid series. It is interesting to note that in both ionic media and for both the acid series, the maximum salting-out occurs for the acids that have intermediate solubility in each series (i.e., vanillic and p-coumaric), which are the ones that respond mainly to ionic strength changes (Figure 2) mostly in NaCl medium.
3. Discussion
The knowledge of the activity coefficients of the neutral species is useful when modeling the dependence of equilibrium constants on the ionic strength [48,49]. For example, to evaluate the sequestering ability of these acids towards biological metal cations in a natural system, such as the ocean or biological fluids, the activity coefficients must be used to extrapolate their equilibrium constants at the infinite dilution reference state from those determined in constant ionic medium [50,51].
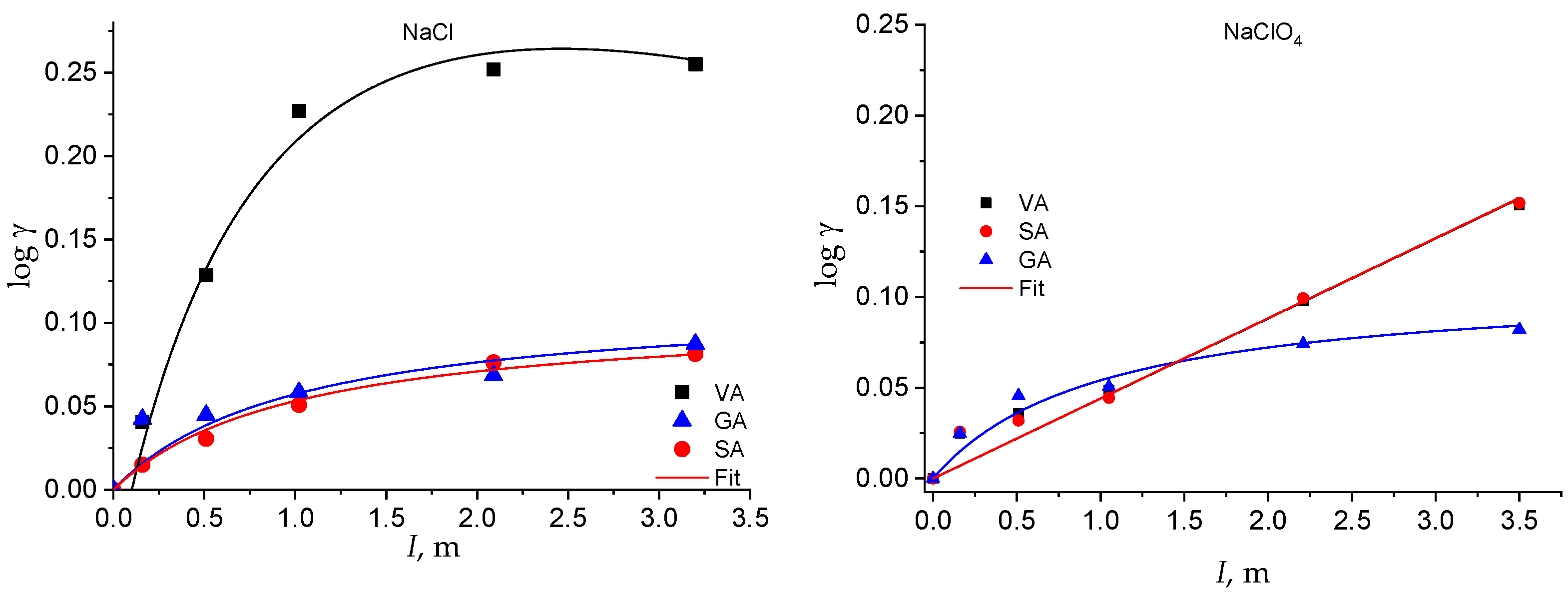
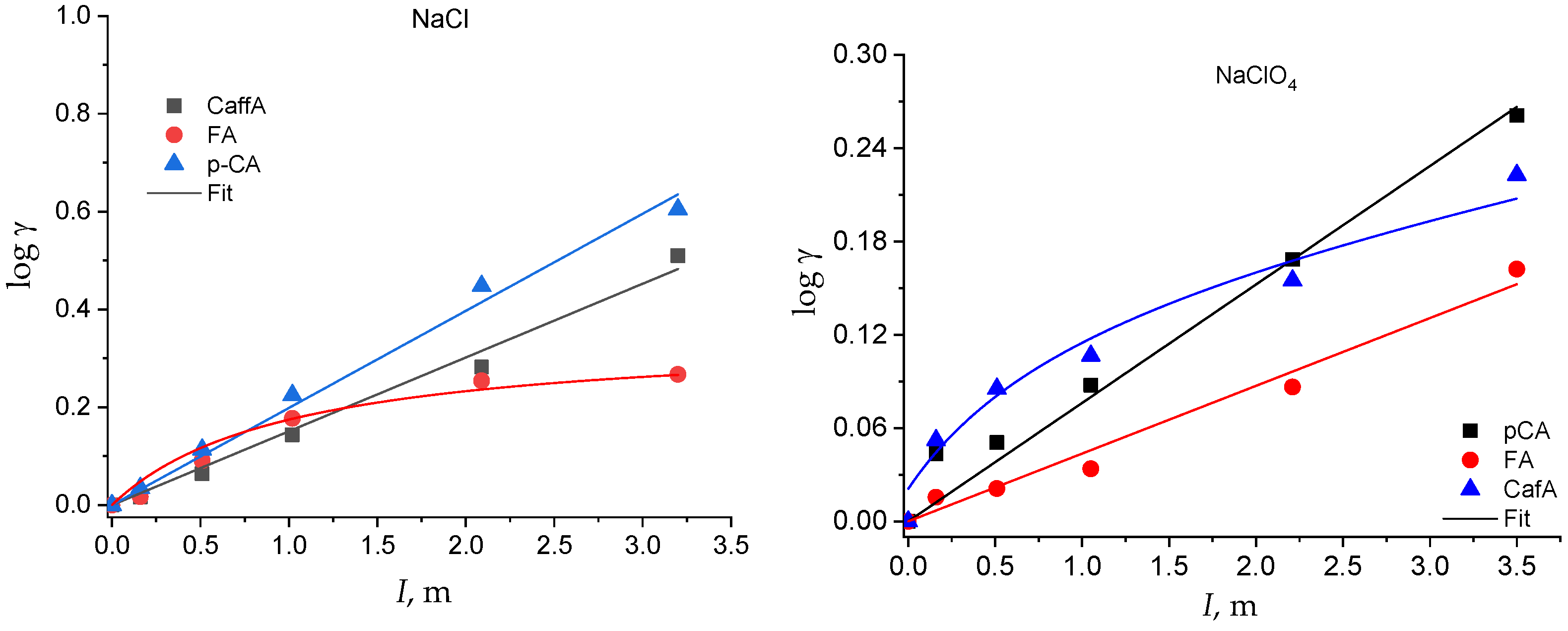
The activity coefficients of the neutral species as a function of I and of the solvent electrolyte are plotted in Figure 4 and Figure 5. The solubility of the neutral species at all the ionic strengths investigated is reported in Table S2 in the Supplementary Material.
