Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment
Abstract
:1. Introduction
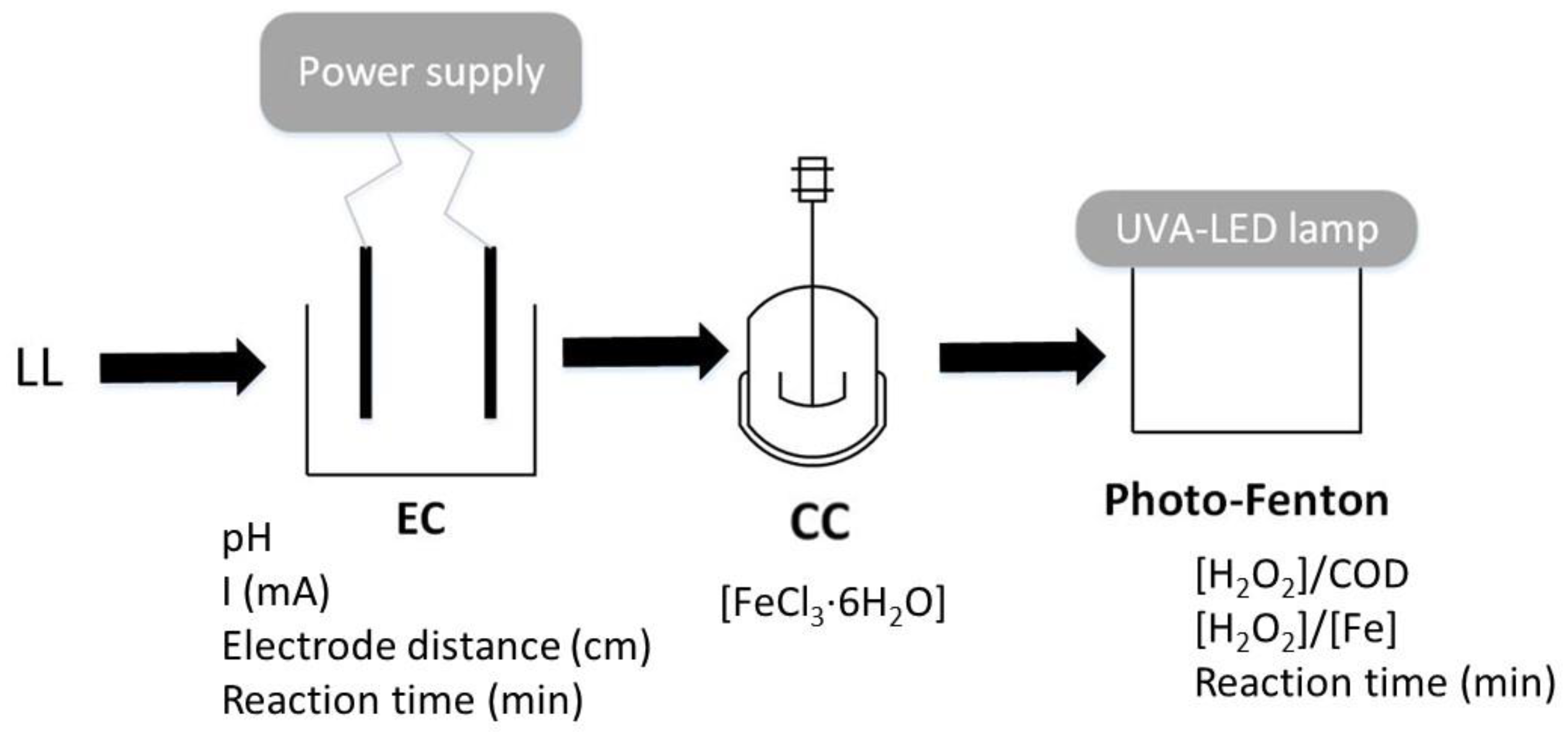
2. Materials and Methods
2.1. Chemicals
2.2. Landfill Leachate (LL)
2.3. Conventional Coagulation Followed by Electrocoagulation
2.4. Electrocoagulation Followed by Conventional Coagulation
2.5. UVA-LED Photo-Fenton
2.6. Analytical Determinations
3. Results and Discussion
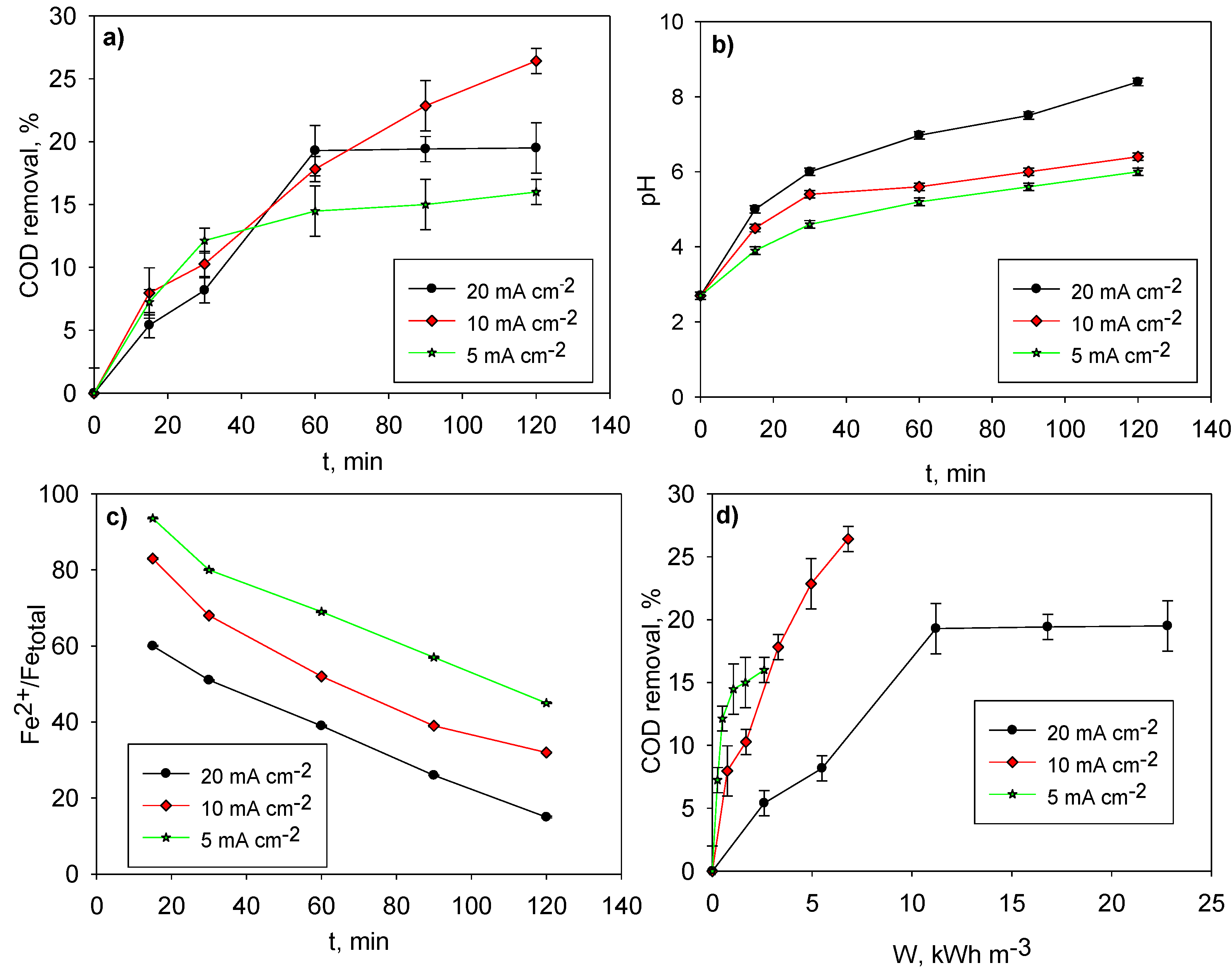
3.1. Electrocoagulation of Conventionally Pre-Coagulated LL
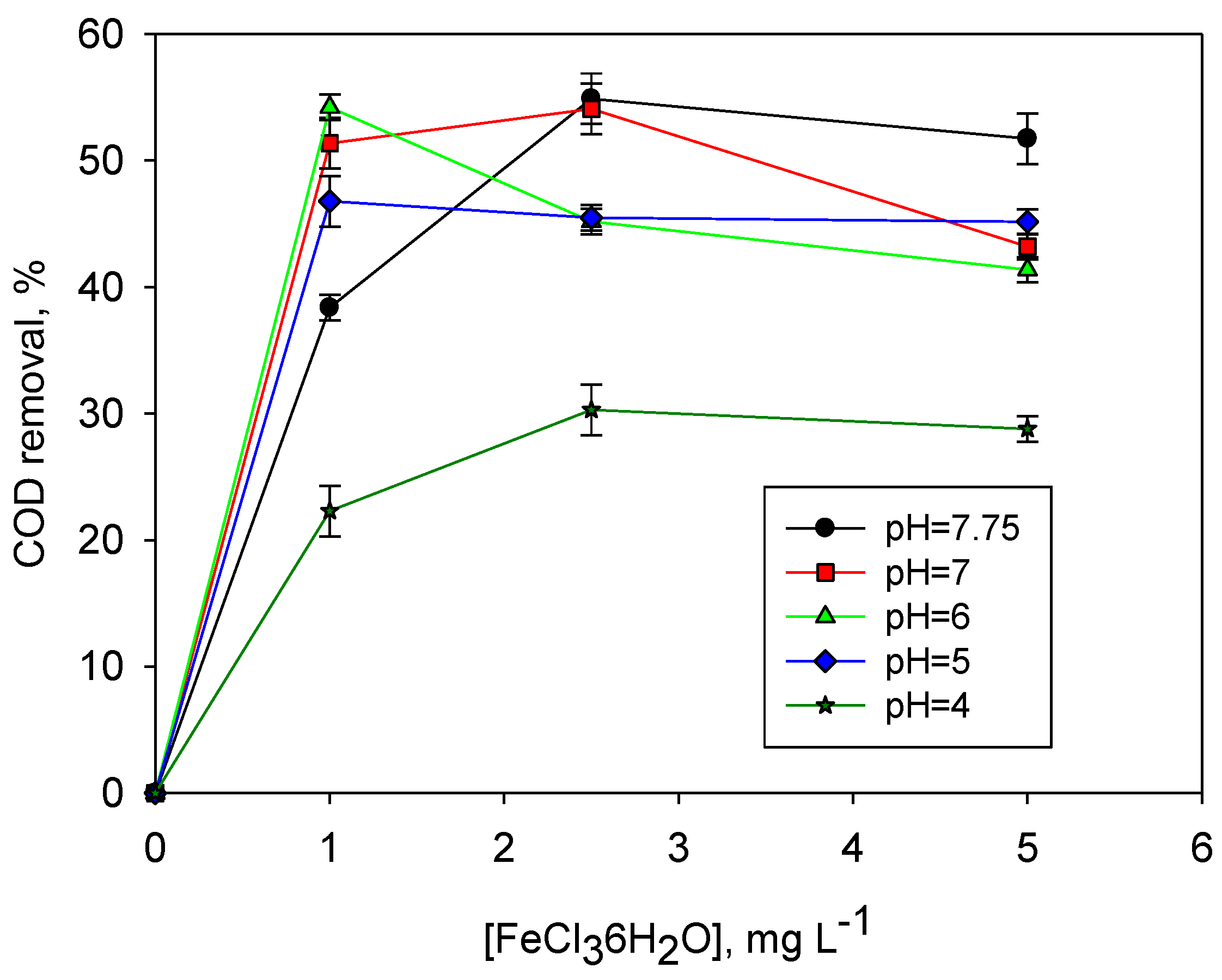
3.2. Conventional Coagulation of Pre-Electrocoagulated LL
3.3. Comparison of CC-EC and EC-CC Combinations
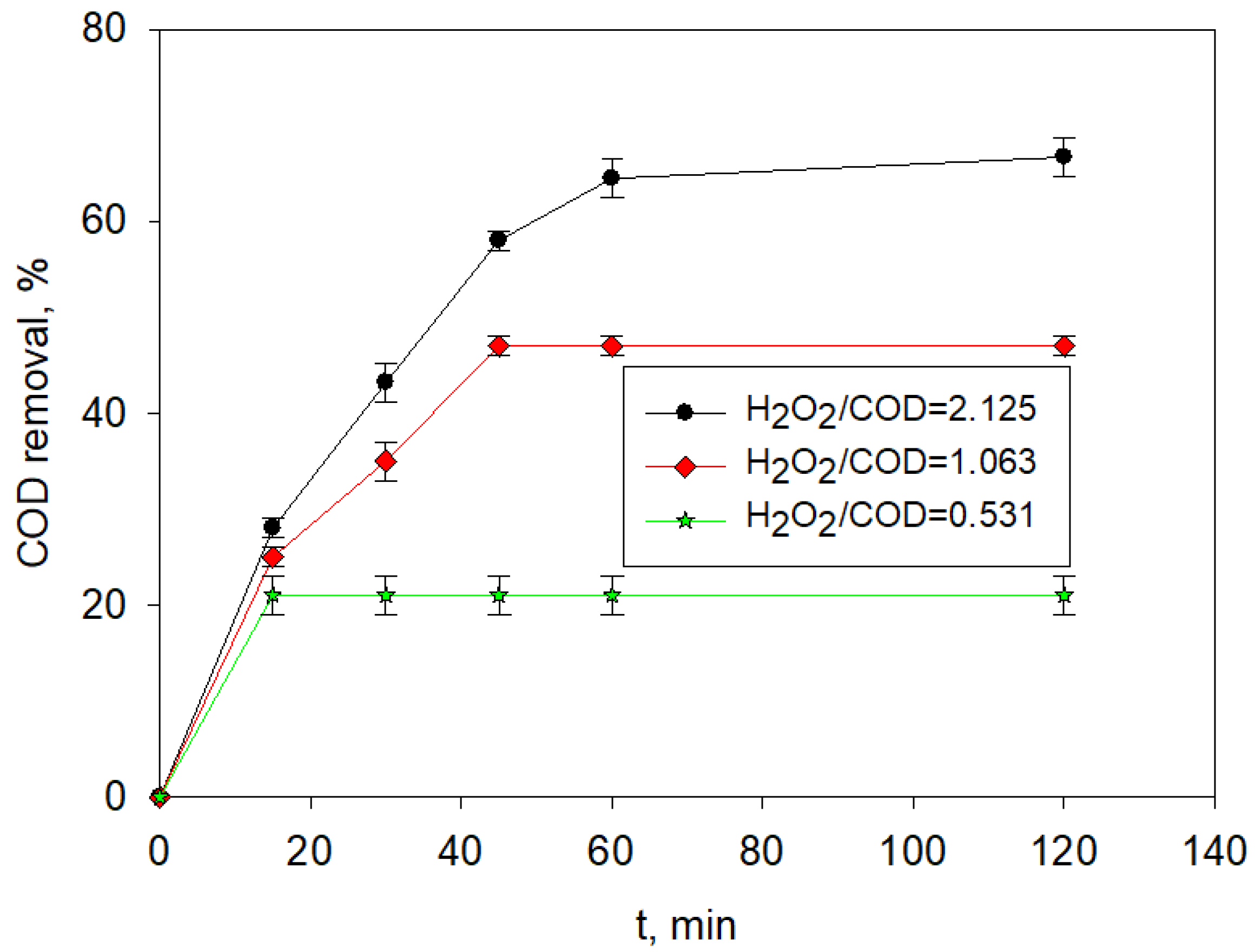
3.4. UVA-LED Photo-Fenton
3.5. Treatment Cost
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Iskander, M.S.; Zhao, R.; Pathak, A.; Gupta, A.; Pruden, A.; Novak, J.T.; He, Z.A. review of landfill leachate induced ultraviolet quenching substances: Sources, characteristics, and treatment. Water Res. 2018, 145, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Publication: Washington, DC, USA, 2018. [Google Scholar]
- Deng, Y.; Englehardt, J.D. Treatment of landfill leachate by the Fenton process. Water Res. 2006, 40, 3683–3694. [Google Scholar] [CrossRef]
- Chou, J.D.; Wey, M.Y.; Liang, H.H.; Chang, S.H. Biotoxicity evaluation of fly ash and bottom ash from different municipal solid waste incinerators. J. Hazard. Mater. 2009, 168, 197–202. [Google Scholar] [CrossRef]
