Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect
Abstract
1. Introduction
2. Results
2.1. VOCs Collection with CAR/PDMS Fiber
2.2. VOCs Collection with PEG Fiber
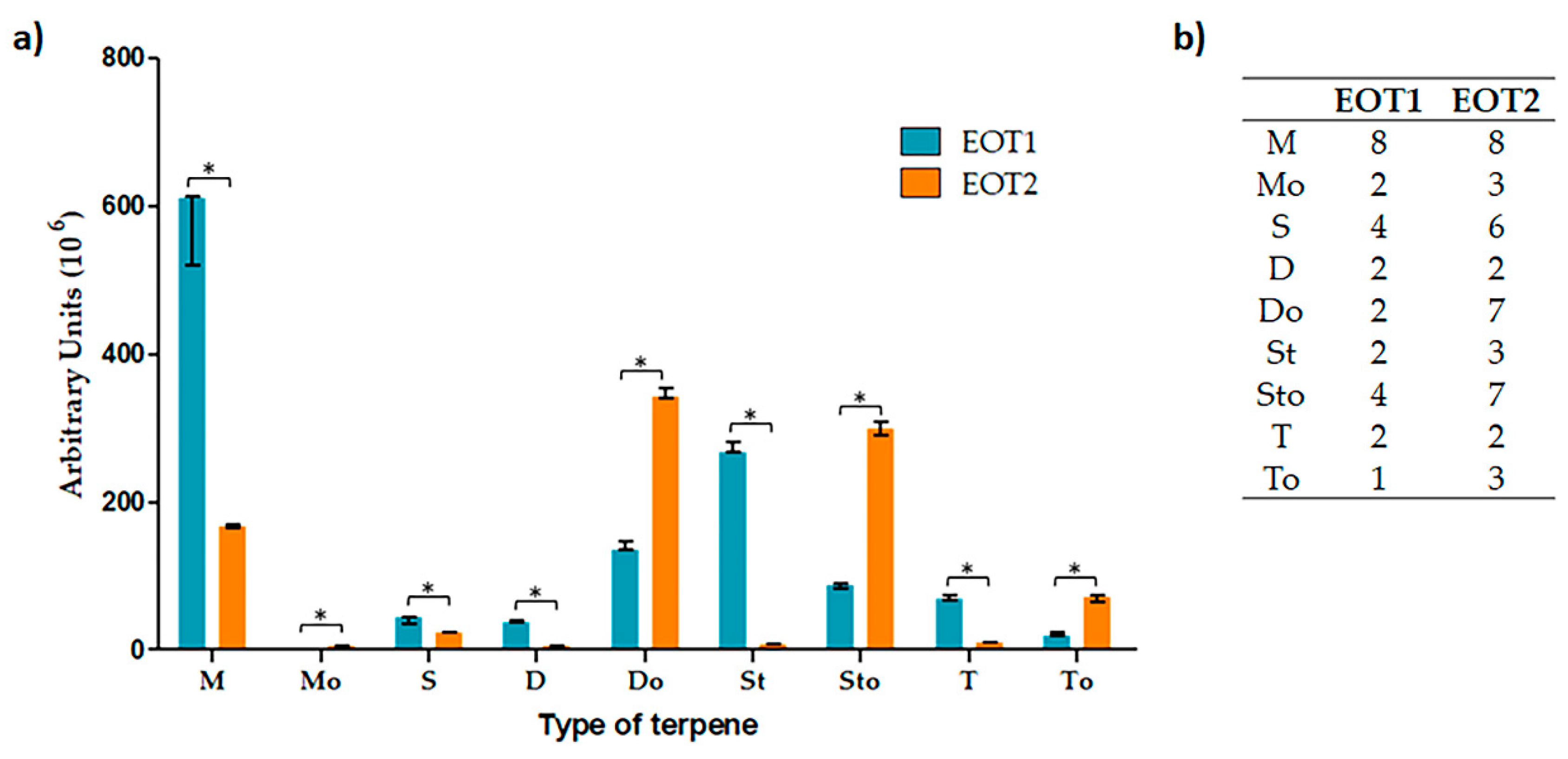
2.3. Obtention and Characterization of Essential Oils
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Insects
4.3. Sample Preparation
- Treatment 1 (T1): manually shaking the vial for 5 min at room temperature to stimulate the release of defense secretion.
- Treatment 2 (T2): 1.5 mL of the PBET solution was added to the vial with the insects and incubated at 37 °C with constant agitation (130 rpm). The PBET solution consisted of 0.5 mg/mL sodium citrate, 0.5 mg/mL malic acid, 0.5 µL/mL acetic acid, 0.4 µL/mL lactic acid and ~800 U/mL pepsin at pH 3. The PBET solution simulates the leaching of a solid matrix in the human gastrointestinal tract in order to determine the bioaccessibility of a particular element, such as the total fraction available for adsorption during transit through the small intestine [53]. This digestion simulant solution allowed emulation of the conditions of the insects being ingested and digested by gastric fluid.
