1. Introduction
The advantages of nuclear medicine methods have led to its sustainable development over the past few decades and its transformation into an integral part of clinical practice in developed countries. The active introduction of positron emission tomography (PET) and radionuclide therapy (RNT) in medical institutions, together with constantly developing new radiopharmaceuticals (RP), has made it possible to raise the possibilities of diagnostics and therapy in a variety of socially significant diseases to new levels.
68Ga (T
½ = 67.83 min., β
+—88.9%, E
βmax = 1899.1 keV [
1]), obtained from the
68Gе/
68Ga radionuclide generator, is the most actively used radiometal for synthesis of PET radiopharmaceuticals in the world today. For all its merits, its short physical half-life limits the available imaging period to just a few hours. PET scans at later times after injection (when the contrast of images increases due to biodistribution), as well as reliable dosimetry calculations and therapy planning (for long-lived therapeutic radionuclides, such as
177Lu) cannot be performed with gallium-68. In the light of these considerations, the interest of researchers in scandium-44 has increased [
2].
44Sc (T
½ = 3.97 h, β
+—94.3%, E
βmax = 1474.3 keV [
1]) has a physical half-life almost four times longer than
68Ga. This fact allows for imaging in a longer time interval (up to 24 h after administration), which increases the efficiency of lesion imaging by increasing the target to nontarget ratio. Clinical studies have demonstrated that PET/CT with [
44Sc]Sc-DOTATOC 4 h after injection allows the detection of tumor foci that were not visualized with [
68Ga]Ga-DOTATOC [
3]. The mean positron energy of
44Sc (Ē
β—0.63 MeV) is lower than that of
68Ga (Ē
β—0.83 MeV), which leads to better spatial image resolutions of
44Sc-PET [
4,
5,
6]. Additionally, the coordination chemistry of
44Sc is similar to that of
90Y and lanthanides, so it forms stable radioconjugates with a similar structures and, apparently, similar receptor affinities [
7]. Thus,
44Sc is a very promising radiometal with significant practical potential that may be used in preclinical and clinical PET studies, and most importantly, as a diagnostic match to therapeutical radionuclides of rare-earth elements (e.g.,
47Sc,
90Y,
153Sm,
161Tb,
177Lu) in a theragnostic approach [
8]. The results of clinical applications of [
44Sc]Sc-DOTATOC [
3] for the diagnosis of neuroendocrine tumors, as well as [
44Sc]Sc-PSMA-617 [
9,
10] for the diagnosis of prostate cancer have been published. It is hypothesized, however, that the clinical application of
44Sc may be compromised by the high dose exposure caused by the co-emission of 1157 keV γ-rays (99.9%) [
11]. These concerns of radiation safety may be addressed by using tungsten-based containers that are employed for other commercial PET nuclides with high energy γ-emission (such as
89Zr). On the other hand, this γ-co-emission of
44Sc could be relevant to a new imaging approach based on the three-dimensional measurement of the emitter location using β
+/γ emitters [
8,
12].
44Sc can be obtained by both using a cyclotron from a calcium target [
13,
14,
15] and using a radionuclide generator from the parent radionuclide
44Ti (T
½ = 60.0 a [
1]). Regarding the potentially wide clinical application of
44Sc (up to the current level of
68Ga use), the cyclotron method of production is preferable, considering the cross sections and yields of
44Ca(p,n)
44Sc and
45Sc(p,2n)
44Ti→
44Sc reactions [
16]. At the same time, a
44Ti/
44Sc generator could be a useful tool for universities and research centers wishing to start working with
44Sc but without a cyclotron of their own, and/or those who are far enough from suitable accelerators. Based on the ratio of the decay constants of
44Ti (λ
1 = 3.66 × 10
−10·s
−1) and
44Sc (λ
2 = 4.85 × 10
−5·s
−1), the maximum time required to reach the secular equilibrium in this pair is ∼67.5 h. At the same time, after 4 h, the level of accumulation of the daughter radionuclide is already 50%, after 12 h—87.7%, after 24 h—98.5%, and after 40 h—99.9 %. Thus, in the presence of a sufficiently effective separation method for
44Ti and
44Sc, characterized by low
44Ti breakthrough and a high
44Sc yield, the separation of the daughter radionuclide for research purposes can be carried out daily. The half-life of
44Ti, in turn, provides, in theory, an extremely long possible generator operation period.
The earliest published (and probably the first) work about
44Ti/
44Sc generators belongs to Margaret Greene [
17], who, based on data on the anion-exchange separation of scandium, vanadium, and titanium [
18], suggested using Dowex 1×8 resin as a sorbent for
44Ti and a mixture of 0.1 M oxalic acid in 0.2 M HCl for
44Sc elution. The yield of
44Sc was 60–70%, while the breakthrough of titanium-44 was at the level of 0.02% and increased to 0.1% after 40 elutions. Later, different prototypes of
44Ti/
44Sc generators were described using hydrous zirconium oxide [
19], Bio-Rad AG 1×8 anion-exchange resin [
20], and ZR hydroxamate resin [
21] as a sorbent (see Discussion). The generator presented by Filosofov et al. [
20] has the highest load of titanium-44 to date (185 MBq). The yield of
44Sc is 97% in 20 mL of eluate, which is a mixture of 0.005 M oxalic acid/0.07 M HCl (preconcentration of activity for further radiolabeling is required), and for the stability of the sorption front of titanium-44 it is necessary to use reverse elution. This generator was used for the first in-human study of [
44Sc]Sc-PSMA-617 for PET imaging of metastasized castrate-resistant prostate cancer [
10].
This article is devoted to the evaluation of a
44Ti/
44Sc generator prototype based on Greene’s concept [
17], but using TEVA resin (
TEtra
Valent
Actinides; quaternary ammonium salt, also called Aliquat
® 336 as extractant) [
22,
23]. Evaluation of suitability of the obtained scandium-44 solutions for radiolabeling was carried out using PSMA-617 and PSMA-I&T precursors (
Figure S1).
2. Results
Two generator columns (PEEK 100 mm × Ø2.1 mm, 150 mg of TEVA resin) with an initial load of 0.5 and 5.0 MBq
44Ti were prepared for this study and used throughout the work. During the study, several additional generator columns with ∼1.5 MBq
44Ti were prepared. They were used to evaluate and optimize the separation parameters of
44Ti and
44Sc in dynamic conditions, as well as to collect data for statistics, but they have not been operated for a long time. This work was started with one tentative experiment as proof-of-concept for Greene’s method [
17] with 0.1 M H
2C
2O
4/0.2 M HCl as eluent but with TEVA resin instead of Dowex 1 × 8 as sorbent. The very first generator prototype with an initial
44Ti load of 0.5 MBq demonstrated 90%
44Sc yield and ≤5.5 × 10
−5% of
44Ti breakthrough. The results obtained made it possible to continue further work, starting with the determination of the distribution coefficients of
44Ti and
44Sc on TEVA resin.
2.1. Distribution Coefficients of 44Ti and 44Sc on TEVA Resin in HCl-Oxalic Acid Media
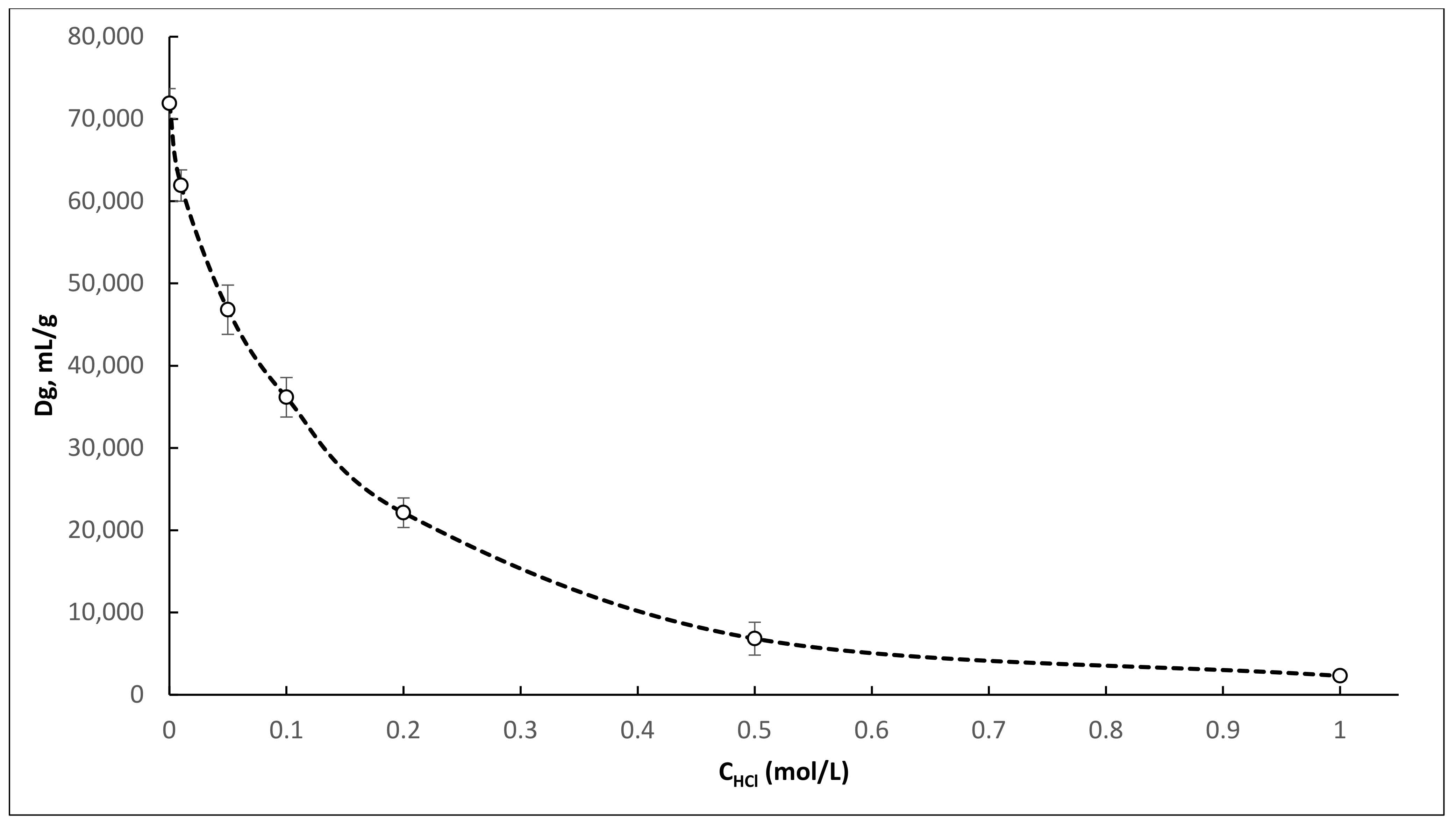
Results of the Dg values obtained for
44Ti and
44Sc on TEVA resin in HCl-oxalic acid media are shown in
Table 1. Since we took the Greene’s concept as a basis, attention was mainly paid to the oxalic acid concentration of 0.1 M and various concentrations of hydrochloric acid. The determination of the distribution coefficients of
44Ti and
44Sc in pure 4 M HCl was carried out to assess the possibility of
44Ti recovery from the resin. Blends of 0.005 M H
2C
2O
4/0.007 M HCl and 0.025 M H
2C
2O
4/0.125 M HCl presented by Filosofov et al. [
20] have been added for comparison.
