Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes †
Abstract
:1. Introduction
2. N-Doped Polycyclic Aromatic Hydrocarbons
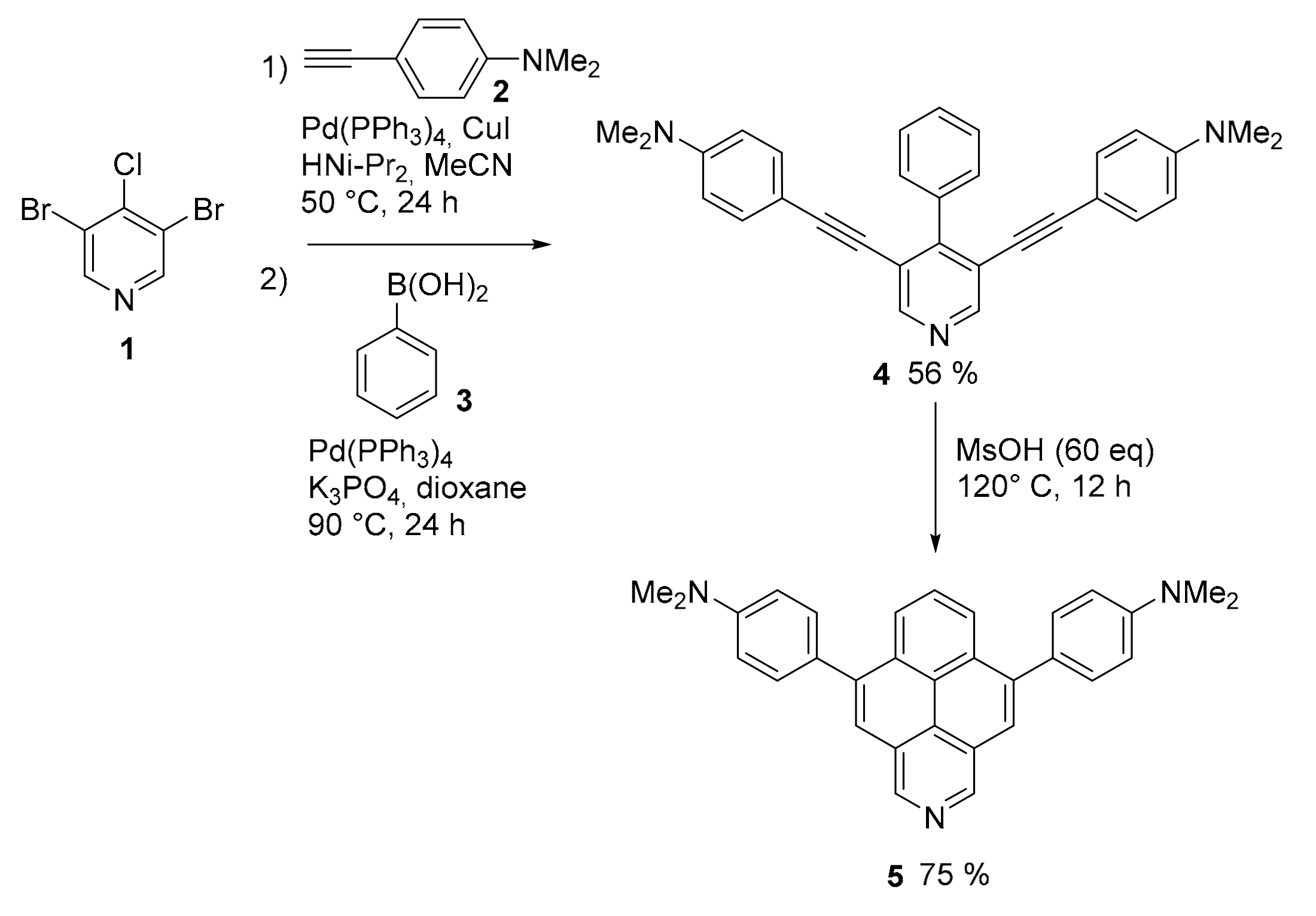
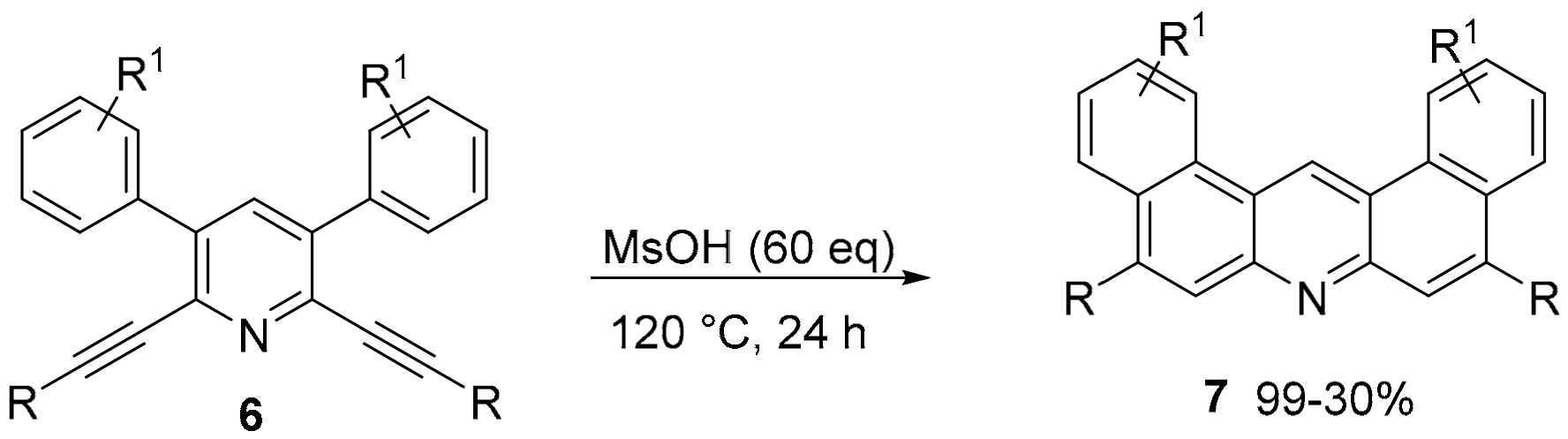
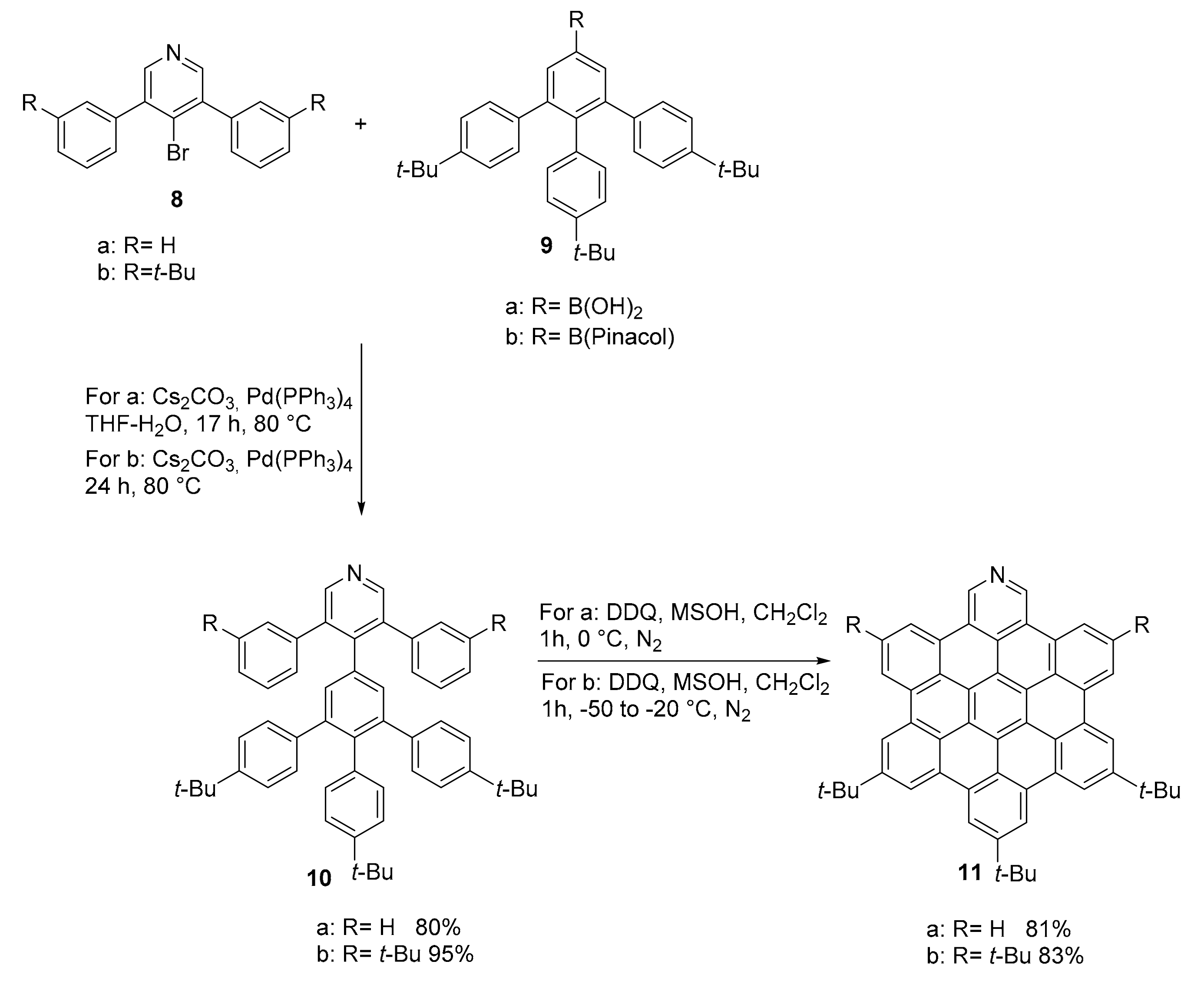
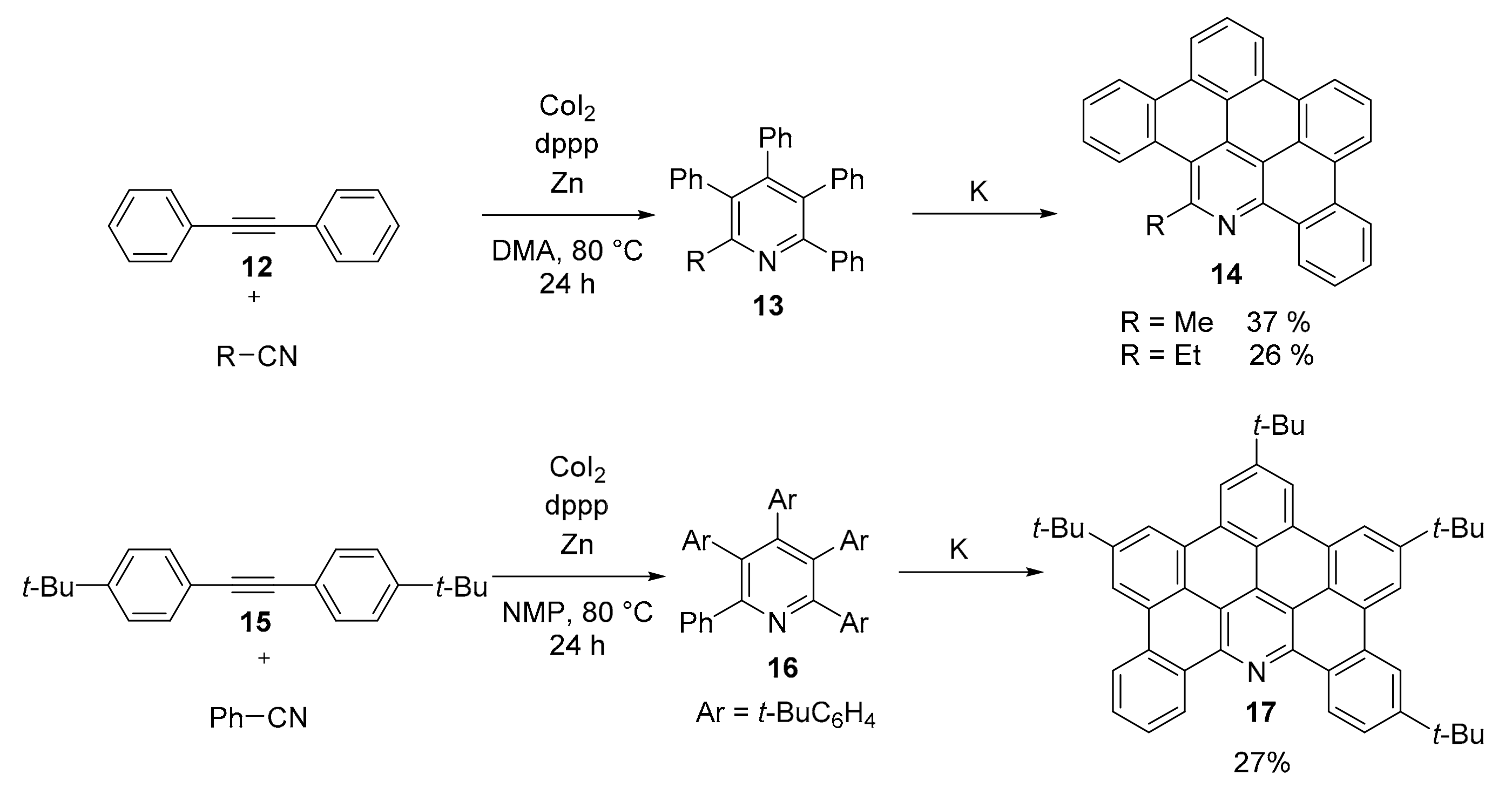
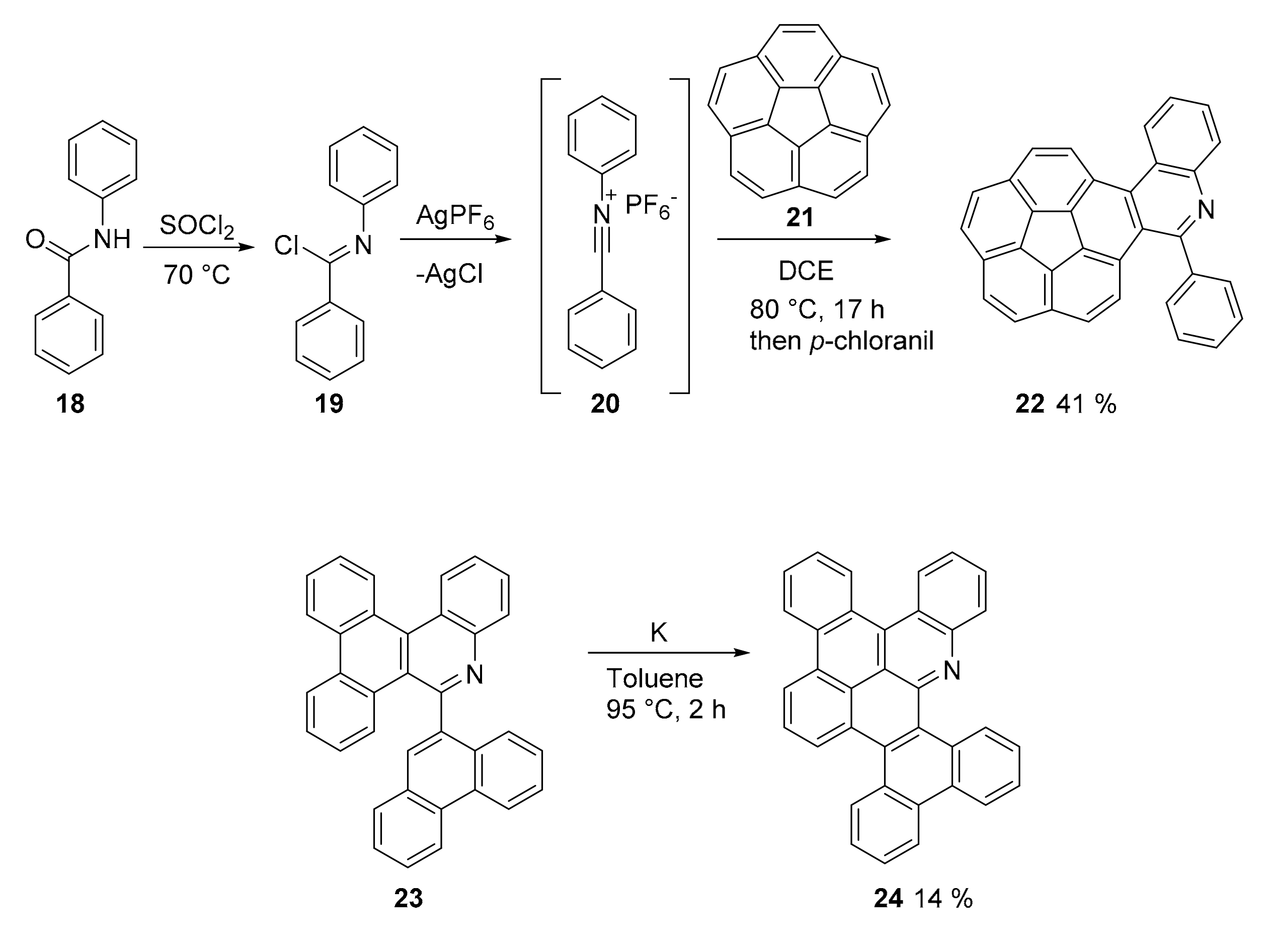
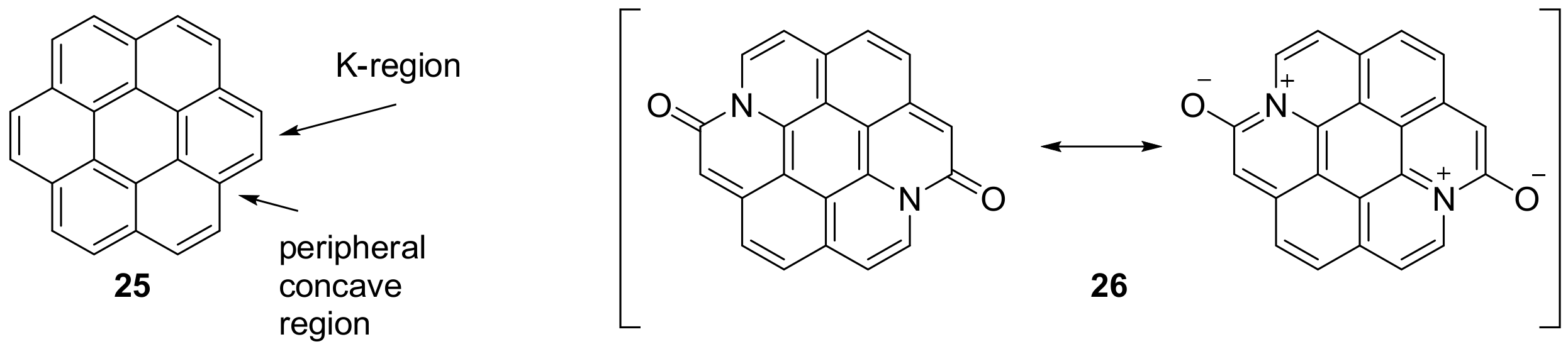
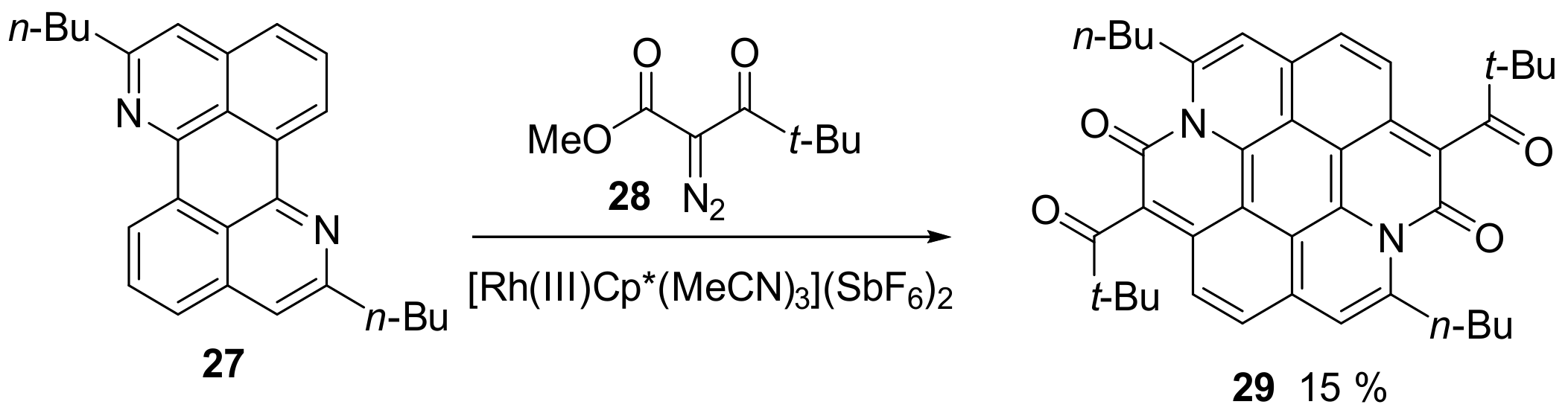
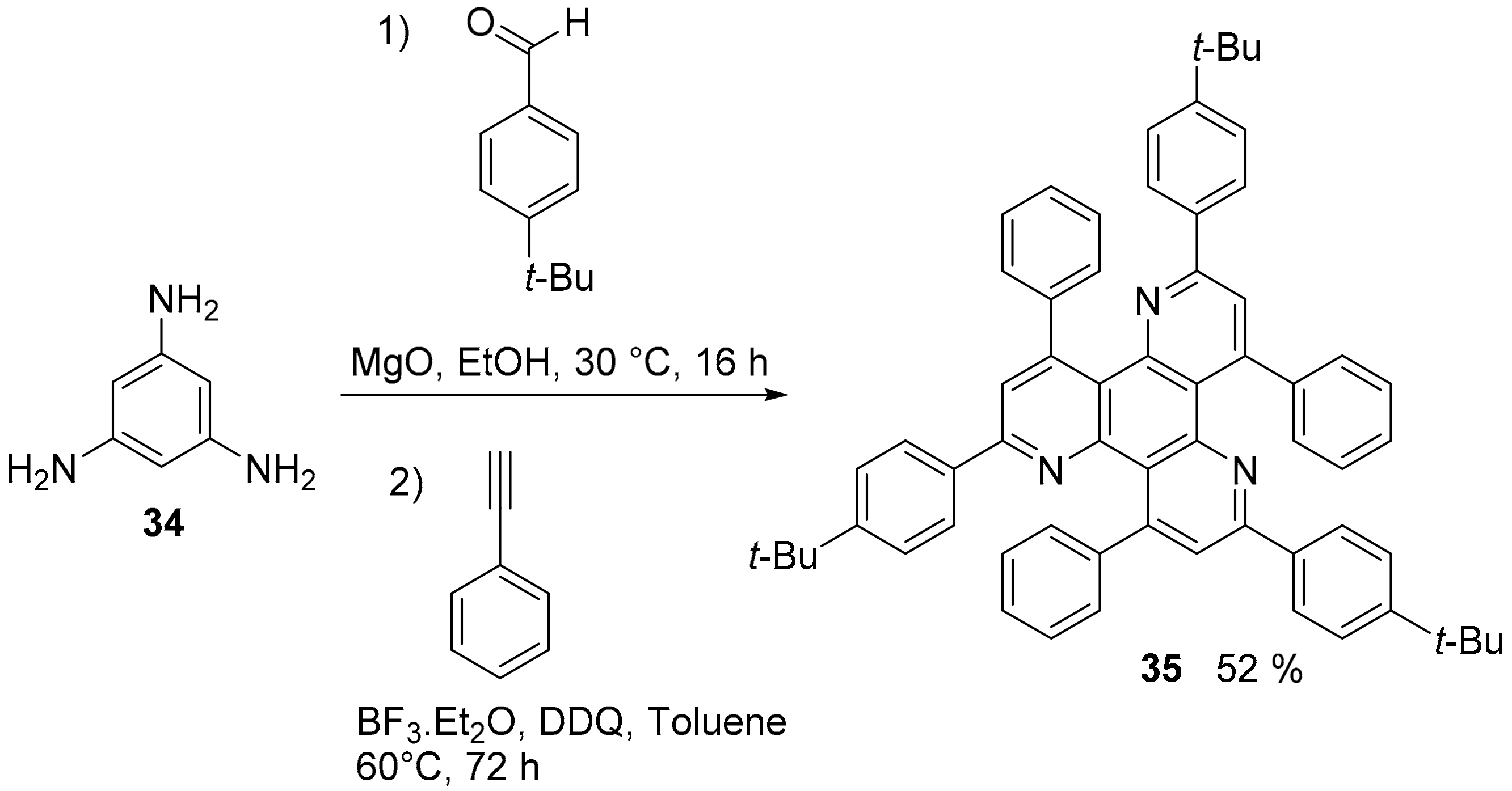
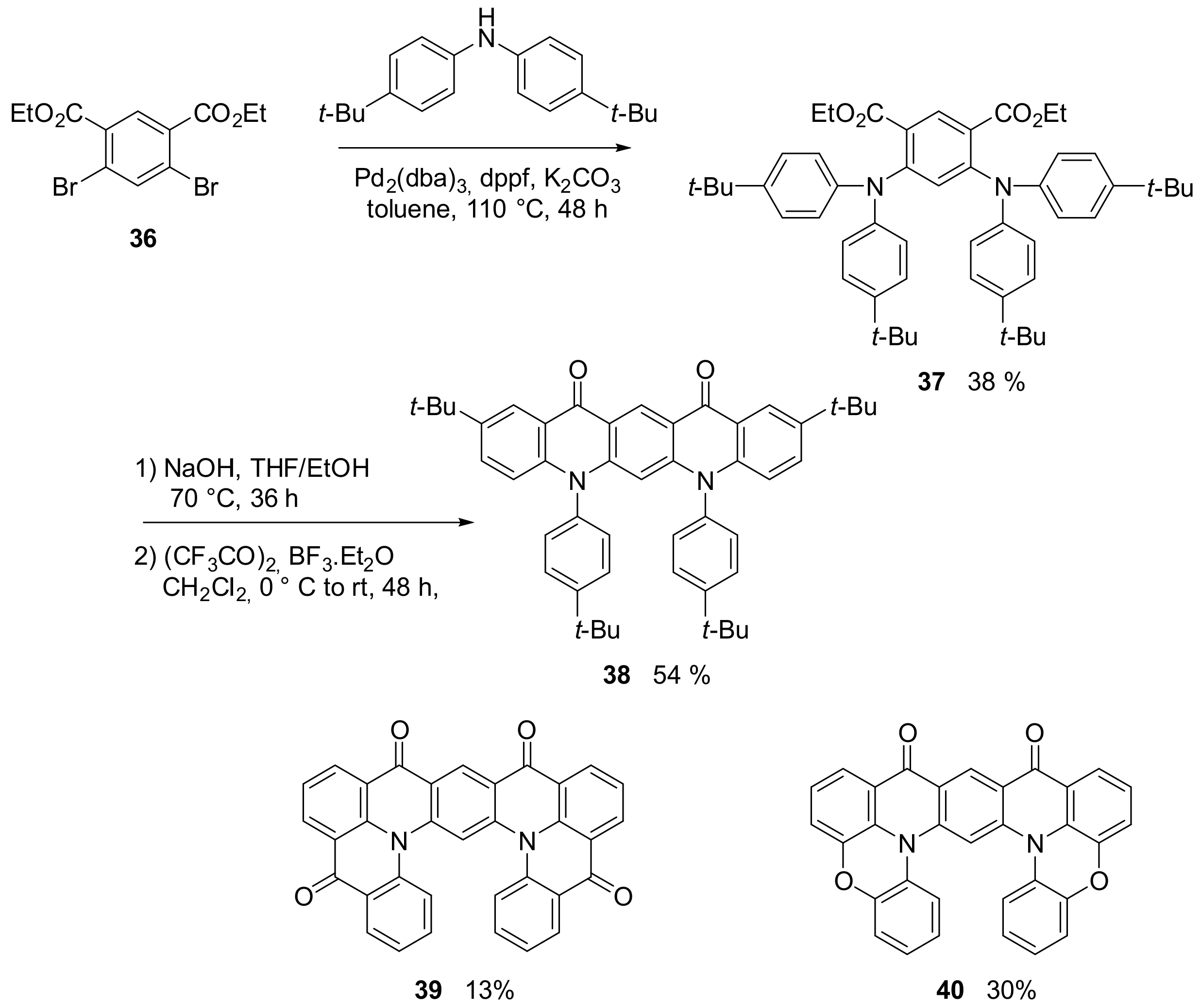
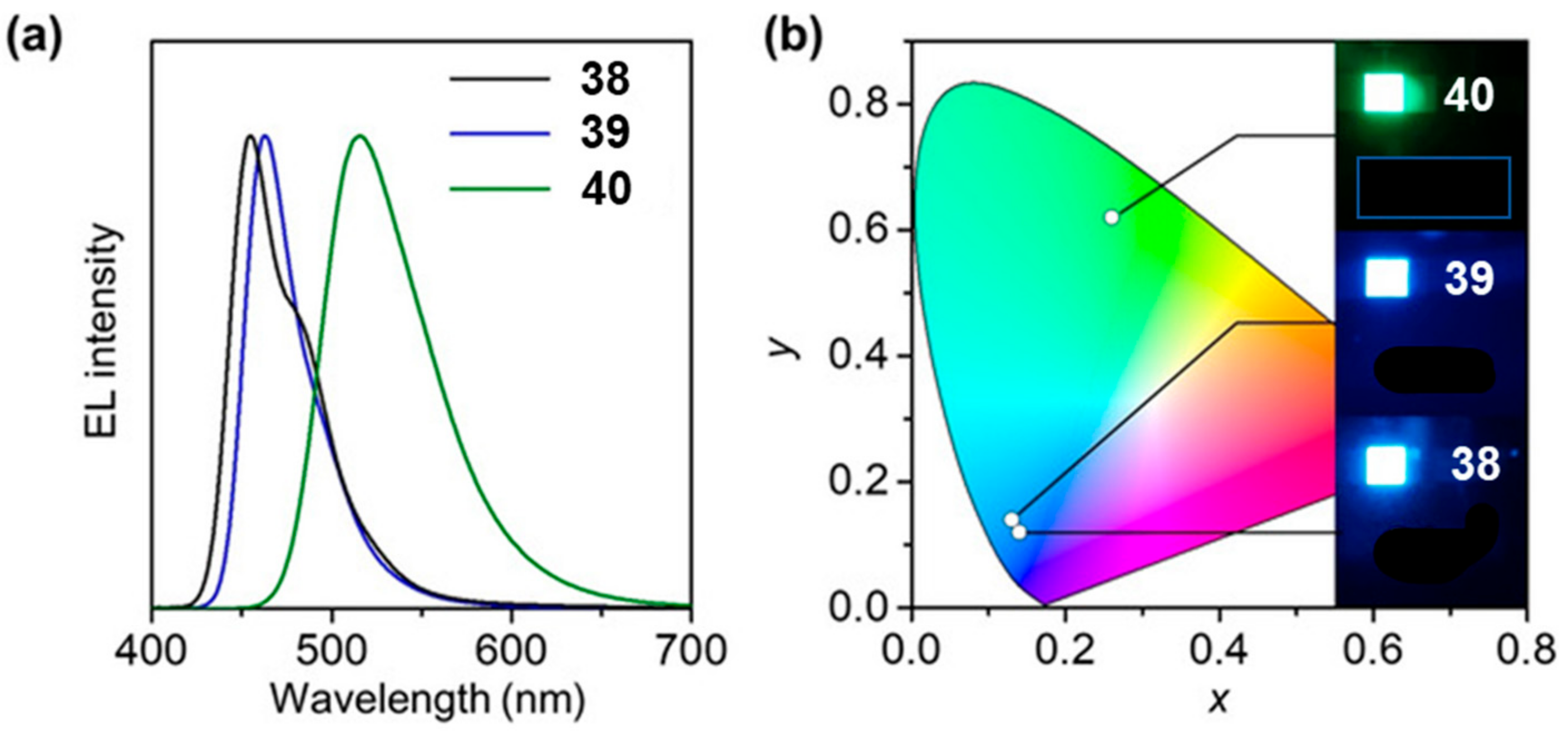
2.1. N-Doped Polycyclic Aromatic Hydrocarbons Embedding Pyridine Rings
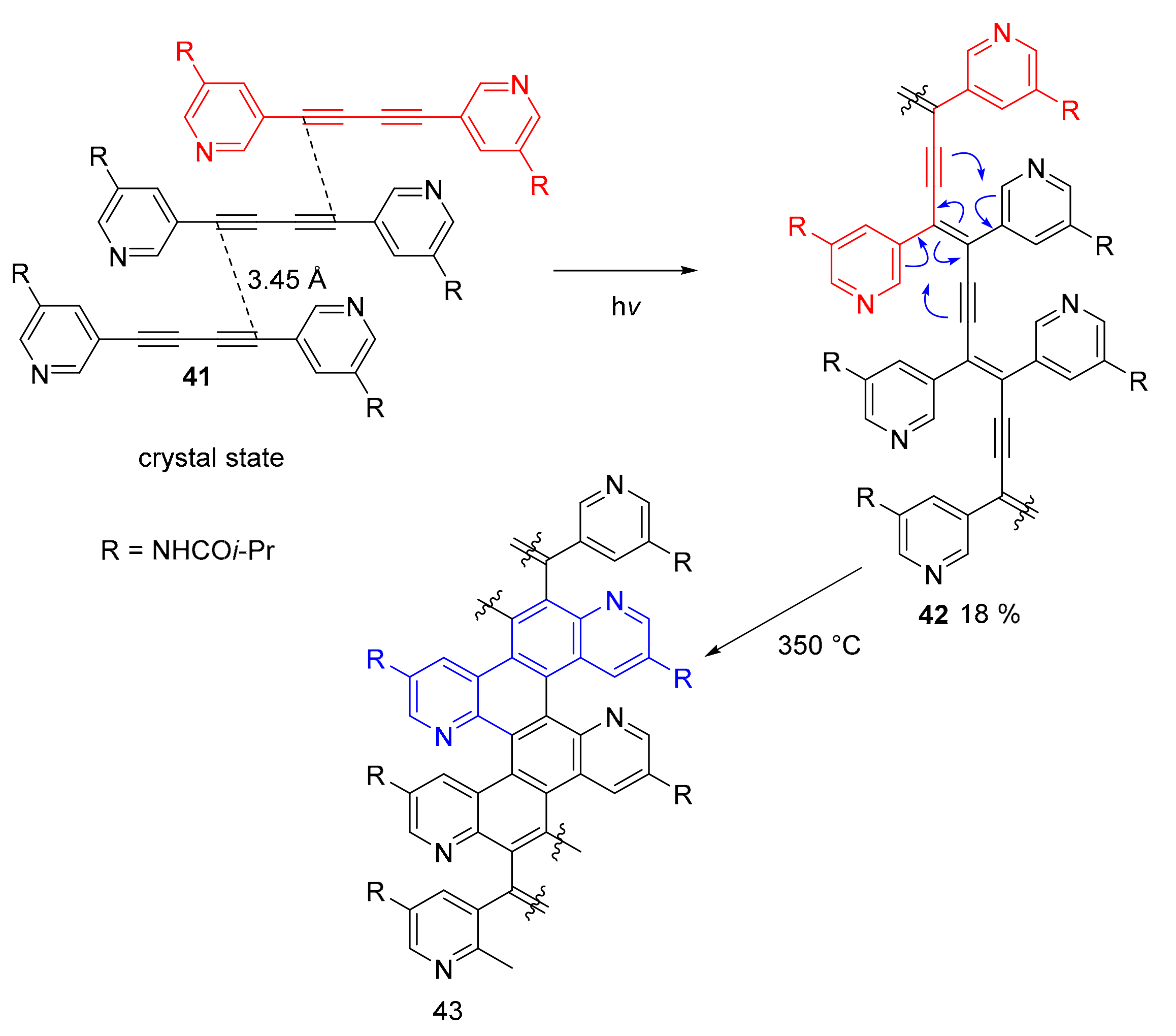
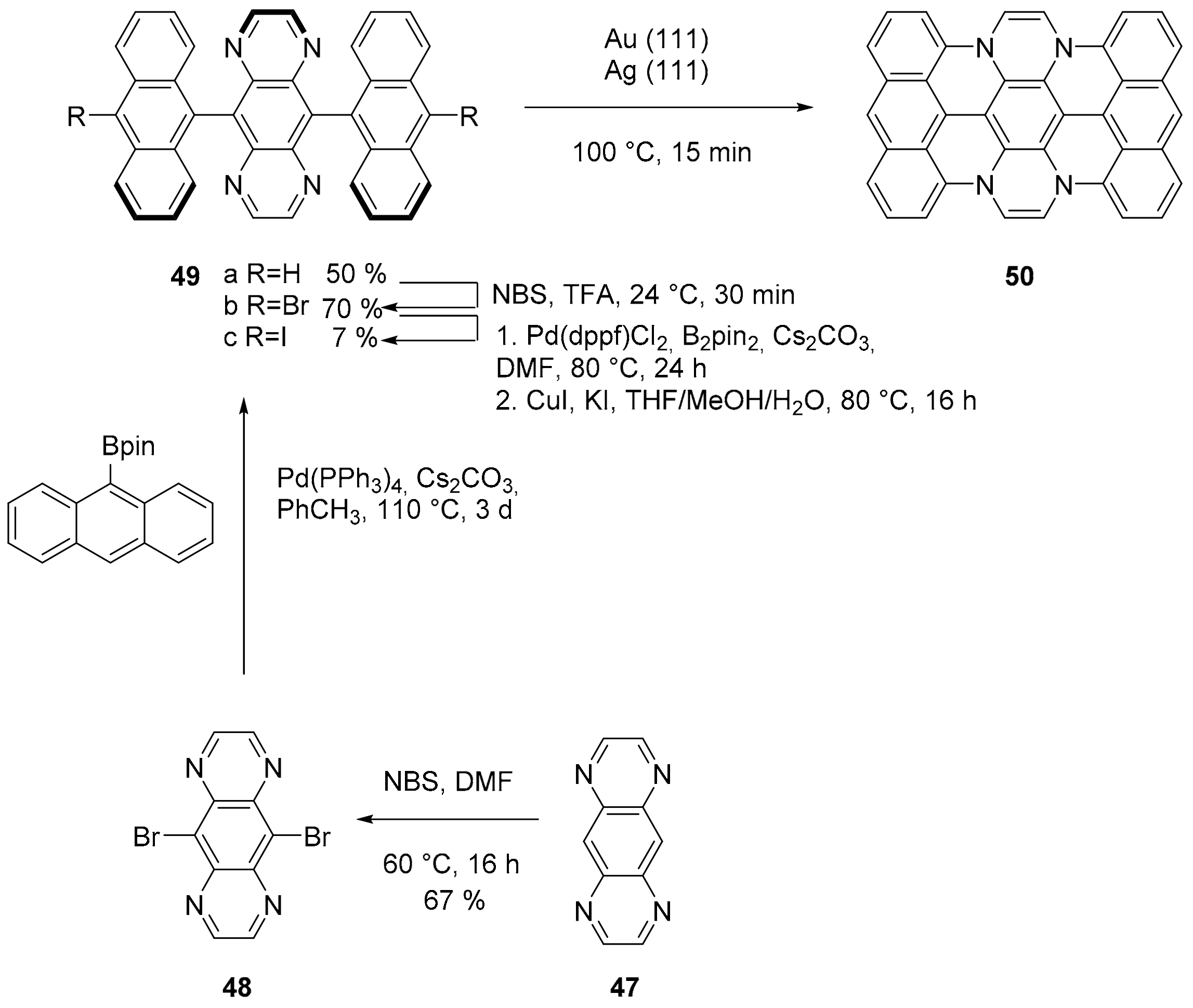
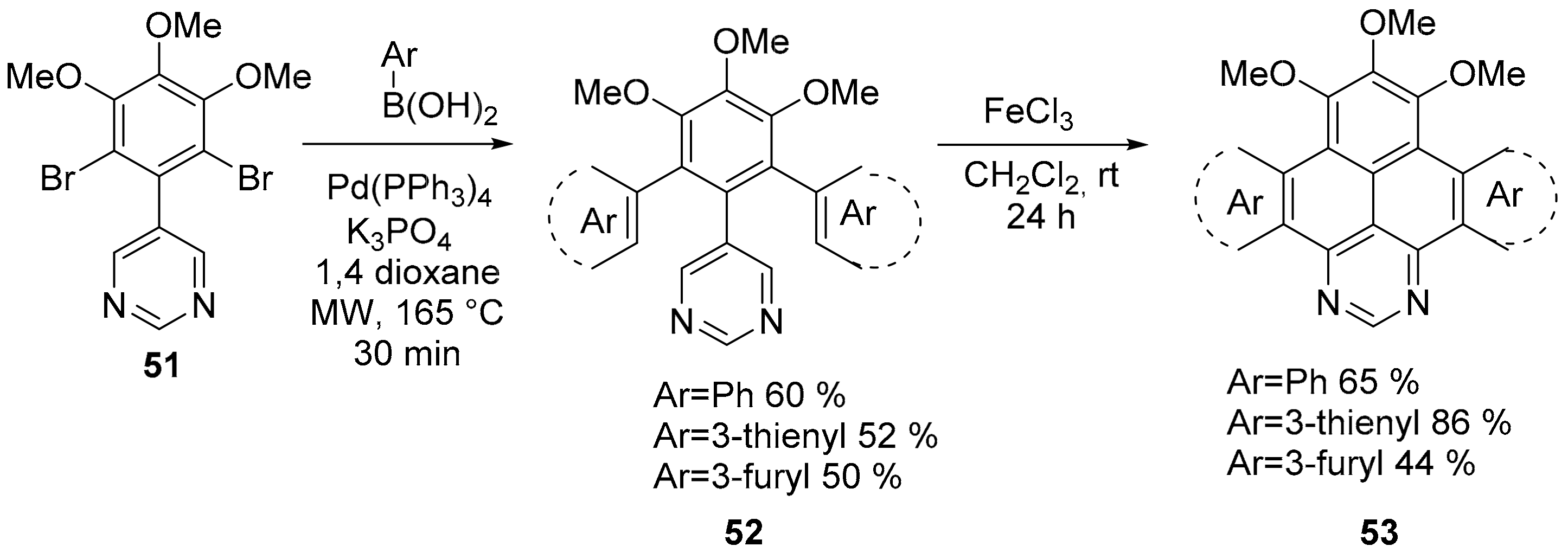
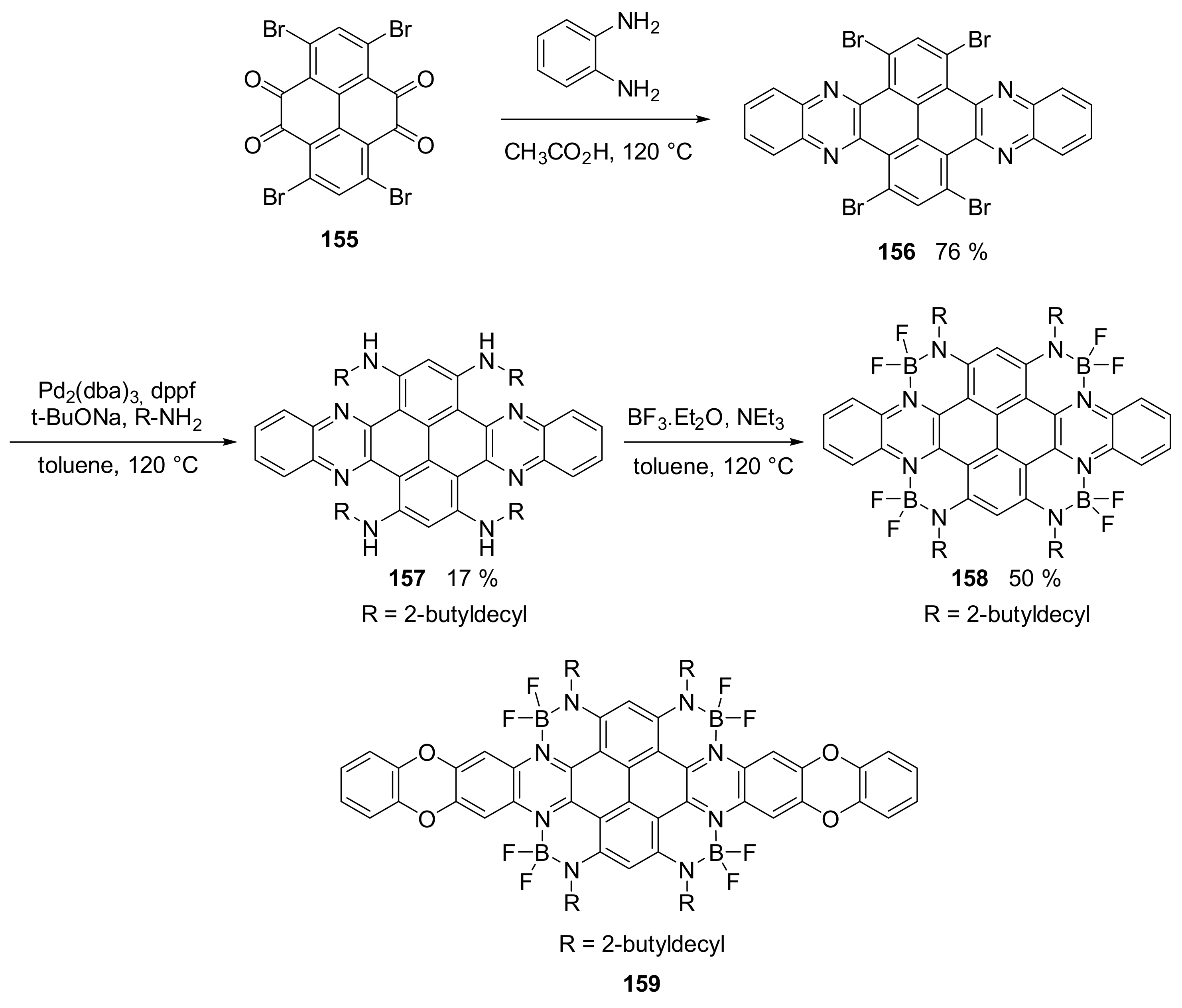
2.2. N-Doped Polycyclic Aromatic Hydrocarbons Embedding Pyrazine or Pyrimidine Rings
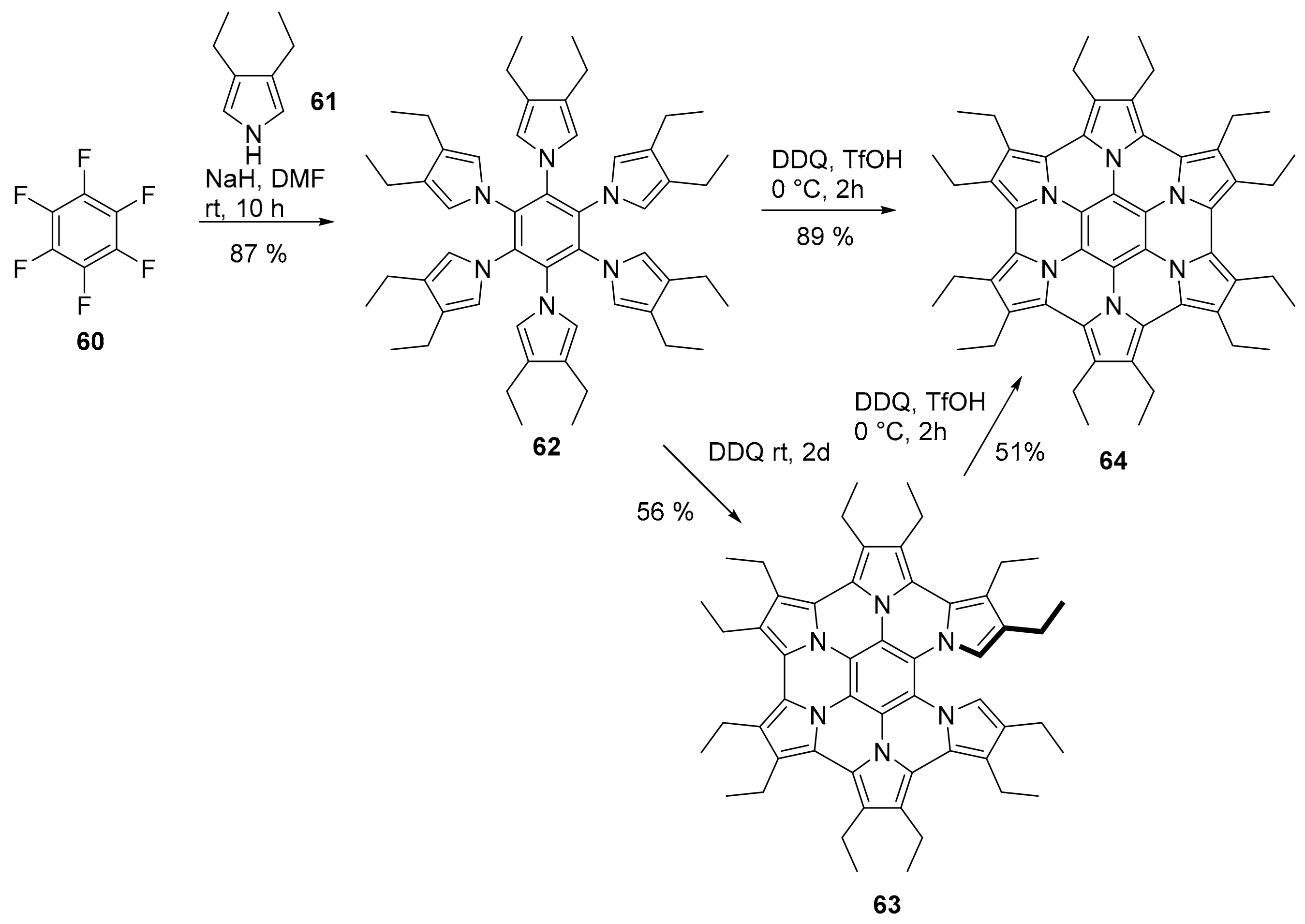
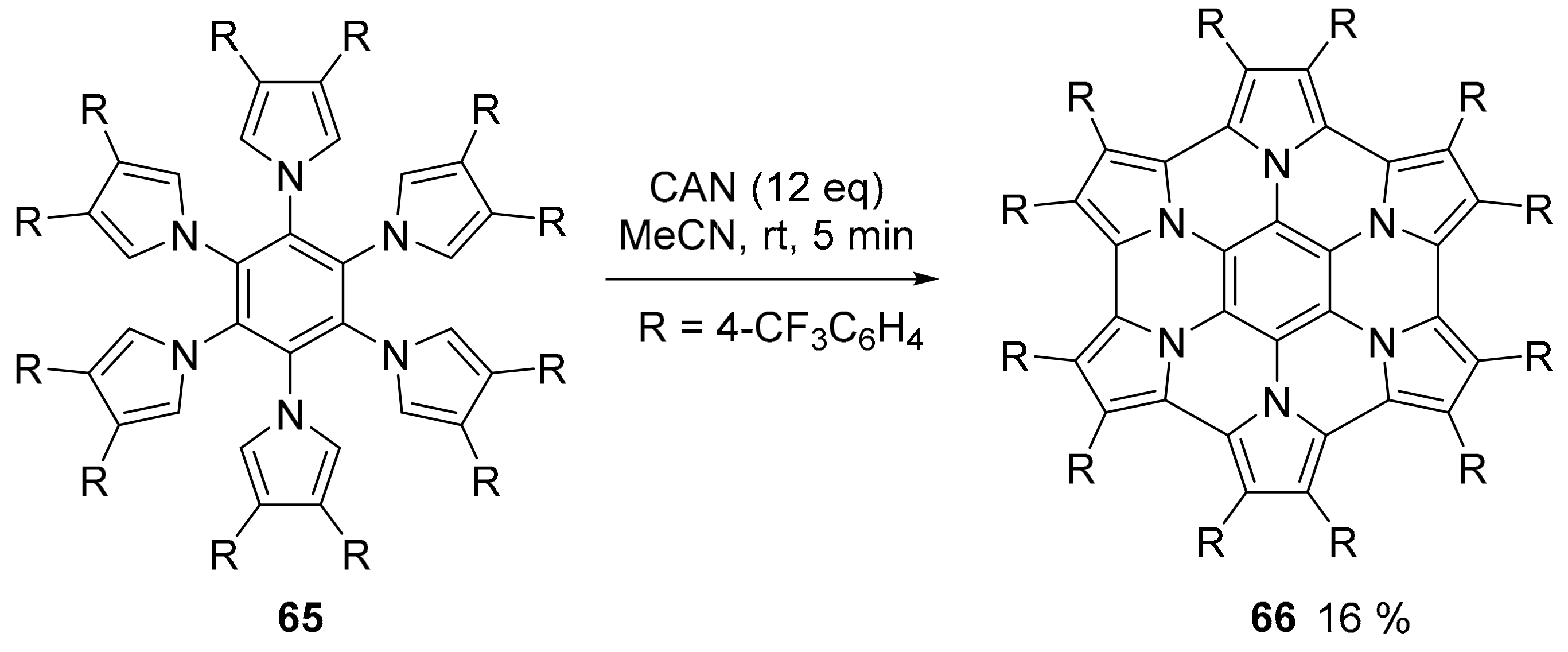
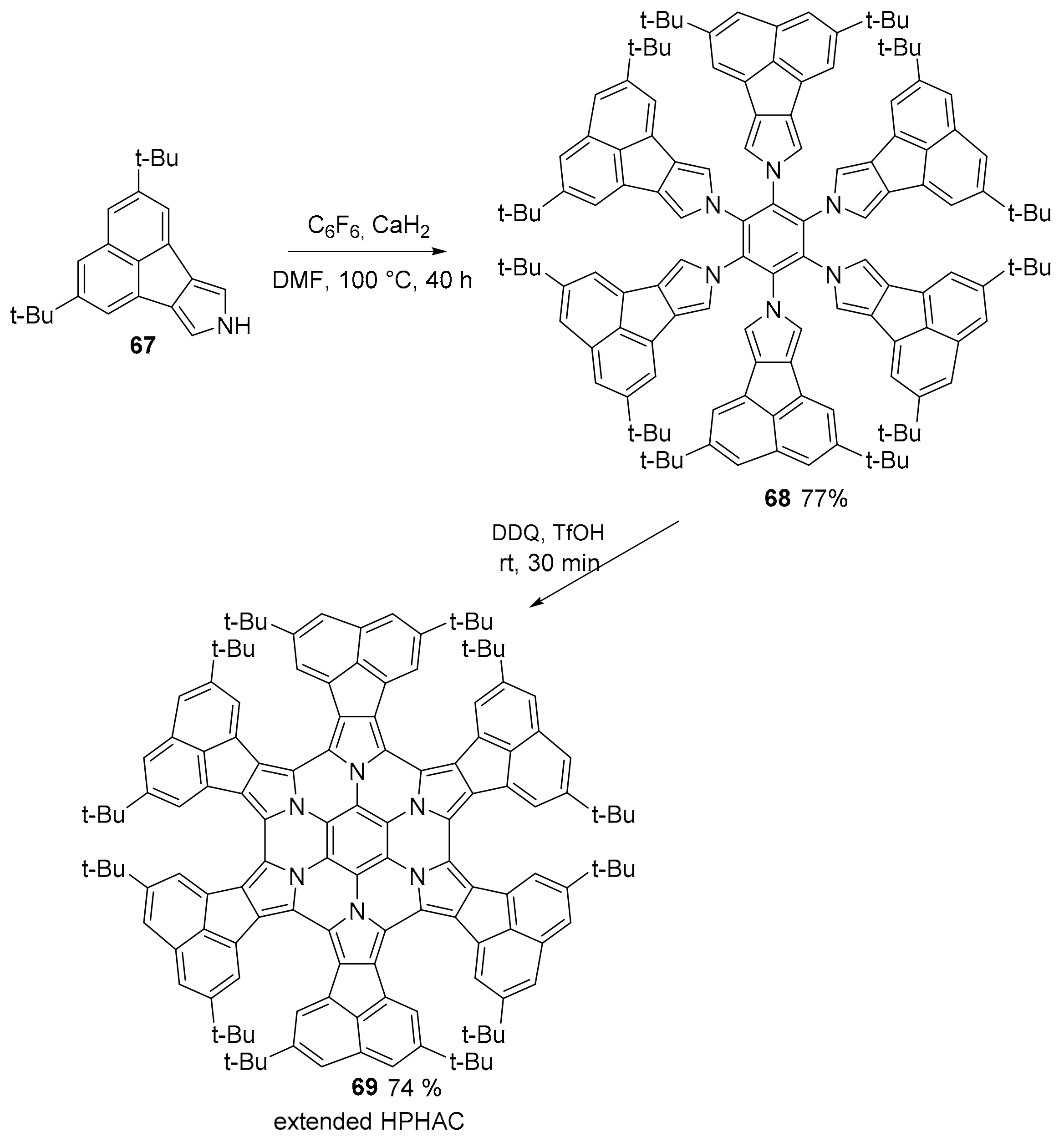
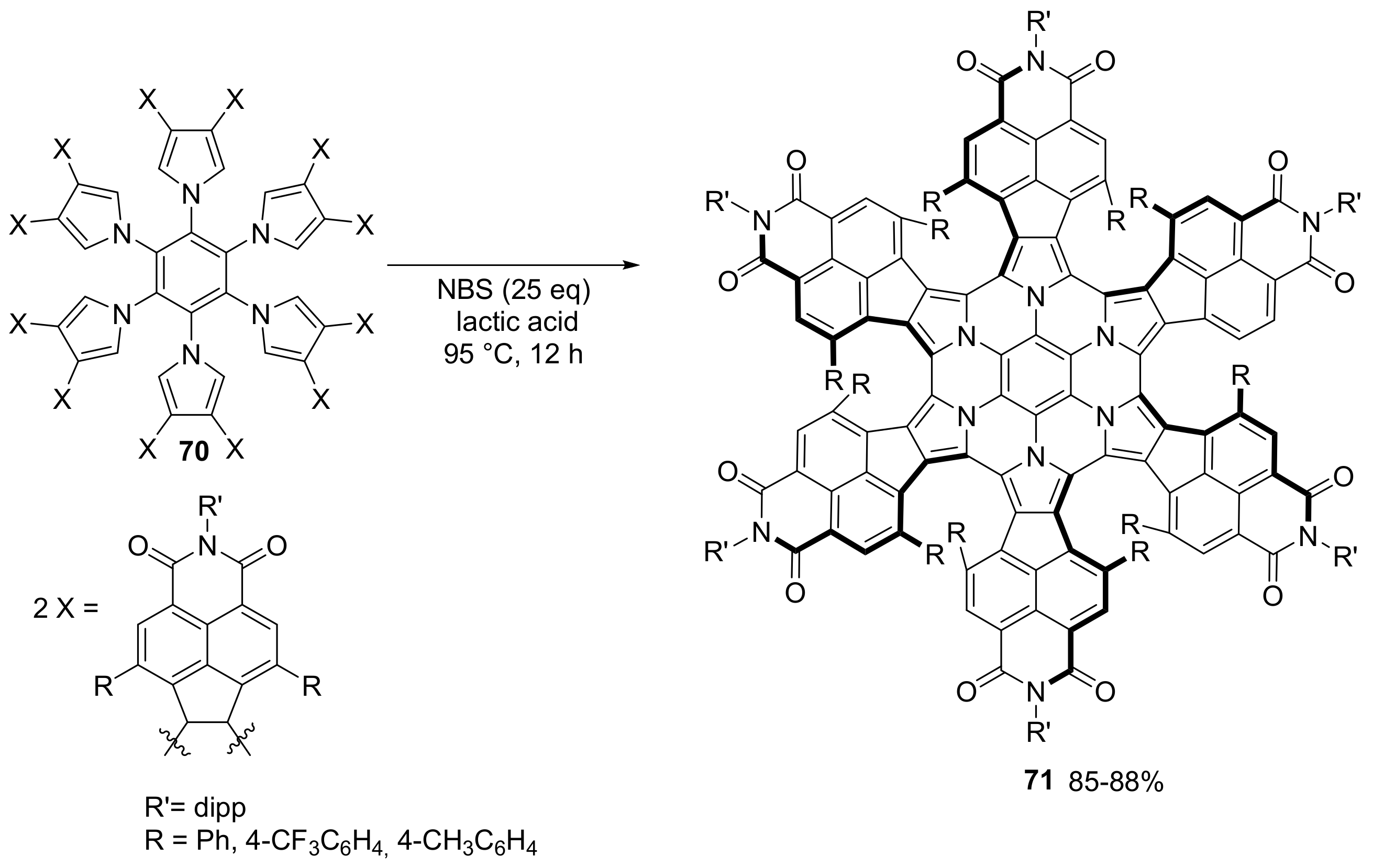
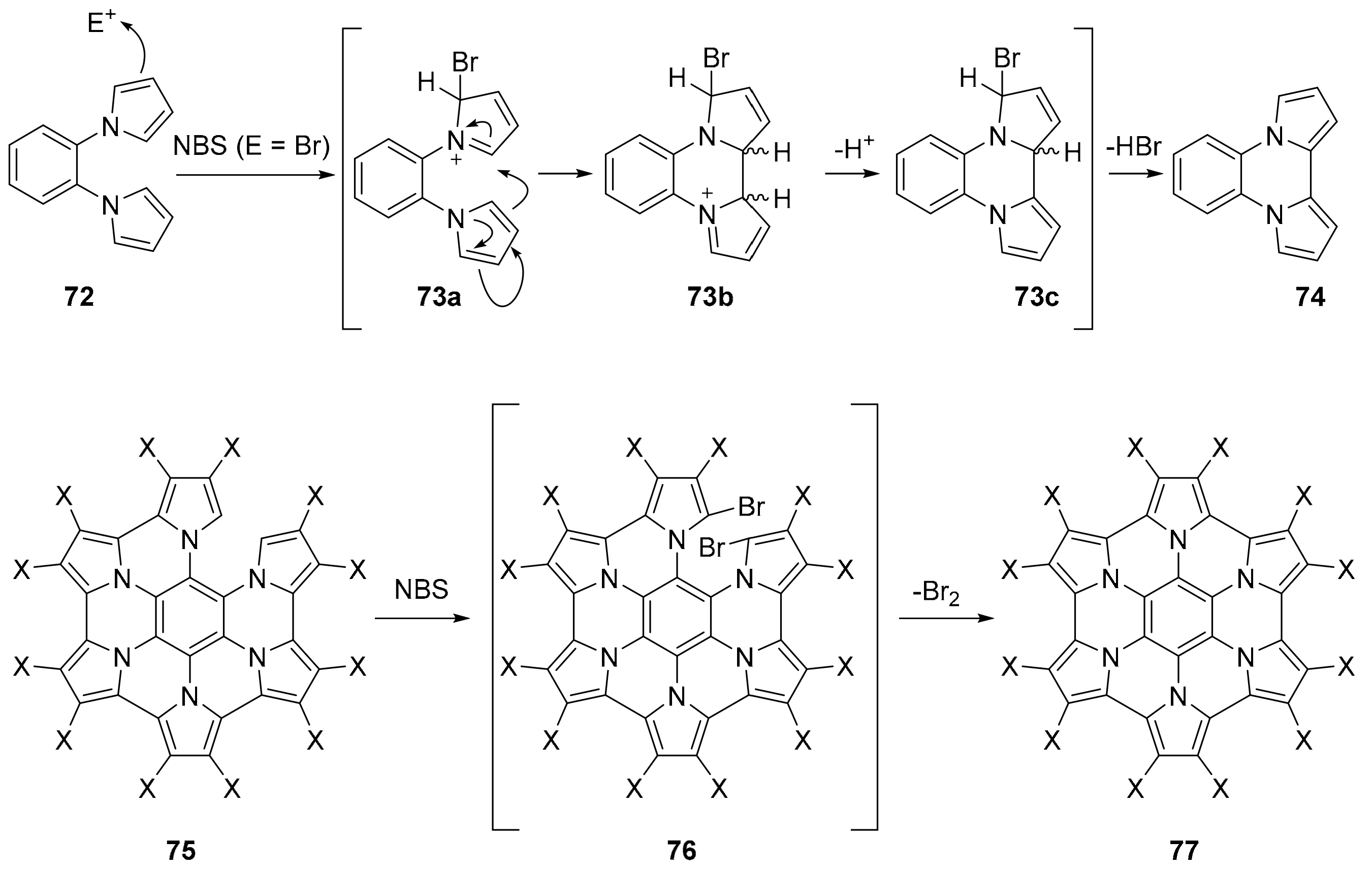
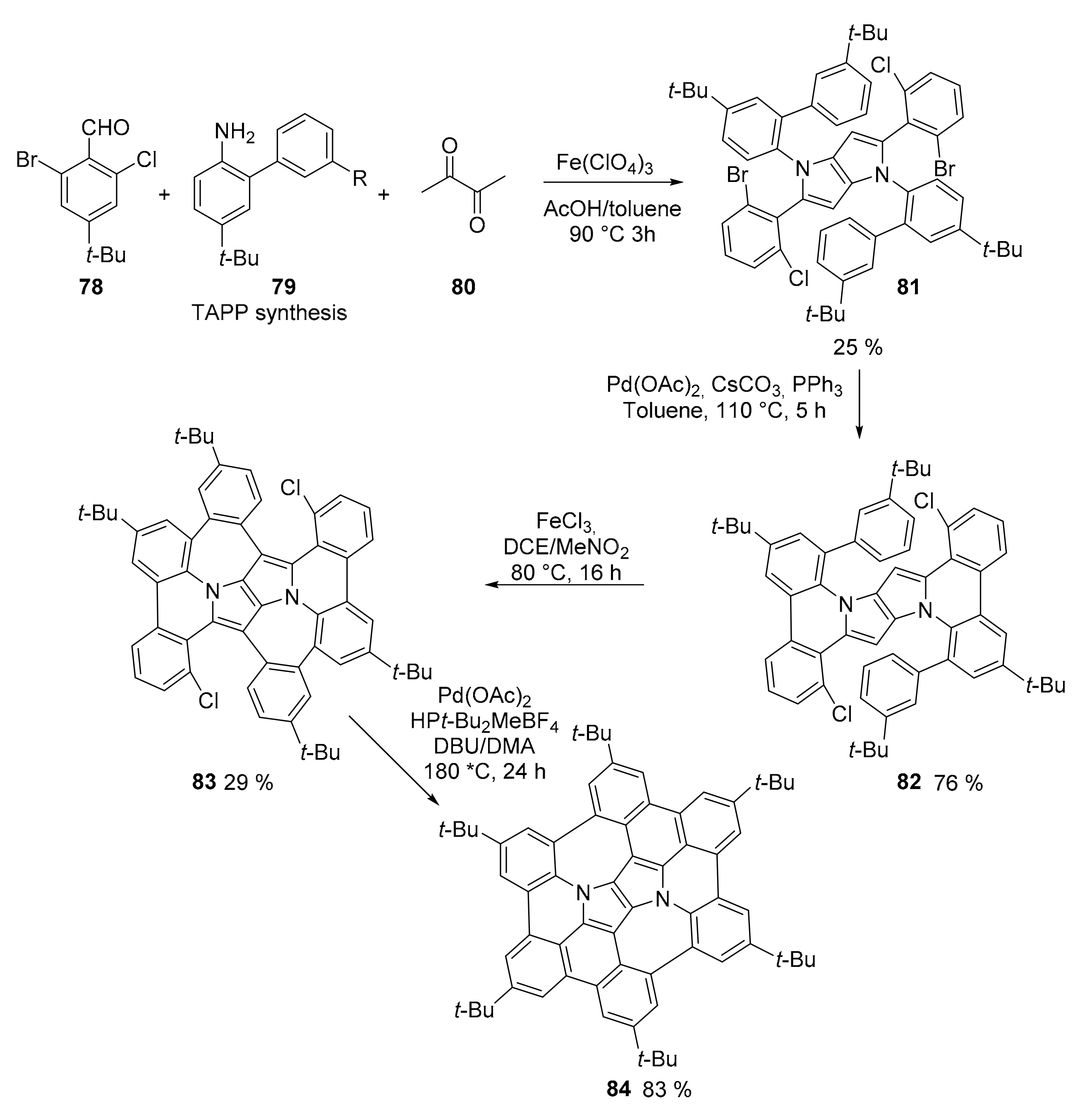
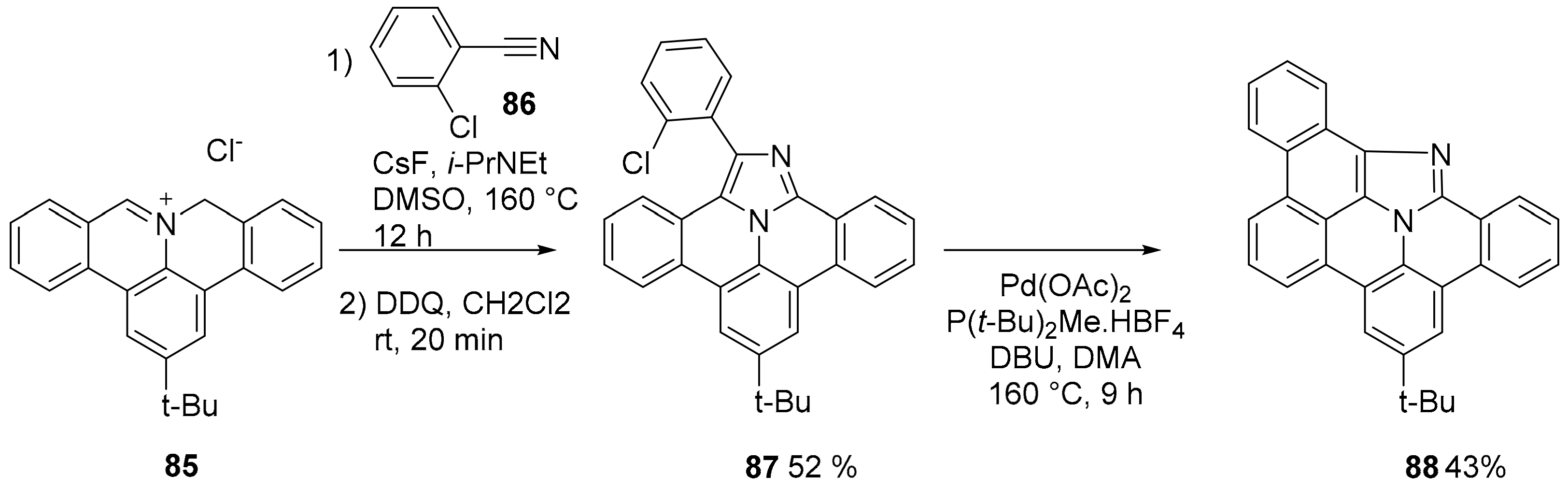
2.3. N-Doped Polycyclic Aromatic Hydrocarbons Embedding Pyrrole or Imidazole Rings
2.4. N-Doped Polycyclic Aromatic Hydrocarbons Embedding An Azepine Ring
3. B-Doped Polycyclic Aromatic Hydrocarbons
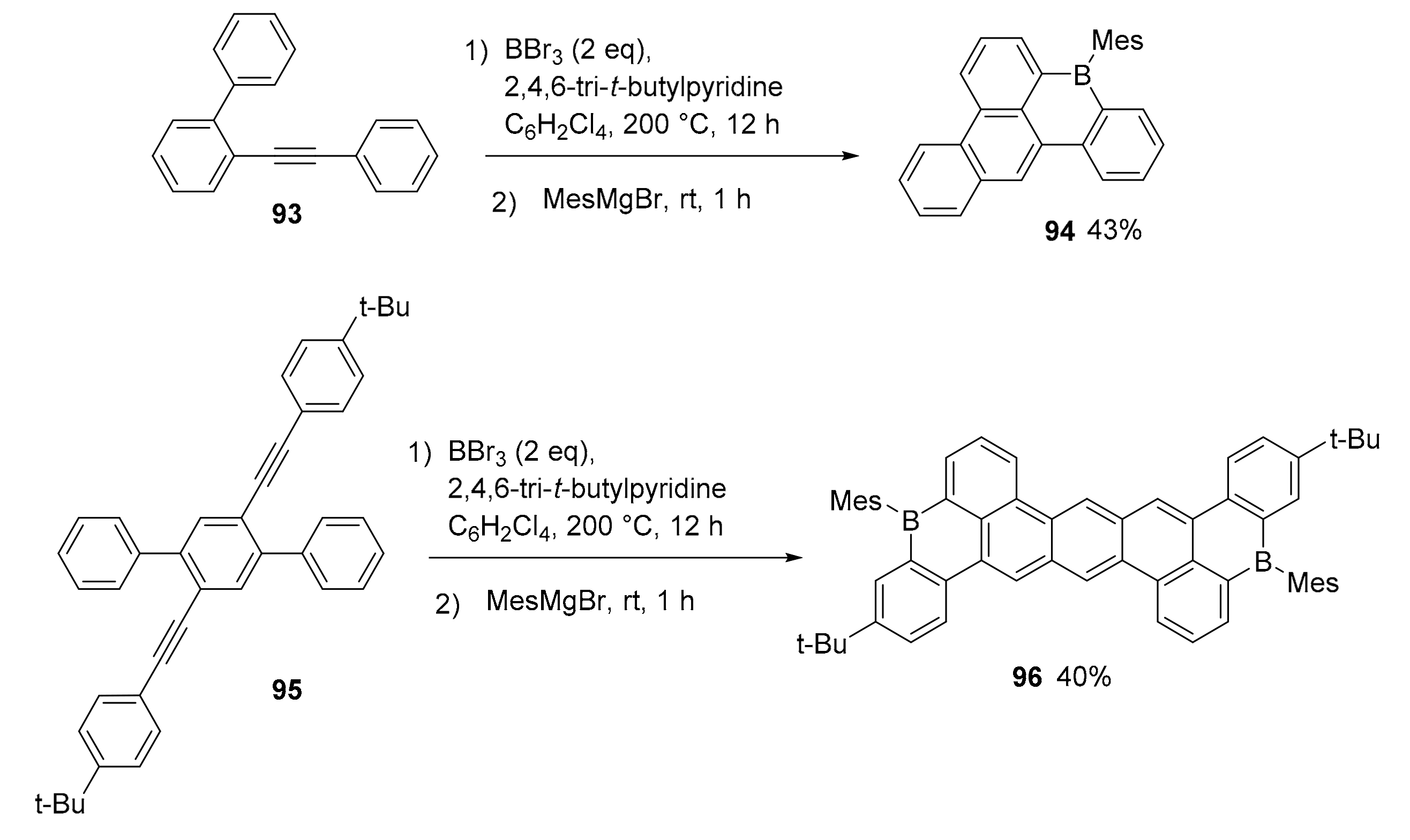
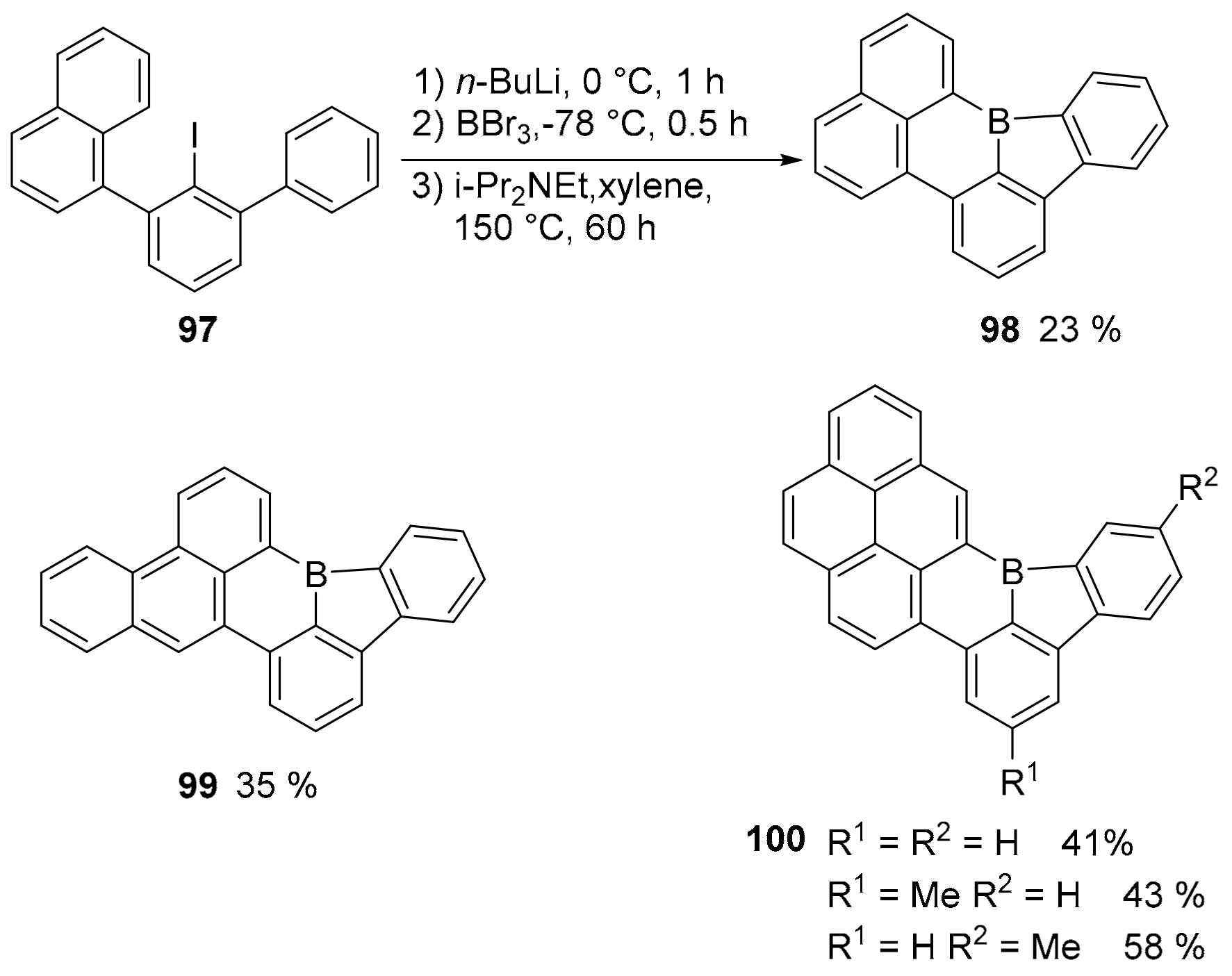
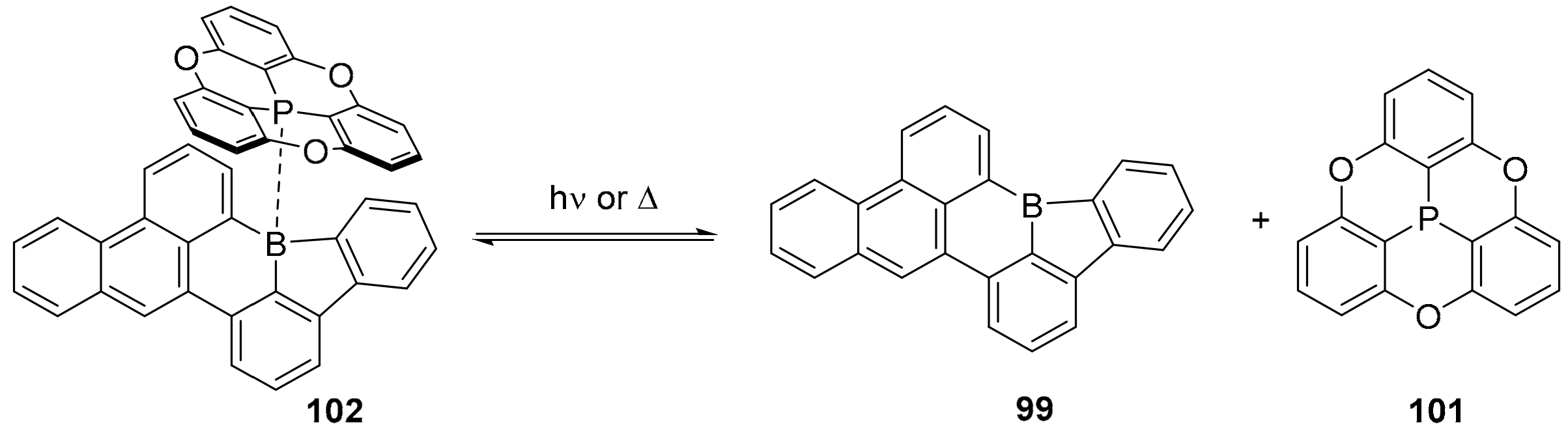
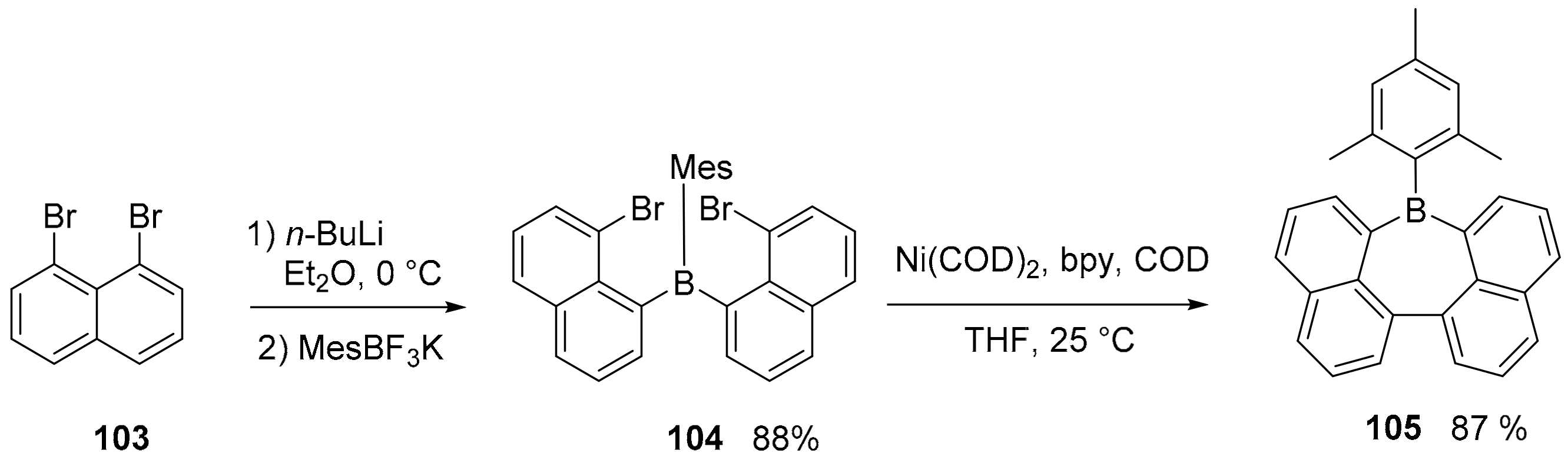
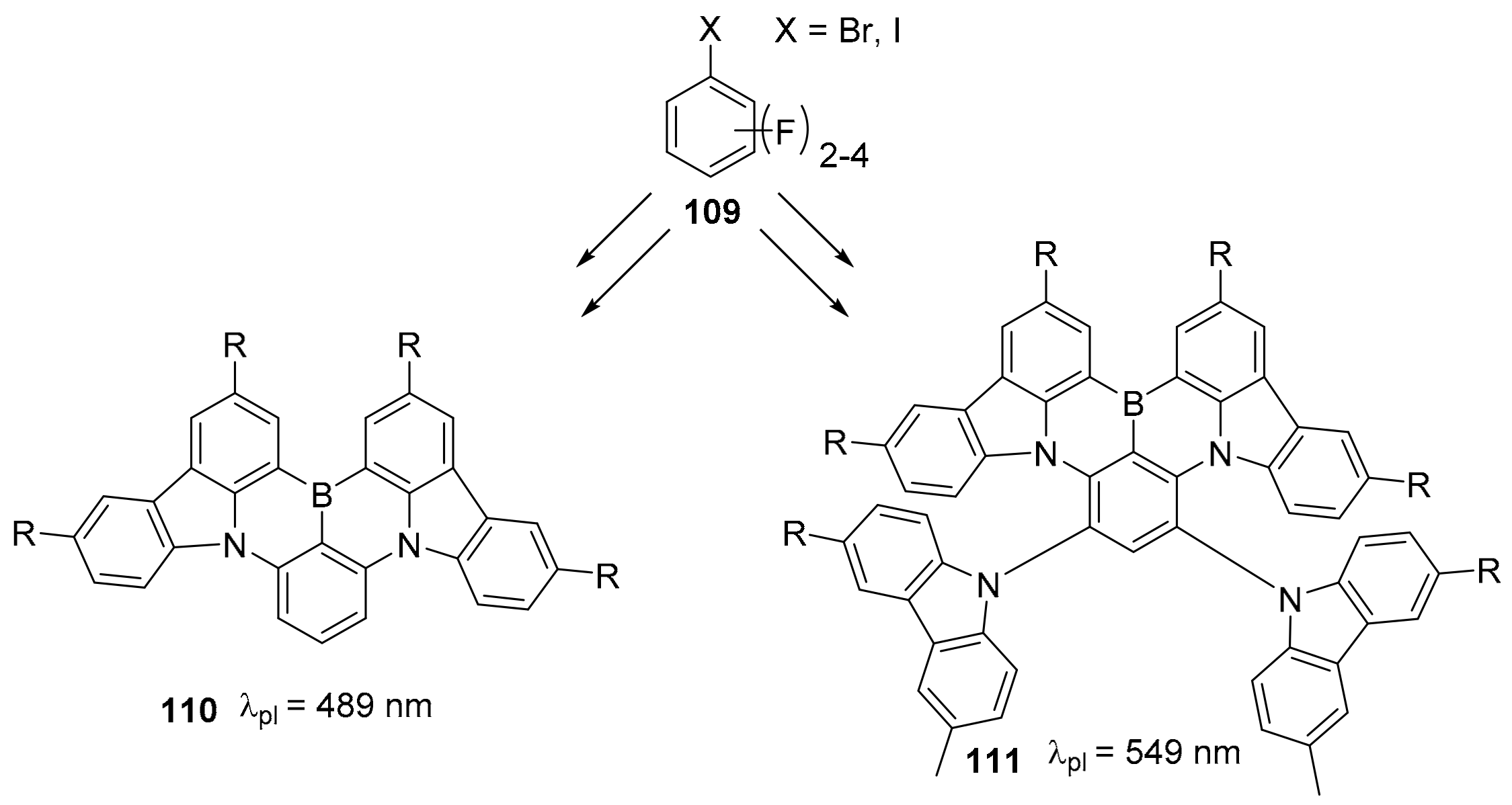
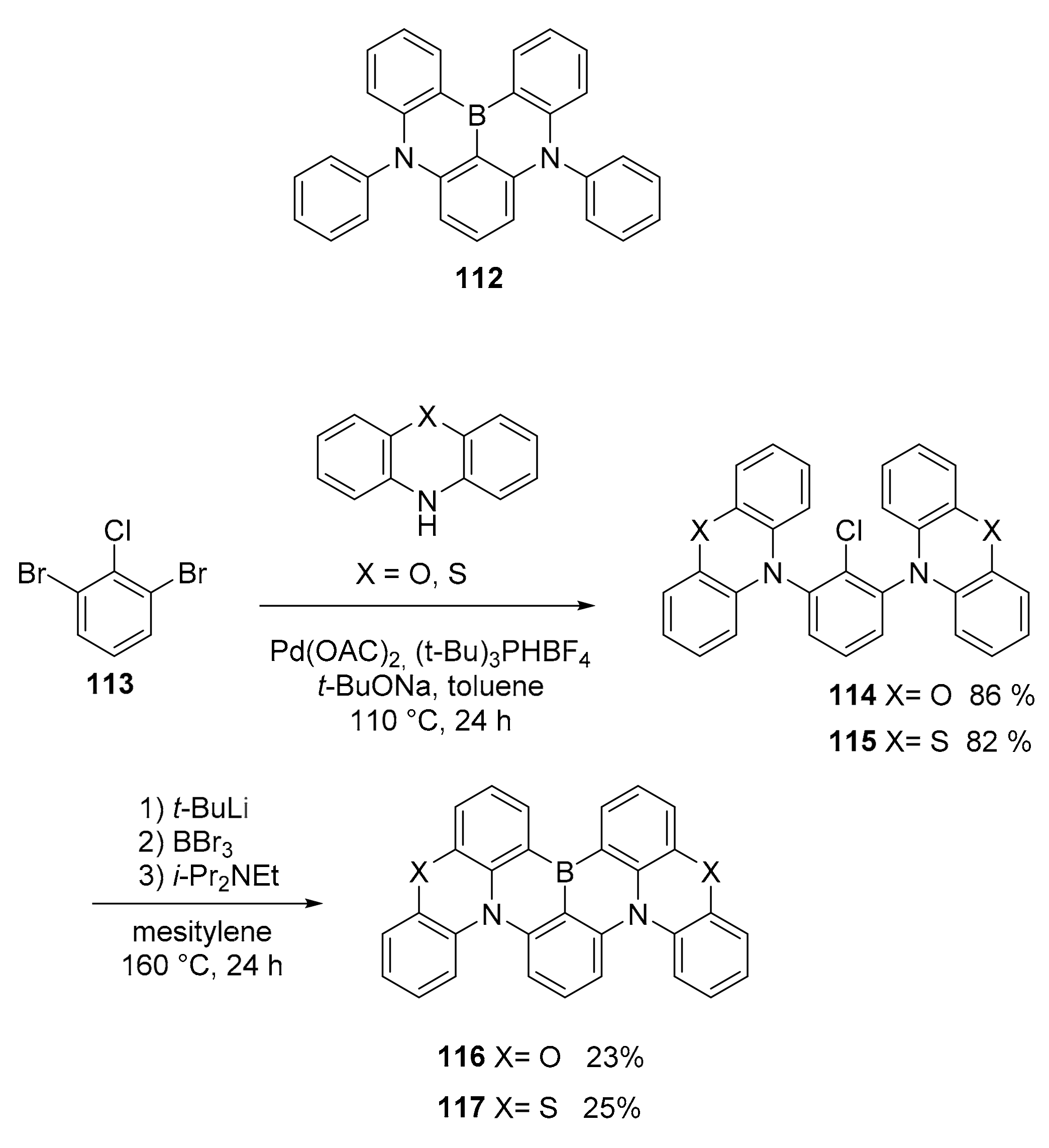
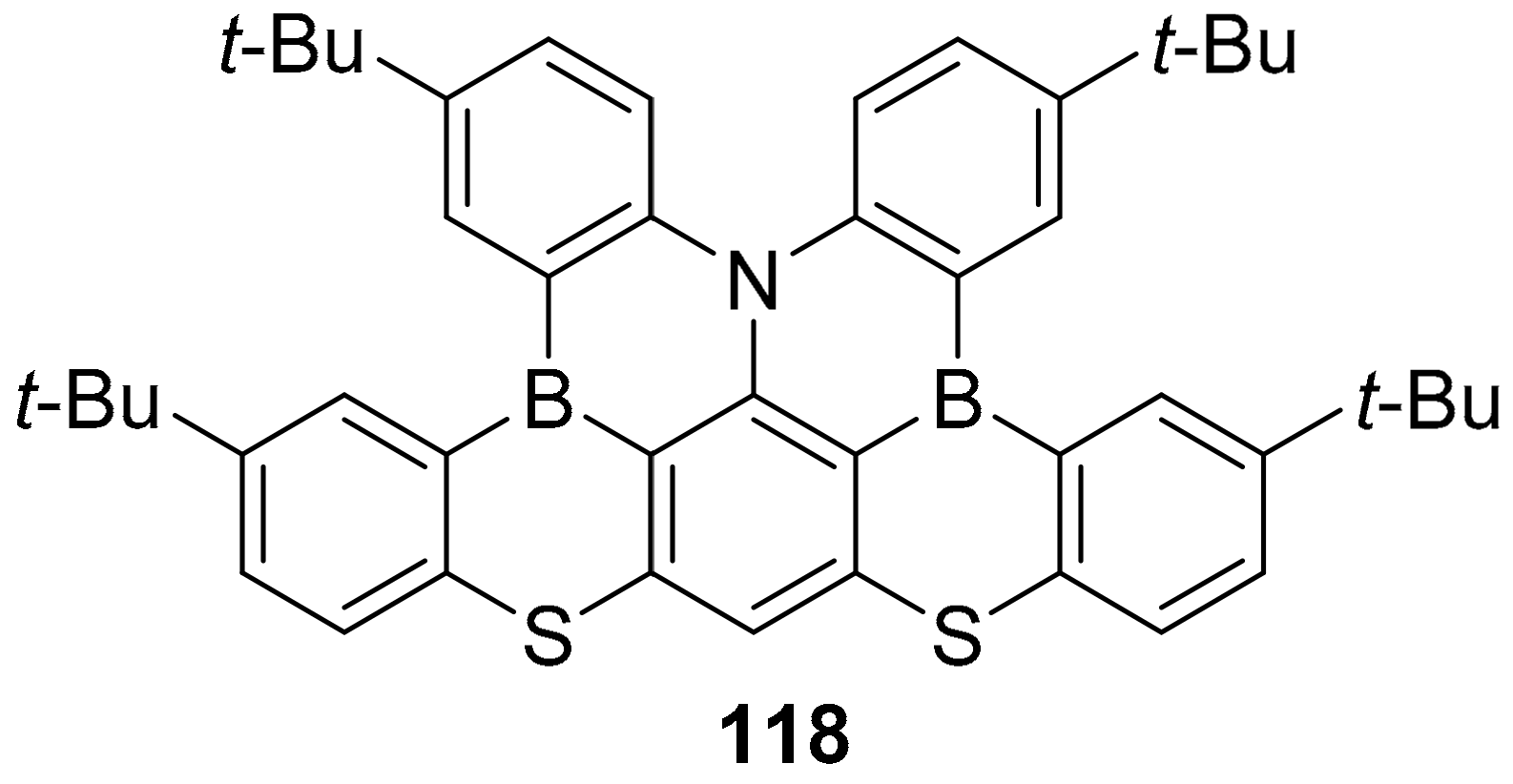
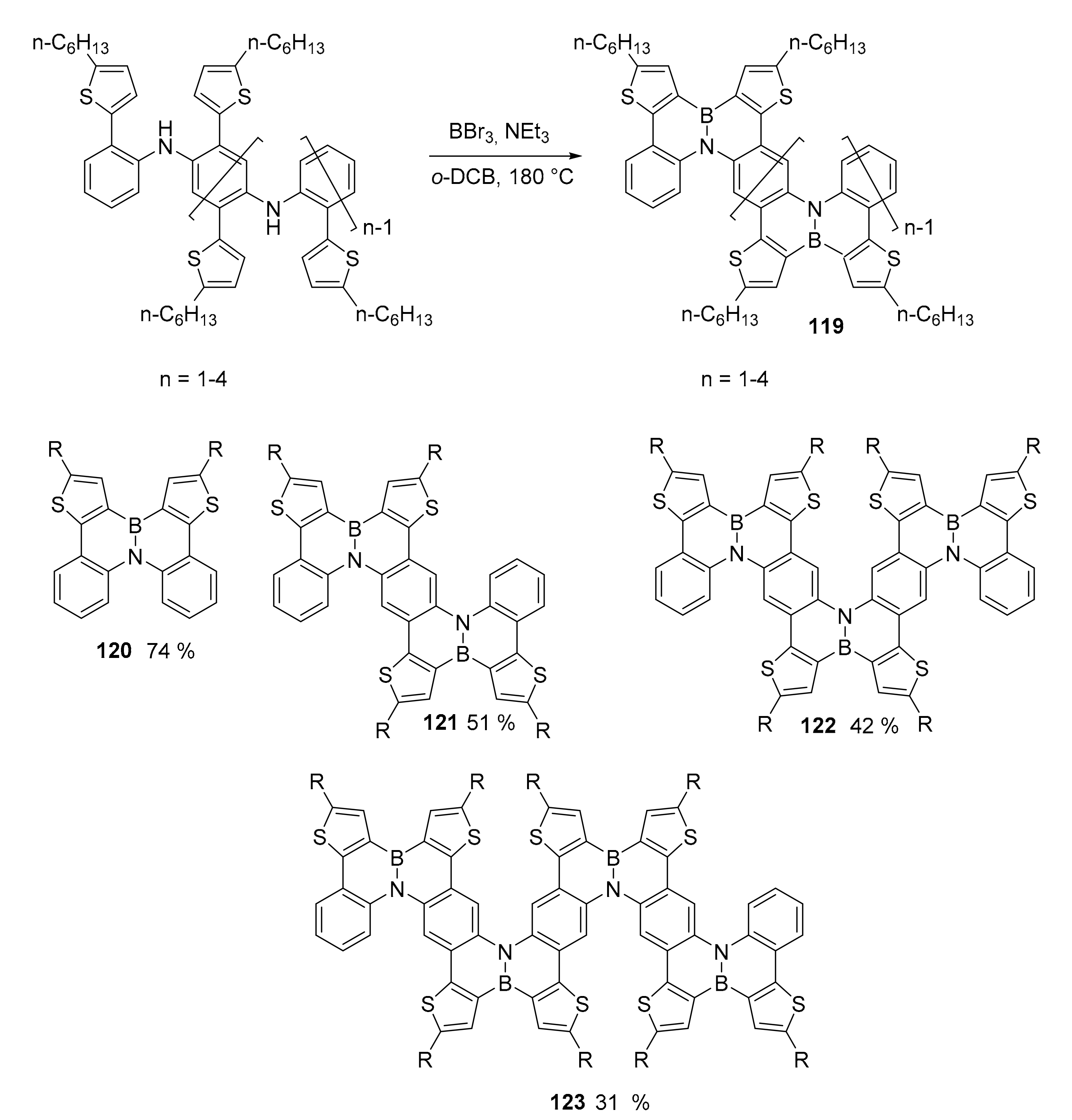
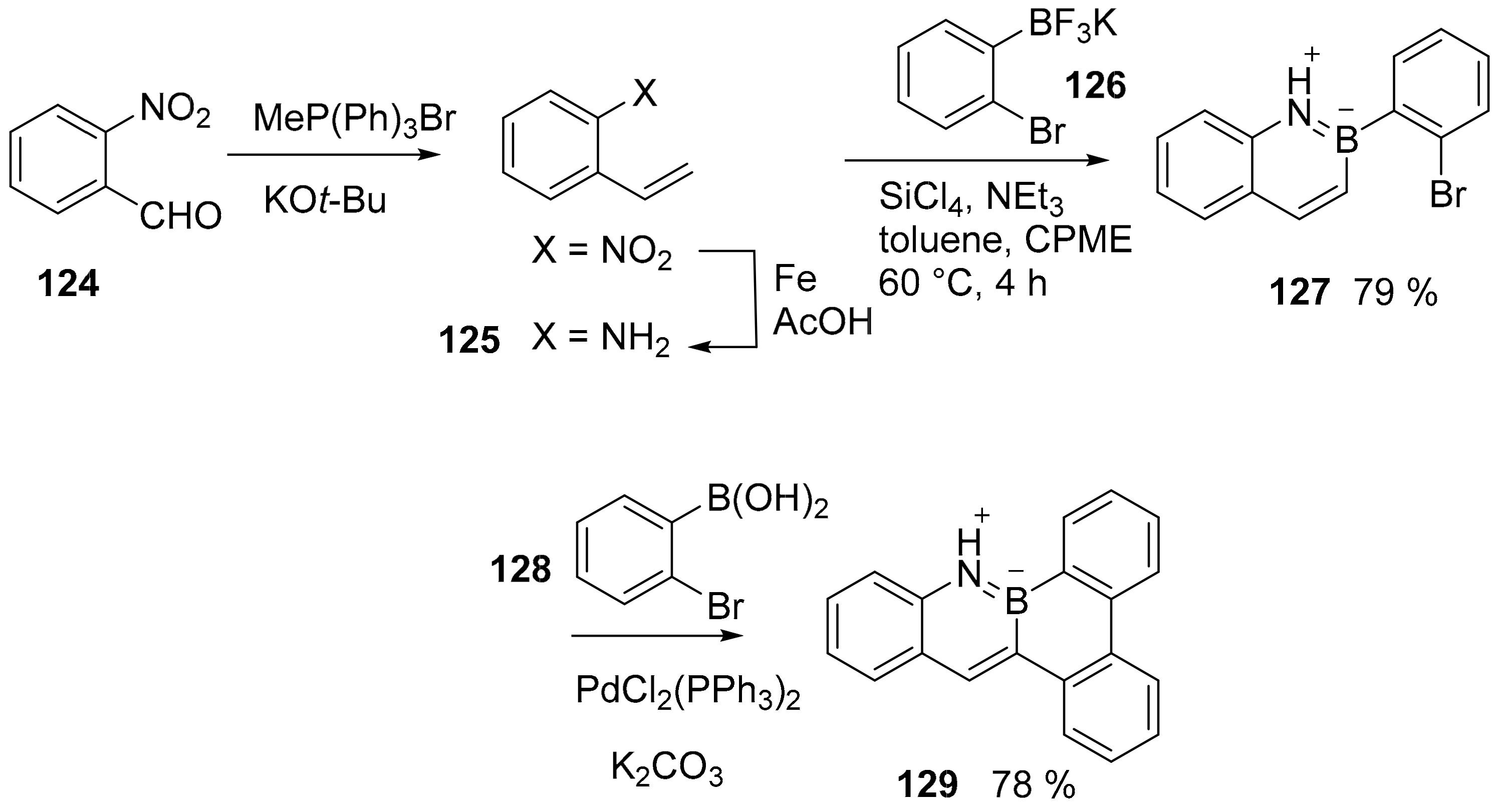
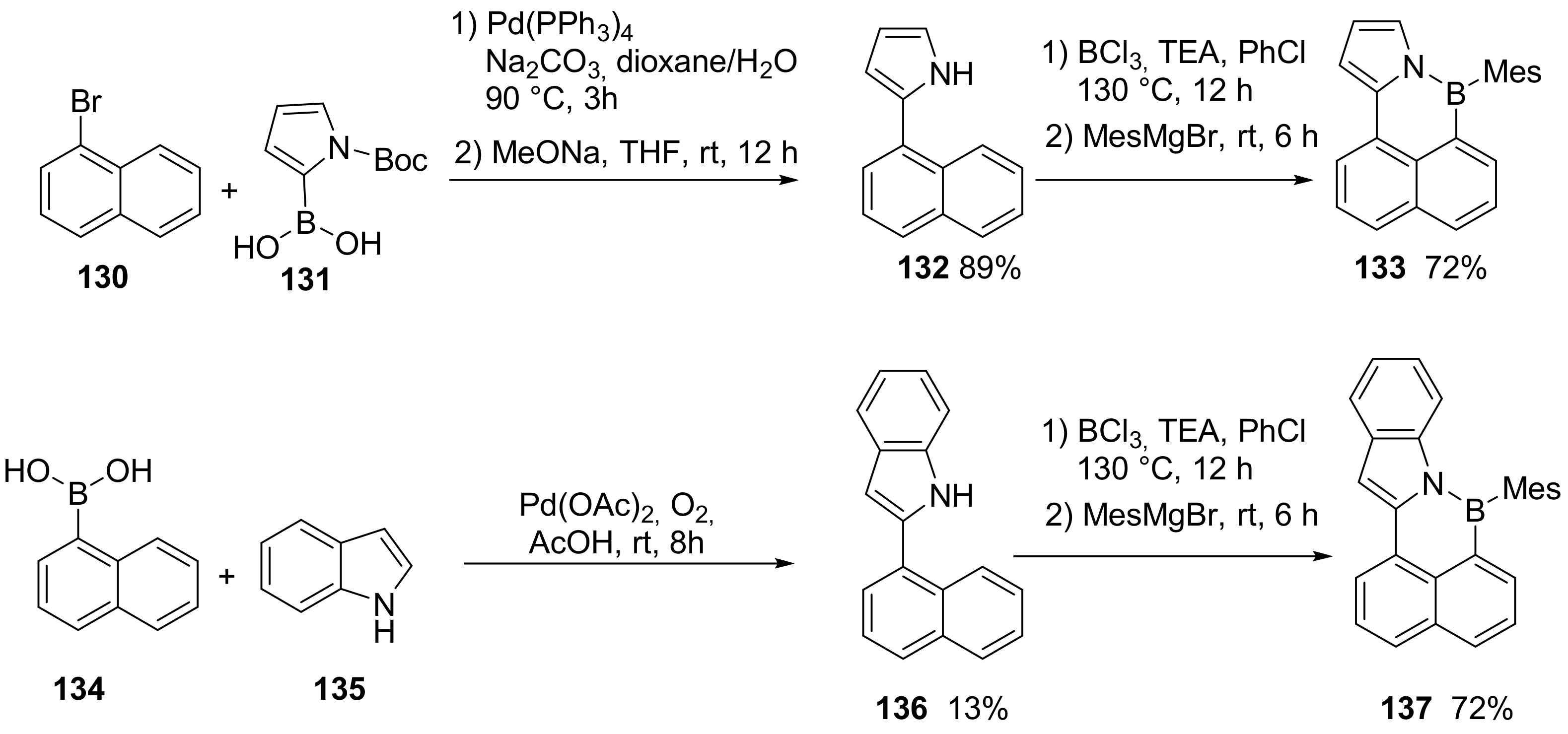
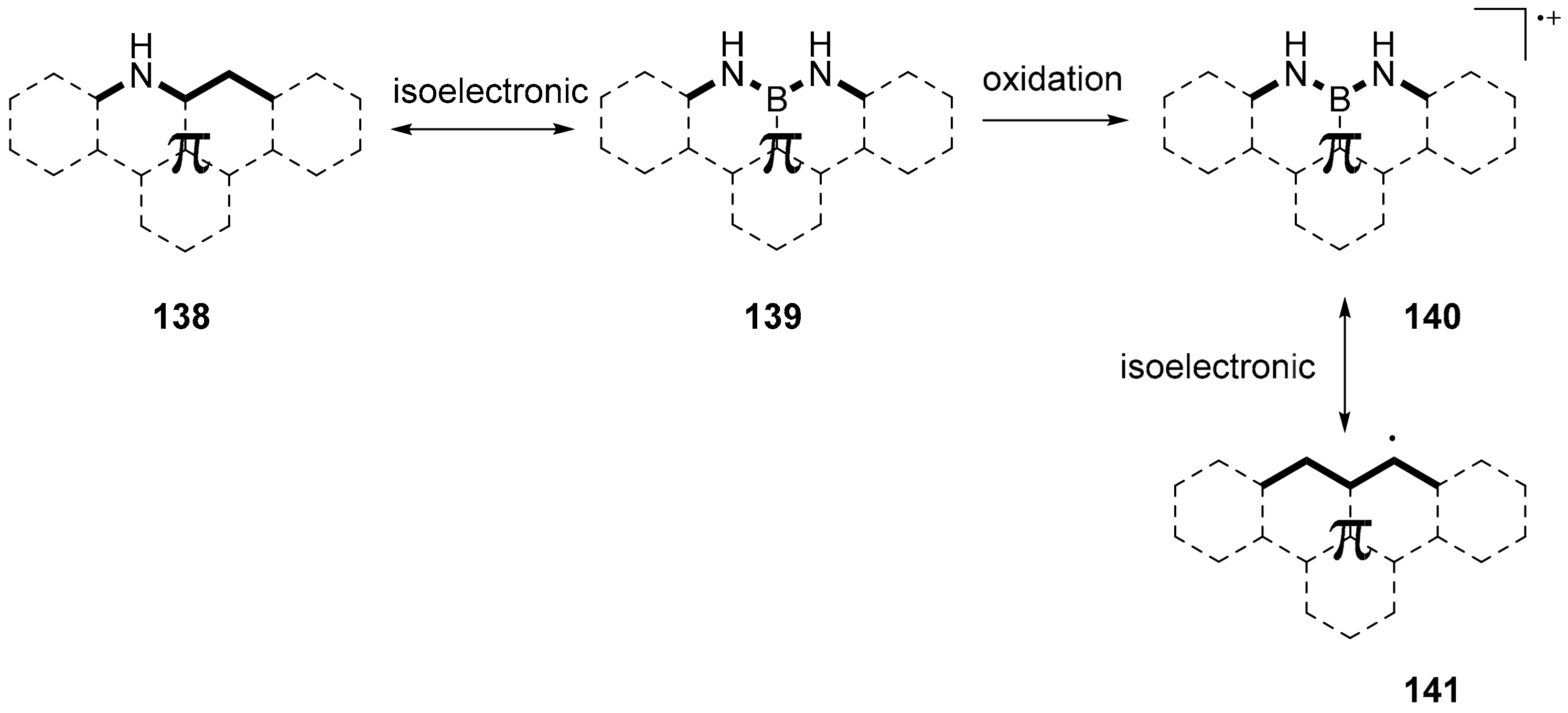
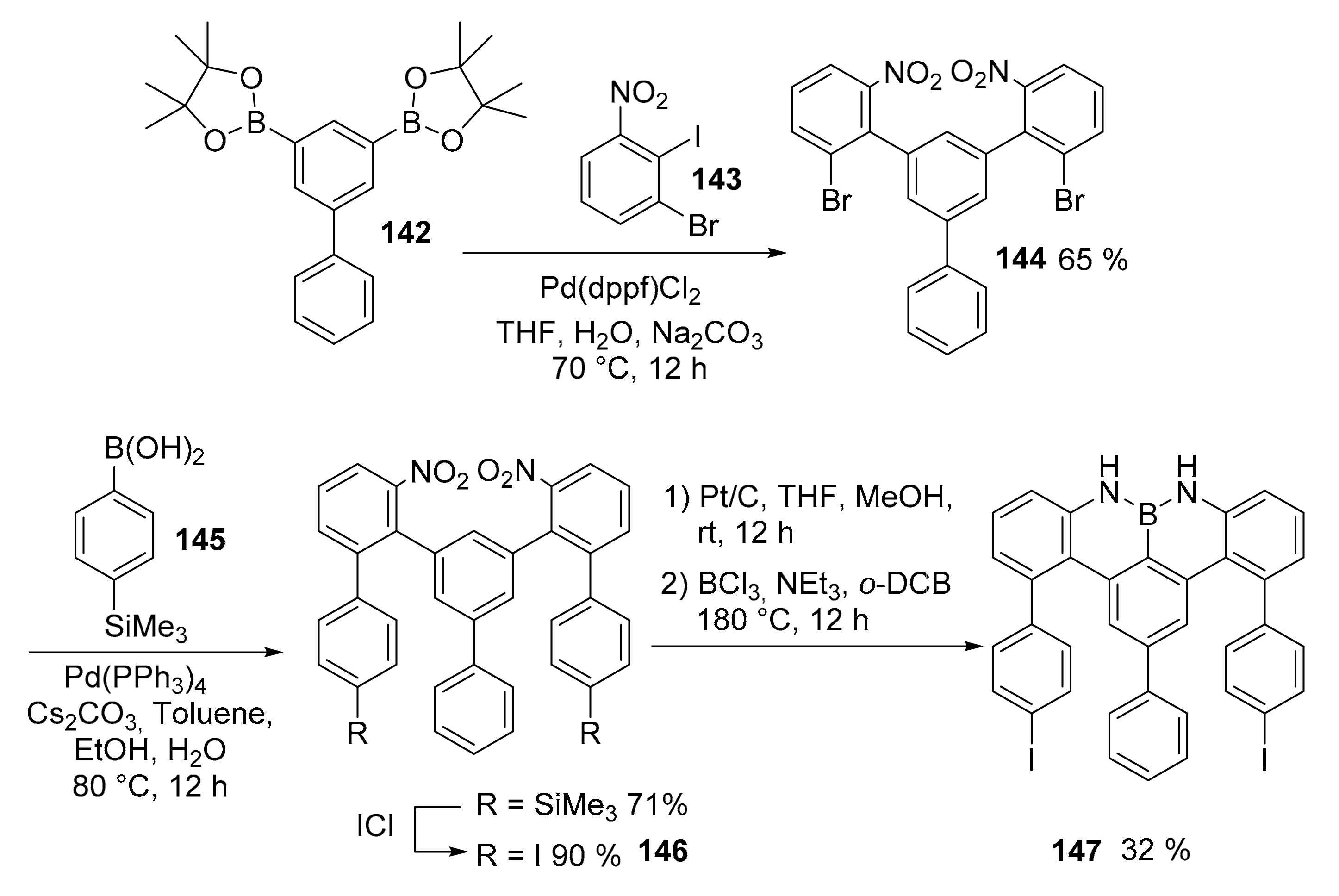
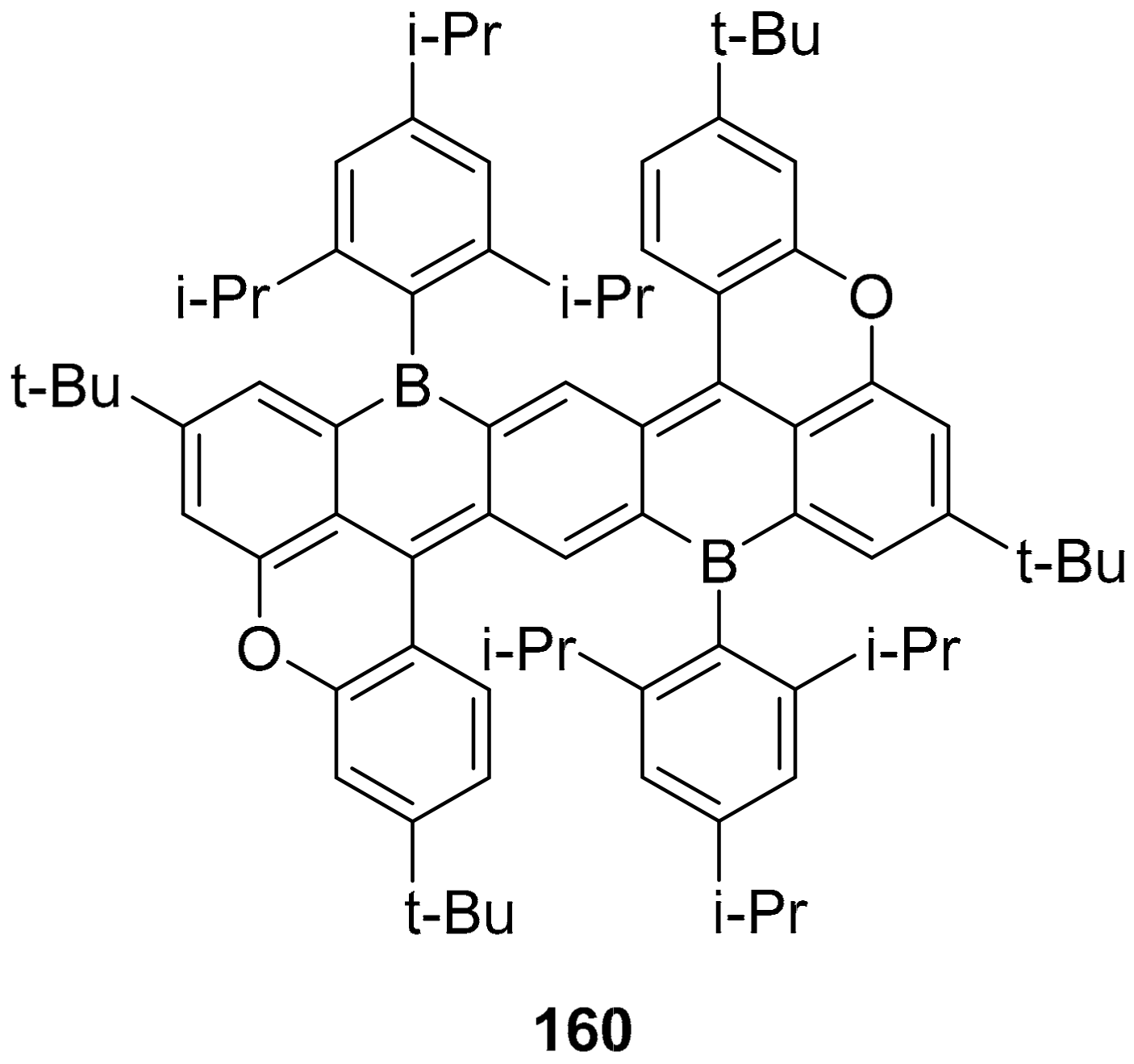
3.1. B-Doped Polycyclic Aromatic Hydrocarbons Embedding Borine and Borole and Borepin Rings
3.2. B-Doped Polycyclic Aromatic Hydrocarbons Embedding 1,4-Azaborine Rings
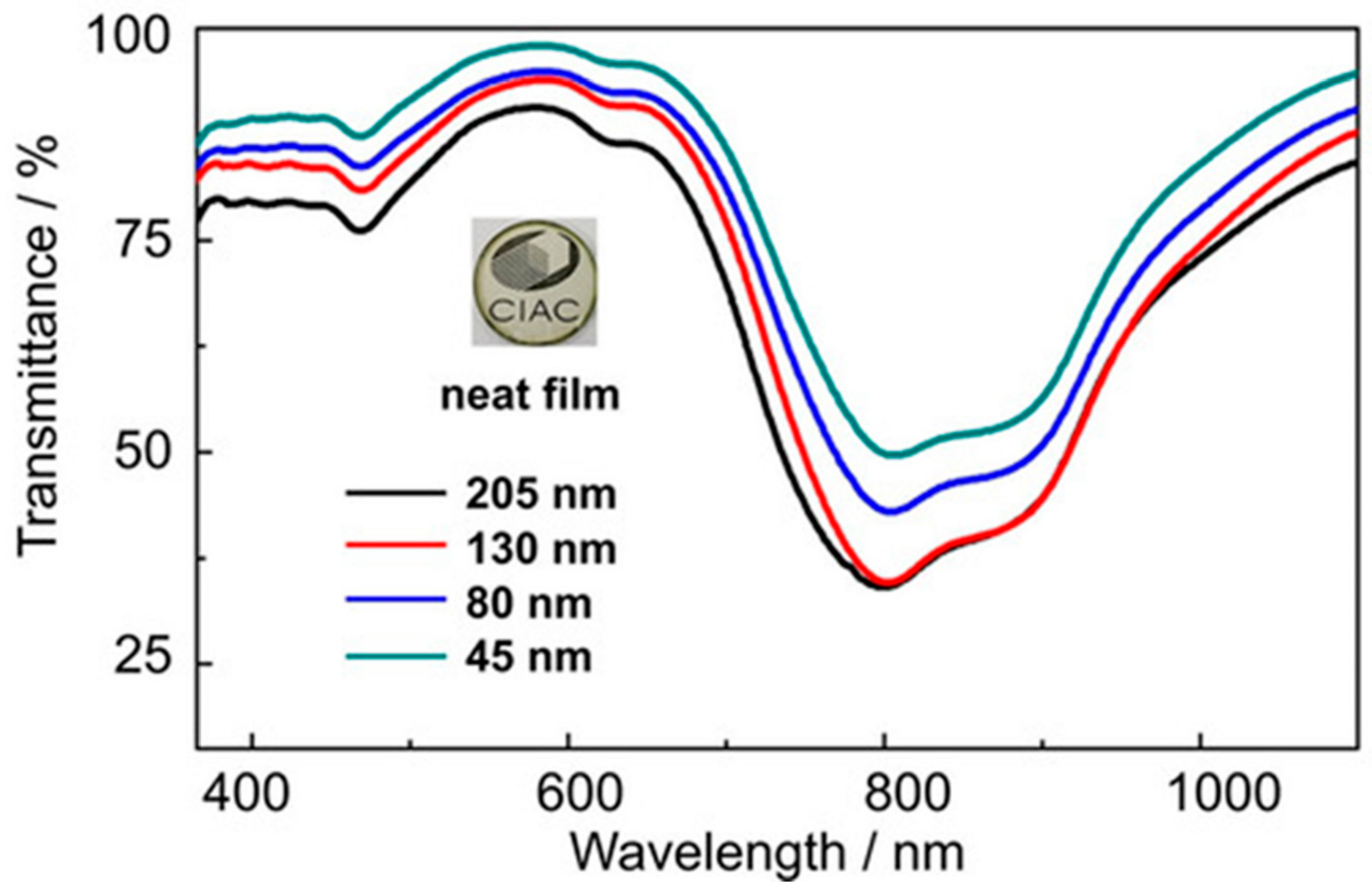
3.3. B-Doped Polycyclic Aromatic Hydrocarbons Embedding 1,2-Azaborine and 1,3,2-Diazaborine Rings
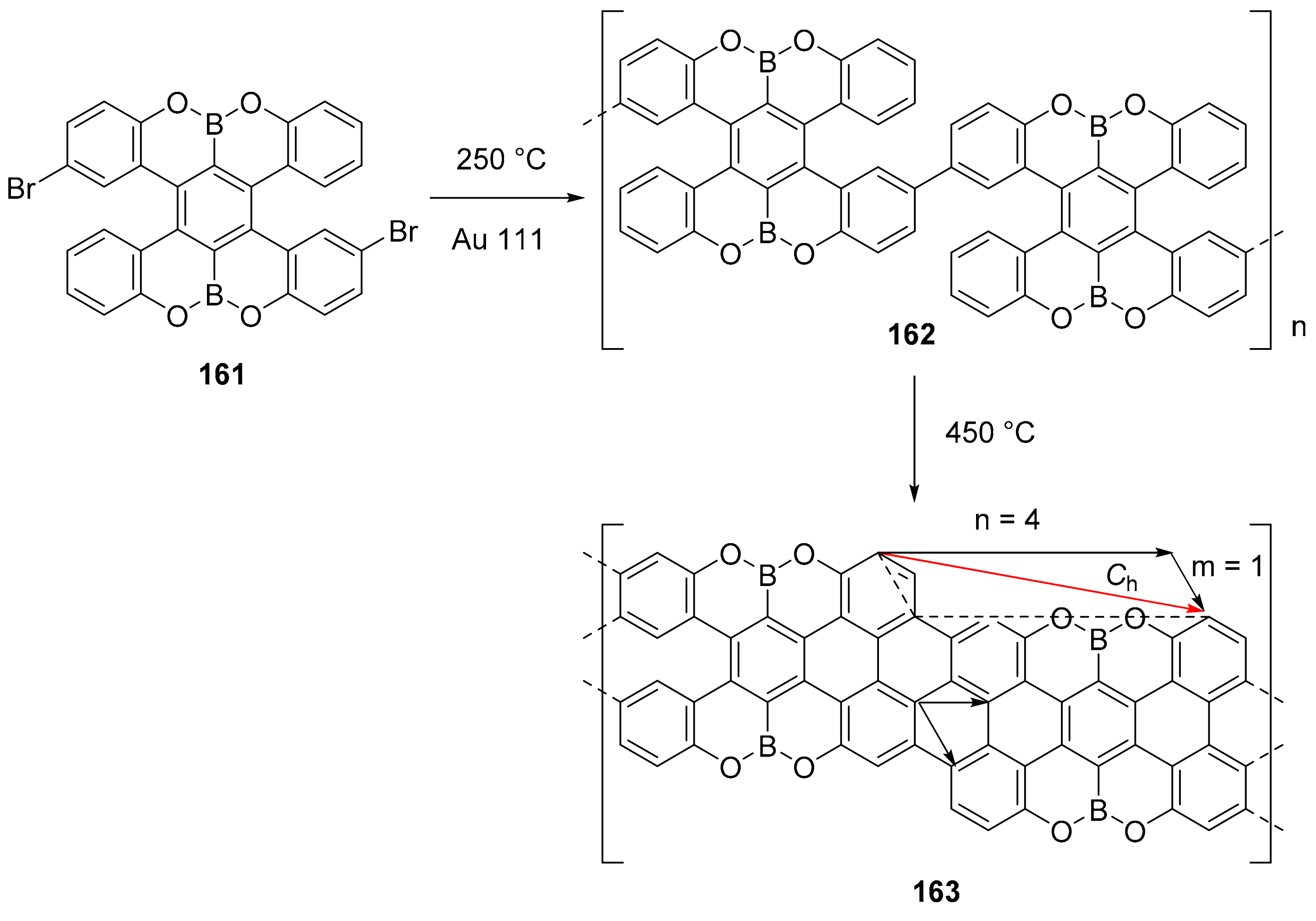
3.4. B-O and B-S Doped Polycyclic Aromatic Hydrocarbons Embedding Miscellaneous Rings
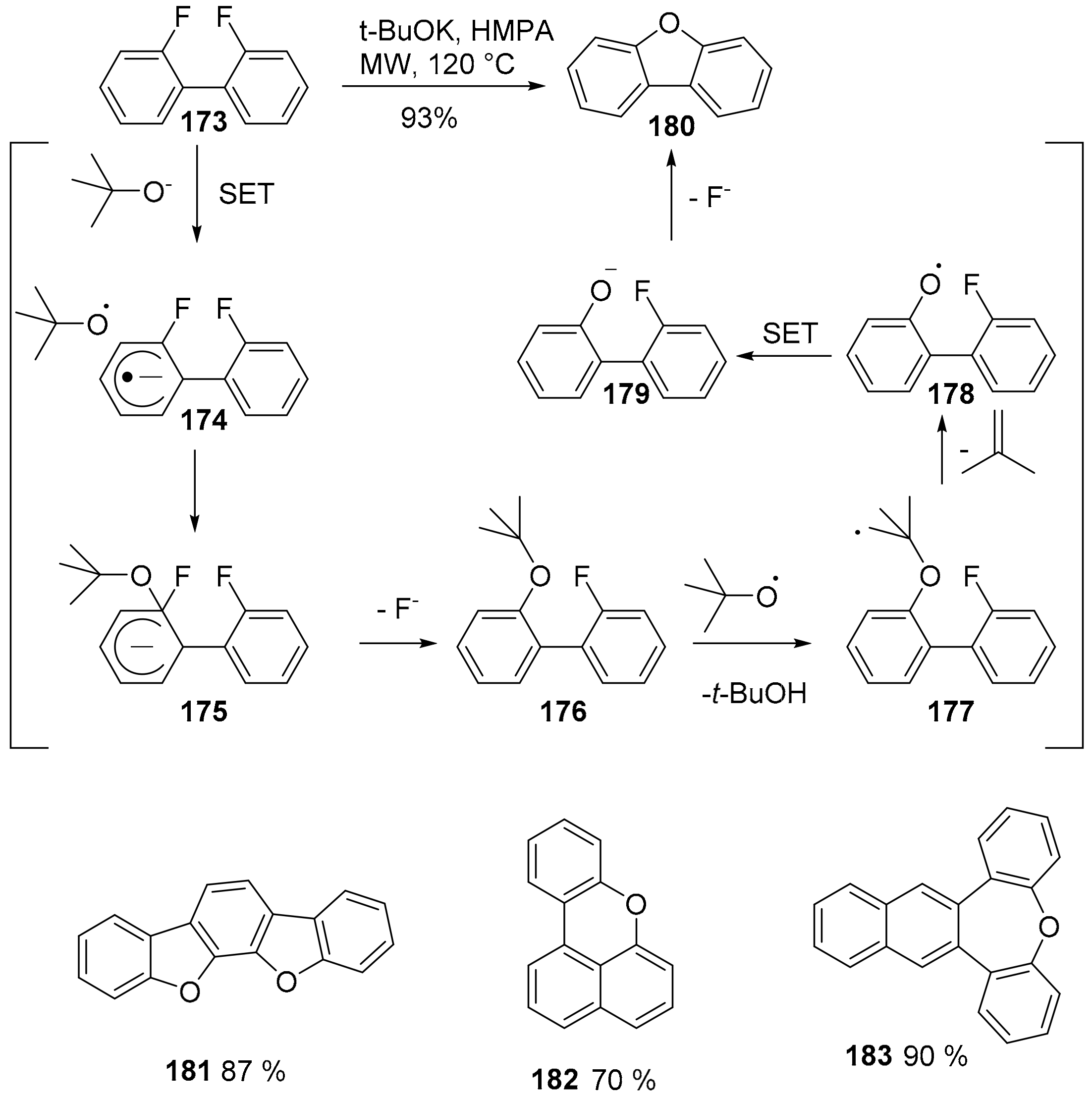
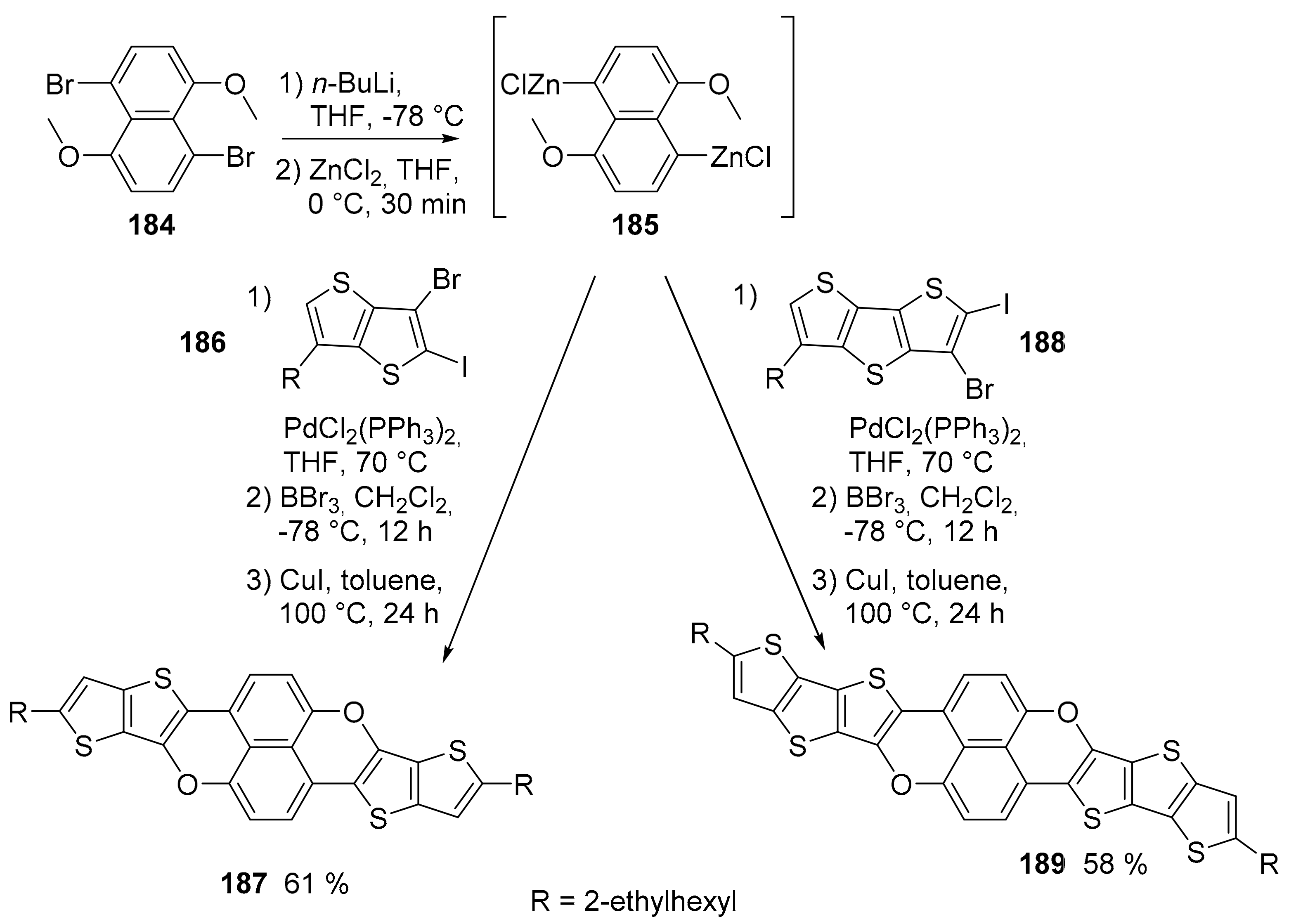
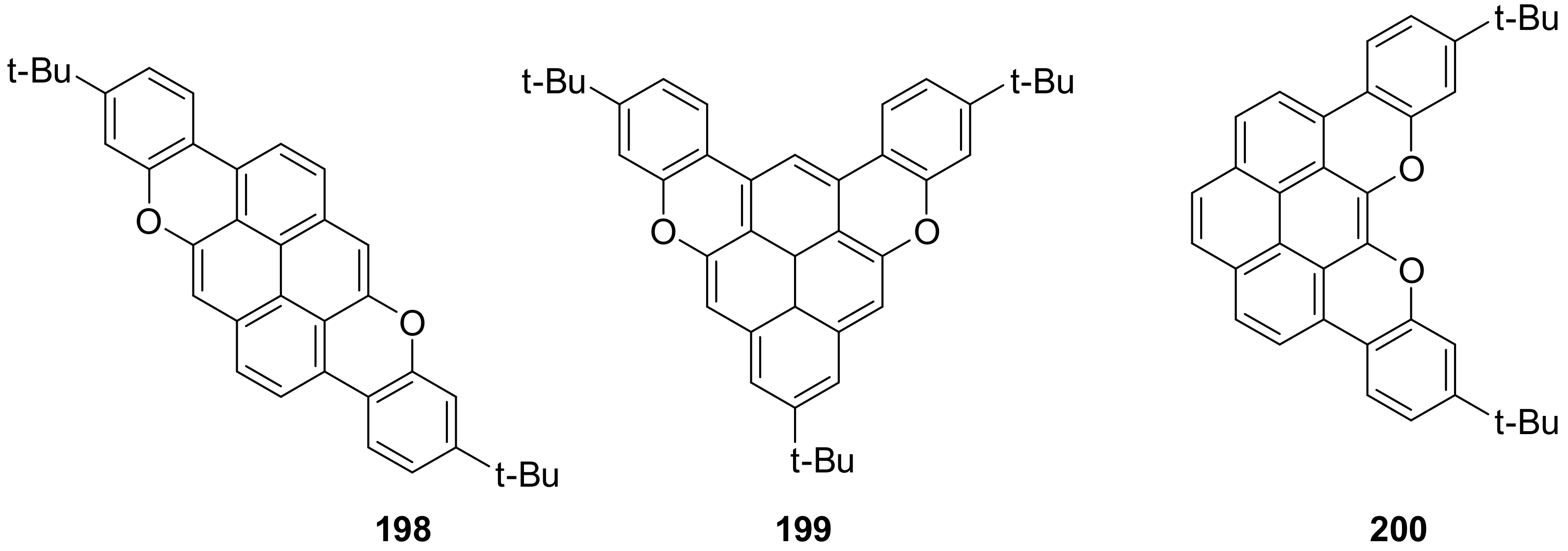
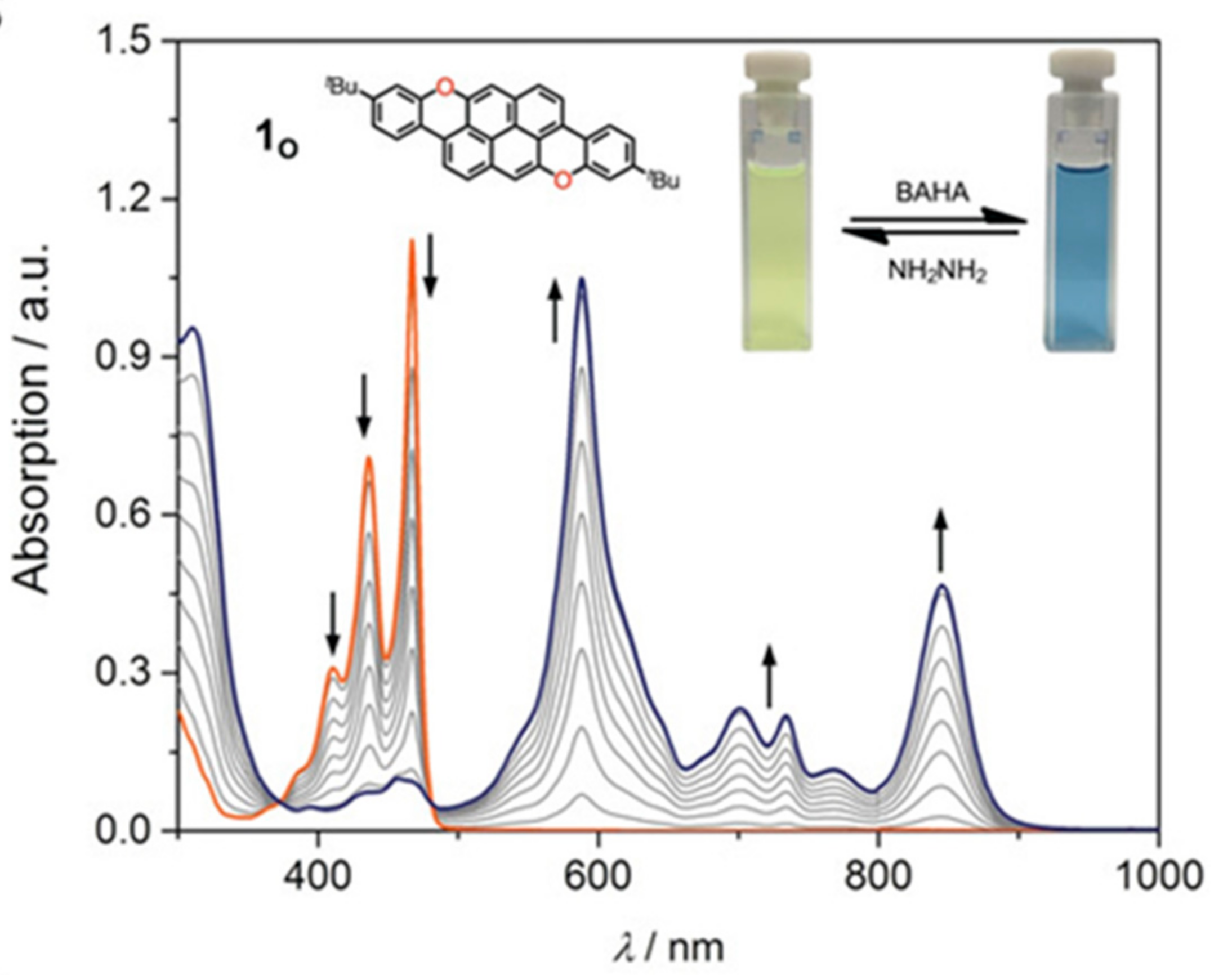
4. O-Doped Polycyclic Aromatic Hydrocarbons Embedding Furan, Pyran and Oxepine Rings
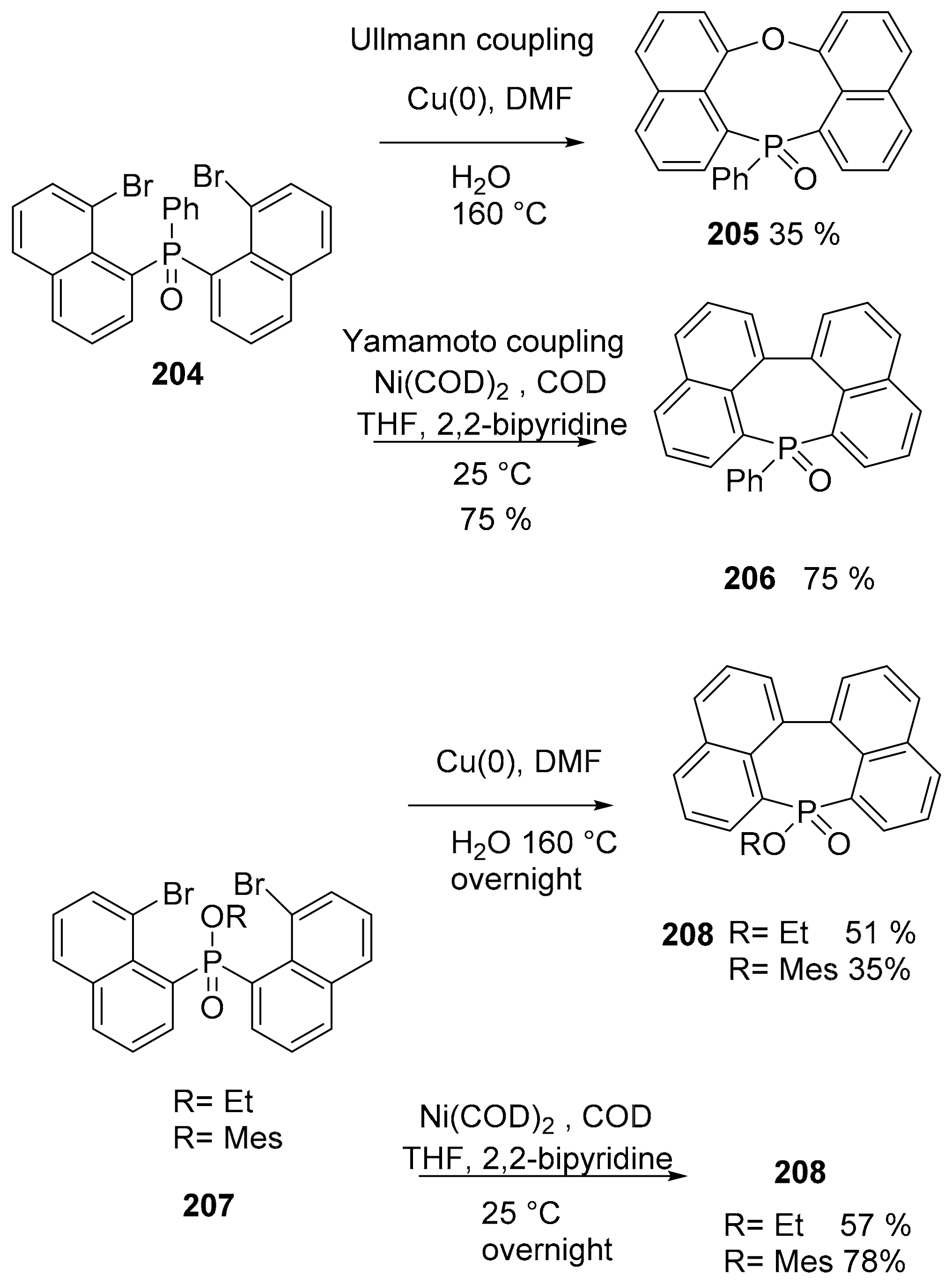
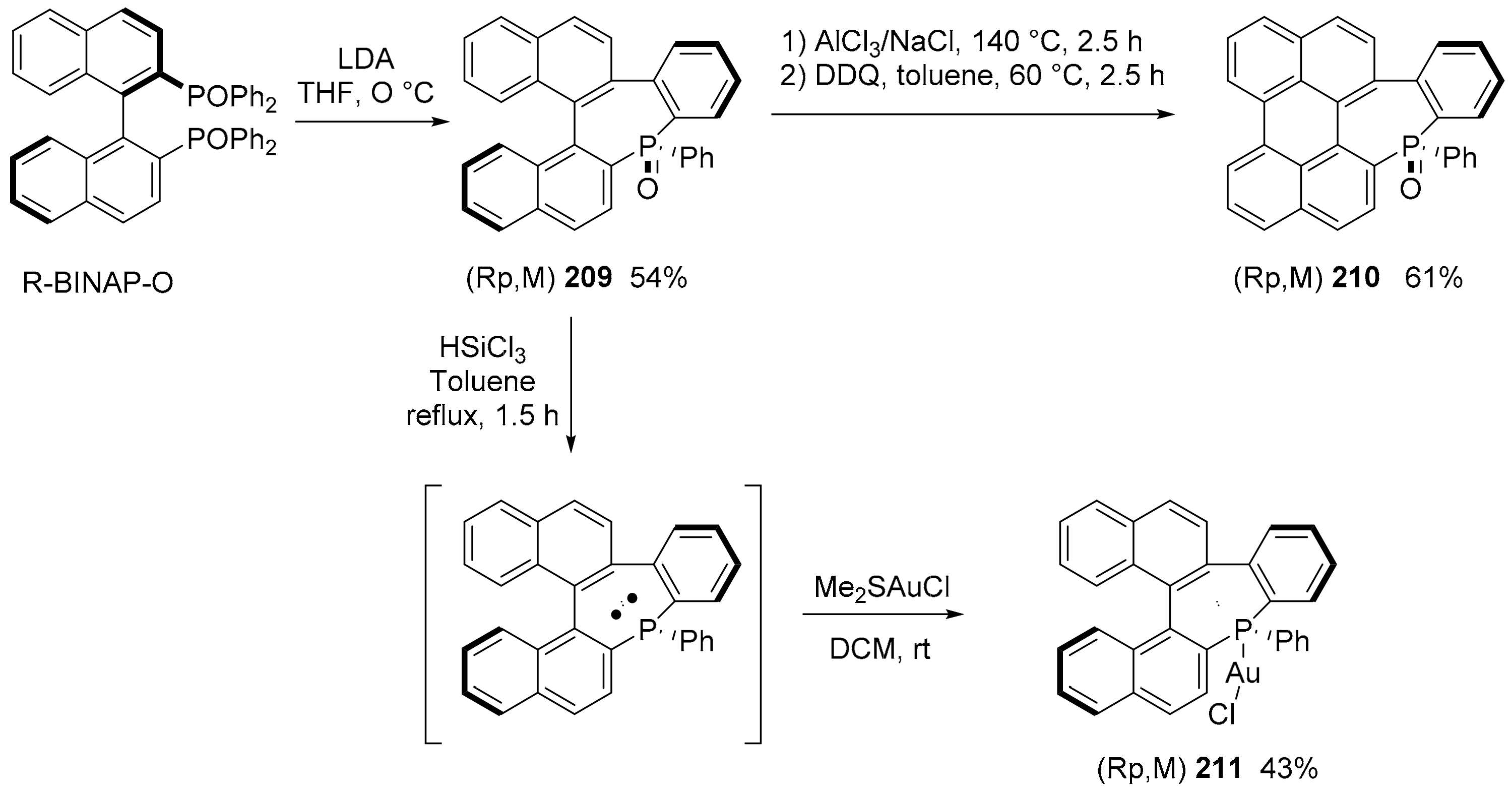
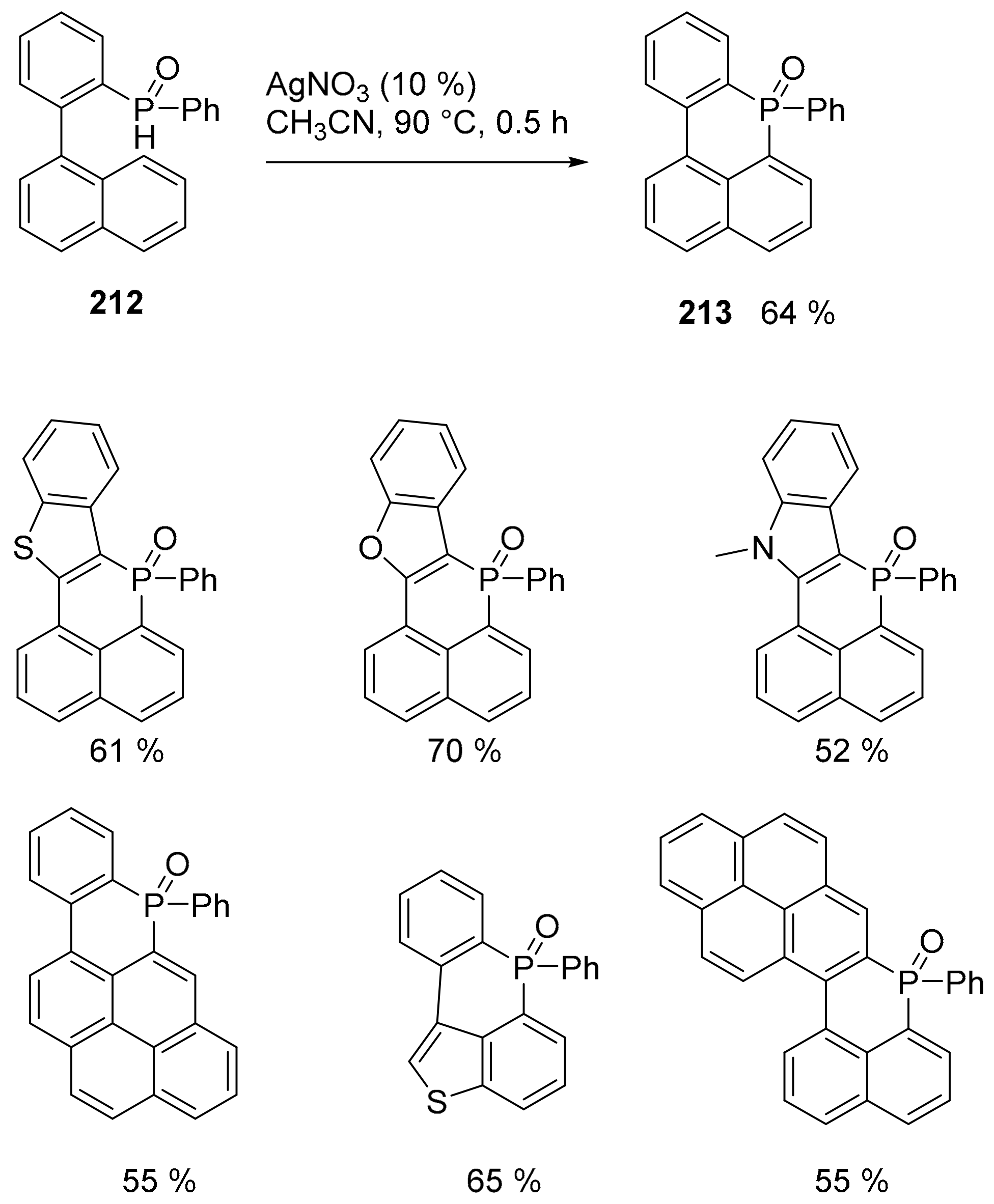
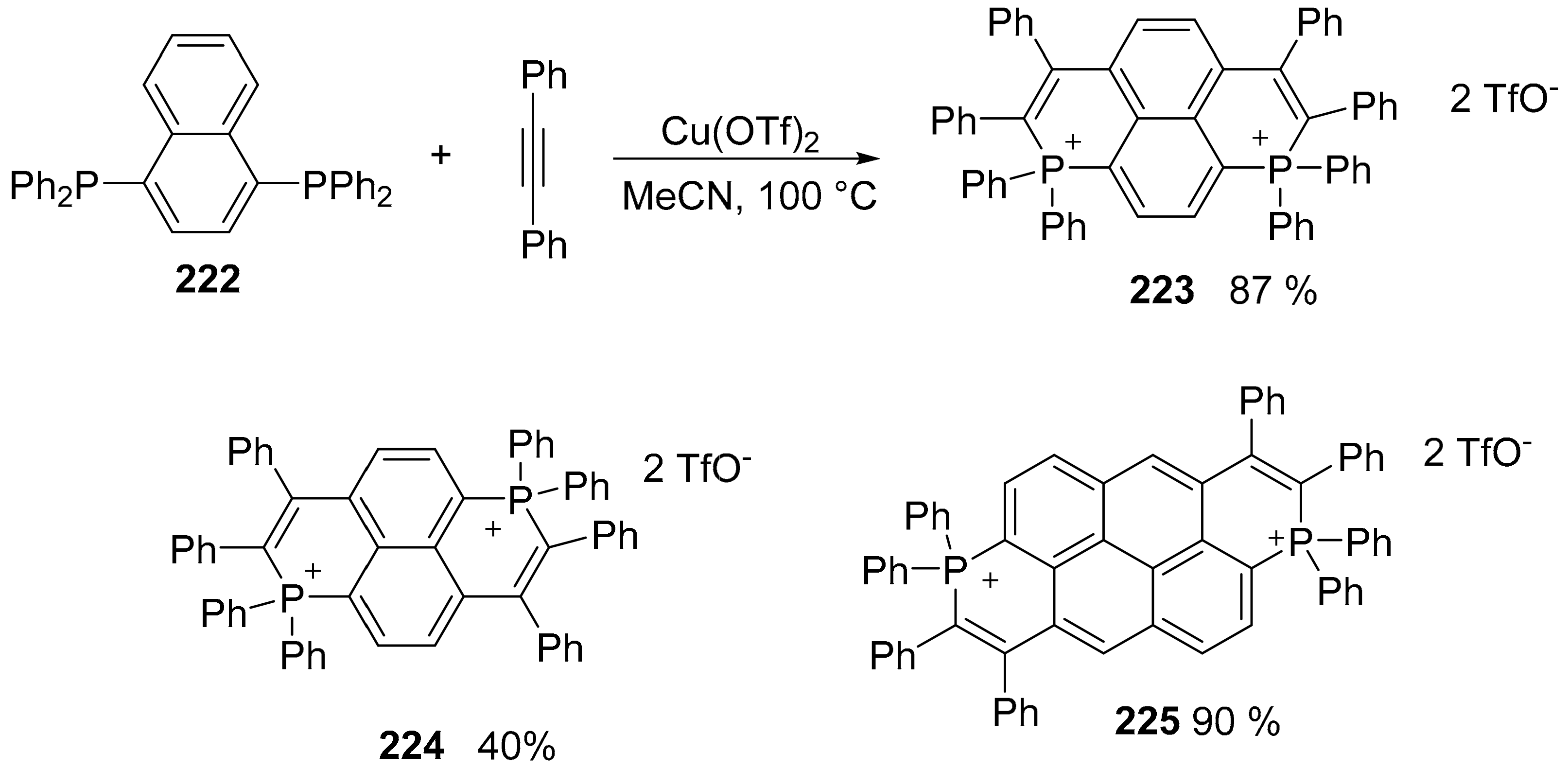
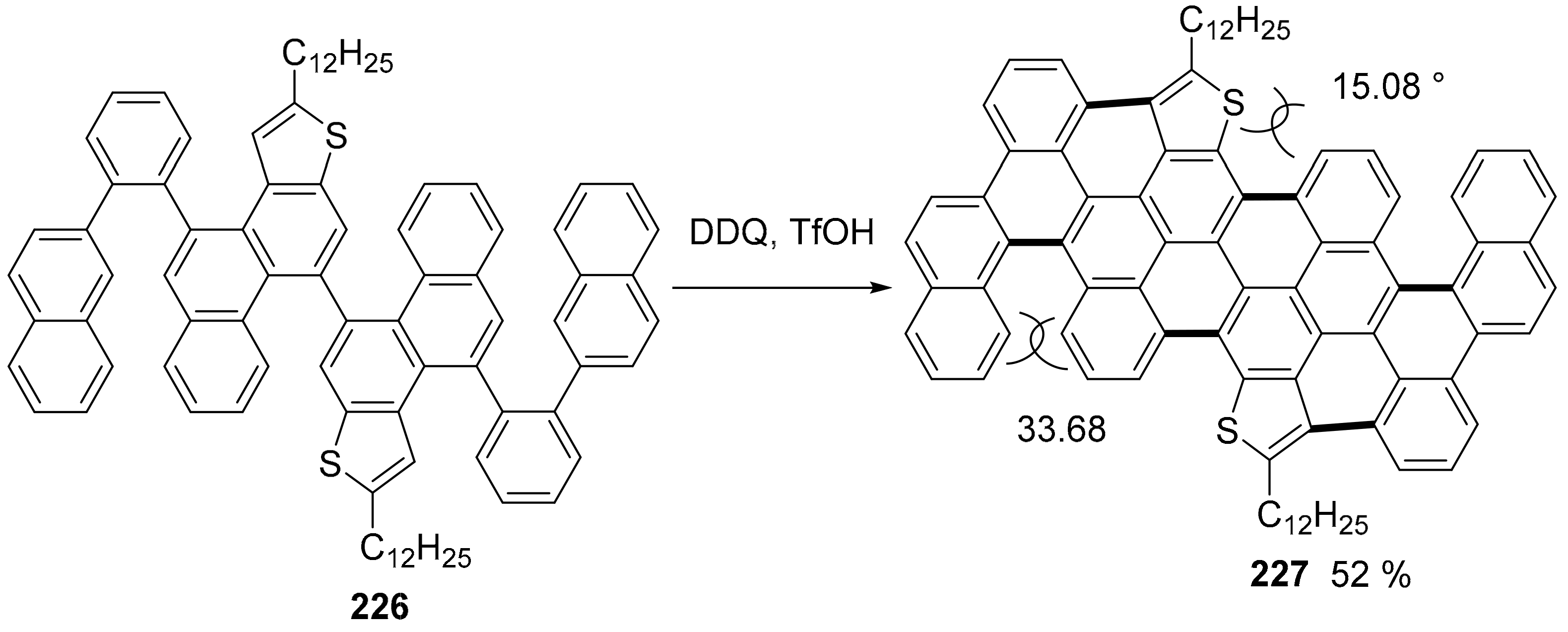
5. P or S-Doped Polycyclic Aromatic Hydrocarbons