- Dastjerdi, B.; Strezov, V.; Kumar, R.; He, J.; Behnia, M. Comparative life cycle assessment of system solution scenarios for residual municipal solid waste management in NSW. Aust. Sci. Total Environ. 2021, 767, 144355. [Google Scholar] [CrossRef] [PubMed]
- Abbas, A.A.; Jingsong, G.; Ping, L.Z.; Ya, P.Y.; Al-Rekabi, W.S. Review on LandWll Leachate Treatments. Res. J. Appl. Sci. 2009, 5, 534–545. [Google Scholar]
- Mukherjee, S.; Mukhopadhyay, S.; Hashim, M.A.; Sen Gupta, B. Contemporary environmental issues of landfill leachate: Assessment and remedies. Crit. Rev. Environ. Sci. Technol. 2015, 45, 472–590. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, P.; Thakur, L.S.; Kaushik, A. Bioassays for toxicological risk assessment of landfill leachate: A review. Ecotoxicol. Environ. Saf. 2017, 141, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Zolfaghari, M.; Jardak, K.; Drogui, P.; Brar, S.K.; Buelna, G.; Dubé, R. Landfill leachate treatment by sequential membrane bioreactor and electro-oxidation processes. J. Environ. Manag. 2016, 184, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef]
- Tejera, J.; Miranda, R.; Hermosilla, D.; Urra, I.; Negro, C.; Blanco, A. Treatment of a Mature Landfill Leachate: Comparison between Homogeneous and Heterogeneous Photo-Fenton with Different Pretreatments. Water 2019, 11, 1849. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.N.; Lan, C.Q. Treatment of landfill leachate using membrane bioreactors: A review. Desalination 2012, 287, 41–54. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and long-term composition of MSW landfill leachate: A review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 2020, 143131. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhu, X.; Chen, N.; Feng, C.; Wang, H.; Kuang, P.; Hu, W. Review on electrochemical system for landfill leachate treatment: Performance, mechanism, application, shortcoming, and improvement scheme. Sci. Total Environ. 2020, 745, 140768. [Google Scholar] [CrossRef] [PubMed]
- Boumechhour, F.; Rabah, K.; Lamine, C.; Said, B.M. Treatment of landfill leachate using Fenton process and coagulation/flocculation. Water Environ. J. 2013, 27, 114–119. [Google Scholar] [CrossRef]
- Amor, C.; De Torres-Socías, E.; Peres, J.A.; Maldonado, M.A.; Oller, I.; Malato, S.; Lucas, M.S. Mature landfill leachate treatment by coagulation/flocculation combined with Fenton and solar photo-Fenton processes. J. Hazard. Mater. 2015, 286, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, L.P.; Arana, V.A.; Trilleras, J.; Barros, G.E.; González-Solano, A.J.; Maury-Ardila, H. Efficiency of Combined Processes Coagulation/Solar Photo Fenton in the Treatment of Landfill Leachate. Water 2019, 11, 1351. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Q. The role of coagulation in water treatment. Curr. Opin. Chem. Eng. 2015, 8, 36–44. [Google Scholar] [CrossRef]
- Litter, M.I.; Slodowicz, M. An overview on heterogeneous Fenton and photoFenton reactions using zerovalent iron materials. J. Adv. Oxid. Technol. 2017, 20, 19. [Google Scholar] [CrossRef]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Asaithambi, P.; Govindarajan, R.; Yesuf, M.B.; Selvakumar, P.; Alemayehu, E. Enhanced treatment of landfill leachate wastewater using sono (US)-ozone (O3)–electrocoagulation (EC) process: Role of process parameters on color, COD and electrical energy consumption. Process Saf. Environ. 2020, 142, 212–218. [Google Scholar] [CrossRef]