4.4. VOCs Collection by HS-SPME
4.5. Volatilome GS-MS Analysis
4.6. Obtention of Essential oil of U. dermestoides (EOT1)
4.7. Obtention of Essential oil of U. dermestoides Post PBET Digestion (EOT2)
4.8. Derivatization for Alcohols and Carboxylic Acid Detection
4.9. Derivatization for Aldehydes and Alkyne Detection
4.10. Essential Oil GS-MS Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Flores, G.E.; Padín, S.B.; Stetson, R.E. First records of the Oriental species Ulomoides dermestoides (Coleoptera: Tenebrionidae) in Argentina. Rev. Soc. Entomol. Argent. 2002, 61, 48–50. [Google Scholar]
- Deloya-Brito, G.G.; Deloya, C. Sustancias Producidas Por El Coleóptero Ulomoides Dermestoides (Chevrolat, 1878) (Insecta: Coleoptera: Tenebrionidae): Efecto Anti-Inflamatorio Y Citotóxico. Acta Zoológica Mex. 2014, 30, 655–661. [Google Scholar] [CrossRef]
- Vianna-Santos, R.C.; Lunardelli, A.; Caberlon, E.; Maria, C.; Bastos, A.; Nunes, F.B.; Guerra, M.; Pires, S.; Biolchi, V.; Paul, E.L.; et al. Anti-inflammatory and Immunomodulatory Effects of Ulomoides dermestoides on Induced Pleurisy in Rats and Lymphoproliferation In Vitro. Inflammation 2010, 33, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Crespo, R.; Villaverde, M.L.; Girotti, J.R.; Güerci, A.; Juárez, M.P.; De Bravo, M.G. Cytotoxic and genotoxic effects of defence secretion of Ulomoides dermestoides on A549 cells. J. Ethnopharmacol. 2011, 136, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Jasso-Villagomez, E.; Garcia-Lorezana, M.; Almanza-Perez, J.C.; Fortis-Barrera, M.A.; Blancas-Flores, G.; Roman-Ramos, R.; Prado-Barragan, L.A.; Alarcon-Aguilar, F.J. Beetle (Ulomoides dermestoides) fat improves diabetes: Effect on liver and pancreatic architecture and on PPARg expression. Braz. J. Med. Biol. Res. 2018, 51, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Villaverde, M.L.; Girotti, J.R.; Mijailovsky, S.J.; Pedrini, N.; Juárez, M.P. Volatile secretions and epicuticular hydrocarbons of the beetle Ulomoides dermestoides. Comp. Biochem. Physiol. Part B 2009, 154, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, N.; Ortiz-Urquiza, A.; Huarte-Bonnet, C.; Fan, Y.; Juárez, M.P.; Keyhani, N.O. Tenebrionid secretions and a fungal benzoquinone oxidoreductase form competing components of an arms race between a host and pathogen. Proc. Natl. Acad. Sci. USA 2015, 112, 651–660. [Google Scholar] [CrossRef]
- Dávila-vega, J.P.; Duarte-martínez, H.E.; López-aguirre, C.A.; Pérez-arteaga, E.; Zagal-salinas, A.A.; Karla, G.E.; Martínez-elizalde, S. Efecto antiproliferativo del extracto metanólico de Ulomoides dermestoides Chevrolat (Coleoptera: Tenebrionidae) en linfocitos humanos. Entomol. Mex. 2017, 4, 560–565. [Google Scholar]
- Flores, D.R.; Casados, L.E.; Velasco, S.F.; Ramírez, A.C.; Velázquez, G. Comparative study of composition, antioxidant and antimicrobial activity of two adult edible insects from Tenebrionidae family. BMC Chem. 2020, 14, 1–9. [Google Scholar] [CrossRef]
- Kartika, T.; Shimizu, N.; Yoshimura, T. Identification of esters as novel aggregation pheromone components produced by the male powder-post beetle, Lyctus africanus lesne (Coleoptera: Lyctinae). PLoS ONE 2015, 10, 1–13. [Google Scholar] [CrossRef]
- Uzakah, R.P.; Odebiyi, J.A.; Chaudhury, M.F.B.; Hassanali, A. Evidence for the presence of a female produced sex pheromone in the banana weevil Cosmopolites sordidus Germar (Coleoptera: Curculionidae). Sci. Res. Essays 2015, 10, 471–481. [Google Scholar] [CrossRef]
- Silva, W.D.; Millar, J.G.; Hanks, L.M.; Bento, J.M.S. (6E,8Z)-6,8-Pentadecadienal, a Novel Attractant Pheromone Produced by Males of the Cerambycid Beetles Chlorida festiva and Chlorida costata. J. Chem. Ecol. 2016, 42, 1082–1085. [Google Scholar] [CrossRef] [PubMed]
- Lyons-Yerion, C.D.; Barbour, J.D.; Mongold-Diers, J.A.; Williams, C.J.; Cook, S.P. Identification of a male-produced volatile pheromone for Phymatodes dimidiatus (Coleoptera: Cerambycidae) and seasonal flight phenology of four phymatodes species endemic to the north american intermountain west. Environ. Entomol. 2020, 49, 1077–1087. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Fujiwara-Tsujii, N.; Yasuda, T. Detection of volatile pheromone candidates from the white-spotted longicorn beetle, Anoplophora malasiaca (Coleoptera: Cerambycidae). Appl. Entomol. Zool. 2019, 54, 203–211. [Google Scholar] [CrossRef]
- Pizzolante, G.; Cordero, C.; Tredici, S.M.; Vergara, D.; Pontieri, P.; Del Giudice, L.; Capuzzo, A.; Rubiolo, P.; Kanchiswamy, C.N.; Zebelo, S.A.; et al. Cultivable gut bacteria provide a pathway for adaptation of Chrysolina herbacea to Mentha aquatica volatiles. BMC Plant Biol. 2017, 17, 1–20. [Google Scholar] [CrossRef]