6. Conclusions and Future Perspective
Funding
Acknowledgments
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Jiang, D.; Schedin, F.; Booth, T.J.; Khotkevich, V.V.; Morozov, S.V.; Geim, A.K. Two-Dimensional Atomic Crystals. Proc. Natl. Acad. Sci. USA 2005, 102, 10451–10453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirsch, A. The Era of Carbon Allotropes. Nat. Mater. 2010, 9, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K.; Novoselov, K.S. The Rise of Graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical Properties of Graphene and Graphene-Based Nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal Properties of Graphene and Nanostructured Carbon Materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Morozov, S.V.; Mohinddin, T.M.G.; Ponomarenko, L.A.; Elias, D.C.; Yang, R.; Barbolina, I.I.; Blake, P.; Booth, T.J.; Jiang, D.; et al. Electronic Properties of Graphene. Phys. Status Solidi 2007, 244, 4106–4111. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Pang, J.; Cheng, Q.; Zhang, S.; Li, Y.; Zhang, C.; Sun, D.; Ibarlucea, B.; Li, Y.; Chen, D.; et al. Synthesis of Wafer-Scale Graphene with Chemical Vapor Deposition for Electronic Device Applications. Adv. Mater. Technol. 2021, 6, 2000744. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically Derived, Ultrasmooth Graphene Nanoribbon Semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Ponomarenko, L.A.; Schedin, F.; Katsnelson, M.I.; Yang, R.; Hill, E.W.; Novoselov, K.S.; Geim, A.K. Chaotic Dirac Billiard in Graphene Quantum Dots. Science 2008, 320, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon Nanodots: Synthesis, Properties and Applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-Doped Graphene Materials: Syntheses, Properties and Applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putri, L.K.; Ong, W.-J.; Chang, W.S.; Chai, S.-P. Heteroatom Doped Graphene in Photocatalysis: A Review. Appl. Surf. Sci. 2015, 358, 2–14. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Maegawa, K.; Tan, W.K.; Kawamura, G.; Kar, K.K.; Matsuda, A. Heteroatom Doped Graphene Engineering for Energy Storage and Conversion. Mater. Today 2020, 39, 47–65. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Pal Singh, P. Heteroatom-Doped Graphene as Sensing Materials: A Mini Review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Narita, A.; Müllen, K. Precision Synthesis versus Bulk-Scale Fabrication of Graphenes. Nat. Rev. Chem 2017, 2, 0100. [Google Scholar] [CrossRef]