Obtained distribution coefficients for
44Ti on TEVA resin in a mixture of 0.1 M oxalic acid with 0.01–1.0 M HCl (
Figure 1) are significantly higher than those presented by McAlister [
24] for Ti on TEVA resin in a mixture of 0.05 M H
2C
2O
4/0.01–1.0 M HCl. However, the obtained dependences have a similar form and correlate well with each other. Based on the obtained Dg values, the mixtures of 0.1 M H
2C
2O
4/0.05–0.2 M HCl, and 0.025 M H
2C
2O
4/0.125 M HCl are the most suitable for the separation of
44Ti and
44Sc. These mixtures were selected for further study under dynamic conditions.
2.2. Dynamic Column Separation Experiments
The results of
44Ti and
44Sc separation under dynamic conditions for the selected eluents are presented in
Table 2 (a mixture of 0.005 M H
2C
2O
4/0.007 M HCl was also added for comparison). For each selected composition, a separate column with a load of ∼1.5 MBq
44Ti was used.
Unlike the Bio-Rad AG 1 × 8 anion exchange resin [
20], in the case of TEVA resin, mixtures of 0.005 M H
2C
2O
4/0.007 M HCl and 0.025 M H
2C
2O
4/0.125 M HCl did not show satisfactory scandium-44 yield (despite the good Dg values obtained under static conditions). At the same time, eluents with compositions of 0.1 M H
2C
2O
4/0.05–0.2 M HCl made it possible to achieve a sufficiently high yield of
44Sc. The breakthrough of
44Ti was about ≤5.5 × 10
−5% when those compositions of eluent were used (except the very first elution directly after column loading). This corresponds to an exceptionally good separation factor of ≥1.8 × 10
6.
Using pure 4.0 M HCl as eluent, almost quantitative (≥ 99 %) recovery of 44Ti from TEVA resin can be achieved (in 0.5 mL of eluate). No statistically significant difference in desorption rate of 44Ti from the columns that have been in use for 1 to 11 months was observed. This fact facilitating the regeneration and loading of new generator columns is also noteworthy.
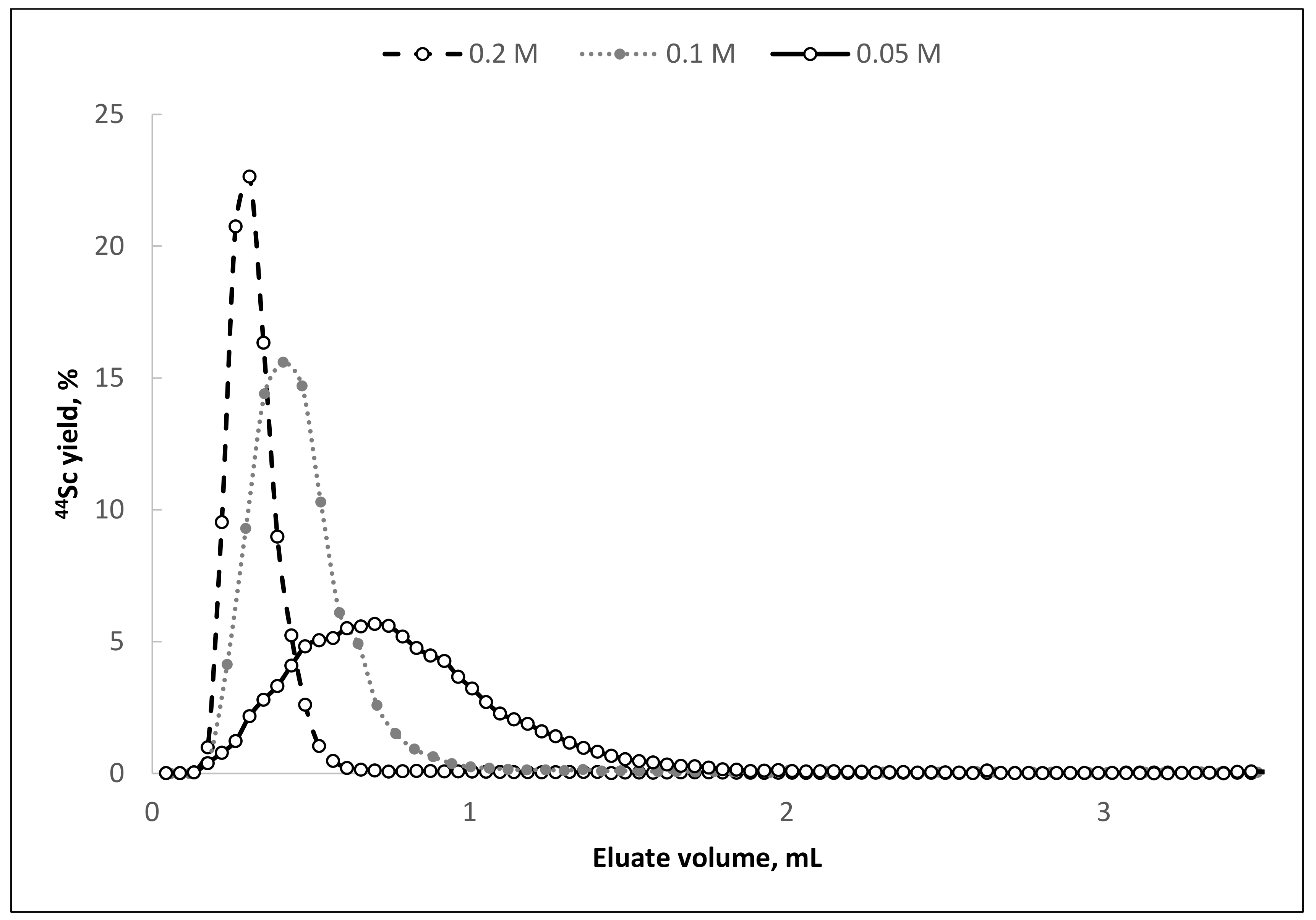
All three investigated HCl concentrations with 0.1 M oxalic acid content are attractive for further study. However, based on the elution profiles of the generator columns with different concentrations of hydrochloric acid (
Figure 2), the mixture 0.1 M H
2C
2O
4/0.2 M HCl (giving the highest average value of scandium-44 yield in the smallest eluate volume) was chosen to continue the study. This choice was made with the assumption that if, during long-term evaluation the behavior of titanium-44 will be satisfactory, then for eluents with a lower content of hydrochloric acid, the behavior of titanium-44 will be the same or better.
2.3. Elution Characteristics of TEVA-Based 44Ti/44Sc Generator Prototype (Long-Term Evaluation)
The 5.0 MBq TEVA-based
44Ti/
44Sc generator prototype was used for long-term evaluation of its elution characteristics. The typical elution profile of
44Sc (as well as content of
44Ti in eluate fractions) are presented in
Figure 3.
