Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Thymoquinone (TQ) Preparation
3.2. Microorganism
3.3. Antibacterial Activity Assay of Thymoquinone (TQ) against Tested Organisms
3.4. Minimum Inhibitory Concentration (MIC) Test
3.5. Minimum Bactericidal Concentration (MBC) Test
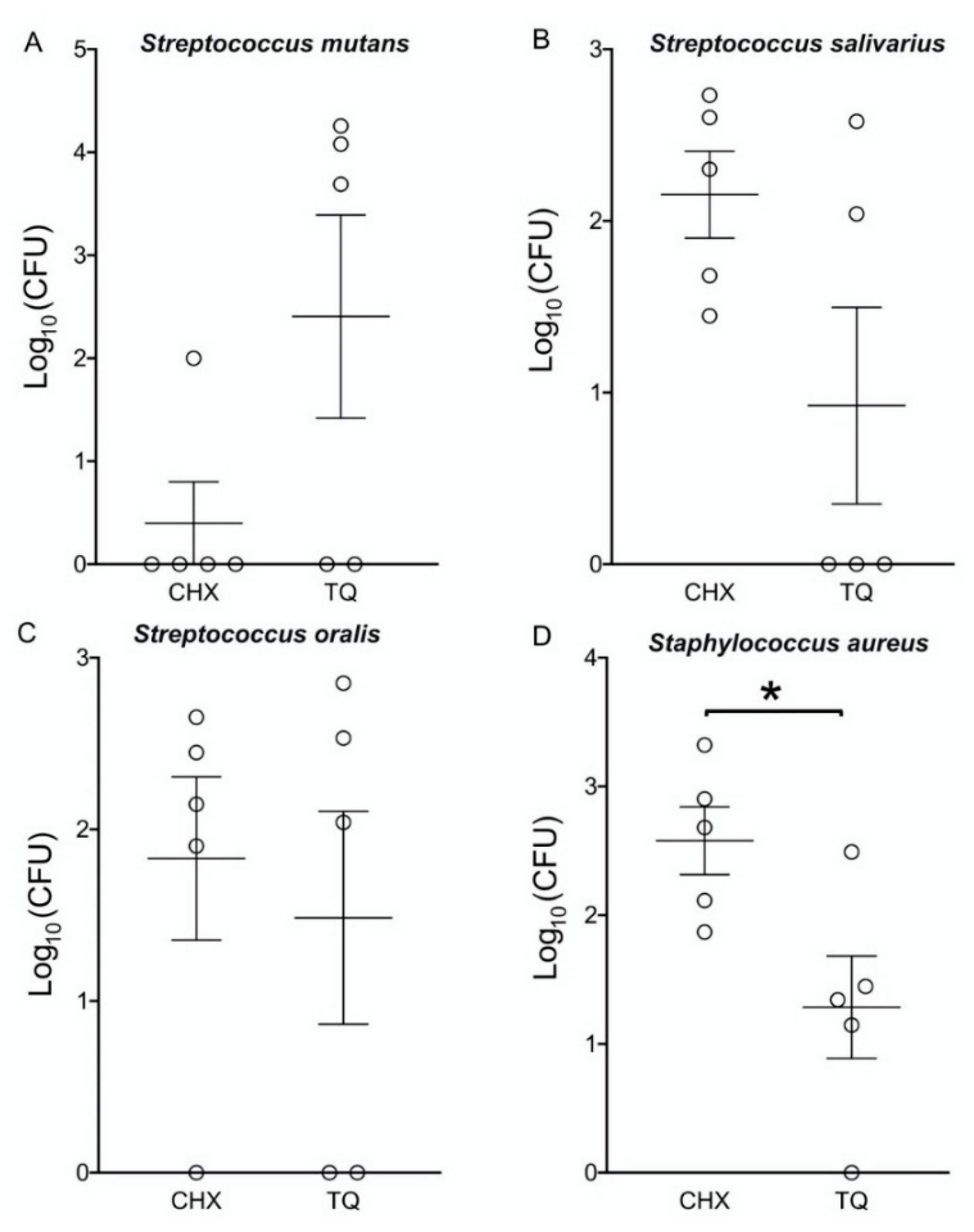
3.6. The Bacterial Count of MBC Values during Different Periods
3.7. Well Diffusion Test
4. Statistical Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wang, H.; Ren, D. Controlling Streptococcus mutans and Staphylococcus aureus biofilms with direct current and chlorhexidine. AMB Express 2017, 7, 204. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Soda, Y.; Hayashi, F.; Doi, T.; Suzuki, J.; Miura, K.; Kozai, K. Longitudinal study of dental caries incidence associated with Streptococcus mutans and Streptococcus sobrinus in pre-school children. J. Med. Microbiol. 2005, 54, 661–665. [Google Scholar] [CrossRef]
- Kouidhi, B.; Fdhila, K.; Ben Slama, R.; Mahdouani, K.; Hentati, H.; Najjari, F.; Bakhrouf, A.; Chaieb, K. Molecular detection of bacteria associated to dental caries in 4–12-year-old Tunisian children. Microb. Pathog. 2014, 71–72, 32–36. [Google Scholar] [CrossRef]
- Cummins, D. Dental caries: A disease which remains a public health concern in the 21st century—The exploration of a breakthrough technology for caries prevention. J. Clin. Dent. 2013, 24, A1–A14. [Google Scholar]
- Mathur, V.P.; Dhillon, J.K. Dental Caries: A Disease Which Needs Attention. Indian J. Pediatrics 2018, 85, 202–206. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of untreated caries: A systematic review and metaregression. J. Dent. Res. 2015, 94, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Dye, B.A.; Arevalo, O.; Vargas, C.M. Trends in paediatric dental caries by poverty status in the United States, 1988–1994 and 1999–2004. Int. J. Paediatr. Dent. 2010, 20, 132–143. [Google Scholar] [CrossRef]
- Dye, B.A.; Vargas, C.M.; Fryar, C.D.; Ramos-Gomez, F.; Isman, R. Oral health status of children in Los Angeles County and in the United States, 1999–2004. Community Dent. Oral Epidemiol. 2017, 45, 135–144. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nedumgottil, B.M. Relative presence of Streptococcus mutans, Veillonella atypica, and Granulicatella adiacens in biofilm of complete dentures. J. Indian Prosthodont. Soc. 2018, 18, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.; Gujjari, A.K.; Gowda, V.; Angadi, S. Antifungal response of oral-associated candidal reference strains (American Type Culture Collection) by supercritical fluid extract of nutmeg seeds for geriatric denture wearers: An in vitro screening study. J. Indian Prosthodont. Soc. 2017, 17, 267–272. [Google Scholar] [CrossRef] [PubMed]
- LuIs, H.S.; Luis, L.S.; Bernardo, M. In vitro study of the effect of an essential oil and a delmopinol mouth rinse on dental plaque bacteria. Indian J. Dent. Res. Off. Publ. Indian Soc. Dent. Res. 2016, 27, 648–651. [Google Scholar] [CrossRef] [PubMed]
- Dattner, A.M. From medical herbalism to phytotherapy in dermatology: Back to the future. Dermatol. Ther. 2003, 16, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.H. Integration of herbal medicine into modern medical practices: Issues and prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef]
- Oskouei, Z.; Akaberi, M.; Hosseinzadeh, H. A glance at black cumin (Nigella sativa) and its active constituent, thymoquinone, in ischemia: A review. Iran. J. Basic Med. Sci. 2018, 21, 1200–1209. [Google Scholar] [CrossRef]
- Al-Attass, S.A.; Zahran, F.M.; Turkistany, S.A. Nigella sativa and its active constituent thymoquinone in oral health. Saudi Med. J. 2016, 37, 235–244. [Google Scholar] [CrossRef]
- Al-Bayaty, F.H.; Kamaruddin, A.A.; Ismail, M.A.; Abdulla, M.A. Formulation and Evaluation of a New Biodegradable Periodontal Chip Containing Thymoquinone in a Chitosan Base for the Management of Chronic Periodontitis. J. Nanomater. 2013, 5. [Google Scholar] [CrossRef]
- Alhebshi, A.H.; Gotoh, M.; Suzuki, I. Thymoquinone protects cultured rat primary neurons against amyloid beta-induced neurotoxicity. Biochem. Biophys. Res. Commun. 2013, 433, 362–367. [Google Scholar] [CrossRef]
- Ugur, A.R.; Dagi, H.T.; Ozturk, B.; Tekin, G.; Findik, D. Assessment of In vitro Antibacterial Activity and Cytotoxicity Effect of Nigella sativa Oil. Pharmacogn. Mag. 2016, 12, S471–S474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dyrda, G.; Boniewska-Bernacka, E.; Man, D.; Barchiewicz, K.; Słota, R. The effect of organic solvents on selected microorganisms and model liposome membrane. Mol. Biol. Rep. 2019, 46, 3225–3232. [Google Scholar] [CrossRef] [Green Version]
- Institute, C.L.S. Methods for Dilution Antimicrobia Susceptibility Tests for Bacteria That Grow Aerobically Approved Standard. CLSI 2012, M07-A9, 13–61. [Google Scholar]