- Djelal, H.; Lelievre, Y.; Ricordel, C. Combination of Electro-Coagulation and biological treatment by bioaugmentation for landfill leachate. Desalin. Water Treat. 2015, 54, 2986–2993. [Google Scholar] [CrossRef]
- Le, S.T.; Le, K.C. Reduction of COD in Nam Son landfill leachate by electro-Fenton as secondary treatment after electrocoagulation pretreatment. Vietnam J. Sci. Technol. 2019, 57, 724. [Google Scholar] [CrossRef] [Green Version]
- Nidheesh, P.V.; Scaria, J.; Babu, D.S.; Kumar, M.S. An overview on combined electrocoagulation/degradation processes for the effective treatment of water and wastewater. Chemosphere 2020, 263, 127907. [Google Scholar] [CrossRef]
- Phalakornkule, C.; Mangmeemak, J.; Intrachod, K.; Nuntakumjorn, B. Pretreatment of palm oil mill effluent by electrocoagulation and coagulation. ScienceAsia 2010, 36, 142–149. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Mostafapour, F.K.; Farzadkia, M.; Ownagh, K.A.; Mahvi, A.H. Slaughterhouse wastewater treatment by combined chemical coagulation and electrocoagulation process. PLoS ONE 2012, 7, e40108. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin. Water Treat. 2016, 57, 9203–9215. [Google Scholar] [CrossRef]
- Tejera, J.; Hermosilla, D.; Gascó, A.; Miranda, R.; Alonso, V.; Negro, C.; Blanco, A. Treatment of mature landfill leachate by electrocoagulation followed by Fenton or UVA-LED photo-Fenton processes. J. Taiwan Inst. Chem. Eng. 2021, 119, 33–44. [Google Scholar] [CrossRef]