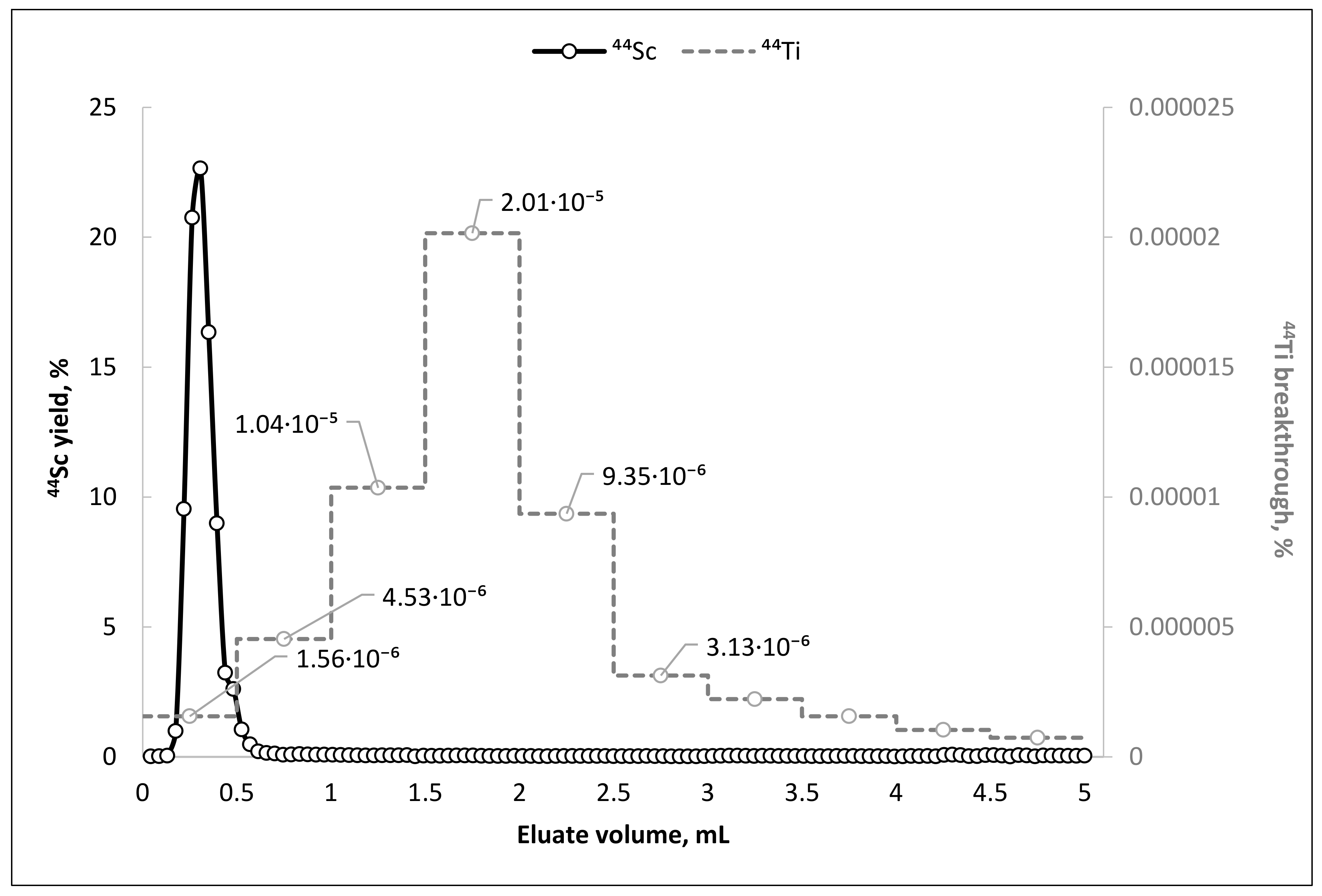
The experimental data show that, as in the case of pilot dynamic column separation experiments, elution of the main 44Sc activity (≥95% of all eluted activity) occurs in the first 0.5 mL. The 44Ti breakthrough strongly depends on the eluate volume and has a pronounced maximum (∼2 × 10−5%) at 1.5–2.0 mL fraction. This feature, in combination with the ability to elute scandium-44 in the first 1.0 mL of the eluate, makes it possible to achieve an extremely high separation factor of 1.6 × 107.
During 1 year of regular
44Sc elutions (at least 3 times a week, no more than 1 time a day), the
44Sc yield was 91 ± 6%, and the breakthrough of titanium-44 did not exceed 1.5 × 10
−5% (
Figure 4). The TEVA-based
44Ti/
44Sc generator prototype had consistently high yields of
44Sc with no significant decreases with an increasing number of elutions. The
44Sc yield SD of 6% is due to the fact that elution was performed manually using a syringe and cannula, which, in turn, led to deviations in the eluent flow rate (decreases in the
44Sc yield are due to slightly faster elution rates; this aspect was subsequently fixed by the use of a syringe pump).
Only the very first eluate obtained directly after column loading contained relatively high amounts of 44Ti (3.5 × 10−4%). 44Ti breakthrough of 1.5 × 10−4% was obtained as soon as within the second elution. After 10 elutions, no more than 1.5 × 10−5% of 44Ti breakthrough was detected in the eluate. It is noteworthy that unlike other published 44Ti/44Sc generator prototypes (in the “direct” elution mode), there was a tendency towards decreasing the average value of 44Ti breakthrough for TEVA resin.
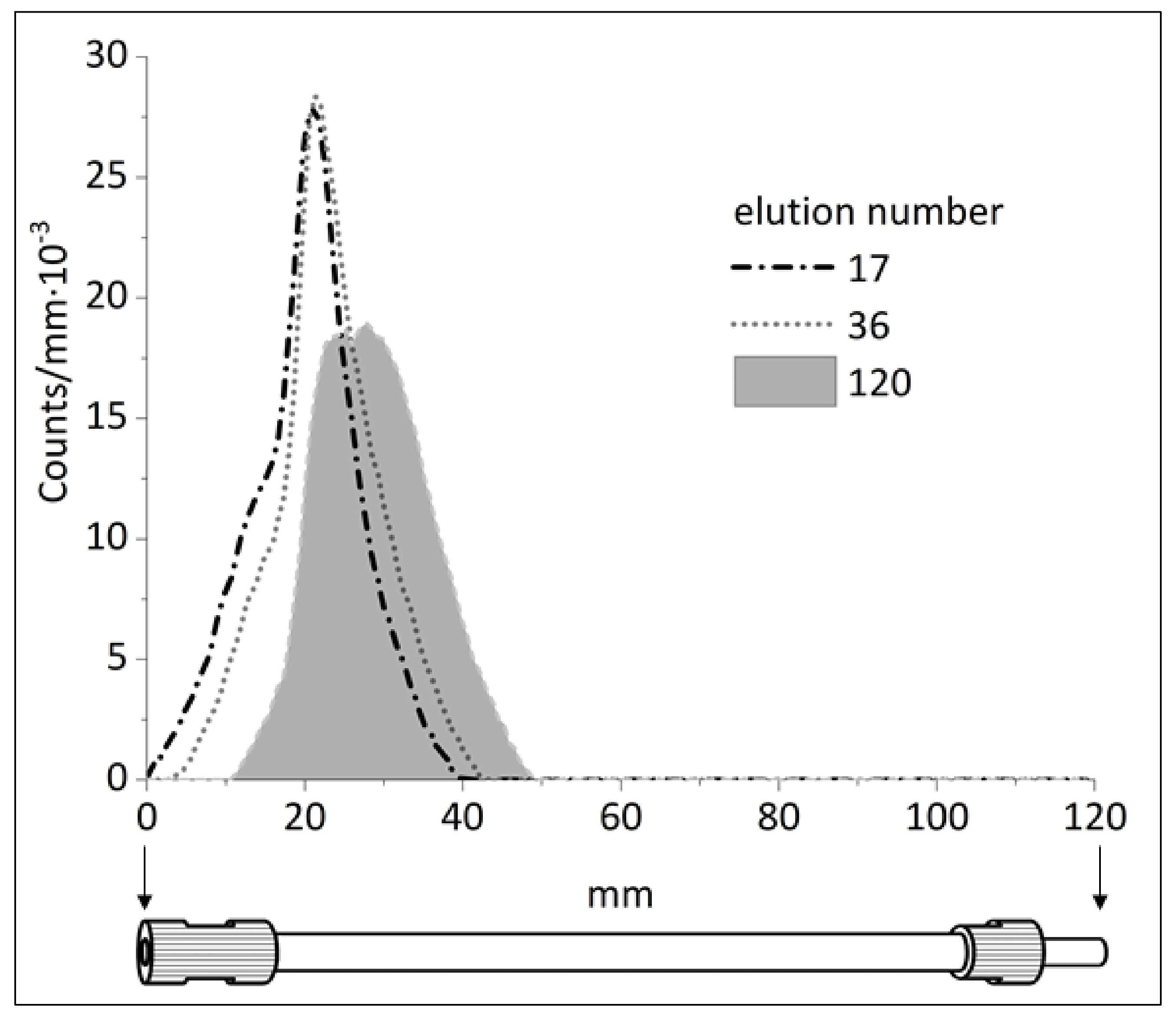
The autoradiography image of the
44Ti/
44Sc generator column after 120 elutions showed main activity disposition in the top of the column (
Figure S2), i.e., no dramatic spreading and blurring of
44Ti sorption zone in the column was observed for 1 year of testing.
The results of regular radiometry of the generator column demonstrates that some drifting of
44Ti sorption zone is nonetheless observed (
Figure 5). Аfter 120 elutions, the maximum activity of the
44Ti zone shifted by almost 7 mm. Furthermore, after 120 elutions the full width at half maximum of the
44Ti zone increased from 13 to 19 mm.
Given that the column was eluted continuously only in direct mode, this shift of the 44Ti sorption zone still corresponds to good 44Ti retention on the resin. Based on the data on 44Sc elution yield and 44Ti breakthrough, as well as the absence of significant changes in the elution profile, the shift of 44Ti zone did not have any negative effect on the basic characteristics of the generator. The column is currently being monitored to establish the critical elution number after which the column will need to be regenerated.