- Đukić, N.; Andrić, G.; Glinwood, R.; Ninkovic, V.; Andjelković, B.; Radonjić, A. The effect of 1-pentadecene on Tribolium castaneum behaviour: Repellent or attractant? Pest Manag. Sci. 2021, 77, 4034–4039. [Google Scholar] [CrossRef]
- Lečić, S.; Ćurčić, S.; Vujisić, L.; Ćurčić, B.; Ćurčić, N.; Nikolić, Z.; Anđelković, B.; Milosavljević, S.; Tešević, V.; Makarov, S. Defensive Secretions in Three Ground-Beetle Species (Insecta: Coleoptera: Carabidae). Ann. Zool. Fennici 2014, 51, 285–300. [Google Scholar] [CrossRef]
- Hassemer, M.J.; Sant’Ana, J.; De Oliveira, M.W.M.; Borges, M.; Laumann, R.A.; Caumo, M.; Blassioli-Moraes, M.C. Chemical Composition of Alphitobius diaperinus (Coleoptera: Tenebrionidae) Abdominal Glands and the Influence of 1,4-Benzoquinones on Its Behavior. J. Econ. Entomol. 2015, 108, 2107–2116. [Google Scholar] [CrossRef]
- Brown, W.V.; Doyen, J.T.; Moore, B.P.; Lawrence, J.F. Chemical composition and taxonomic significance of defensive secretions of some australian Tenebrionidae (Coleoptera). Aust. J. Entomol. 1992, 31, 79–89. [Google Scholar] [CrossRef]
- Mohebat, R.; Bidoki, M.Z. Comparative chemical analysis of volatile compunds of Echinops ilicifolius using hydrodestillation and headspce solid-phase microextraction and the actibacterial acivities of its essential oils. R. Soc. Open Sci. 2018, 5, 1–8. [Google Scholar] [CrossRef]
- Paniandy, J.C.; Chane-Ming, J.; Pieribattesti, J.C. Chemical composition of the essential oil and headspace solid-phase microextraction of the guava fruit (psidium guajava l.). J. Essent. Oil Res. 2000, 12, 153–158. [Google Scholar] [CrossRef]
- Skalicka-Wozniak, K.; Los, R.; Glowniak, K.; Malm, A. Comparison of hydrodistillation and headspace solid-phase microextraction techniques for antibacterial volatile compounds from the fruits of Seseli libanotis. Nat. Prod. Commun. 2010, 5, 1427–1430. [Google Scholar] [CrossRef]
- Monks, T.; Jones, D. The Metabolism and Toxicity of Quinones, Quinonimines, Quinone Methides, and Quinone-Thioethers. Curr. Drug Metab. 2005, 3, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.L.; Dunlap, T. Formation and Biological Targets of Quinones: Cytotoxic versus Cytoprotective Effects. Chem. Res. Toxicol. 2016, 30, 13–37. [Google Scholar] [CrossRef]
- Das, A.; Chakrabarty, S.; Choudhury, D.; Chakrabarti, G. 1,4-benzoquinone (PBQ) induced toxicity in lung epithelial cells is mediated by the disruption of the microtubule network and activation of caspase-3. Chem. Res. Toxicol. 2010, 23, 1054–1066. [Google Scholar] [CrossRef] [PubMed]
- Beran, F.; Köllner, T.G.; Gershenzon, J.; Tholl, D. Chemical convergence between plants and insects: Biosynthetic origins and functions of common secondary metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [PubMed]
- Burse, A.; Boland, W. Deciphering the route to cyclic monoterpenes in Chrysomelina leaf beetles: Source of new biocatalysts for industrial application? Z. Naturforsch.—Sect. C J. Biosci. 2017, 72, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Burse, A.; Schmidt, A.; Frick, S.; Kuhn, J.; Gershenzon, J.; Boland, W. Iridoid biosynthesis in Chrysomelina larvae: Fat body produces early terpenoid precursors. Insect Biochem. Mol. Biol. 2007, 37, 255–265. [Google Scholar] [CrossRef]
- Frick, S.; Nagel, R.; Schmidt, A.; Bodemann, R.R.; Rahfeld, P.; Pauls, G.; Brandt, W.; Gershenzon, J.; Boland, W.; Burse, A. Metal ions control product specificity of isoprenyl diphosphate synthases in the insect terpenoid pathway. Proc. Natl. Acad. Sci. USA 2013, 110, 4194–4199. [Google Scholar] [CrossRef] [PubMed]
- Lancaster, J.; Khrimian, A.; Young, S.; Lehner, B.; Luck, K.; Wallingford, A.; Ghosh, S.K.B.; Zerbe, P.; Muchlinski, A.; Marek, P.E.; et al. De novo formation of an aggregation pheromone precursor by an isoprenyl diphosphate synthase-related terpene synthase in the harlequin bug. Proc. Natl. Acad. Sci. USA 2018, 115, E8634–E8641. [Google Scholar] [CrossRef]
- Gilg, A.B.; Tittiger, C.; Blomquist, G.J. Unique animal prenyltransferase with monoterpene synthase activity. Naturwissenschaften 2009, 96, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Lü, S.; Zhang, Y. The Potential Organ Involved in Cantharidin Biosynthesis in Epicauta chinensis Laporte (Coleoptera: Meloidae). J. Insect Sci. 2017, 17, 1–9. [Google Scholar] [CrossRef]
- Banerjee, A.; Hamberger, B. P450s controlling metabolic bifurcations in plant terpene specialized metabolism. Phytochem. Rev. 2018, 17, 81–111. [Google Scholar] [CrossRef] [PubMed]
- Eksi, G.; Kurbanoglu, S.; Erdem, S.A. Analysis of diterpenes and diterpenoids. In Recent Advances in Natural Products Analysis; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 313–345. ISBN 9780128164556. [Google Scholar]