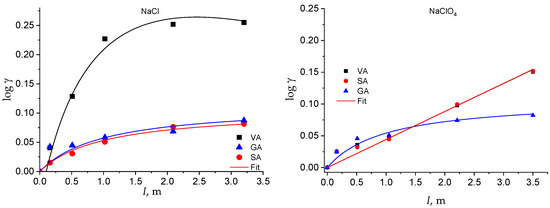
Figure 4.
log γ of the hydroxybenzoic acids as a function of the solvent salt and of the ionic strength.
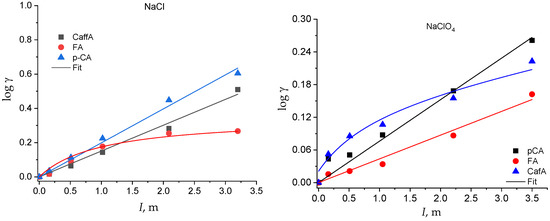
Figure 5.
log γ of the hydroxycinnamic acids as a function of the solvent salt and of the ionic strength.
The activity coefficient of the neutral species of all the acids increases with the ionic strength in both the solvent electrolytes (Figure 4 and Figure 5). However, some of them show a linear trend that can be well fitted by Equation (1), with Setschenow coefficients ranging from 0.7 up to 0.53 kg mol−1, while others show a nonlinear trend that is modeled by Equation (7) (Table S3 in Supplementary Material). In the latter cases, the Setschenow coefficient depends on the concentration of the solvent electrolyte and the log γ tends towards a plateau value for ionic strengths larger than around 2 molal. These data show that a salting-out effect in the total solubility corresponds to a salting-in effect on the activity coefficients, as already observed for other acids in aqueous solution [48].