- Kim, S.M.; Geissen, S.U.; Vogelpohl, A. Landfill leachate treatment by a photoassisted Fenton reaction. Water Sci. Technol. 1997, 35, 239–248. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Hatchard, C.; Parker, C.A. Mathematical and Physical Sciences. P. Roy. Soc. Lond. A Mat. 1956, 235, 518–538. [Google Scholar]
- APHA, A. Standard Methods for the Examination of Water and Wastewater; WPCF Publisher: Washington, DC, USA, 2005. [Google Scholar]
- Barndõk, H.; Merayo, N.; Blanco, L.; Hermosilla, D.; Blanco, A. Application of on-line FTIR methodology to study the mechanisms of heterogeneous advanced oxidation processes. Appl. Catal. B 2016, 185, 344–352. [Google Scholar] [CrossRef] [Green Version]
- Bacardit, J.; Stötzner, J.; Chamarro, E.; Esplugas, S. Effect of salinity on the photo-Fenton process. Ind. Eng. Chem. Res. 2007, 46, 7615–7619. [Google Scholar] [CrossRef]
- Hermosilla, D.; Cortijo, M.; Huang, C.P. Optimizing the treatment of landfill leachate by conventional Fenton and photo-Fenton processes. Sci. Total Environ. 2009, 407, 3473–3481. [Google Scholar] [CrossRef]
- Ertugay, N.; Kocakaplan, N.; Malkoc, E. Investigation of pH Effect with Fenton-Like Oxidation using ZVI in Treatment of the Landfill Leachate. In Proceedings of the 16th International Symposium on Environmental Issues and Waste Management in Energy and Mineral Production, Istanbul, Turkey, 5–7 October 2016. [Google Scholar]