- Liu, J.; Feng, X. Synthetic Tailoring of Graphene Nanostructures with Zigzag-Edged Topologies: Progress and Perspectives. Angew. Chem. Int. Ed. 2020, 59, 23386–23401. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Narita, A.; Müllen, K. Spiers Memorial Lecture. Faraday Discuss. 2021, 227, 8–45. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Yao, X.; Müllen, K. Polycyclic Aromatic Hydrocarbons in the Graphene Era. Sci. China Chem. 2019, 62, 1099–1144. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Y.; Yao, X.; Narita, A.; Müllen, K. Heteroatom-Doped Nanographenes with Structural Precision. Acc. Chem. Res. 2019, 52, 2491–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Müllen, K.; Narita, A. Syntheses and Characterizations of Functional Polycyclic Aromatic Hydrocarbons and Graphene Nanoribbons. Bull. Chem. Soc. Jpn. 2020, 93, 490–506. [Google Scholar] [CrossRef]
- Ten, Y.A.; Troshkova, N.M.; Tretyakov, E.V. From Spin-Labelled Fused Polyaromatic Compounds to Magnetically Active Graphene Nanostructures. Russ. Chem. Rev. 2020, 89, 693–712. [Google Scholar] [CrossRef]
- Drummer, M.C.; Singh, V.; Gupta, N.; Gesiorski, J.L.; Weerasooriya, R.B.; Glusac, K.D. Photophysics of Nanographenes: From Polycyclic Aromatic Hydrocarbons to Graphene Nanoribbons. Photosynth. Res. 2021. [Google Scholar] [CrossRef]
- Houtsma, R.S.K.; de la Rie, J.; Stöhr, M. Atomically Precise Graphene Nanoribbons: Interplay of Structural and Electronic Properties. Chem. Soc. Rev. 2021, 50, 6541–6568. [Google Scholar] [CrossRef]
- Mathew, B.P.; Kuram, M.R. Emerging C H Functionalization Strategies for Constructing Fused Polycyclic Aromatic Hydrocarbons and Nanographenes. Inorg. Chim. Acta 2019, 490, 112–129. [Google Scholar] [CrossRef]
- Stępień, M.; Gońka, E.; Żyła, M.; Sprutta, N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. [Google Scholar] [CrossRef]
- Narita, A.; Wang, X.-Y.; Feng, X.; Müllen, K. New Advances in Nanographene Chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirai, M.; Tanaka, N.; Sakai, M.; Yamaguchi, S. Structurally Constrained Boron-, Nitrogen-, Silicon-, and Phosphorus-Centered Polycyclic π-Conjugated Systems. Chem. Rev. 2019, 119, 8291–8331. [Google Scholar] [CrossRef]
- Oda, S.; Hatakeyama, T. Development of One-Shot/One-Pot Borylation Reactions toward Organoboron-Based Materials. BCSJ 2021, 94, 950–960. [Google Scholar] [CrossRef]
- Chen, L.; Hernandez, Y.; Feng, X.; Müllen, K. From Nanographene and Graphene Nanoribbons to Graphene Sheets: Chemical Synthesis. Angew. Chem. Int. Ed. 2012, 51, 7640–7654. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on Recent Progress in Nitrogen-Doped Graphene: Synthesis, Characterization, and Its Potential Applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Aksenov, A.V.; Borovlev, I.V.; Aksenova, I.V.; Pisarenko, S.V.; Kovalev, D.A. A New Method for [c,d]Pyridine Peri-Annelation: Synthesis of Azapyrenes from Phenalenes and Their Dihydro Derivatives. Tetrahedron Lett. 2008, 49, 707–709. [Google Scholar] [CrossRef]