- Costa, M.S.; Rego, A.; Ramos, V.; Afonso, T.B.; Freitas, S.; Preto, M.; Lopes, V.; Vasconcelos, V.; Magalhães, C.; Leaõ, P.N. The conifer biomarkers dehydroabietic and abietic acids are widespread in Cyanobacteria. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Itoh, H.; Tago, K.; Hayatsu, M.; Kikuchi, Y. Detoxifying symbiosis: Microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 2018, 35, 434–454. [Google Scholar] [CrossRef]
- Brooks, S.N.; Pettinger, W.A. Defensive use by an insect of a plant resin. Science 1973, 184, 996–999. [Google Scholar]
- Blomquist, G.J.; VOgt, R.G. Insect Pheromone Biochemistry and Molecular Biology: The Biosynthesis and Detection of Pheromones and Plant Volatiles; Elsevier: London, UK, 2003; pp. 205–235. [Google Scholar]
- Blomquist, G.J.; Tittiger, C.; Jurenka, R. Cuticular Hydrocarbons and Pheromones of Arthropods. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1–32. [Google Scholar]
- Jurenka, R.A.; Subchev, M. Identification of cuticular hydrocarbons and the alkene precursor to the pheromone in hemolymph of the female gypsy moth, Lymantria dispar. Arch. Insect Biochem. Physiol. 2000, 43, 108–115. [Google Scholar] [CrossRef]
- Provost, E.; Blight, O.; Tirard, A.; Renucci, M. Hydrocarbons and insects’ social physiology. In Social Insects: Structure, Function, and Behavior; Nova Science Publishers: Hauppauge, NY, USA, 2011; pp. 19–69. ISBN 9781617614668. [Google Scholar]
- Holze, H.; Schrader, L.; Buellesbach, J. Advances in deciphering the genetic basis of insect cuticular hydrocarbon biosynthesis and variation. Heredity 2021, 126, 219–234. [Google Scholar] [CrossRef]
- Qiu, Y.; Tittiger, C.; Wicker-Thomas, C.; Le Goff, G.; Young, S.; Wajnberg, E.; Fricaux, T.; Taquet, N.; Blomquist, G.J.; Feyereisen, R. An insect-specific P450 oxidative decarbonylase for cuticular hydrocarbon biosynthesis. Proc. Natl. Acad. Sci. USA 2012, 109, 14858–14863. [Google Scholar] [CrossRef]
- Chung, H.; Carroll, S.B. Wax, sex and the origin of species: Dual roles of insect cuticular hydrocarbons in adaptation and mating. BIoEssays 2015, 37, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Rao, R.; Yadav, P. Azelaic Acid: A Promising Agent for Dermatological Applications. Curr. Drug Ther. 2019, 15, 181–193. [Google Scholar] [CrossRef]
- Chai, W.M.; Liu, X.; Hu, Y.H.; Feng, H.L.; Jia, Y.L.; Guo, Y.J.; Zhou, H.T.; Chen, Q.X. Antityrosinase and antimicrobial activities of furfuryl alcohol, furfural and furoic acid. Int. J. Biol. Macromol. 2013, 57, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Khan, A.L.; Ali, L.; Khan, A.R.; Waqas, M.; Hussain, J.; Lee, I.J.; Shin, J.H. Benzaldehyde as an insecticidal, antimicrobial, and antioxidant compound produced by Photorhabdus temperata M1021. J. Microbiol. 2015, 53, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Kurashov, E.A.; Fedorova, E.V.; Krylova, J.V.; Mitrukova, G.G. Assessment of the potential biological activity of low molecular weight metabolites of freshwater macrophytes with QSAR. Scientifica 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2016, 96, 1856–1866. [Google Scholar] [CrossRef]
- González, M.A. Aromatic abietane diterpenoids: Their biological activity and synthesis. Nat. Prod. Rep. 2015, 32, 684–704. [Google Scholar] [CrossRef]
- Vieira, A.J.; Beserra, F.P.; Souza, M.C.; Totti, B.M.; Rozza, A.L. Limonene: Aroma of innovation in health and disease. Chem. Biol. Interact. 2018, 283, 97–106. [Google Scholar] [CrossRef]
- KIM, J.I.; JUNG, B.H. Contribution to the Tribes Diaperini doyen in Korea (Coleoptera: Tenebrionidae: Diaperinae). Entomol. Res. 2005, 35, 95–100. [Google Scholar] [CrossRef]
- Ruby, M.V.; Davis, A.; Schoof, R.; Eberle, S.; Sellstone, C.M. Estimation of lead and arsenic bioavailability using a physiologically based extraction test. Environ. Sci. Technol. 1996, 30, 422–430. [Google Scholar] [CrossRef]

| No. | Compounds | KI | Relative Abundance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Exp | 5 min | 1 h | 6 h | 18 h | 24 h | |||||||
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | ||||
| Quinones | |||||||||||||
| 1 | p-Benzoquinone | 912 | 920 | 6.11 | 2.9 | 0.83 | |||||||
| 2 | Methyl-1,4-benzoquinone | 1015 | 1016 | 14.3 | 0.07 | 13.6 | 0.95 | 3.59 | 0.68 | 1.94 | 1.42 | 12.1 | 0.97 |
| 3 | Ethyl-1,4-benzoquinone | 1215 | 1112 | 39.2 | 1.9 | 33.3 | 4.89 | 9.9 | 2.65 | 7.44 | 6.71 | 34.1 | 2.56 |
| 4 | Hydroquinone | 1241 | 1291 | 0.67 | 0.11 | 0.16 | |||||||
| 5 | 2-Methylhydroquinone | 1378 | 1359 | 0.4 | 0.37 | 0.11 | 0.13 | 0.65 | |||||
| 6 | 2-Ethylhydroquinone | 1413 | 1440 | 1.16 | 1.08 | 0.25 | 0.46 | 0.18 | 2.02 | ||||