2.4. Post-Elution Processing of Generator Eluate for Radiolabeling
For successful application in the field of radiopharmaceutical R&D, a generator should not only give satisfactory results in the separation of the parent and daughter radionuclides, but the resulting solution of the daughter radionuclide should also be suitable for the synthesis of RP with a high yield. The TEVA-based 44Ti/44Sc generator prototype has exceptionally good separation characteristics, but unfortunately its eluate is not suitable for direct use in radiopharmaceutical synthesis: after incubation of buffered eluate (pH 4.5, 1 mL) for 30 min at 95 °C with 50 nmol of PSMA-617 or PSMA-I&T precursors, no complex ([44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T) formation was observed. The high concentration of oxalic acid in the eluate interferes with the incorporation of scandium-44 into the structure of chelator-conjugated vector molecules. Thus, post-processing of the eluate is necessary for further use in radiopharmaceutical synthesis.
2.4.1. Decomposition (Decarboxylation) of Oxalic Acid with H2O2 Followed by Evaporation
One of the classical approaches to remove oxalate from metal solutions, including solutions of n.c.a. metal radionuclides, is decarboxylation using hydrogen peroxide [
25,
26]. This method was successfully used, for example, in the case of
89Zr solutions [
27], and was also proposed by Green in her work on the
44Sc generator [
17].
After two evaporations of TEVA-based 44Ti/44Sc generator eluate (1 mL) with 500 μL of 30% H2O2, 44Sc was dissolved in 100 μL of 1.0 M HCl and then diluted to 1.0 mL with water (0.1 M HCl in final solution). As a result of this procedure, the concentration of residual oxalic acid in the final solution was 0.0001–0.0003 mol/L. The 44Sc recovery was about 87 ± 3%.
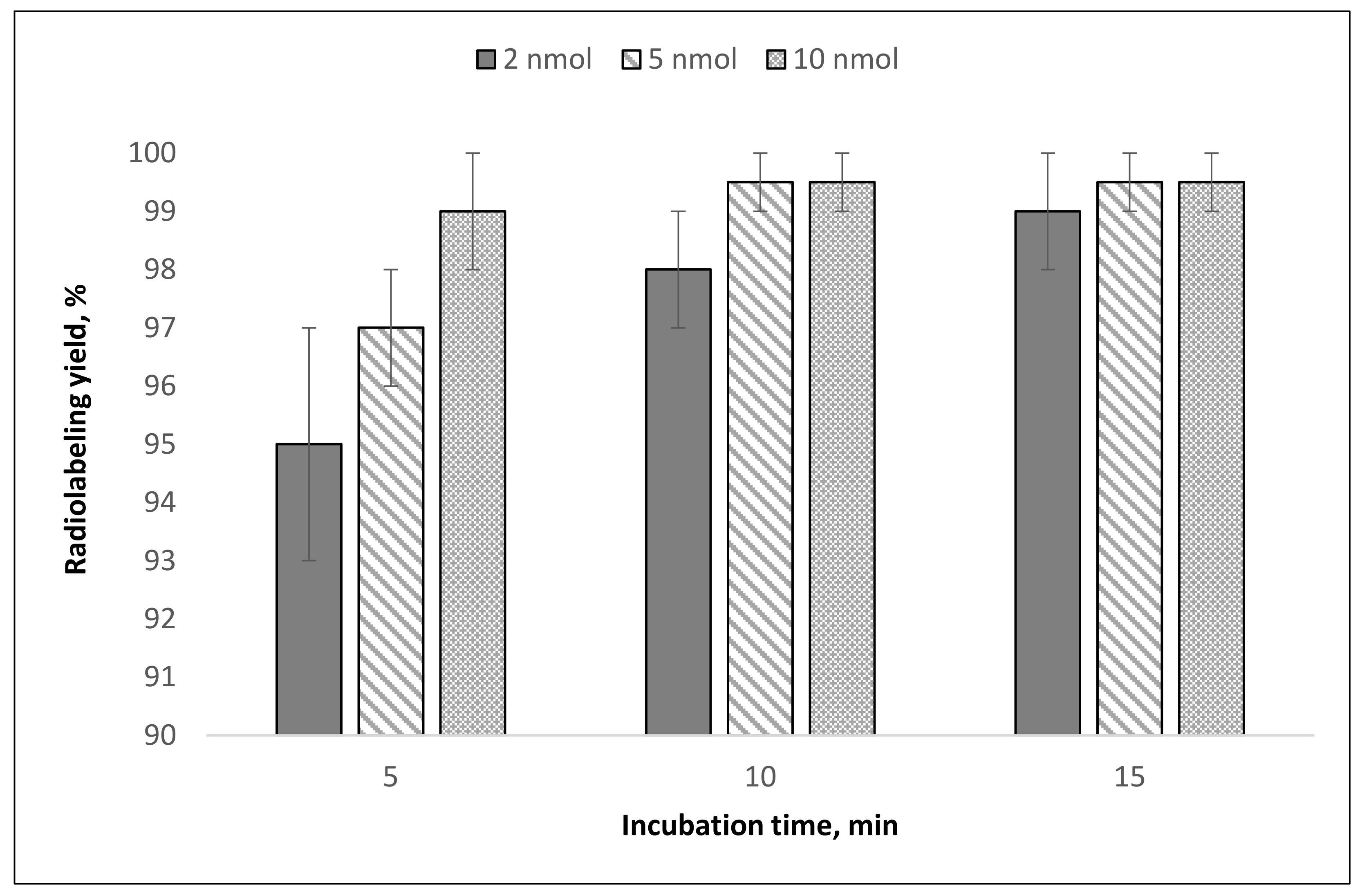
The results of [
44Sc]Sc-PSMA-617 synthesis demonstrates that even with 2 nmol of precursor, a radiolabeling yield of >95% can be achieved within 15 min (
Figure 6).
Using these solutions of
44Sc spiked with oxalic acid, the maximum permissible concentration of oxalic acid for sufficient radiolabeling yield was determined (
Figure 7). According to experimental data, for a radiolabeling yield of [
44Sc]Sc-PSMA-617 higher than 95%, the content of oxalate anion in reaction mixture should be ≤0.001 mol/L. Even though the decarboxylation allows us to achieve good
44Sc-radiolabeling yields (it was also successfully used in a study on the complexation of
44Sc with oxabis(ethylenenitrilo)tetramethylenephosphonic acid [
28]) this approach is not very technological and not very convenient for automatization and implementation in a synthesis module. It was decided to replace this approach with SPE chromatographic methods.
2.4.2. Presep® PolyChelate Resin
Various ion-exchange- and SPE-resins have been tested in search of one suitable for efficient trapping of
44Sc directly from the generator eluate (
Table 3). Unfortunately, none of them showed satisfactory retention of scandium-44 in this case.
Considering the published results on the successful extraction of n.c.a. scandium radionuclides (
43,44,47Sc) using chelating resins Chelex 100 [
13,
29,
30,
31] and NOBIAS Chelate-PA1 [
32], as well as our results on the efficient isolation of medical radionuclides from oxalate solutions with Chelex 100 (e.g.,
89Zr [
33]), this type of resin has also been tested for the recovery of scandium-44 from the generator eluate.
Unfortunately, it turned out that Chelex 100 (iminodiacetic acid) is not capable of retaining scandium-44 from the generator eluate with sufficient efficiency (
Table 4). NOBIAS Chelate-PA1 resin (iminodiacetic acid and ethylenediaminetriacetic acid) was not available for use. However, fortunately, we had a different resin at our disposal—Presep
® PolyChelate, а chelate resin immobilizing carboxymethylated pentaethylenehexamine [
34,
35]. Published data suggests that its performance is comparable to those of Nobias Chelate-PA1 and superior to those of some other commercially available aminocarboxilyc acid-type chelating resins [
36,
37].
Experiments under static conditions have shown that 44Sc distribution coefficient for this resin is: in 0.1 M HCl—3.8 ± 1.1 mL/g; in 0.2 M HCl—1.9 ± 0.7 mL/g; and for mixture of 0.1 M oxalic acid / 0.2 M HCl—>3 × 104 mL/g.
Experiments under dynamic conditions (80 mg of the resin, column 50 mm × Ø2 mm, 1 mL/min) showed that 44Sc from the generator eluate is quantitatively trapped on this resin (sorption rate is ≥99%). To remove the residues of the sorption solution, the resin could be washed with both water and ethanol (1–5 mL). In both cases, the loss of activity during washing did not exceed 1%.