- Molenda, R.; Boldt, S.; Villinger, A.; Ehlers, P.; Langer, P. Synthesis of 2-Azapyrenes and Their Photophysical and Electrochemical Properties. J. Org. Chem. 2020, 85, 12823–12842. [Google Scholar] [CrossRef]
- Ammon, E.; Ohlendorf, L.; Villinger, A.; Ehlers, P.; Langer, P. Synthesis and Properties of Dibenzo[a,j]Acridines. Eur. J. Org. Chem. 2020, 2020, 5867–5875. [Google Scholar] [CrossRef]
- Reger, D.; Schöll, K.; Hampel, F.; Maid, H.; Jux, N. Pyridinic Nanographenes by Novel Precursor Design. Chem. A Eur. J. 2021, 27, 1984–1989. [Google Scholar] [CrossRef] [PubMed]
- Draper, S.M.; Gregg, D.J.; Madathil, R. Heterosuperbenzenes: A New Family of Nitrogen-Functionalized, Graphitic Molecules. J. Am. Chem. Soc. 2002, 124, 3486–3487. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, L.P.; Lankage, B.S.; Máille, G.M.Ó.; Perera, S.D.; Nolan, D.; Wang, L.; Draper, S.M. Methoxy Functionalisation: Exerting Synthetic Control of the Supramolecular and Electronic Structure of Nitrogen-Doped Nanographenes. Chem. Commun. 2014, 50, 10637–10640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-S.; Sun, Q.; García, F.; Wang, C.; Yoshikai, N. Robust Cobalt Catalyst for Nitrile/Alkyne [2 + 2 + 2] Cycloaddition: Synthesis of Polyarylpyridines and Their Mechanochemical Cyclodehydrogenation to Nitrogen-Containing Polyaromatics**. Angew. Chem. Int. Ed. 2021, 60, 9627–9634. [Google Scholar] [CrossRef]
- Nehl, H. (H3-Allyl)(H5-Pentamethylcyclopentadienyl)Cobalt—Ein Selektiver Katalysator Für Die Pyridinsynthese. Chem. Ber. 1994, 127, 2535–2537. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, C.; Jia, C.; Tung, C.-H.; Wang, W. Iron-Cobalt-Catalyzed Heterotrimerization of Alkynes and Nitriles to Polyfunctionalized Pyridines. Org. Chem. Front. 2020, 7, 2196–2201. [Google Scholar] [CrossRef]
- Kawahara, K.P.; Matsuoka, W.; Ito, H.; Itami, K. Synthesis of Nitrogen-Containing Polyaromatics by Aza-Annulative π-Extension of Unfunctionalized Aromatics. Angew. Chem. Int. Ed. 2020, 59, 6383–6388. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Kawasumi, K.; Shibata, M.; Ito, H.; Itami, K. One-Shot K-Region-Selective Annulative π-Extension for Nanographene Synthesis and Functionalization. Nat. Commun. 2015, 6, 6251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greßies, S.; Ito, M.; Sakai, M.; Osaki, H.; Kim, J.H.; Gensch, T.; Daniliuc, C.; Ando, N.; Yamaguchi, S.; Glorius, F. Twofold C−H Activation Enables Synthesis of a Diazacoronene-Type Fluorophore with Near Infrared Emission Through Isosteric Replacement. Chem. A Eur. J. 2021, 27, 2753–2759. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Jiang, W.; Wang, Z. Integration of Nitrogen into Coronene Bisimides. Tetrahedron 2012, 68, 9234–9239. [Google Scholar] [CrossRef]