Therefore, while, in NaCl medium, the solubility of the phenolic acids tends towards zero (and the activity coefficient tends towards infinity) at infinite I, in NaClO4, the solubility tends towards a minimum value (and the activity coefficient towards a maximum value) at very high I. This different behavior in the two electrolyte media is intriguing and deserves further study to be explained by changing the experimental conditions (i.e., the nature of inert salt) and extending the evaluation to other classes of analytes.
Table 2 collects literature data [6,30,32,34,39,41,43,46,52,53,54,55] for a comparison with our solubility results. All data are expressed as mass fraction (χ).

Table 2.
Solubility of phenolic acids, as mass fraction (χ), in water and in 1 molal NaCl at 37 °C from this work and from the literature.
Our results are in line with previous literature data for vanillic, gallic, caffeic and p-coumaric acids. The agreement is also satisfactory for syringic and ferulic acids in pure water. There is less agreement with the data at 1 m NaCl and those reported previously in sulphate [30] and nitrate media [54], where the salting-out effect is higher than in chloride for syringic and vanillic acid and lower for gallic. This behavior has been already observed previously for syringic acid at room temperature [54] and clearly highlights the significant effect of the anion of the electrolyte salt. Moreover, the above discrepancies may be related to a substantial difference in the preparation of the saturated solutions: in our experimental approach, the direct contact of the analytes with the magnetic stirring is avoided and this is mainly reflected in the less soluble analytes.
4. Materials and Methods
4.1. Materials
All solutions were prepared by means of analytical-grade reagents and ultrapure water (Millipore MilliQ system). Vanillic (J&K Scientific, Beijing, China, 99%), syringic and gallic (Sigma Aldrich, St. Louis, MI, USA, ≥98% and ≥99%, respectively), p-coumaric (Sigma, St. Louis, MI, USA, ≥98%), ferulic and caffeic (Aldrich, St. Louis, MI, USA, 99%) acids were used without further purification. Sodium perchlorate and sodium chloride stock solutions were prepared and standardized according to previous works [56,57].
4.2. Solubility Apparatus and Procedure
The absorption spectra in the UV region were recorded with a Varian Cary 50 Scan UV–Visible spectrophotometer on a series of hydroxybenzoic and hydroxycinnamic acid test solutions, prepared by 100-fold dilution of 50 microliters of saturated solutions. These were prepared with a leaching apparatus suitable to prevent solid particles from coming into contact with the magnetic stirrer. To prevent grinding by the stirrer, hydroxybenzoic and hydroxycinnamic acids were wrapped in highly retentive filter paper (Whatman 42) bags. These were retained in glass cylinders containing pure water as well as sodium chloride and sodium perchlorate solutions at pre-established ionic strength values while continuously stirring with a magnetic bar. The cylinders were then placed in a thermostatic water bath at (37.0 ± 0.1) °C, and the hydroxybenzoic and hydroxycinnamic acid concentrations were monitored over time, until they reached a constant value, which usually occurred in around 4 days. Matched quartz cells of thickness 1 cm and 100 microliter volume were employed. The absorbance, Aλ, was recorded to ±0.001 units. The formulations of the parameters and the acquisition of data were achieved with the aid of a computer connected to the instrument.
4.3. Thermodynamic Modeling
Total solubility data in the molal concentration scale were fitted by the smoothing empirical Equation (5):
where is the total solubility in pure water, and and are empirical parameters at infinite and at zero ionic strength, respectively. Equation (5) becomes linear for a∞ = a0 ≡ a.
The solubility of the neutral species, S0, was calculated by Equation (6), considering the deprotonation equilibrium of the phenolic acids (Equation (7)), behaving as a monoprotic acid with constant (Equation (8)):
where, according to the specific ion interaction model (SIT) [58,59], the activity coefficients of the ionic species and of the neutral one are given by Equations (9) and (10), respectively.
with and is the Setschenow coefficient.
The dependence of the ionization constant of the acids on the ionic strength was explicitly considered in the calculation. Unfortunately, data on the protonation constant of these acids are only reported at a few ionic strengths and in the low molal range (up to 0.1 mol/kg) [60,61,62,63,64,65,66]. Therefore, here, the for each acid was measured at 0.16 mol/kg and 0.51 mol/kg and its values at higher ionic strength, up to 3.5 mol/kg, were estimated by the SIT model at 37 °C (Equations (8)–(11)).
where is the value of at infinite dilution and is the extended Debye term. was calculated by Equation (11) using the experimental values at I = 0.51 mol/kg.