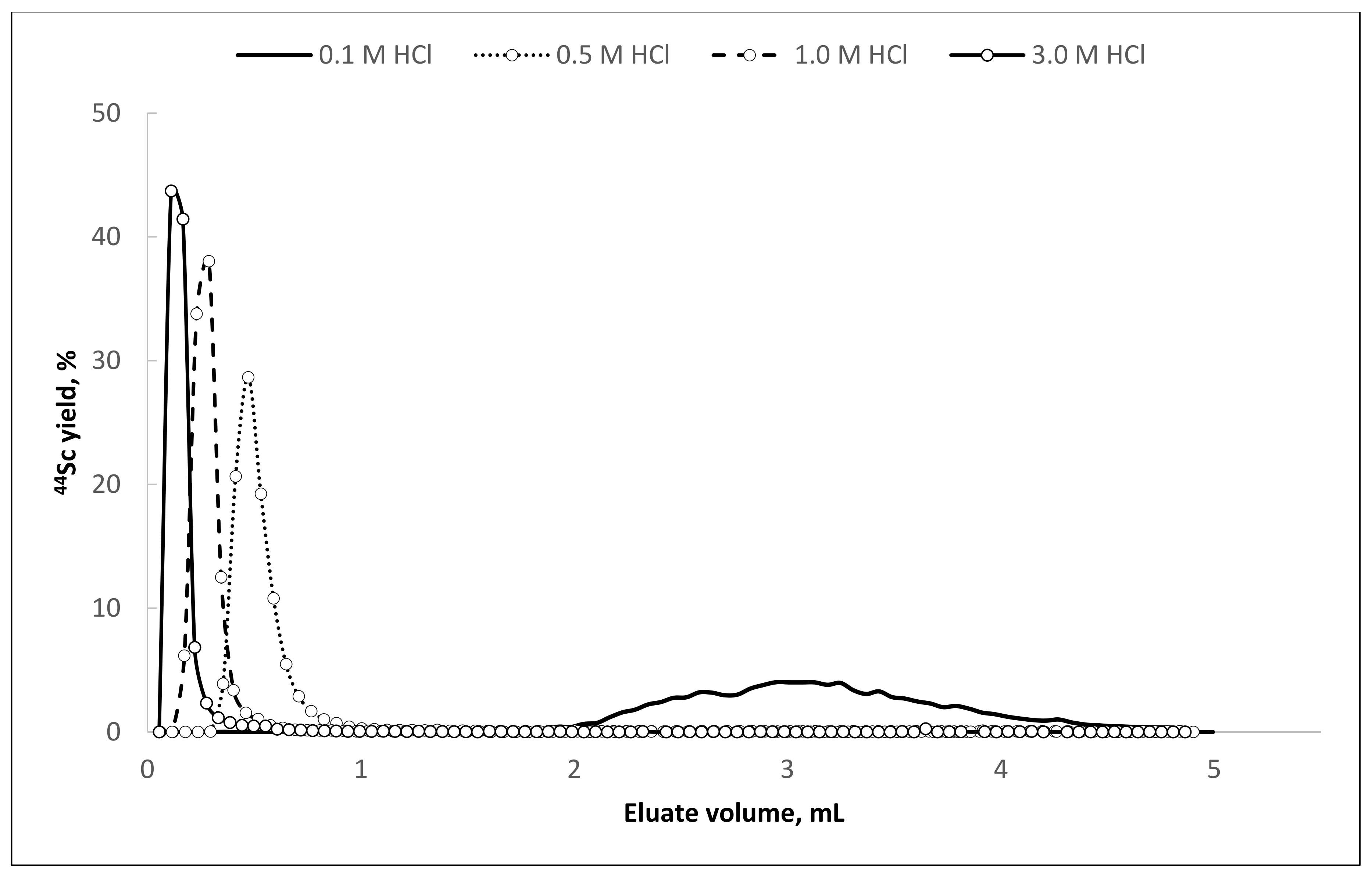
It is noteworthy that
44Sc desorption can be performed using hydrochloric acid in the concentration range from 0.1 to 3.0 mol/L. With an increase of hydrochloric acid concentration in the eluent,
44Sc recovery rate increases, and the required volume of the eluate decreases (
Table 5,
Figure 8).
Experimental data show that Presep
® PolyChelate resin is very promising for recovery of
44Sc from oxalate media, e.g., from TEVA-based
44Ti/
44Sc generator eluate. At the same time, an unexpected fact was discovered: relatively high amounts of oxalic acid still remain present in the obtained
44Sc solutions. This amount is equal to the maximum permissible concentration of oxalic acid for sufficient radiolabeling and even exceeds it. Increased volumes of water and ethanol used for resin washing (till 10–15 mL) did not lead to significant improvements in residual oxalic acid content. The highest yield of [
44Sc]Sc-PSMA-617 synthesis with
44Sc in 0.1 M HCl after Presep
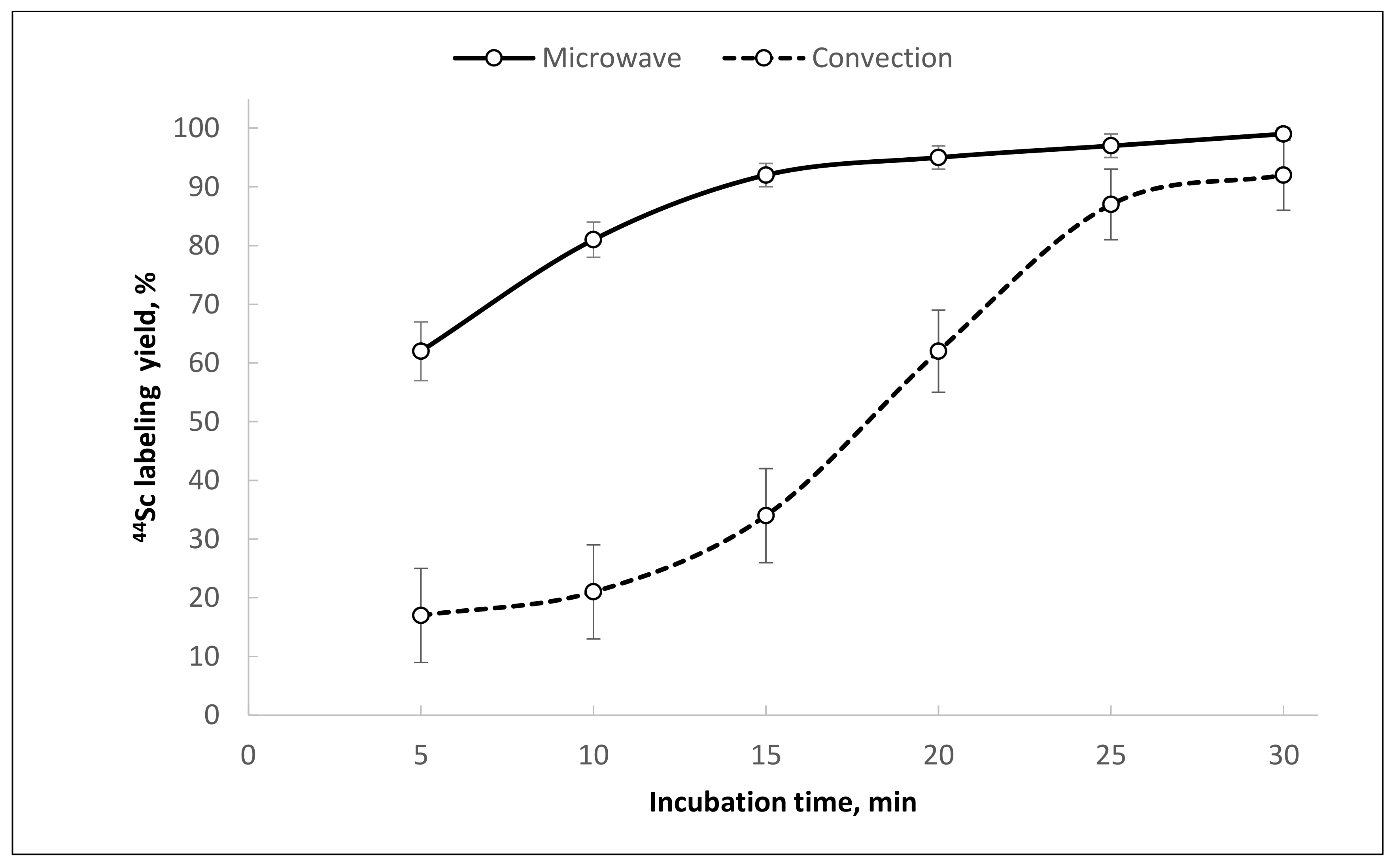
® PolyChelate resin was about 92% (95 °C, 30 min, V = 1.0 mL, pH = 4.5, 20 nmol of PSMA-617). However, the average radiolabeling reaction yield at these conditions was only 75 ± 10%. It was found that microwave heating could significantly improve the radiolabeling reaction yield in this case (
Figure 9).
The use of microwave heating allowed a consistently high [
44Sc]Sc-PSMA-617 synthesis yield (>95%) to be achieved in comparison to convective heating and stirring. This effect has already been demonstrated with
68Ga during synthesis of [
68Ga]Ga-DOTATOC [
38]. The kinetics of [
44Sc]Sc-PSMA-617 formation indicate that even under microwave heating, the reaction is hampered and takes at least 25 min to reach sufficient yield (
Figure 10).