| Terpenes | |||||||||||||
| 1 | α-Pinene | 922 | 936 | 4.35 | 8.95 | 7.97 | 2.35 | 22.2 | 6.89 | 31.6 | 11.9 | 6.72 | 9.09 |
| 2 | Camphene | 958 | 952 | 0.13 | 0.18 | 0.43 | 0.75 | 0.06 | |||||
| 3 | Carene | 1008 | 1001 | 1.27 | 1.15 | 1.6 | 1.24 | 3.91 | 2.79 | 3.58 | 3.9 | 1.88 | 2.94 |
| 4 | α-Phellandrene | 1007 | 1006 | 0.26 | 0.33 | 0.63 | 0.62 | 0.43 | 0.66 | 0.59 | 0.31 | 0.94 | |
| 5 | o-Cymene | 1025 | 1029 | 0.91 | 0.67 | 0.97 | 0.48 | 1.8 | 0.95 | 1.53 | 0.92 | 0.63 | 1.18 |
| 6 | Limonene | 1020 | 1033 | 21.8 | 29.2 | 28.4 | 33 | 44.4 | 47.8 | 36.9 | 47.7 | 29.9 | 42.8 |
| 7 | γ-Terpinene | 1053 | 1063 | 0.08 | 0.05 | 0.13 | 0.17 | 0.12 | 0.15 | 0.16 | 0.06 | 0.2 | |
| 8 | p-Cymenene | 1081 | 1095 | 0.64 | 0.55 | 0.53 | 0.71 | 0.33 | 0.66 | 0.7 | 0.32 | 0.75 | |
| 9 | Di-epi-α-cedrene-(I) | 1388 | 1414 | 0.15 | 0.05 | 0.69 | 0.08 | 0.29 | 0.1 | 0.1 | |||
| 10 | α-Guaiene | 1457 | 1456 | 0.11 | |||||||||
| 11 | cis-(-)-2,4a,5,6,9a-Hexahydro-3,5,5,9-tetramethyl(1H)benzocycloheptene | 1478 | 1486 | 0.3 | |||||||||
| 12 | Cuparene | 1539 | 1540 | 0.09 | 0.02 | 1.04 | 0.03 | 0.19 | 0.07 | 0.37 | |||
| Alkenes | |||||||||||||
| 1 | 1-Tridecene | 1287 | 1295 | 1.13 | 2.63 | 1.17 | 7.1 | 1.3 | 3.24 | 1.34 | 2.06 | 1.02 | 4.51 |
| 2 | 1,13-Tetradecadiene | 1393 | 1384 | 0.11 | |||||||||
| 3 | 1-Tetradecene | 1388 | 1391 | 0.64 | |||||||||
| 4 | 1,14-Pentadecadiene | 1480 | 1479 | 0.46 | 0.19 | 1.12 | 0.1 | 0.45 | 0.04 | 0.24 | 0.42 | ||
| 5 | 1-Pentadecene | 1486 | 1494 | 4.33 | 53.7 | 4.63 | 40.7 | 9.06 | 31.7 | 12 | 22.4 | 6.28 | 31.5 |
| Aromatic compounds | |||||||||||||
| 1 | 2,2′-Bifuran | 1334 | 1335 | 0.25 | 0.65 | 0.39 | 0.44 | 0.4 | 0.09 | ||||
| 2 | Nonylbenzene | 1554 | 1584 | 0.18 | |||||||||
| No. | Compounds | KI | Relative Abundance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ref | Exp | 5 min | 1 h | 6 h | 18 h | 24 h | |||||||
| T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | T1 | T2 | ||||
| Quinones | |||||||||||||
| 1 | p-Benzoquinone | 912 | 920 | 3.08 | 0.11 | 0.62 | 1.29 | ||||||
| 2 | Methyl-1,4-benzoquinone | 1015 | 1016 | 17 | 1.16 | 15.7 | 1.46 | 10.8 | 1.09 | 7.42 | 1.69 | 17.1 | 2.61 |
| 3 | Ethyl-1,4-benzoquinone | 1215 | 1112 | 54.3 | 11 | 54.7 | 14.7 | 49 | 16.9 | 49.8 | 22.4 | 58.5 | 26.9 |
| 4 | Hydroquinone | 1241 | 1291 | 3.54 | 0.13 | 0.94 | 0.21 | 0.43 | 2.34 | ||||
| 5 | 2-Methylhydroquinone | 1378 | 1359 | 1.49 | 0.46 | 2.76 | 0.61 | 2.28 | 1.2 | 2.55 | 2.03 | 2.96 | 1.47 |
| 6 | 2-Ethylhydroquinone | 1413 | 1440 | 7.45 | 4.89 | 12.1 | 7.74 | 15.8 | 12.3 | 21.6 | 22.4 | 12.5 | 16.4 |
| Terpenes | |||||||||||||
| 1 | α-Pinene | 922 | 936 | 0.54 | 0.5 | 0.29 | 0.66 | ||||||
| 3 | Carene | 1008 | 1001 | 0.19 | 0.15 | 0.07 | 0.17 | ||||||
| 6 | Limonene | 1020 | 1033 | 1 | 10.2 | 1.18 | 10.5 | 2.98 | 10.2 | 0.64 | 9.26 | 0.54 | 13.6 |
| 13 | cis-Verbenol | 1148 | 1158 | 0.32 | 0.19 | 0.08 | |||||||
| 14 | p-Cymen-8-ol | 1172 | 1196 | 0.11 | 0.3 | 0.16 | 0.17 | ||||||
| 15 | Verbenone | 1204 | 1204 | 0.02 | |||||||||
| 16 | Myrtenol | 1213 | 1212 | 0.35 | 0.28 | ||||||||
| 17 | Perillol | 1297 | 1318 | 0.1 | |||||||||
| 9 | Di-epi-α-cedrene-(I) | 1414 | 1414 | 0.06 | |||||||||
| 12 | Cuparene | 1502 | 1540 | 0.66 | 0.03 | 0.4 | 0.13 | 0.04 | |||||
| Aromatic compounds | |||||||||||||
| 3 | m-Cresol | 1053 | 1088 | 0.25 | 2.6 | ||||||||
| 4 | 3,4-Dimethylphenol | 1167 | 1180 | 0.2 | 2.11 | ||||||||
| 1 | 2,2′-Bifuran | 1334 | 1335 | 0.09 | |||||||||
| Alkenes | |||||||||||||
| 1 | 1-Tridecene | 1287 | 1295 | 1.94 | 0.5 | 1.37 | 0.45 | 0.31 | 0.47 | ||||
| 4 | 1,14-Pentadecadiene | 1480 | 1479 | 3.06 | 1.04 | 0.59 | 0.59 | 1.14 | 0.88 | 0.74 | |||
| 5 | 1-Pentadecene | 1486 | 1494 | 5.64 | 67.6 | 10.2 | 62 | 14.5 | 58.3 | 10.5 | 42.2 | 2.16 | 38.2 |
| No. | Compounds | EOUd1 | EOUd2 | KI Ref | ||
|---|---|---|---|---|---|---|
| RA (%) | KI Exp | RA (%) | KI Exp | |||
| Terpenes | 4.31% | 2.13% | ||||
| 1 | α-Pinene | 0.239 ± 0.007 | 903.6 | 0.106 ± 0.002 | 903.6 | 922 |
| 18 | β-thujene | 0.015 ± 0.000 | 958.1 | 968 | ||
| 19 | Isolimonene | 0.008 ± 0.000 | 967.6 | 0.005 ± 0.001 | 967.6 | 974 |
| 3 | 2-Carene | 0.074 ± 0.002 | 995.7 | 0.037 ± 0.001 | 995.7 | 996 |
| 4 | α-Phellandrene | 0.002 ± 0.001 | 1000.2 | 997 | ||
| 20 | α-Terpinene | 0.037 ± 0.001 | 1013 | 0.084 ± 0.002 | 1012.9 | 1008 |
| 5 | o-Cymene | 0.029 ± 0.001 | 1021.3 | 0.024 ± 0.001 | 1021.1 | 1025.4 |
| 6 | D-Limonene | 2.898 ± 0.075 | 1026.7 | 1.435 ± 0.016 | 1025.4 | 1033 |
| 8 | p-Cymenene | 0.006 ± 0.001 | 1085.1 | 0.004 ± 0.001 | 1086.2 | 1081 |
| 21 | Terpinen-4-ol | 0.002 ± 0.001 | 1174.9 | 0.006 ± 0.000 | 1174.4 | 1161 |
| 14 | p-Cymen-8-ol | 0.002 ± 0.001 | 1185.1 | 0.018 ± 0.001 | 1183.4 | 1172 |