In the SIT model (Equation (9)), the specific ion interaction coefficients relative to the proton with the anion of the electrolyte salt (i.e., Cl−, ClO4−) and that relative to the conjugate base of the acid (A−) with Na+ were kept constant in the iterations used for fitting the experimental data, respectively, at 0.12 (in NaCl)/0.14 (in NaClO4) [58] and 0.06 (in both media) [67]. For the specific ion interaction coefficient of A− with Na+, we chose a value identical to that of the hydrogen salicylate ion with Na+, by considering the similarity of the anion structures. Figure S1 displays the dependence of the first ionization acidic constant vs. the ionic strength for all the investigated acids.
Equations (2) and (12), which consider the dependence of the Setschenow coefficients k on the ionic strength, were used to fit the activity coefficient data:
where k∞ and k0 are the Setschenow coefficients at infinite and at zero ionic strength, respectively.
4.4. Statistical Analysis
All the experiments were performed in triplicate, and the data were expressed as means ± standard deviation. To test statistical differences among solubility values of each compound under different experimental conditions, data were evaluated with one-way ANOVA followed by Tukey’s multiple comparison test. A p value of < 0.05 was considered statistically significant.
5. Conclusions
The study of the solubility of benzoic and cinnamic acid derivatives is important for at least two aspects: one is on a fundamental thermodynamic basis, and concerns the knowledge of the solubility behavior at different temperatures and in different aqueous media, such as those containing electrolyte salts that may act as co-solvents. Calculation of the activity coefficients from the solubility values allows us to obtain a more complete picture of different aspects of the thermodynamic properties of hydroxybenzoic and hydroxycinnamic acids. Solubility measurements were analyzed to determine the Setschenow coefficients at infinite and at zero ionic strength and the solubility of the neutral species in pure water. Knowledge of these data simplifies the calculation of the activity coefficients of the charged and uncharged species. For instance, environmental partitioning of these acids in the aqueous phase is determined by their solubility in different conditions. Therefore, solubility studies of these compounds are of crucial interest for modeling the behavior of natural aquatic systems. The other aspect is of practical interest because the acids studied here are biologically active compounds that find applications in different industrial fields, such as pharmaceutical, cosmetic, food and biological wastewater treatment applications. In these cases, it is important to know the solubility in water and in other media in order to better design, for instance, processes aimed at their extraction from different matrices.
In this work, the solubility of hydroxybenzoic and hydroxycinnamic acids was studied in aqueous solutions of different ionic strengths at 37 °C. Here, we have verified that the higher polarity of the first class of phenolic acids reflects their higher solubility in aqueous solutions, independently of the ionic medium and the ionic strength. According to Kruyt and Robinson [24], this trend is due to the lower availability of the water molecules to the solvation of the phenolic acids because they are mainly involved in the ion’s hydration. The solubility of hydroxybenzoic acids decreases linearly when the inert salt is NaCl, while in NaClO4, the dependence on the ionic strength is smoothed. The behavior of hydroxycinnamic acids is different; in fact, the solubility of this class of acids decreases linearly with the ionic strength independently of the ionic medium.
Supplementary Materials
The following are available online, Figure S1. Dependence of the ionization constant on the ionic strength in the two salts media. Experimental values (symbols) and values modeled by the SIT theory (lines) with the equation 11. For all of the fitting, an adjusted R2 > 0.998 and a reduced χ2 as low as 10−5 were obtained. Table S1. Smoothed total solubility data as a function of the ionic strength in NaCl and NaClO4. Table S2. Solubility of the neutral species at 37 °C of phenolic acids in water and in aqueous solutions of NaCl and NaClO4 at different ionic strength. The uncertainties represent standard deviation. Table S3. Setschenow coefficients of hydroxybenzoic and hydroxycinnamic acids in NaCl and NaClO4.