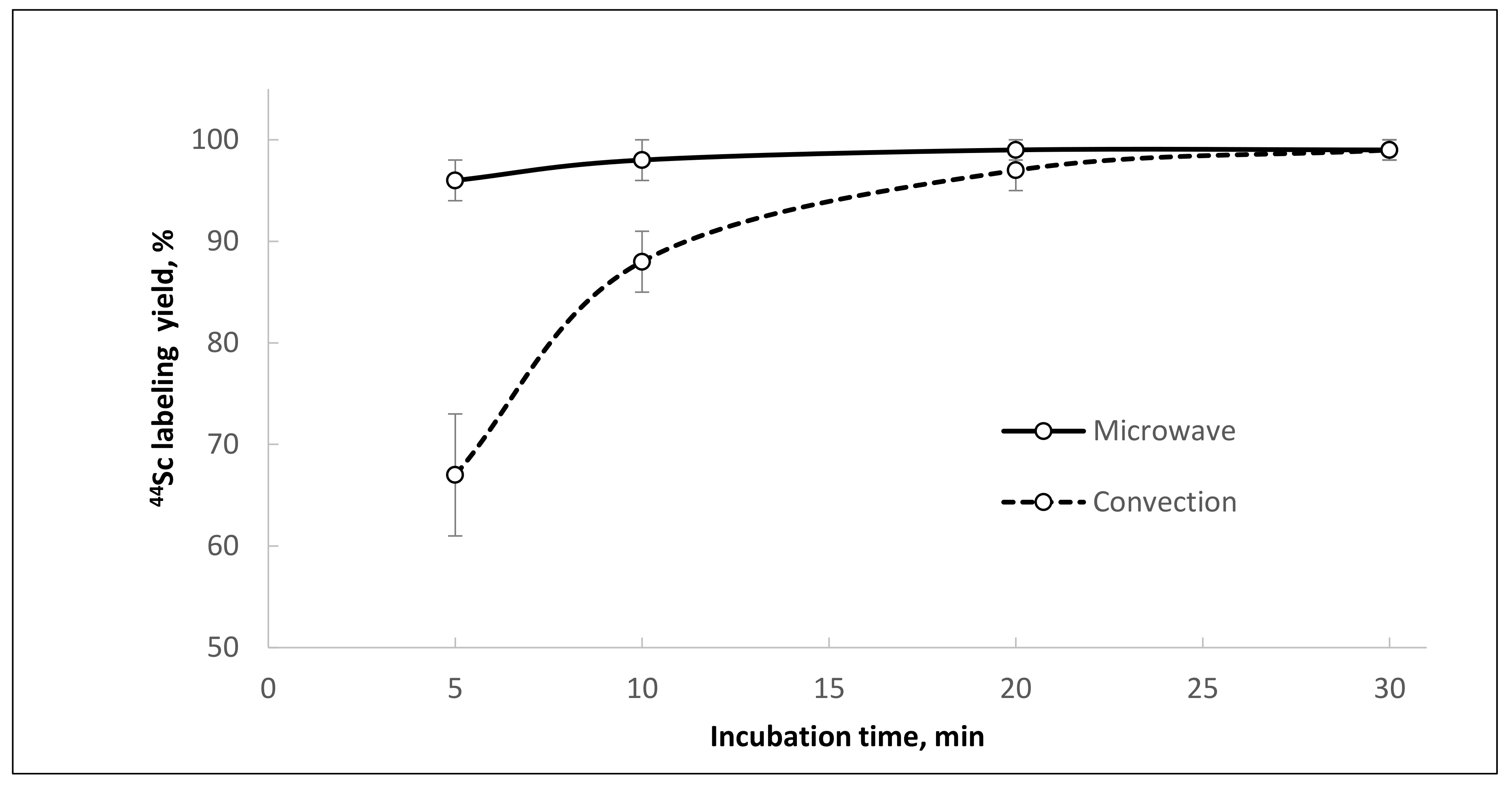
Of particular interest is the fact that synthesis of [
44Sc]Sc-PSMA-I&T under similar conditions (after reformulation of
44Sc with Presep
® PolyChelate into 0.1 M HCl, 95 °C, 30 min, V = 1.0 mL, pH = 4.5) gives consistently high yields (≥95%) with convective heating. The chelating agent DOTAGA (used in the structure of PSMA-I&T) appears to be less sensitive to the presence of chemical impurities compared to DOTA (used in the structure of PSMA-617) and much more readily forms complex with scandium-44 (
Table 6).
Using
44Sc after reformulation with Presep
® PolyChelate into 0.1 M HCl and microwave heating for synthesis of [
44Sc]Sc-PSMA-I&T achieved a labeling yield of >95% in only five minutes (with 5 nmol of precursor), and in 20 min with convective heating and stirring (
Figure 11).
Since [44Sc]Sc-PSMA-I&T synthesis is less dependent on 44Sc solution quality, [44Sc]Sc-PSMA-617 labelling yield was further used to indicate its suitability. In this context, the solution obtained using Presep® PolyChelate resin is much inferior to those obtained after decarboxylation. So, in this way an additional processing stage is required.
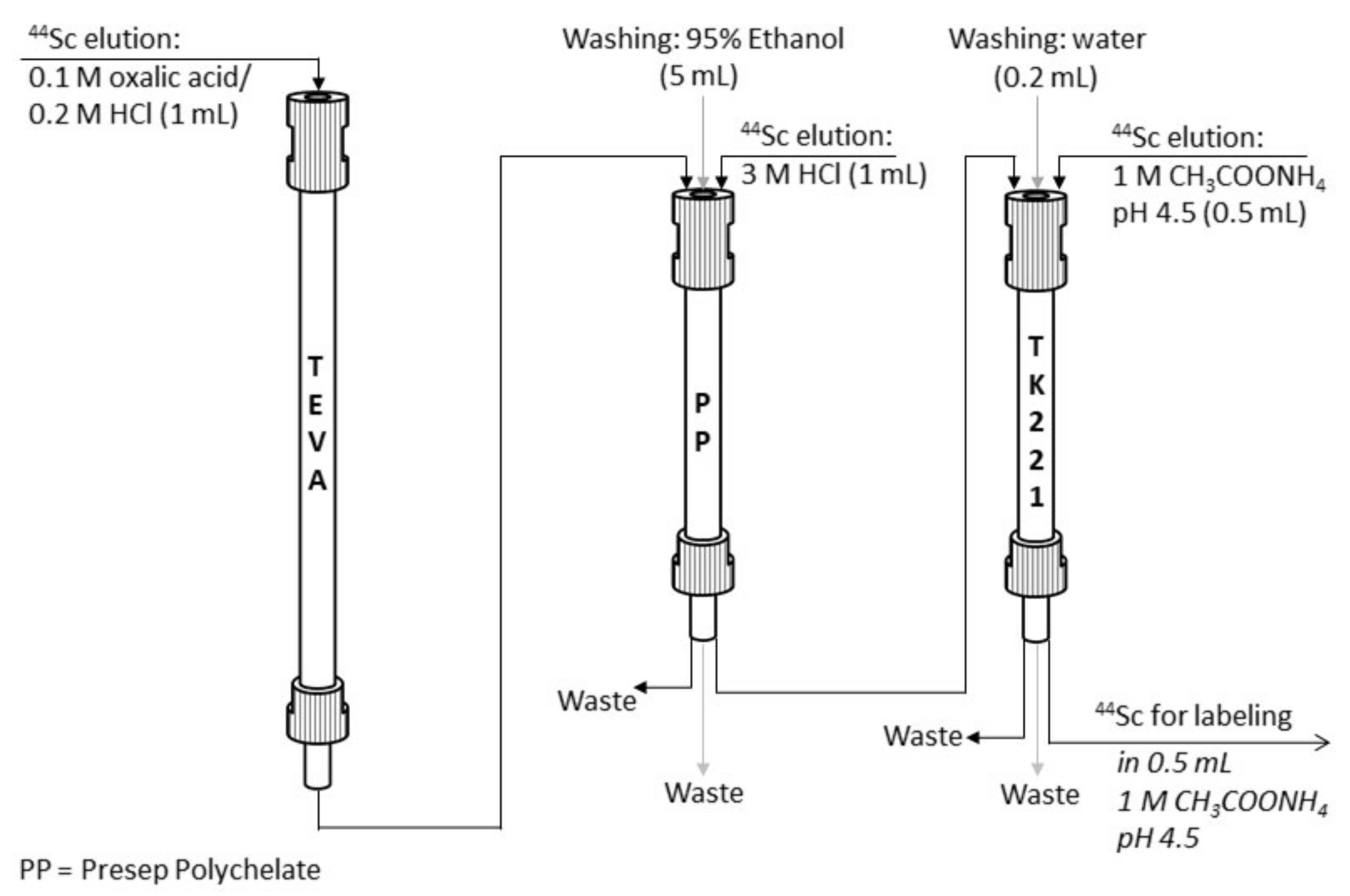
2.4.3. Additional Reformulation Stage (Presep® PolyChelate Resin + TK221 Resin)
The fact that
44Sc desorption from Presep
® PolyChelate resin can be carried out with hydrochloric acid of different concentrations (0.1–3.0 mol/L) opens a wide range of possible options for an additional purification stage, which may be either a cation exchange resin or a solid-phase extractant. In the course of this work, it was decided to test TK221 resin for this purpose [
39,
40].
Experiments under static conditions have shown that 44Sc distribution coefficients for TK221 resin in HCl media are as follows (mL/g): 0.01 M—24 ± 3, 0.1 M—33 ± 7, 0.5 M—41 ± 4, 1.0 M—61 ± 8, 2.0 M—1300 ± 200, 3.0 M—>1.5 × 104. Experiments under dynamic conditions (80 mg of the resin, column 50 mm × Ø2 mm, 1 mL/min) showed that 44Sc in 3.0 M HCl after Presep® PolyChelate resin is quantitatively trapped by TK221 resin (sorption rate is ≥99%).
As for desorption of scandium from TK221 resin it is necessary to use eluents with reduced acidity (mainly dilute acids according to the manufacturers data), several different eluents were investigated during this work (
Table 7).