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.C.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.; Fasih, N.; Jabeen, K.; Jamil, B. Rothia dentocariosa endocarditis with mitral valve prolapse: Case report and brief review. Infection 2011, 39, 177–179. [Google Scholar] [CrossRef]
- Kojima, A.; Nomura, R.; Naka, S.; Okawa, R.; Ooshima, T.; Nakano, K. Aggravation of inflammatory bowel diseases by oral streptococci. Oral Dis. 2014, 20, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.I.; Reynolds, H.S.; Sreenivasan, P.K.; Subramanyam, R.; Cummins, D.; Zambon, J.J. Media- and method-dependent variations in minimal inhibitory concentrations of antiplaque agents on oral bacteria. Lett. Appl. Microbiol. 2006, 43, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Aikemu, A.; Xiaerfuding, X.; Shiwenhui, C.; Abudureyimu, M.; Maimaitiyiming, D. Immunomodulatory and anti-tumor effects of Nigella glandulifera freyn and sint seeds on ehrlich ascites carcinoma in mouse model. Pharmacogn. Mag. 2013, 9, 187–191. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, S.S.; Rao, M.V.; Kaneez, F.S.; Qadri, S.; Al-Marzouqi, A.H.; Chandranath, I.S.; Adem, A. Nigella sativa extract as a potent antioxidant for petrochemical-induced oxidative stress. J. Chromatogr. Sci. 2011, 49, 321–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, I.; Ali, S.; Siddiqui, I.A.; Al-Khalifa, K.S.; Al-Hariri, M. Influence of Thymoquinone Exposure on the Micro-Hardness of Dental Enamel: An In Vitro Study. Eur. J. Dent. 2019, 13, 318–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negi, P.; Rathore, C.; Sharma, G.; Singh, B.; Katare, O.P. Thymoquinone a Potential Therapeutic Molecule from the Plant Nigella sativa: Role of Colloidal Carriers in its Effective Delivery. Recent Pat. Drug Deliv. 2018, 12, 3–22. [Google Scholar] [CrossRef]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [Green Version]
- Hartanti, A.W.; Tjandrawinata, R.R. Antibacterial activity of Nigella sativa L.seed oil in water emulsion against dental cariogenic bacteria. IJPSR 2017, 8, 3155–3161. [Google Scholar]
- Kouidhi, B.; Zmantar, T.; Jrah, H.; Souiden, Y.; Chaieb, K.; Mahdouani, K.; Bakhrouf, A. Antibacterial and resistance-modifying activities of thymoquinone against oral pathogens. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 29. [Google Scholar] [CrossRef] [Green Version]