Author Contributions
Conceptualization, validation, writing—review and editing, supervision, A.B., E.F. and P.P.; Methodology, software, visualization, data curation, formal analysis, investigation, A.B., E.F., L.M. and C.L.T.; Writing—original draft preparation, A.B., E.F., A.F. and P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the University of Calabria for financial support.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Malacaria, L.; Corrente, G.A.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. A review on coordination properties of Al(III) and Fe(III) towards natural antioxidant molecules: Experimental and theoretical insights. Molecules 2021, 26, 2603. [Google Scholar] [CrossRef]
- Furia, E.; Beneduci, A.; Russo, N.; Marino, T. Structural characterization of aluminium (III) and iron (III) complexes of coumarinic acid in aqueous solution from combined experimental and theoretical investigations. New J. Chem. 2018, 42, 11006–11012. [Google Scholar] [CrossRef]
- Beneduci, A.; Corrente, G.A.; Marino, T.; Aiello, D.; Bartella, L.; Di Donna, L.; Napoli, A.; Russo, N.; Romeo, I.; Furia, E. Insight on the chelation of aluminum(III) and iron(III) by curcumin in aqueous solution. J. Mol. Liq. 2019, 296, 111805–111814. [Google Scholar] [CrossRef]
- Corrente, G.A.; Malacaria, L.; Beneduci, A.; Furia, E.; Marino, T.; Mazzone, G. Experimental and theoretical study on the coordination properties of quercetin towards aluminum(III), iron(III) and copper(II) in aqueous solution. J. Mol. Liq. 2021, 325, 115171–115182. [Google Scholar] [CrossRef]
- Boskou, D. Sources of natural phenolic antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar]
- Queimada, A.J.; Mota, F.L.; Pinho, S.P.; Macedo, E.A. Solubilities of Biologically Active Phenolic Compounds: Measurements and Modeling. J. Phys. Chem. B 2009, 113, 3469–3476. [Google Scholar] [CrossRef]
- Du, L.; Yu, P.; Rossnagel, B.G.; Christensen, D.A.; McKinnon, J.J. Physicochemical Characteristics, Hydroxycinnamic Acids (Ferulic Acid, p-Coumaric Acid) and Their Ratio, and in Situ Biodegradability: Comparison of Genotypic Differences among Six Barley Varieties. J. Agric. Food Chem. 2009, 57, 4777–4783. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Cione, E.; La Torre, C.; Cannataro, R.; Caroleo, M.C.; Plastina, P.; Gallelli, L. Quercetin, epigallocatechin gallate, curcumin and resveratrol: From dietary sources to human microRNA modulation. Molecules 2020, 25, 63. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C.M.; Goulas, V.; Tsakona, S.; Manganaris, G.A.; Gekas, V. A Knowledge Base for The Recovery of Natural Phenols with Different Solvents. Int. J. Food Prop. 2013, 16, 382–396. [Google Scholar] [CrossRef]
- Petrucci, R.; Astolfi, P.; Greci, L.; Firuzi, O.; Saso, L.; Marrosu, G. A spectroelectrochemical and chemical study on oxidation of hydroxycinnamic acids in aprotic medium. Electrochim. Acta 2007, 52, 2461–2470. [Google Scholar] [CrossRef]
- Fernandes, C.I.S.; Rebelo, M.J.F. Polyphenolic Biosensors. Application in Red Wines. Port. Electrochim. Acta 2009, 27, 457–462. [Google Scholar] [CrossRef]
- Arribas, A.S.; Martìnez-Fernàndez, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates–nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Singh, S.K.; Kundu, A.; Kishore, N. Interactions of some amino acids and glycine peptides with aqueous sodium dodecyl sulfate and cetyltrimethylammonium bromide at T = 298.15 K: A volumetric approach. J. Chem. Thermodyn. 2004, 36, 7–16. [Google Scholar] [CrossRef]
- Soto, A.; Arce, A.; Khoshkbarchi, M.K. Thermodynamics of Diglycine and Triglycine in Aqueous NaCl Solutions: Apparent Molar Volume, Isentropic Compressibility, and Refractive Index. J. Sol. Chem. 2004, 33, 11–21. [Google Scholar] [CrossRef]