- Jin, E.; Yang, Q.; Ju, C.-W.; Chen, Q.; Landfester, K.; Bonn, M.; Müllen, K.; Liu, X.; Narita, A. A Highly Luminescent Nitrogen-Doped Nanographene as an Acid- and Metal-Sensitive Fluorophore for Optical Imaging. J. Am. Chem. Soc. 2021, 143, 10403–10412. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Thoms, S.; Stöttinger, S.; Schollmeyer, D.; Müllen, K.; Narita, A.; Basché, T. Dibenzo[Hi,St]Ovalene as Highly Luminescent Nanographene: Efficient Synthesis via Photochemical Cyclodehydroiodination, Optoelectronic Properties, and Single-Molecule Spectroscopy. J. Am. Chem. Soc. 2019, 141, 16439–16449. [Google Scholar] [CrossRef] [Green Version]
- Dempsey, G.T.; Vaughan, J.C.; Chen, K.H.; Bates, M.; Zhuang, X. Evaluation of Fluorophores for Optimal Performance in Localization-Based Super-Resolution Imaging. Nat. Methods 2011, 8, 1027–1036. [Google Scholar] [CrossRef]
- Yamamoto, S.; Zhou, Z.Y.; Hiruta, G.; Takeuchi, K.; Choi, J.-C.; Yasuda, T.; Kanbara, T.; Kuwabara, J. One-Pot Synthesis of Triazatriphenylene Using the Povarov Reaction. J. Org. Chem. 2021, 86, 7920–7927. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Chen, W.; Wei, J.; Liu, Y.; Wang, Y. Achieving High Power Efficiency and Low Roll-off OLEDs Based on Energy Transfer from Thermally Activated Delayed Excitons to Fluorescent Dopants. Chem. Commun. 2015, 51, 11972–11975. [Google Scholar] [CrossRef]
- Song, H.-J.; Kim, D.-H.; Lee, E.-J.; Heo, S.-W.; Lee, J.-Y.; Moon, D.-K. Conjugated Polymer Consisting of Quinacridone and Benzothiadiazole as Donor Materials for Organic Photovoltaics: Coplanar Property of Polymer Backbone. Macromolecules 2012, 45, 7815–7822. [Google Scholar] [CrossRef]
- Min, H.; Park, I.S.; Yasuda, T. Cis-Quinacridone-Based Delayed Fluorescence Emitters: Seemingly Old but Renewed Functional Luminogens. Angew. Chem. Int. Ed. 2021, 60, 7643–7648. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Mao, Z.; Xie, Z.; Zhang, Y.; Liu, S.; Zhao, J.; Xu, J.; Chi, Z.; Aldred, M.P. Recent Advances in Organic Thermally Activated Delayed Fluorescence Materials. Chem. Soc. Rev. 2017, 46, 915–1016. [Google Scholar] [CrossRef]
- Lauher, J.W.; Fowler, F.W.; Goroff, N.S. Single-Crystal-to-Single-Crystal Topochemical Polymerizations by Design. Acc. Chem. Res. 2008, 41, 1215–1229. [Google Scholar] [CrossRef]
- Jordan, R.S.; Wang, Y.; McCurdy, R.D.; Yeung, M.T.; Marsh, K.L.; Khan, S.I.; Kaner, R.B.; Rubin, Y. Synthesis of Graphene Nanoribbons via the Topochemical Polymerization and Subsequent Aromatization of a Diacetylene Precursor. Chem 2016, 1, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.L.; Zee, C.-T.; Lin, J.B.; Basile, V.M.; Muni, M.; Flores, M.D.; Munárriz, J.; Kaner, R.B.; Alexandrova, A.N.; Houk, K.N.; et al. Fjord-Edge Graphene Nanoribbons with Site-Specific Nitrogen Substitution. J. Am. Chem. Soc. 2020, 142, 18093–18102. [Google Scholar] [CrossRef] [PubMed]