- Gawron, G.; Krzyczkowski, W.; Lemke, K.; Ołdak, A.; Kadziński, L.; Banecki, B. Nigella sativa seed extract applicability in preparations against methicillin-resistant Staphylococcus aureus and effects on human dermal fibroblasts viability. J. Ethnopharmacol. 2019, 244, 112135. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.T.; Khan, R.A.; Shukla, I. Antimicrobial activity of Nigella sativa Linn. seed oilagainst multi-drug resistant bacteria from clinical isolates. Nat. Prod. Radian Nat. Prod. Radiance 2008, 7, 10–14. [Google Scholar]
- Hannan, A.; Saleem, S.; Chaudhary, S.; Barkaat, M.; Arshad, M.U. Anti bacterial activity of Nigella sativa against clinical isolates of methicillin resistant Staphylococcus aureus. JAMC 2008, 20, 72–74. [Google Scholar] [PubMed]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Mouwakeh, A.; Kincses, A.; Nové, M.; Mosolygó, T.; Mohácsi-Farkas, C.; Kiskó, G.; Spengler, G. Nigella sativa essential oil and its bioactive compounds as resistance modifiers against Staphylococcus aureus. Phytother. Res. 2019, 33, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Emeka, L.B.; Emeka, P.M.; Khan, T.M. Antimicrobial activity of Nigella sativa L. seed oil against multi-drug resistant Staphylococcus aureus isolated from diabetic wounds. Pak. J. Pharm. Sci. 2015, 28, 1985–1990. [Google Scholar]
- Filogônio, C.e.F.; Soares, R.V.; Horta, M.C.; Penido, C.V.; Cruz, R.e.A. Effect of vegetable oil (Brazil nut oil) and mineral oil (liquid petrolatum) on dental biofilm control. Braz. Oral. Res. 2011, 25, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, P.L.; Fonteles, C.S.; Marques, L.A.; Jamacaru, F.V.; Fonseca, S.G.; de Carvalho, C.B.; de Moraes, M.E. The efficacy of three formulations of Lippia sidoides Cham. essential oil in the reduction of salivary Streptococcus mutans in children with caries: A randomized, double-blind, controlled study. Phytomedicine 2014, 21, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Charugundla, B.R.; Anjum, S.; Mocherla, M. Comparative effect of fluoride, essential oil and chlorhexidine mouth rinses on dental plaque and gingivitis in patients with and without dental caries: A randomized controlled trial. Int. J. Dent. Hyg. 2015, 13, 104–109. [Google Scholar] [CrossRef] [PubMed]
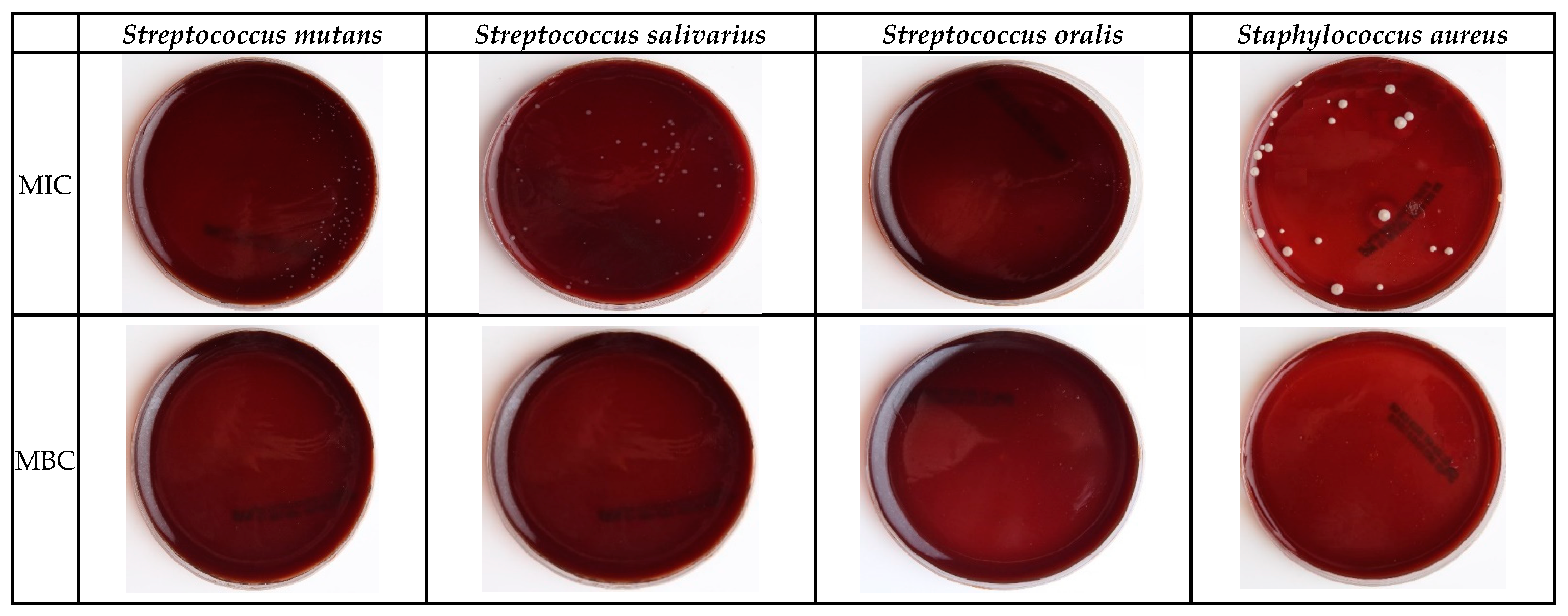
| Concentration (mg/)mL | Inhibition Zone (Mean and SD in mm) | |||