- Poulson, S.R.; Harrington, R.R.; Drever, J.I. The solubility of toluene in aqueous salt solutions. Talanta 1999, 48, 633–641. [Google Scholar] [CrossRef]
- Goȑgényi, M.; Dewulf, J.; Van Langenhove, H.; Héberger, K. Aqueous salting-out effect of inorganic cations and anions on non-electrolytes. Chemosphere 2006, 65, 802–810. [Google Scholar] [CrossRef]
- Bullister, J.L.; Wisegarver, D.P.; Menzia, F.A. The solubility of sulfur hexafluoride in water and seawater. Deep Sea Res. Part I Oceanogr. Res. Pap. 2002, 49, 175–187. [Google Scholar] [CrossRef]
- Falcone, G.; Giuffrè, O.; Sammartano, S. Acid-base and UV properties of some aminophenol ligands and their complexing ability towards Zn2+ in aqueous solution. J. Mol. Liquids 2011, 159, 146–151. [Google Scholar] [CrossRef]
- Bretti, C.; Giuffrè, O.; Lando, G.; Sammartano, S. Solubility, protonation and activity coefficients of some aminobenzoic acids in NaClaq and (CH3)4NClaq, at different salt concentrations, at T = 298.15K. J. Mol. Liq. 2015, 212, 825–832. [Google Scholar] [CrossRef]
- Long, F.A.; McDevit, W.F. Activity coefficients of nonelectrolyte solutes in aqueous salt solutions. Chem. Rev. 1952, 50, 119–169. [Google Scholar] [CrossRef]
- Debye, P. Das elektrische Ionenfeld und das Aussalzen. Z. Phys. Chem. 1927, 130, 56–65. [Google Scholar] [CrossRef]
- Kruyt, H.R.; Robinson, C. The Stability of Suspensoids under Influence of Electrolyte Mixture on Lyotropie. Proc. Acad. Sci. Amst. 1926, 29, 1244. [Google Scholar]
- Porto, R.; Furia, E. On the complexation of copper (II) ion with 2-hydroxybenzamide. Ann. Chim. 2007, 97, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Furia, E.; Falvo, M.; Porto, R. Solubility and Acidic Constants of l-Cystine in NaClO4 Aqueous Solutions at 25 °C. J. Chem. Eng. Data 2009, 54, 3037–3042. [Google Scholar] [CrossRef]
- Furia, E.; Nardi, M.; Sindona, G. Standard Potential and Acidic Constants of Oleuropein. J. Chem. Eng. Data 2010, 55, 2824–2828. [Google Scholar] [CrossRef]
- Furia, E.; Napoli, A.; Tagarelli, A.; Sindona, G. Speciation of 2-Hydroxybenzoic Acid with Calcium(II), Magnesium(II), and Nickel(II) Cations in Self-Medium. J. Chem. Eng. Data 2013, 58, 1349–1353. [Google Scholar] [CrossRef]
- Furia, E.; Sindona, G.; Tagarelli, A. Solubility and acidic constants at 25 °C in NaClO4 aqueous solutions of 1-(2-hydroxyphenyl)ethenone. Monatsh. Chem. 2016, 147, 1009–1014. [Google Scholar] [CrossRef]
- Noubigh, A.; Cherif, M.; Provost, E.; Abderrabba, M. Solubility of Gallic Acid, Vanillin, Syringic Acid, and Protocatechuic Acid in Aqueous Sulfate Solutions from (293.15 to 318.15) K. J. Chem. Eng. Data 2008, 53, 1675–1678. [Google Scholar] [CrossRef]
- Calado, M.S.; Branco, A.S.H.; Najdanovic-Visak, V.; Visak, Z.P. Solubility of high-value compounds in environmentally friendly solvents-liquid poly(ethylene glycol) and ionic liquids: Experimental study and thermodynamic analysis. J. Chem. Thermodyn. 2014, 70, 154–159. [Google Scholar] [CrossRef]
- Mota, F.L.; Queimada, A.J.; Pinho, S.P.; Macedo, E.A. Aqueous Solubility of Some Natural Phenolic Compounds. Ind. Eng. Chem. Res. 2008, 47, 5182–5189. [Google Scholar] [CrossRef]
- Alevizou, E.I.; Voutsas, E.C. Solubilities of p-coumaric and caffeic acid in ionic liquids and organic solvents. J. Chem. Thermodyn. 2013, 62, 69–78. [Google Scholar] [CrossRef]
- Lu, L.-L.; Lu, X.-Y. Solubilities of Gallic Acid and Its Esters in Water. J. Chem. Eng. Data 2007, 52, 37–39. [Google Scholar] [CrossRef]
- Murga, R.; Sanz, M.T.; Beltrán, S.; Cabezas, J.L. Solubility of three hydroxycinnamic acids in supercritical carbon dioxide. J. Supercrit. Fluids 2003, 27, 239–245. [Google Scholar] [CrossRef]
- Murga, R.; Sanz, M.T.; Beltrán, S.; Cabezas, J.L. Solubility of Syringic and Vanillic Acids in Supercritical Carbon Dioxide. J. Chem. Eng. Data 2004, 49, 779–782. [Google Scholar] [CrossRef]