- Dia, O.; Drogui, P.; Buelna, G.; Dubé, R.; Ihsen, B.S. Electrocoagulation of bio-filtrated landfill leachate: Fractionation of organic matter and influence of anode materials. Chemosphere 2017, 168, 136–1141. [Google Scholar] [CrossRef]
- Gomes, A.I.; Santos, S.G.S.; Silva, T.F.C.V.; Boaventura, R.A.R.; Vilar, V.J.P. Treatment train for mature landfill leachates: Optimization studies. Sci. Total Environ. 2019, 673, 470–479. [Google Scholar] [CrossRef]
- Dogaris, I.; Ammar, E.; Philippidis, G.P. Prospects of integrating algae technologies into landfill leachate treatment. World J. Microbiol. Biotechnol. 2020, 36, 1–25. [Google Scholar] [CrossRef]
- Tejera, J.; Hermosilla, D.; Miranda, R.; Gascó, A.; Alonso, V.; Negro, C.; Blanco, A. Assessing an integral treatment for landfill leachate reverse osmosis concentrate. Catalysts 2020, 10, 1389. [Google Scholar] [CrossRef]
- Blanco, L.; Hermosilla, D.; Merayo, N.; Blanco, A. Assessing the use of zero-valent iron microspheres to catalyze Fenton treatment processes. J. Taiwan Inst. Chem. Eng. 2016, 69, 54–60. [Google Scholar] [CrossRef]
| Parameter (LL) | Value |
|---|---|
| pH | 8.2 ± 0.1 |
| Conductivity, mS cm–1 | 20.1 ± 0.8 |
| [Cl−], mg L–1 | 2800 ± 200 |
| UV-254, cm–1 | 60 ± 1 |
| Color, mg Pt L−1 | 19,600 ± 1200 |
| COD, mg O2 L−1 | 5025 ± 500 |
| BOD5, mg O2 L−1 | 50 ± 10 |
| BOD5/COD | 0.01 ± 0.01 |
| TOC, mg C L−1 | 1900 ± 50 |
| SUVA, L mg−1 m−1 | 3.2 ± 0.4 |
| TSS, mg L−1 | 1250 ± 50 |
| NH4+, mg L−1 | 1500 ± 100 |
| TNb, mg N L−1 | 1680 ± 50 |
| CC (pH = 5.0 + 2 g L−1 FeCl36H2O) + EC (pH = 2.4, 10 mA cm−2, 3 cm of Electrode Distance) | EC (pH = 5.0; 10 mA cm−2; 3 cm of Electrode Distance) + CC (pH = 6 + 1 g L−1 FeCl36H2O) | |