| 9 | Di-epi-α-cedrene-(I) | 0.213 ± 0.007 | 1383.1 | 0.111 ± 0.001 | 1382.7 | 1388.2 |
| 22 | β-Cedrene | 0.012 ± 0.001 | 1418.2 | 0.005 ± 0.002 | 1418 | 1423 |
| 23 | cis-Thujopsene | 0.005 ± 0.000 | 1429.3 | 0.002 ± 0.001 | 1428.5 | 1435 |
| 12 | Cuparene | 0.004 ± 0.002 | 1518.7 | 0.075 ± 0.002 | 1510.9 | 1502 |
| 24 | Phytan | 0.026 ± 0.002 | 1807.9 | 1811 | ||
| 25 | Squalene | 0.045 ± 0.002 | 2826.8 | 2847 | ||
| 26 | 28-Nor-17β(H)-hopane | 0.395 ± 0.022 | 3044.9 | 0.038 ± 0.005 | 3033.9 | |
| 27 | 22R-17alpha(h),21beta(H)-bishomohopane | 0.097 ± 0.007 | 3313.6 | |||
| 28 | γ-Sitosterol | 0.248 ± 0.028 | 3331.9 | 0.128 ± 0.020 | 3333.7 | 3351.3 |
| Alkanes | 6.01% | 14.74% | ||||
| 1 | 4-Propylheptane | 0.002 ± 0.000 | 920.2 | 0.046 ± 0.002 | 920.2 | 945 |
| 2 | 4-Ethyloctane | 0.004 ± 0.002 | 934.4 | 0.114 ± 0.003 | 934.4 | 954 |
| 3 | 4-Methylnonane | 0.012 ± 0.001 | 943.1 | 0.185 ± 0.003 | 943.1 | 963.8 |
| 4 | 5-Methyldecane | 0.022 ± 0.013 | 1056.8 | 0.181 ± 0.010 | 1055.2 | 1057.4 |
| 5 | Undecane | 0.003 ± 0.001 | 1098.7 | 0.017 ± 0.001 | 1098 | 1100 |
| 6 | 5-Ethyldecane | 0.024 ± 0.000 | 1142.6 | 1146 | ||
| 7 | 6-Methylundecane | 0.028 ± 0.001 | 1152.8 | 1157 | ||
| 8 | Dodecane | 0.004 ± 0.001 | 1198.9 | 0.009 ± 0.001 | 1198.2 | 1200 |
| 9 | Hexadecane | 0.020 ± 0.002 | 1599.4 | 0.015 ± 0.002 | 1598.3 | 1600 |
| 10 | Heptadecane | 0.097 ± 0.009 | 1701 | 0.038 ± 0.002 | 1700.3 | 1700 |
| 11 | Octadecane | 0.062 ± 0.005 | 1798.3 | 0.012 ± 0.001 | 1797.8 | 1800 |
| 12 | Eicosane | 0.241 ± 0.011 | 2001.2 | 0.166 ± 0.003 | 2002.4 | 2000 |
| 13 | Heneicosane | 0.169 ± 0.011 | 2103.5 | 2100 | ||
| 14 | Docosane | 0.161 ± 0.010 | 2202.2 | 0.066 ± 0.012 | 2199.5 | 2200 |
| 15 | Tricosane | 1.551 ± 0.066 | 2306.5 | 2.789 ± 0.017 | 2302.9 | 2300 |
| 16 | Tetracosane | 0.545 ± 0.035 | 2405.2 | 0.230 ± 0.007 | 2399.1 | 2400 |
| 17 | Pentacosane | 1.833 ± 0.084 | 2511.1 | 2.270 ± 0.020 | 2503.5 | 2500 |
| 18 | 1-Hexadecyloctahydro-1H-indene | 0.802 ± 0.068 | 2553.2 | |||
| 19 | 3-Ethyltetracosane | 0.015 ± 0.002 | 2572.8 | 2567 | ||
| 20 | Hexacosane | 0.081 ± 0.008 | 2599 | 2600 | ||
| 21 | Heptacosane | 0.324 ± 0.005 | 2709.6 | 0.406 ± 0.003 | 2700 | 2700 |
| 22 | 1-cyclohexyleicosane | 0.123 ± 0.009 | 2704.2 | |||
| 23 | Octacosane | 0.042 ± 0.001 | 2797.5 | 2800 | ||
| 24 | Nonacosane | 0.642±0.006 | 2899.7 | 2900 | ||
| 25 | Triacontane | 0.084±0.013 | 2996.9 | 3000 | ||
| 26 | Hentriacontane | 0.032±0.004 | 3104.9 | 6.537±0.015 | 3107.5 | 3100 |
| 27 | Dotriacontane | 0.107±0.006 | 3198.4 | 3200 | ||
| 28 | Tritriacontane | 0.638±0.005 | 3300.3 | 3300 | ||
| Alkenes | 82.78% | 61.51% | ||||
| 6 | Decene | 0.003±0.000 | 985 | 0.005±0.001 | 985 | 987 |
| 7 | Dodecene | 0.006±0.001 | 1190.8 | 0.011 ± 0.000 | 1190.1 | 1187 |
| 1 | 1-Tridecene | 3.020 ± 0.126 | 1295 | 1.755 ± 0.006 | 1293.4 | 1287 |
| 3 | 1-Tetradecene | 0.434 ± 0.019 | 1392.2 | 0.231 ± 0.002 | 1391.6 | 1385 |
| 4 | 1,14-Pentadecadiene | 0.456 ± 0.056 | 1479.2 | 0.714 ± 0.007 | 1477.4 | 1480 |
| 5 | 1-Pentadecene | 77.671 ± 0.906 | 1517 | 57.965 ± 0.240 | 1507.1 | 1486 |
| 8 | 1-Hexadecene | 0.278 ± 0.017 | 1592.6 | 0.183 ± 0.006 | 1591.3 | 1587 |
| 9 | (Z,Z)-1,8,11-Heptadecatriene | 0.083 ± 0.007 | 1663.3 | 0.054 ± 0.002 | 1662.6 | 1664.6 |
| 10 | Heptadecadiene | 0.318 ± 0.020 | 1670.8 | 0.196 ± 0.002 | 1670.1 | 1671 |
| 11 | Heptadecene | 0.513 ± 0.030 | 1694.4 | 0.354 ± 0.021 | 1693.5 | 1687 |
| 12 | Pentacosene | 0.037 ± 0.001 | 2473.2 | 2488 | ||
| Alkyl disulphides | 0.02% | 1.16% | ||||
| 1 | Methyl n-butyl disulfide | 0.004 ± 0.001 | 1027.8 | 1016 | ||
| 2 | Ethyl n-butyl disulfide | 0.006 ± 0.001 | 1110.9 | 1120 | ||
| 3 | Propyl n-butyl disulfide | 0.004 ± 0.001 | 1202.2 | 1207 | ||
| 4 | Methyl n-heptyl disulfide | 0.007 ± 0.002 | 1269 | 0.206 ± 0.001 | 1268.6 | |
| 5 | Ethyl n-heptyl disulfide | 0.003 ± 0.001 | 1360.4 | 0.028 ± 0.003 | 1359.5 | |
| 6 | Propyl n-heptyl disulfide | 0.002 ± 0.001 | 1425.6 | 0.057 ± 0.002 | 1424.7 | |
| 7 | Butyl n-heptyl disulfide | 0.057 ± 0.001 | 1524.4 | |||
| 8 | Pentyl n-heptyl disulfide | 0.019 ± 0.002 | 1552.2 | |||
| 9 | Diheptyl disulfide | 0.007 ± 0.001 | 1738.5 | 0.776 ± 0.003 | 1738.2 | |
| Aldehydes | 0.001% | 0.18% | ||||
| 1 | Phenylacetaldehyde | 0.001 ± 0.000 | 1041 | 0.105 ± 0.004 | 1040.6 | 1048 |
| 2 | Hexadecanal | 0.079 ± 0.005 | 1815.7 | 1820 | ||
| Alcohols | ||||||
| 1 | 1-Heptanol | 0.003 ± 0.001 | 1090 | 0.007 ± 0.001 | 1089.4 | 1092 |
| Quinones | 0.00% | 0.11% | ||||
| 3 | Ethyl-1,4-benzoquinone | 0.083 ± 0.002 | 1102.6 | |||
| 6 | 2-Ethylhydroquinone | 0.026 ± 0.003 | 1437.9 | 1427 | ||
| Carboxylic acids and derivatives | 0.58% | 13.12% | ||||