- Dubey, R.K.; Melle-Franco, M.; Mateo-Alonso, A. Twisted Molecular Nanoribbons with up to 53 Linearly-Fused Rings. J. Am. Chem. Soc. 2021, 143, 6593–6600. [Google Scholar] [CrossRef]
- Piskun, I.; Blackwell, R.; Jornet-Somoza, J.; Zhao, F.; Rubio, A.; Louie, S.G.; Fischer, F.R. Covalent C-N Bond Formation through a Surface Catalyzed Thermal Cyclodehydrogenation. J. Am. Chem. Soc. 2020, 142, 3696–3700. [Google Scholar] [CrossRef] [Green Version]
- Verbitskiy, E.V.; Dinastiya, E.M.; Eltsov, O.S.; Zhilina, E.F.; Schepochkin, A.V.; Rusinov, G.L.; Chupakhin, O.N.; Charushin, V.N. Assembly of Annulated 1,3-Diazapyrenes by Consecutive Cross-Coupling and Cyclodehydrogenation of (Het)Arene Moieties. Mendeleev Commun. 2020, 30, 142–144. [Google Scholar] [CrossRef]
- Moshniaha, L.; Żyła-Karwowska, M.; Cybińska, J.; Chmielewski, P.J.; Favereau, L.; Stępień, M. Bipyrrole Boomerangs via Pd-Mediated Tandem Cyclization–Oxygenation. Controlling Reaction Selectivity and Electronic Properties. Beilstein J. Org. Chem. 2020, 16, 895–903. [Google Scholar] [CrossRef]
- Qian, Y.; Shang, J.; Lyu, Z.; Huang, X.; Guan, A.; Xu, L.; Gong, H. Synthesis of π-Extended Carbazoles via One-Pot C—C Coupling and Chlorination Promoted by FeCl3. Chin. J. Chem. 2020, 38, 1538–1544. [Google Scholar] [CrossRef]
- Oki, K.; Takase, M.; Kobayashi, N.; Uno, H. Synthesis and Characterization of Peralkylated Pyrrole-Fused Azacoronene. J. Org. Chem. 2021, 86, 5102–5109. [Google Scholar] [CrossRef]
- Zhai, L.; Shukla, R.; Rathore, R. Oxidative C−C Bond Formation (Scholl Reaction) with DDQ as an Efficient and Easily Recyclable Oxidant. Org. Lett. 2009, 11, 3474–3477. [Google Scholar] [CrossRef]
- Sasaki, Y.; Takase, M.; Mori, S.; Uno, H. Synthesis and Properties of NitroHPHAC: The First Example of Substitution Reaction on HPHAC. Molecules 2020, 25, 2486. [Google Scholar] [CrossRef]
- Pandey, S.K.; Gupta, V.; Singh, R.P. CAN-Mediated Oxidative Cyclodehydrogenation of Hexapyrrolylbenzenes. Synlett 2020, 31, 1268–1272. [Google Scholar] [CrossRef]
- Sasaki, Y.; Takase, M.; Kobayashi, N.; Mori, S.; Ohara, K.; Okujima, T.; Uno, H. Radially π-Extended Pyrrole-Fused Azacoronene: A Series of Crystal Structures of HPHAC with Various Oxidation States. J. Org. Chem. 2021, 86, 4290–4295. [Google Scholar] [CrossRef] [PubMed]
- Navakouski, M.; Zhylitskaya, H.; Chmielewski, P.J.; Żyła-Karwowska, M.; Stȩpień, M. Electrophilic Aromatic Coupling of Hexapyrrolylbenzenes. A Mechanistic Analysis. J. Org. Chem. 2020, 85, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Krzeszewski, M.; Dobrzycki, Ł.; Sobolewski, A.L.; Cyrański, M.K.; Gryko, D.T. Bowl-Shaped Pentagon- and Heptagon-Embedded Nanographene Containing a Central Pyrrolo[3,2-b]Pyrrole Core. Angew. Chem. Int. Ed. 2021, 60, 14998–15005. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Krzeszewski, M.; Pignedoli, C.A.; Ruffieux, P.; Fasel, R.; Gryko, D.T. On-Surface Synthesis of a Nitrogen-Embedded Buckybowl with Inverse Stone–Thrower–Wales Topology. Nat. Commun. 2018, 9, 1714. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.-Q.; Ochiai, K.; Lee, C.-A.; Ito, S. Synthesis of π-Extended Imidazoles by 1,3-Dipolar Cycloaddition of Polycyclic Aromatic Azomethine Ylides with Nitriles. Org. Lett. 2020, 22, 6132–6137. [Google Scholar] [CrossRef]
- Hayakawa, S.; Kawasaki, A.; Hong, Y.; Uraguchi, D.; Ooi, T.; Kim, D.; Akutagawa, T.; Fukui, N.; Shinokubo, H. Inserting Nitrogen: An Effective Concept To Create Nonplanar and Stimuli-Responsive Perylene Bisimide Analogues. J. Am. Chem. Soc. 2019, 141, 19807–19816. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-J.; Tang, M.-C.; Fu, Y.; Low, K.-H.; Ma, J.; Yang, L.; Weigand, J.J.; Liu, J.; Yam, V.W.-W.; Feng, X. One-Pot Synthesis of Boron-Doped Polycyclic Aromatic Hydrocarbons via 1,4-Boron Migration. Angew. Chem. Int. Ed. 2021, 60, 2833–2838. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Yamada, T.; Narita, H.; Oehlmann, N.N.; Wagner, M.; Yamaguchi, S. Boron-Doped Polycyclic π-Electron Systems with an Antiaromatic Borole Substructure That Forms Photoresponsive B-P Lewis Adducts. J. Am. Chem. Soc. 2021, 9944. [Google Scholar] [CrossRef]
- Schickedanz, K.; Radtke, J.; Bolte, M.; Lerner, H.-W.; Wagner, M. Facile Route to Quadruply Annulated Borepins. J. Am. Chem. Soc. 2017, 139, 2842–2851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messersmith, R.E.; Tovar, J.D. Borepin Rings as “Sigma-Free” Reporters of Aromaticity within Polycyclic Aromatic Scaffolds. J. Phys. Chem. A 2019, 123, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Ruiz, L.; Rodríguez-González, S.; Fallaque, J.G.; Márquez, I.R.; Agraït, N.; Díaz, C.; Leary, E.; Cuerva, J.M.; Campaña, A.G.; Martín, F.; et al. Single-Molecule Conductance of 1,4-Azaborine Derivatives as Models of BN-Doped PAHs. Angew. Chem. Int. Ed. 2021, 60, 6609–6616. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Park, I.S.; Yasuda, T. Full-Color, Narrowband, and High-Efficiency Electroluminescence from Boron and Carbazole Embedded Polycyclic Heteroaromatics. J. Am. Chem. Soc. 2020, 142, 19468–19472. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Shiren, K.; Nakajima, K.; Nomura, S.; Nakatsuka, S.; Kinoshita, K.; Ni, J.; Ono, Y.; Ikuta, T. Ultrapure Blue Thermally Activated Delayed Fluorescence Molecules: Efficient HOMO–LUMO Separation by the Multiple Resonance Effect. Adv. Mater. 2016, 28, 2777–2781. [Google Scholar] [CrossRef] [PubMed]
- Hua, T.; Zhan, L.; Li, N.; Huang, Z.; Cao, X.; Xiao, Z.; Gong, S.; Zhou, C.; Zhong, C.; Yang, C. Heavy-Atom Effect Promotes Multi-Resonance Thermally Activated Delayed Fluorescence. Chem. Eng. J. 2021, 426, 131169. [Google Scholar] [CrossRef]