|---|---|---|
| pH | 6.4 ± 0.1 | 3.4 ± 0.1 |
| Conductivity mS cm−1 | 16.9 ± 0.8 (16%) * | 16.5 ± 0.8 (18%) |
| Color mg Pt L−1 | 5300 ± 500 (73%) | 4900 ± 500 (75%) |
| COD mg O2 L−1 | 1005 ± 90 (80%) | 854 ± 70 (83%) |
| SUVA L mg−1 m−1 | 2.3 ± 0.4 (27%) | 2.1 ± 0.5 (34%) |
| Fetotal mg L−1 | 100 ± 40 | 210 ± 30 |
| Raw LL | UVA-LED Photo-Fenton [H2O2]:COD = 2.125, 120 min | UVA-LED Photo-Fenton [H2O2]:COD = 1.063, 45 min | |
|---|---|---|---|
| pH | 8.1 ± 0.1 | 4.4 ± 0.1 | 3.9 ± 0.1 |
| Conductivity mS cm−1 | 20.1 ± 0.8 | 17.1 ± 0.8 (15%) * | 16.7 ± 0.8 (17%) |
| Color mg Pt L−1 | 19,600 ± 1200 | 90 ± 20 (99%) | 120 ± 30 (99%) |
| COD mg O2 L−1 | 5025 ± 500 | 290 ± 30 (94%) | 453 ± 50 (89%) |
| SUVA L mg−1 m−1 | 3.1 ± 0.4 | 1.0 ± 0.2 (67%) | 1.2 ± 0.3 (60%) |
| Fetotal mg L−1 | 4.5 ± 1 | 210 ± 30 | 210 ± 30 |
| BOD5/COD | 0.01 ± 0.01 | 0.40 ± 0.10 | 0.40 ± 0.10 |
| EC Costs | EC (120 min; pH = 5; 10 mA cm−2) | |
| H2SO4, EUR m−3 | 0.95 (7.3 g L−1) | |
| Iron electrode, EUR m−3 | 1.00 (2.0 g L−1) | |
| EC power consumption, EUR m−3 | 0.75 | |
| EC total cost, EUR m−3 | 2.70 | |
| EC total cost, EUR kg−1 of COD removed | 0.86 | |
| Conventional coagulation (CC) costs | CC (pH = 6; 1 g L−1 FeCl3∙6H2O) | |
| CC. Total cost, EUR m−3 | 0.20 | |
| CC. Total cost, EUR kg−1 of COD | 0.24 | |
| Oxidation costs | UVA-LED photo-Fenton [H2O2]:COD = 2.125, 120 min | UVA-LED photo-Fenton [H2O2]:COD = 1.063, 45 min |
| H2O2, EUR m−3 | 1.90 (1.81 g L−1) | 0.95 (0.91 g L−1) |