| 1 | 2,4-Dimethyl-5-hexanolide | 0.002 ± 0.000 | 1181.6 | 0.020 ± 0.001 | 1180.1 | |
| 2 | Dodecanoic acid | 0.012 ± 0.001 | 1567.2 | 1556 | ||
| 3 | n-Hexyl salicylate | 0.004 ± 0.001 | 1677.4 | 1684 | ||
| 4 | Myristic acid | 0.263 ± 0.019 | 1767.9 | 1765 | ||
| 5 | Ethyl myristate | 0.021 ± 0.002 | 1794.2 | 1780 | ||
| 6 | Methyl palmitate | 0.021 ± 0.001 | 1929 | 0.033 ± 0.001 | 1928.5 | 1927 |
| 7 | Pentadecanoic acid | 0.065 ± 0.014 | 1945.8 | 1942 | ||
| 8 | Palmitic acid | 0.219 ± 0.031 | 1967.6 | 6.475 ± 0.160 | 1982.2 | 1964 |
| 9 | Ethyl palmitate | 0.022 ± 0.002 | 1996.5 | 0.212 ± 0.002 | 1996.2 | 1982 |
| 10 | Linolenic acid | 0.029 ± 0.002 | 2058.6 | 2102 | ||
| 11 | γ-Palmitolactone | 0.086 ± 0.005 | 2104.4 | 2106 | ||
| 12 | Linoleic acid | 2.795 ± 0.142 | 2148.2 | 2140 | ||
| 13 | Oleic Acid | 1.724 ± 0.026 | 2154 | 2140 | ||
| 14 | Ethyl-9,12-octadecadienoate | 0.066 ± 0.008 | 2165.9 | 0.345 ± 0.019 | 2164.2 | |
| 15 | Ethyl oleate | 0.015 ± 0.001 | 2170.9 | 0.364 ± 0.069 | 2170.9 | 2149 |
| 16 | Stearic acid | 0.077 ± 0.004 | 2175 | 0.511 ± 0.013 | 2173.5 | 2179 |
| 17 | Ethyl stearate | 0.039 ± 0.008 | 2196.3 | 2180 | ||
| 18 | Stearyl acetate | 0.156 ± 0.017 | 2213.2 | 0.119 ± 0.002 | 2211.3 | 2211 |
| Aromatic compounds | 0.00% | 0.04% | ||||
| 5 | Benzothiazole | 0.014 ± 0.003 | 1220.9 | 1221 | ||
| 6 | 6-tert-Butyl-3-Methylanisole | 0.026 ± 0.001 | 1235.7 | |||
| No. | Compounds | EOUd1 | EOUd2 | KI Ref | ||
|---|---|---|---|---|---|---|
| RA (%) | KI Exp | RA (%) | KI Exp | |||
| Carboxylic acids and derivatives | 0.60% | 2.71% | ||||
| 19 | Butanoic acid | 0.04 ± 0.005 | 871.5 | 891 | ||
| 20 | Valeric acid | 0.010 ± 0.000 | 982.4 | 975 | ||
| 21 | Peracetic acid | 0.030 ± 0.007 | 1006 | |||
| 22 | Lactic acid | 0.058 ± 0.001 | 1070.3 | 0.056 ± 0.001 | 1072.5 | 1057 |
| 23 | Caproic acid | 0.009 ± 0.001 | 1076 | 0.032 ± 0.002 | 1078.1 | 1071 |
| 24 | 2-Ethylhexanoic acid | 0.002 ± 0.001 | 1168 | 0.011 ± 0.001 | 1168.7 | |
| 25 | Heptanoic acid | 0.006 ± 0.000 | 1184.9 | 0.047 ± 0.002 | 1185.9 | 1166 |
| 26 | Benzoic acid | 0.089 ± 0.001 | 1247 | 0.028 ± 0.001 | 1247.4 | 1232 |
| 27 | 2-Octanoic acid | 0.001 ± 0.006 | 1322.5 | 1313.2 | ||
| 28 | Succinic acid | 0.007 ± 0.001 | 1325.6 | 0.213 ± 0.027 | 1325.6 | 1314 |
| 29 | Propionylglycine | 0.006 ± 0.001 | 1333.9 | 1341 | ||
| 30 | Nonanoic acid | 0.019 ± 0.004 | 1366.2 | 0.038 ± 0.002 | 1366.2 | 1358 |
| 31 | Decanoic acid | 0.03 ± 0.006 | 1468.1 | 0.049 ± 0.003 | 1467 | 1455 |
| 32 | m-Hydroxybenzoic acid | 0.009 ± 0.001 | 1528 | 0.553 ± 0.007 | 1526.4 | 1559 |
| 33 | 10-Undecenoic acid | 0.006 ± 0.001 | 1545.2 | 1542.2 | ||
| 34 | Pimelic acid | 0.003 ± 0.001 | 1614.2 | 0.001 ± 0.000 | 1614.9 | 1608 |
| 35 | Suberic acid | 0.012 ± 0.004 | 1710.3 | 1689 | ||
| 36 | Tridecanoic acid | 0.008 ± 0.001 | 1755.3 | 1748 | ||
| 37 | Azelaic acid | 0.061 ± 0.019 | 1806.9 | 0.041 ± 0.022 | 1807.7 | 1787 |
| 38 | β-Resorcylic acid | 0.005 ± 0.002 | 1833.9 | 1822 | ||
| 39 | 9-Tetradecenoic acid | 0.018 ± 0.003 | 1841.4 | |||
| 40 | Tetradecanoic acid | 0.231 ± 0.004 | 1854.1 | 1.138 ± 0.025 | 1856.2 | 1845 |
| 41 | Sebacic acid | 0.005 ± 0.006 | 1907 | 0.002 ± 0.000 | 1907.5 | 1920 |
| 42 | Pentadecanoic acid | 0.004 ± 0.001 | 1925.1 | 0.015 ± 0.001 | 1924.8 | 1942 |
| 43 | 13-methyltetradec-9-enoic acid | 0.010 ± 0.001 | 1946.9 | |||
| 44 | 9-Hexadecenoic acid | 0.002 ± 0.001 | 1974.5 | 1977 | ||
| 45 | cis-9-Hexadecenoic acid | 0.005 ± 0.001 | 2024.9 | 0.100 ± 0.003 | 2023.7 | 2017 |
| 46 | cis-10-Heptadecenoic acid | 0.002 ± 0.001 | 2127.5 | 2126.2 | ||
| 47 | Margaric acid | 0.041 ± 0.006 | 2152.6 | 0.085 ± 0.002 | 2152 | 2140 |
| 48 | cis-11,14-Eicosadienoic acid | 0.036 ± 0.001 | 2414.8 | 2413.2 | ||
| 49 | cis-11-Eicosenoic acid | 0.014 ± 0.001 | 2420.2 | 2419.7 | ||
| 50 | Arachidic acid | 0.039 ± 0.002 | 2447 | 2437 | ||
| 51 | 1-Monopalmitin | 0.007 ± 0.001 | 2608.9 | 2606 | ||
| 52 | Docosanoic acid | 0.016 ± 0.002 | 2645 | 2638 | ||
| 53 | Triacontadienoic acid | 0.033 ± 0.005 | 3433.1 | |||
| 54 | Dotriacontadienoic acid | 0.025 ± 0.005 | 3639.9 | |||
| Alcohols | 0.18% | 1.15% | ||||
| 2 | 2,2-Dimethyl-3-pentanol | 0.025 ± 0.001 | 993.8 | |||
| 3 | Furfuryl alcohol | 0.102 ± 0.017 | 1003.8 | |||
| 4 | 2,4-Dimethyl-3-pentanol | 0.011 ± 0.000 | 1009.8 | 975.3 | ||
| 5 | 3-heptanol | 0.016 ± 0.001 | 1018.7 | 0.095 ± 0.010 | 1018.7 | |
| 6 | 2-heptanol | 0.034 ± 0.014 | 1025.2 | 0.394 ± 0.018 | 1024.8 | 1008.9 |
| 7 | 2,3-Butanediol | 0.282 ± 0.006 | 1044 | 1040 | ||
| 1 | 1-Heptanol | 0.004 ± 0.001 | 1088.4 | 0.019 ± 0.002 | 1090 | 1092 |
| 8 | 3-Ethylphenol | 0.001 ± 0.001 | 1223.1 | 0.025 ± 0.002 | 1223.1 | 1220 |