The highest
44Sc recovery was achieved with 1 M CH
3COONH
4 (0.5 mL, pH 4.5) after column flushing with a small (0.2 mL = 8 column bed volumes) volume of water. The characteristics of the final
44Sc solution are suitable for direct use in radioconjugate synthesis. The results of [
44Sc]Sc-PSMA-617 synthesis demonstrate that a high radiolabeling yield (≥95%) can be achieved with 5 nmol of precursor after 30 min of incubation at 95 °C (V = 1.0 mL, pH = 4.5), and increasing the amount of PSMA-617 increased radiochemical yield (
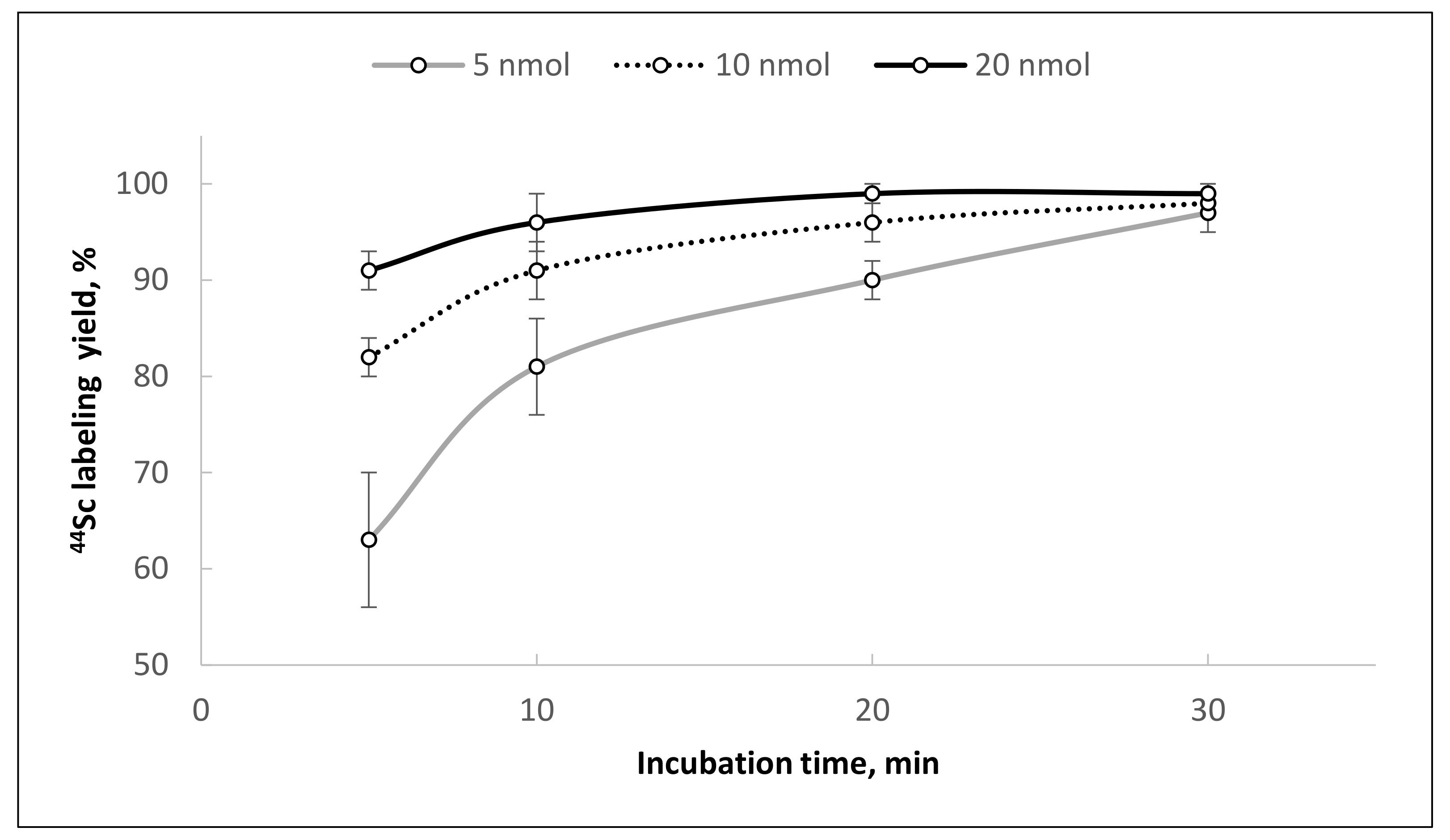
Figure 12).
The obtained data indicate that
44Sc solutions obtained by Presep
® PolyChelate + TK221 reformulation are suitable for synthesis of
44Sc-radioconjugates with sufficient yields. No
44Ti was detected in all samples of
44Sc solutions after combined post-processing. Thus, at this stage of our research, we can suggest that this method of TEVA-based
44Ti/
44Sc generator eluate post-processing is preferable for
44Sc-radiopharmaceutical synthesis (
Figure 13). The proposed method is simple to perform; it takes no more than 15 min (in manual mode), and the decay-corrected yield is ≥90% relative to the activity eluted from the generator.
3. Discussion
Today
44Sc seems to be an attractive and very promising radionuclide for small-molecule-based PET imaging. Growing interest in scandium-44 motivated research on different methods of its production, of course, mainly by using cyclotrons. However, development of the
44Ti/
44Sc generator concept can be characterized by significant progress in recent years as well (
Table 8).
After the first research by Greene [
17], a 5 mCi
44Ti/
44Sc radionuclide generator presented by Filosofov et al. [
20], which was used in the first in-human use of [
44Sc]Sc-PSMA-617 [
10], became a real breakthrough in this area. An advanced generator concept involving alternating directions of elution flow, also proposed by Filosofov et al. [
20], was an impressive solution. Only due to this, the characteristics of the generator reached the required values for further use. In the direct elution mode, the (0.1 M H
2C
2O
4/0.2 M HCl) Bio-Rad AG 1 × 8 based generator results in increasing breakthrough of
44Ti with 50% desorption of
44Ti after 30 elutions, and an almost complete release of
44Ti after 50 elutions. In contrast to this, the TEVA-based
44Ti/
44Sc generator, evaluated in this work, demonstrates high titanium-44 retention over 120 elutions with high
44Sc yield in small volumes and exceptionally low
44Ti breakthrough. Some drifting of
44Ti sorption zone is nonetheless observed. This
44Ti shift did not have any negative effect on the main characteristics of the generator so far. The generator column is currently being monitored.
Based on the experimental data under static and dynamic conditions obtained during this work, we believe that the use of increased column length, as well as an eluent with a lower hydrochloric acid concentration (0.1 M H2C2O4/0.1–0.05 M HCl instead of 0.1 M H2C2O4/0.2 M HCl) will significantly extend the generator’s shelf-life. These studies are being carried out by our team at the moment. Meanwhile, it is proposed that obtained separation characteristics for the TEVA-based 44Ti/44Sc generator demonstrate its ease of use and prospects for further work.
The investigation of described 5 MBq generator prototype may be considered as proof-of-concept for a TEVA-based 44Ti/44Sc generator. The radiolitic stability of TEVA and, therefore, the possibility to scale up 44Ti activity in the generator to at least 200 MBq remains to be addressed.
Considering all the difficulties associated with the presence of oxalic acid in the eluate and its negative effect on the radioconjugation efficiency (established in the course of this study), the
44Ti/
44Sc generator proposed by Radchenko et al. [
21] seems to be the most convenient for radiopharmaceutical purposes (since the eluent is 0.05 M HCl). At the same time, a ZR-based generator also requires alternating directions of elution flow, and
44Ti breakthrough in reverse mode exceeded that for Bio-Rad AG 1×8 and TEVA resins (although this was likely due to an off-center
44Ti loading activity placement). In only the direct elution mode did
44Ti breakthrough reach a value of 0.23% after ∼40 resin bed volumes. Thus, if we compare only the data for direct elution modes, TEVA-based generators provide the best retention of
44Ti. To our best knowledge, unfortunately, no additional studies have appeared with ZR-based
44Ti/
44Sc generator so far. Additional comparison of ZR- and TEVA-based generators is also in our plans.
Radiolabeling experiments show that scandium-44 obtained from the generator is suitable for the synthesis of radiopharmaceuticals with high yield only after some post-processing because of the oxalic acid content. Apart from the problems with residual oxalate, a lucky find was that Presep
® PolyChelate resin captures scandium-44 quantitatively from the TEVA-based generator eluate. This resin looks very promising for recovery of
44Sc from oxalate solutions or similar media. The combination of Presep
® PolyChelate and TK221 resins made it possible to obtain
44Sc-radioconjugates with a high yield (≥95%), even with 5 nmol of precursor. Since
44Sc desorption from Presep
® PolyChelate resin can be carried out with hydrochloric acid of different concentrations, there is still a lot of room for improvement. For example, in addition to a variety of extraction resins, a cation exchange resin could be used if scandium-44 elution from Presep
® PolyChelate were performed with 0.1 M HCl. Or, finally, the eluate of the generator could be easily diluted to make scandium-44 trapping by cation exchange resin possible (by analogy with an already published method [
41]).
Microwave heating of the reaction mixture considerably shortened the reaction time and improved radiolabeling yield and reproducibility. For [
44Sc]Sc-PSMA-617, the radiochemical yield increased from 61 ± 14% to 98 ± 8% (10 nmol of precursor) with microwave heating, even at relatively high levels of oxalate anions. For [
44Sc]Sc-PSMA-I&T, the labeling reaction was complete within 5 min (5 nmol of precursor), and the incorporation of
44Sc was almost quantitative (≥99%). The microwave heating method is still not convenient for radiopharmaceutical preparation, although its advantages in various aspects of radiochemical synthesis have already been shown [
42,
43,
44]. Probably, in the future, with the expansion of production and organization of centralized supplies of radiopharmaceuticals, the use of microwave heating will take its rightful place.
The difference in the labeling efficiency of the DOTAGA-conjugated precursor (PSMA-I&T) over the DOTA-conjugated one (PSMA-617) is noteworthy. According to experimental data, the chelating agent DOTAGA appears to be less sensitive to the presence of chemical impurities compared to DOTA and much more readily forms a complex with scandium-44. Similar differences between DOTAGA- and DOTA-derivates of PSMA-ligands have also been demonstrated for
177Lu [
45]. This fact should be considered in the development of new radiopharmaceuticals for diagnostics and therapy with radionuclides of Sc, Y, and the heaviest lanthanides.