| Oxidation power consumption, EUR m−3 | 8.80 | 3.30 |
| Oxidation total cost, EUR m−3 | 10.70 | 4.25 |
| Oxidation total cost, EUR kg−1 of removed COD | 19.11 | 10.63 |
| Sludge management, EUR m−3 | 8.50 | 8.50 |
| Total process cost, EUR m−3 | 22.10 | 15.65 |
| Total process cost, EUR kg−1 of removed COD | 5.05 | 3.42 |
| Total Treatment Cost EUR kg−1 of Removed COD | Final COD mg O2 L−1 | |
|---|---|---|
| EC + CC + UVA-LED | 1.57 | 450 |
| CC + Hg 450 W photo-Fenton [11] | 1.56 | 826 |
| EC + UVA-LED photo-Fenton [30] | 3.48 | 1000 |
| EC + Conventional Fenton [30] UVA-LED photo-Fenton | 1.09 3.83 | 1000 2000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejera, J.; Hermosilla, D.; Gascó, A.; Negro, C.; Blanco, Á. Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment. Molecules 2021, 26, 6425. https://doi.org/10.3390/molecules26216425
Tejera J, Hermosilla D, Gascó A, Negro C, Blanco Á. Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment. Molecules. 2021; 26(21):6425. https://doi.org/10.3390/molecules26216425
Chicago/Turabian StyleTejera, Javier, Daphne Hermosilla, Antonio Gascó, Carlos Negro, and Ángeles Blanco. 2021. "Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment" Molecules 26, no. 21: 6425. https://doi.org/10.3390/molecules26216425
APA StyleTejera, J., Hermosilla, D., Gascó, A., Negro, C., & Blanco, Á. (2021). Combining Coagulation and Electrocoagulation with UVA-LED Photo-Fenton to Improve the Efficiency and Reduce the Cost of Mature Landfill Leachate Treatment. Molecules, 26(21), 6425. https://doi.org/10.3390/molecules26216425