|---|---|---|---|---|
| S. salivarius | S. mutans | S. oralis | S. aureus | |
| 12.5 | 37 ± 1.5 | 29.5 ± 1.8 | 41.5 ± 1.5 | 36.5 ± 1.5 |
| 25 | 38.5 ± 1.5 | 37.5 ± 1.1 | 44 ± 0.0 | 44 ± 0.0 |
| 50 | 38.5 ± 1.0 | 44 ± 0.0 | 44 ± 0.0 | 44 ± 0.0 |
| 75 | 44 ± 0.0 | 44 ± 0.0 | 44 ± 0.0 | 44 ± 0.0 |
| 100 | 44 ± 0.0 | 44 ± 0.0 | 44 ± 0.0 | 44 ± 0.0 |
| CHX (+ve control) | 11.5 ± 1.0 | 17.5 ± 0.2 | 21.5 ± 1.5 | 12 ± 1.0 |
| Microorganisms | TQ | CHX | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S. salivarius | 4.0 | 5.0 | 0.00857 | 0.0171 |
| S. mutans | 3.0 | 4.0 | ≤0.00002 | 0.0009 |
| S. oralis | 2.0 | 3.0 | 0.00095 | 0.0038 |
| S. aureus | 2.0 | 3.0 | 0.00857 | 0.0171 |
| Time (hours) | S. salivarius | S. mutans | S. oralis | S. aureus | ||||
|---|---|---|---|---|---|---|---|---|
| TQ 5 mg/mL | CHX | TQ 4 mg/mL | CHX | TQ 3 mg/mL | CHX | TQ 3 mg/mL | CHX | |
| 1 h | 0.38 × 103 | 0.54 × 103 | 1.8 × 104 | 1.0 × 102 | 0.71 × 103 | 0.45 × 103 | 0.31 × 103 | 0.21 × 104 |
| 3 h | 0.11 × 103 | 0.40 × 103 | 1.2 × 104 | NG | 0.34 × 102 | 0.28 × 103 | 0.28 × 102 | 0.80 × 103 |
| 6 h | NG | 0.20 × 103 | 4.9 × 103 | NG | 0.11 × 102 | 0.14 × 103 | 0.22 × 102 | 0.48 × 103 |
| 12 h | NG | 0.48 × 102 | NG | NG | NG | 0.80 × 102 | 0.14 × 102 | 0.13 × 103 |
| 24 h | NG | 0.28 × 102 | NG | NG | NG | NG | NG | 0.74 × 102 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khalifa, K.S.; AlSheikh, R.; Al-Hariri, M.T.; El-Sayyad, H.; Alqurashi, M.S.; Ali, S.; Bugshan, A.S. Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study. Molecules 2021, 26, 6451. https://doi.org/10.3390/molecules26216451
Al-Khalifa KS, AlSheikh R, Al-Hariri MT, El-Sayyad H, Alqurashi MS, Ali S, Bugshan AS. Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study. Molecules. 2021; 26(21):6451. https://doi.org/10.3390/molecules26216451
Chicago/Turabian StyleAl-Khalifa, Khalifa S., Rasha AlSheikh, Moahmmed T. Al-Hariri, Hosam El-Sayyad, Maher S. Alqurashi, Saqib Ali, and Amr S. Bugshan. 2021. "Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study" Molecules 26, no. 21: 6451. https://doi.org/10.3390/molecules26216451
APA StyleAl-Khalifa, K. S., AlSheikh, R., Al-Hariri, M. T., El-Sayyad, H., Alqurashi, M. S., Ali, S., & Bugshan, A. S. (2021). Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study. Molecules, 26(21), 6451. https://doi.org/10.3390/molecules26216451