- Manic, M.S.; Villanueva, D.; Fornari, T.; Queimada, A.J.; Macedo, E.A.; Najdanovic–Visak, V. Solubility of high-value compounds in ethyl lactate: Measurements and modelling. J. Chem. Thermodyn. 2012, 48, 93–100. [Google Scholar] [CrossRef]
- Crea, F.; Crea, P.; De Stefano, C.; Giuffrè, O.; Pettignano, A.; Sammartano, S. Thermodynamic Parameters for the Protonation of Poly(allylamine) in Concentrated LiCl(aq) and NaCl(aq). J. Chem. Eng. Data 2004, 49, 658–663. [Google Scholar] [CrossRef]
- Daneshfar, A.; Ghaziaskar, H.S.; Homayoun, N. Solubility of Gallic Acid in Methanol, Ethanol, Water, and Ethyl Acetate. J. Chem. Eng. Data 2008, 53, 776–778. [Google Scholar] [CrossRef]
- Noubigh, A.; Jeribi, C.; Mgaidi, A.; Abderrabba, M. Solubility of gallic acid in liquid mixtures of (ethanol+water) from (293.15 to 318.15)K. J. Chem. Thermodyn. 2012, 55, 75–78. [Google Scholar] [CrossRef]
- Noubigh, A.; Aydi, A.; Mgaidi, A.; Abderrabba, M. Measurement and correlation of the solubility of gallic acid in methanol plus water systems from (293.15 to 318.15) K. J. Mol. Liquids 2013, 187, 226–229. [Google Scholar] [CrossRef]
- De Stefano, C.; Foti, C.; Giuffrè, O.; Sammartano, S. Acid–base and UV behavior of 3-(3,4-dihydroxyphenyl)-propenoic acid (caffeic acid) and complexing ability towards different divalent metal cations in aqueous solution. J. Mol. Liquids 2014, 195, 9–16. [Google Scholar] [CrossRef]
- Noubigh, A.; Mgaidi, A.; Abderrabba, M.; Provost, E.; Fürst, W. Effect of salts on the solubility of phenolic compounds: Experimental measurements and modelling. J. Sci. Food Agric. 2007, 87, 783–788. [Google Scholar] [CrossRef]
- Delmondes, P.H.; Stefani, R. Computational study of natural phenolic acid solubility and their interactions with chitosan. MOL2NET 2016, 2. [Google Scholar] [CrossRef]
- Abraham, M.H. Scales of solute hydrogen-bonding: Their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 1992, 96, 73–83. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Vieira, V.; Brandão, P.; Alves, R.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solvent and temperature effects on the solubility of syringic, vanillic or veratric acids: Experimental, modeling and solid phase studies. J. Mol. Liq. 2019, 289, 111089–111098. [Google Scholar] [CrossRef]
- Vilas-Boas, S.M.; Vieira, V.; Brandão, P.; Alves, R.S.; Coutinho, J.A.P.; Pinho, S.P.; Ferreira, O. Solid-liquid phase equilibrium of trans-cinnamic acid, p-coumaric acid and ferulic acid in water and organic solvents: Experimental and modelling studies. Fluid Phase Equilibria 2020, 521, 112747–112756. [Google Scholar] [CrossRef]
- Bretti, C.; Cigala, R.M.; Crea, F.; Foti, C.; Sammartano, S. Solubility and activity coefficients of acidic and basic non-electrolytes in aqueous salt solutions: 3. Solubility and activity coefficients of adipic and pimelic acids in NaCl(aq), (CH3)4NCl(aq) and (C2H5)4NI(aq) at different ionic strengths and at t = 25 °C. Fluid Phase Equilibria 2008, 263, 43–54. [Google Scholar]
- Furia, E.; Sindona, G. Interaction of Iron(III) with 2-Hydroxybenzoic Acid in Aqueous Solutions. J. Chem. Eng. Data 2012, 57, 195–199. [Google Scholar] [CrossRef]
- Beneduci, A.; Furia, E.; Russo, N.; Marino, T. Complexation behaviour of caffeic, ferulic and p-coumaric acids towards aluminium cations: A combined experimental and theoretical approach. New J. Chem. 2017, 41, 5182–5190. [Google Scholar] [CrossRef]
- Porwal, S.K.; Furia, E.; Harris, M.E.; Viswanathan, R.; Devireddy, L. Synthetic, potentiometric and spectroscopic studies of chelation between Fe(III) and 2,5-DHBA supports salicylate-mode of siderophore binding interactions. J. Inorg. Biochem. 2015, 145, 1–10. [Google Scholar] [CrossRef]