4. Materials and Methods
4.1. Chemicals and Reagents
Only deionized water 18.2 MΩ·cm (Milli-Q Millipore, Merck, Darmstadt, Germany ) was used. All chemicals and solvents were of high purity or pharmaceutical grade. The chemicals were purchased from Sigma-Aldrich/Merck (St. Louis, MO, USA) or Panreac Quimica (Barcelona, Spain), unless otherwise indicated. The SPE resins TEVA (Aliquat
® 336) [
46,
47], TK221 (mixture of a diglocylamide and a phosphine oxide), UTEVA (diamyl, amylphosphonate, DAAP) [
48], DGA (
N,N,N′,N′-tetra-
n-octyldiglycolamide) [
49], and TRU (octylphenyl-
N,N-di-isobutyl carbamoylphosphine oxide (CMPO) dissolved in tri-
n-butyl phosphate (TBP)) [
50] were kindly provided by TrisKem International (Bruz, France). The Presep PolyChelate resin was purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). The precursors PSMA-617 and PSMA-I&T were purchased from ABX advanced biochemical compounds GmbH (Radeberg, Germany) and were also kindly provided by Center of Molecular Research (Moscow, Russia).
4.2. Titanium-44
Titanium-44 as [44Ti]TiCl4 in 4.0 M HCl (36 MBq/mL, 30 MBq, specific activity >3.7×1010 Bq/g, metal impurities (μg/Bq): Sc < 1.05 × 10−4, Cu < 1.35 × 10−5, Fe < 2.07 × 10−7) was purchased from Cyclotron Ltd. (Obninsk, Russia).
4.3. Measurement of Radioactivity
Measurement of 44Sc radioactivity was accomplished using an AtomlabTM 500 Dose Calibrator (Biodex, Shirley, NY, USA) and RIS-A1 (Amplituda Scientific and Technical Center, Moscow, Russia) dose calibrator. The relative activity (count rate) of the samples was measured with Wizard2 2480 automatic γ-counter (PerkinElmer, Waltham, MA, USA). The absolute radioactivity of 44Sc and 44Ti was measured by γ-spectrometry using GR4020 spectrometer with a high-purity germanium detector (Mirion Technologies (Canberra) SAS, San Ramon, CA, USA) at γ-lines 511 and 1157.02 keV for both radionuclides being in secular equilibrium. Measurements of 44Ti breakthrough radioactivity as well as 44Ti distribution coefficients were accomplished using ultra-shielded γ-spectrometer with a highly-sensitive semiconductor detector GC10021 (Mirion Technologies (Canberra) SAS, (San Ramon, CA, USA) with a relative efficiency of 106.8% (60Co), FWHM 1.02 keV (57Co) and 2.03 keV (60Co)), with a DSA-1000 multichannel pulse amplitude analyzer, and Genie-2000 software (V3.2.1) at specific γ-lines 67.9 and 78.3 keV (with a control at 1157.02 keV) at the Laboratory of radiometric and spectroscopic research of human and environment (Burnasyan Federal Medical Biophysical Center). Measurements of 44Ti were performed at least after 300 h after samples preparation. The acquisition time for 44Ti breakthrough measurements was at least 24 h to achieve no less than 1×104 full energy peak net counts in the regions of interest. A 22Na, 44Ti/44Sc, 57Co, 60Co calibration source (LLC “STC Amplituda”, Moscow, Russia) certified with k = 2.1 (Mendeleyev Institute for Metrology, St. Petersburg, Russia) was used. The breakthrough of 44Ti was calculated as the ratio of 44Ti activity in the generator eluate to its activity on the generator column.
Radiography of chromatographic columns (generator prototypes) and TLC-strips was carried out using PET-MiniGita radio-TLC scanner (Elysia-Raytest, Straubenhardt, Germany) as well as Cyclone® Plus storage phosphor system (PerkinElmer, Waltham, MA, USA).
4.4. Distribution Coefficients
The mass distribution coefficient Dg was measured by the batch static method [
51]. The sorption of
44Ti and
44Sc by resins from hydrochloric acid/oxalic acid solutions was measured by equilibration of a known volume (1 mL) of radionuclide spiked solution (80-500 kBq for
44Sc, and ∼12 kBq for
44Ti(
44Sc)) of appropriate concentration with known weight of resin (typically 15 mg). Resins were weighed and used without preconditioning. The samples were mixed in the mechanical mixer with temperature control Bioer Mixing block MB-102 (Bioer, Hangzhou, China) for 24 h at 20 °C with stirring speed 1350 rpm. After that, the samples were centrifuged (Heidolph, Schwabach, Germany) for one minute at 15,000 rpm. The tubes were removed carefully (to avoid phase mixing). Then, the aliquots (100–500 μL) of every solution were taken and their relative activities were measured. The mass distribution coefficient (mL/g) was defined according to the following Equation (1):
where
A0—count rate of the solution aliquot before the contact with the resin (decay corrected);
A—count rate of the solution aliquot after the contact with the resin (decay corrected);
V—solution volume, mL;
m—weight of dry resin, g. Every Dg coefficient was determined at least in 5 parallel experiments.
4.5. Dynamic Column Separation Experiments and 44Ti/44Sc Generators Preparation
Evaluation of the ion exchange behavior of 44Sc and 44Ti in dynamic conditions was performed using PEEK chromatographic columns 100 mm × Ø2.1 mm and 50 mm × Ø2.1 mm (VICI Jour, Schenkon, Switzerland). Transfer of solutions was performed using syringes (BBraun Injekt) manually or with a syringe pump.
For 44Ti/44Sc generators, only 100 mm × Ø2.1 mm columns were used. The columns were filled with TEVA resin (148–150 mg per column) and stuffed with PEEK-encased polyethylene frits and 1/4–28 female to Luer PEEK adapters. A sample of 44Ti solution in 4.0 M HCl with desired activity was evaporated to dryness, and 44Ti was taken up with 150 μL of 0.1 M oxalic acid. The resulting solutions were slightly opalescent, so 5 μL of 1.0 M HCl was added to make them transparent. The obtained solutions were transferred quantitatively to generator columns (using 1–2 additional portions (150 μL) of 0.1 M oxalic acid). The elution of the generators was carried out with the selected solutions of hydrochloric and oxalic acids at least 3 times a week, no more than 1 time a day. Elution was carried out fractionally (each fraction—1 mL, total elution volume—1–5 mL) except the experiments when elution curves were determined (each fraction—45–50 μL).
4.6. Radiolabeling of PSMA-617 and PSMA-I&T
Radiolabeling of PSMA-617 and PSMA-I&T with 44Sc was carried out at pH 4.5 using 1.0–2.0 M CH3COONH4 as buffer. In the case of convective heating, the reaction was performed using a 2.0 mL Eppendorf test tube and Bioer Mixing block MB-102 (Hangzhou, China) at 95 °C (5–30 min). In the case of microwave heating, the reaction was performed with CEM Discover 908005 Microwave Synthesis System in Biotage® Microwave Reaction Vials (Uppsala, Sweden) of 0.5–2 mL (95 °C, 5–30 min). The necessary amount of precursor (2-50 nmol) was mixed in the tube/vial with the 44Sc solution (∼1.0–4.0 MBq of 44Sc in 0.1 M HCl or in 1.0 M CH3COONH4, рН 4.0–4.5). If needed, the required pH was achieved by adjustment with 3.0 M HCl. The CH3COONH4 concentration in the reaction mixture was kept at a level of 1.0 mol/L, and the final volume of the reaction mixture was 1 mL.
A number of radio TLC methods were used to analyze the radiochemical purity (radiochemical yield) of the preparations obtained. The main TLC methods were: (a) ITLC-SG paper (Agilent, Santa Clara, CA, USA) with 0.05 M citric acid water solution as solvent; (b) ITLC-SG paper with 1 M CH3COONH4 in methanol–water mixture (1:1) as solvent; and (c) silica gel coated aluminum TLC plates (5553, Merck, Darmstadt, Germany) with acetonitrile-water mixture (1:1) as solvent. In method (a) the Rf of unbounded 44Sc is 0.9–1.0, and the Rf of [44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T is 0.0-0.1. In method (b), the Rf of unbounded 44Sc is 0.0–0.1, and the Rf of [44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T is 0.8–1.0. In method (c), the Rf of unbound 44Sc is 0.0–0.1 and the Rf of [44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T is 0.9–1.0. For HPLC analysis, one method was used. In short: a Knauer Smartline HPLC system (Berlin, Germany) equipped with an fLumo radiometric detector (Berthold, Bad Wildbad, Germany) and reversed phase C18 column (250 × 4.6 mm column; Jupiter, Phenomenex Inc.,) was used. The column thermostat temperature was 55 °C, gradient flow (0.85 mL·min−1): 0–13–13.5–21 min = 85–28–85–85 % A (A—0.1% TFAaq., B—methanol). Retention time using this method for [44Sc]Sc-PSMA-617 and [44Sc]Sc-PSMA-I&T was 13.2 ± 0.3 min, and for [44Sc]Sc3+ was 4.1 ± 0.2 min.
4.7. Determination of Oxalic Acid
Determination of oxalic acid content in hydrochloric acid samples was accomplished using HPLC. LC-20AD Prominence chromatograph (Shimadzu Co. Ltd., Kyoto, Japan) was used. The analysis was conducted on a Shodex 1C SI-90 4E column (4.0 mm Ø × 250 mm, Shodex Group, Tokyo, Japan). Detection was performed using diode array detector SPD-M20A at 210 nm. The column thermostat temperature was 33 °C, isocratic flow (1.5 mL·min−1): 1.7 mM NaHCO3 + 1.8 mM Na2CO3. Retention time for oxalate anion using this method is 28.2 ± 0.2 min.