| 9 | 4-hydroxybenzenemethanol | 0.003 ± 0.001 | 1520.3 | 1500 | ||
| 10 | 1-Dodecanol | 0.046 ± 0.004 | 1574.5 | 0.070 ± 0.003 | 1574.3 | 1575 |
| 11 | 1-Tetradecanol | 0.039 ± 0.002 | 1768.5 | 0.035 ± 0.002 | 1768.7 | 1768 |
| 12 | 1-Pentadecanol | 0.008 ± 0.000 | 1868.1 | 1866 | ||
| 13 | 2-Pentadecanol | 0.001 ± 0.001 | 1879.4 | |||
| 14 | 1-Hexadecanol | 0.028 ± 0.003 | 1966.4 | 0.022 ± 0.002 | 1966.6 | 1965 |
| 15 | 1-Heptadecanol | 0.013 ± 0.000 | 2069.5 | 2856 | ||
| 16 | Oleyl alcohol | 0.017 ± 0.003 | 2136.3 | 2126 | ||
| 17 | 1-Hexacosanol | 0.007 ± 0.001 | 2949 | 2950 | ||
| 18 | 1-Octacosanol | 0.016 ± 0.004 | 3149 | 3148 | ||
| 19 | 1-Dotriacontanol | 0.019 ± 0.004 | 3532.9 | 3529.9 | ||
| Quinones | 0.01% | 0.05% | ||||
| 4 | Hydroquinone | 0.008 ± 0.001 | 1409.7 | 0.049 ± 0.002 | 1408.4 | 1400 |
| Terpenes | 2.97% | 2.46% | ||||
| 29 | Myrtenoic acid | 0.014 ± 0.000 | 1535.4 | |||
| 30 | 18-Norabieta-8,11,13-triene | 0.004 ± 0.001 | 1978.2 | |||
| 31 | 10,18-Bisnorabieta-8,11,13-triene | 0.014 ± 0.002 | 2040.9 | |||
| 32 | Allopregnane | 0.009 ± 0.003 | 2204.8 | 2175 | ||
| 33 | Levopimaric acid | 0.012 ± 0.001 | 2262.7 | 0.015 ± 0.003 | 2264.6 | |
| 34 | Pimaric acid | 0.020 ± 0.004 | 2281.7 | 2287 | ||
| 35 | 7-Ethyl-1,4a,7-trimethyl-3,4,4b,5,6,8,10,10a-octahydro-2H-phenanthrene-1-carboxylic acid | 0.015 ± 0.004 | 2293.3 | |||
| 36 | 15-Isobutyl-(13α-H)-isocopalane | 0.110 ± 0.001 | 2294.2 | |||
| 37 | Isopimaric acid | 0.010 ± 0.002 | 2337.1 | 2329 | ||
| 38 | 8-Pimarenic acid | 0.102 ± 0.004 | 2353.8 | |||
| 39 | Abiet-8-en-18-oic acid | 0.179 ± 0.003 | 2371.8 | |||
| 40 | Dehydroabietic acid | 0.451 ± 0.011 | 2394 | 0.831 ± 0.010 | 2391.7 | 2385 |
| 41 | 12α-Hydroxy-5α-pregnane | 0.007 ± 0.003 | 2756 | |||
| 42 | Coprostane | 0.010 ± 0.002 | 2835.6 | 2822 | ||
| 43 | 17.alfa.,21β-28,30-Bisnorhopane | 0.177 ± 0.010 | 2873.4 | 0.005 ± 0.000 | 2858.8 | |
| 44 | Gammacerane | 0.674 ± 0.031 | 3135.1 | 0.019 ± 0.004 | 3122.8 | |
| 45 | Cholesterol | 0.053 ± 0.002 | 3151.5 | 3143 | ||
| 46 | Germanicol | 0.012 ± 0.001 | 3208.6 | |||
| 47 | 3-Epimoretenol | 0.182 ± 0.014 | 3244.3 | |||
| 48 | Campesterol | 0.010 ± 0.001 | 3259 | 3220 | ||
| 49 | Stigmasterol | 0.196 ± 0.014 | 3296.4 | 0.090 ± 0.003 | 3291 | 3274.3 |
| 50 | β-Sitosterol | 0.991 ± 0.014 | 3355.2 | 0.606 ± 0.008 | 3349.8 | 3348 |
| 51 | Fucosterol | 0.286 ± 0.004 | 3370.8 | 0.197 ± 0.007 | 3366.3 | |
| 52 | Aven asterol | 0.015 ± 0.001 | 3421.7 | |||
| 53 | 24-Methylenecycloartenol | 0.07 ± 0.009 | 3463.6 | 0.042 ± 0.004 | 3459.4 | 3460 |
| Aromatic compounds | 0.001% | 0.01% | ||||
| 7 | 2,4-Dihydroxyacetophenone | 0.001 ± 0.001 | 1726.1 | 0.007 ± 0.001 | 1726.4 | 1709.3 |
| Compounds | EOUd1 | EOUd2 | KI Ref | |||
|---|---|---|---|---|---|---|
| RA (%) | KI Exp | RA (%) | KI Exp | |||
| Aldehydes | 0.01% | 0.49% | ||||
| 2 | Hexanal | 0.001 ± 0.001 | 971.8 | 0.013 ± 0.000 | 968.5 | 964 |
| 3 | Heptanal | 0.003 ± 0.000 | 1077.2 | 0.015 ± 0.001 | 1077.2 | 1069 |
| 4 | Benzaldehyde | 0.021 ± 0.001 | 1107.6 | 1200 | ||
| 1 | Phenylacetaldehyde | 0.161 ± 0.001 | 1217.2 | 1194 | ||
| 5 | Nonanal | 0.001±0.001 | 1278.5 | 0.041 ± 0.001 | 1278.9 | 1267 |
| 6 | Decanal | 0.014 ± 0.002 | 1377.4 | 1366 | ||
| 7 | Dodecanal | 0.013 ± 0.000 | 1577.4 | |||
| 8 | Tridecanal | 0.011 ± 0.003 | 1676.5 | |||
| 9 | Tetradecanal | 0.001 ± 0.001 | 1774.8 | 0.051 ± 0.002 | 1774.8 | |
| 10 | Pentadecanal | 0.012 ± 0.001 | 1876.8 | |||
| 11 | Hexadecanal | 0.068 ± 0.004 | 1976.9 | |||
| 12 | Octadecanal | 0.065 ± 0.010 | 2177.4 | |||
| Alkynes | 0.00% | 0.07% | ||||
| 1 | Pentadecine | 0.009 ± 0.002 | 1744.4 | |||
| 2 | Hexadecine | 0.007 ± 0.000 | 1849.8 | |||
| 3 | Octadecine | 0.054 ± 0.002 | 2017 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cázares-Samaniego, P.J.; Castillo, C.G.; Ramos-López, M.A.; González-Chávez, M.M. Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect. Molecules 2021, 26, 6311. https://doi.org/10.3390/molecules26206311
Cázares-Samaniego PJ, Castillo CG, Ramos-López MA, González-Chávez MM. Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect. Molecules. 2021; 26(20):6311. https://doi.org/10.3390/molecules26206311
Chicago/Turabian StyleCázares-Samaniego, Paulina J., Claudia G. Castillo, Miguel A. Ramos-López, and Marco M. González-Chávez. 2021. "Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect" Molecules 26, no. 20: 6311. https://doi.org/10.3390/molecules26206311
APA StyleCázares-Samaniego, P. J., Castillo, C. G., Ramos-López, M. A., & González-Chávez, M. M. (2021). Volatilome and Essential Oil of Ulomoides dermestoides: A Broad-Spectrum Medical Insect. Molecules, 26(20), 6311. https://doi.org/10.3390/molecules26206311