- Bitencourt, R.G.; Cabral, F.A.; Meirelles, A.J.A. Ferulic acid solubility in supercritical carbon dioxide, ethanol and water mixtures. J. Chem. Thermodyn. 2016, 103, 285–291. [Google Scholar] [CrossRef]
- Shakeel, F.; Salem-Bekhit, M.M.; Haqa, N.; Siddiqui, N.A. Solubility and thermodynamics of ferulic acid in different neat solvents: Measurement, correlation and molecular interactions. J. Mol. Liq. 2017, 236, 144–150. [Google Scholar] [CrossRef]
- Noubigh, A.; Cherif, M.; Provost, E.; Abderrabba, M. Solubility of some phenolic compounds in aqueous alkali metal nitrate solutions from (293.15 to 318.15) K. J. Chem. Thermodyn. 2008, 40, 1612–1616. [Google Scholar] [CrossRef]
- Noubigh, A.; Abderrabba, M.; Provost, E. Temperature and salt addition effects on the solubility behaviour of some phenolic compounds in water. J. Chem. Thermodyn. 2007, 39, 297–303. [Google Scholar] [CrossRef]
- Furia, E.; Porto, R. 2-Hydroxybenzamide as a Ligand. Complex Formation with Dioxouranium(VI), Aluminum(III), Neodymium(III), and Nickel(II) Ions. J. Chem. Eng. Data 2008, 53, 2739–2745. [Google Scholar] [CrossRef]
- Cardiano, P.; Giuffrè, O.; Napoli, A.; Sammartano, S. Potentiometric, 1H NMR and ESI-MS investigation on dimethyltin(IV) cation–mercaptocarboxylate interaction in aqueous solution. New J. Chem. 2009, 33, 2286–2295. [Google Scholar] [CrossRef]
- Ciavatta, L. The specific interaction theory in evaluating ionic equilibria. Ann. Chim. 1980, 70, 551–567. [Google Scholar]
- Ciavatta, L. The specific interaction theory in equilibrium analysis. Some empirical rules for estimating interaction coefficients of metal ion complexes. Ann. Chim. 1990, 80, 255–263. [Google Scholar]
- Öhman, L.-O.; Sjöberg, S. Equilibrium and structural studies of silicon(IV) and aluminium(III) in aqueous solution. 1. The formation of ternary mononuclear and polynuclear complexes in the system Al3+-gallic acid-OH-. A potentiometric study in 0.6 M Na(Cl). Acta Chem. Scand. 1981, 35, 201–212. [Google Scholar] [CrossRef][Green Version]
- Sedeh, I.F.; Öhman, L.-O.; Sjöberg, S. Equilibrium and structural studies of silicon(IV) and aluminium(III) in aqueous solution. 30. Aqueous complexation between silicic acid and some ortho-Di- and triphenolic compounds. Acta Chem. Scand. 1992, 46, 933–940. [Google Scholar] [CrossRef]
- Sandmann, B.J.; Chien, M.H.; Sandmann, R.A. Stability constants of calcium, magnesium and zinc gallate using a divalent ion-selective electrode. Anal. Lett. 1985, 18, 149–159. [Google Scholar] [CrossRef]
- Kipton, H.; Powell, J.; Taylor, M.C. Interactions of Iron(II) and Iron(III) with Gallic Acid and its Homologues: A Potentiometric and Spectrophotometric Study. Aust. J. Chem. 1982, 35, 739–756. [Google Scholar]
- Ambulkar, R.S.; Marathe, D.G.; Munshi, K.N. Complexes of gallium(III) with pyrocatechol, pyrogallol & protocatechuic, α-resorcylic, gallic & 2,3-dihydroxynaphthalene-6-sulphonic acids in aqueous medium. Ind. J. Chem. 1981, 20, 1044–1046. [Google Scholar]
- Ozkorucuklu, S.P.; Beltrán, J.L.; Fonrodona, G.; Barrón, D.; Alsancak, G.; Barbosa, J. Determination of Dissociation Constants of Some Hydroxylated Benzoic and Cinnamic Acids in Water from Mobility and Spectroscopic Data Obtained by CE-DAD. J. Chem. Eng. Data 2009, 54, 807–811. [Google Scholar] [CrossRef]
- Beltrán, J.L.; Sanli, N.; Fonrodona, G.; Barrón, D.; Özkan, G.; Barbosa, J. Spectrophotometric, potentiometric and chromatographic pKa values of polyphenolic acids in water and acetonitrile–water media. Anal. Chim. Acta 2003, 484, 253–264. [Google Scholar] [CrossRef]
- Furia, E.; Porto, R. The effect of ionic strength on the complexation of copper (II) with salicylate ion. Ann. Chim. 2002, 92, 521–530. [Google Scholar] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).