- Nagata, M.; Min, H.; Watanabe, E.; Fukumoto, H.; Mizuhata, Y.; Tokitoh, N.; Agou, T.; Yasuda, T. Fused-Nonacyclic Multi-Resonance Delayed Fluorescence Emitter Based on Ladder-Thiaborin Exhibiting Narrowband Sky-Blue Emission with Accelerated Reverse Intersystem Crossing. Angew. Chem. Int. Ed. 2021, 60, 20280–20285. [Google Scholar] [CrossRef]
- Liu, Z.; Marder, T.B. B-N versus C-C: How Similar Are They? Angew. Chem. Int. Ed. 2008, 47, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Farrell, J.M.; Mützel, C.; Bialas, D.; Rudolf, M.; Menekse, K.; Krause, A.-M.; Stolte, M.; Würthner, F. Tunable Low-LUMO Boron-Doped Polycyclic Aromatic Hydrocarbons by General One-Pot C–H Borylations. J. Am. Chem. Soc. 2019, 141, 9096–9104. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W.; Qiao, Y.; Lu, X.; Zhou, G. BN-Embedded Polycyclic Aromatic Hydrocarbon Oligomers: Synthesis, Aromaticity, and Reactivity. Angew. Chem. Int. Ed. 2020, 59, 7122–7130. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, Y.; Wang, Y.; Cao, X.; Han, C.; Liu, G.; Xu, Z.; Zhan, C.; Hu, H.; Peng, Y.; et al. Precise Molecular Design for BN-Modified Polycyclic Aromatic Hydrocarbons toward Mechanochromic Materials. J. Mater. Chem. A 2020, 8, 22023–22031. [Google Scholar] [CrossRef]
- Zhang, M.-X.; Zuckerman, N.B.; Pagoria, P.F.; Steele, B.A.; Kuo, I.-F.; Imler, G.H.; Parrish, D. Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization. Molecules 2021, 26, 4209. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Guo, Y.; Ye, J.; Zhen, B.; Chen, Y.; Liu, X. Pyrrolic Type N Directed Borylation Route to BN-PAHs: Tuning the Photophysical Properties by Varying the Conjugation Shape and Size. J. Org. Chem. 2021, 86, 6322–6330. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, H.; Gao, Y.; Huang, L.; Berger, R.; Liu, J.; Lu, H.; Cheng, Z.; Du, S.; Gao, H.; et al. On-Surface Synthesis of NBN-Doped Zigzag-Edged Graphene Nanoribbons. Angew. Chem. Int. Ed. 2020, 59, 8873–8879. [Google Scholar] [CrossRef] [Green Version]
- Ju, C.-W.; Li, B.; Li, L.; Yan, W.; Cui, C.; Ma, X.; Zhao, D. Modular Synthesis of Pentagonal and Hexagonal Ring-Fused NBN-Phenalenes Leading to an Excited-State Aromatization-Induced Structural Planarization Molecular Library. J. Am. Chem. Soc. 2021, 143, 5903–5916. [Google Scholar] [CrossRef]
- Min, Y.; Cao, X.; Tian, H.; Liu, J.; Wang, L. B←N-Incorporated Dibenzo-azaacene with Selective Near-Infrared Absorption and Visible Transparency. Chem. Eur. J. 2021, 27, 2065–2071. [Google Scholar] [CrossRef]
- Petrushenko, I.K.; Tsar’kova, A.I.; Petrushenko, K.B. Hydrogen Adsorption on BN-Embedded Tetrabenzopentacene as a Promising Nanoflake for Energy Storage: Theoretical Insights. Diam. Relat. Mater. 2020, 108, 107968. [Google Scholar] [CrossRef]
- Karamanis, P.; Otero, N.; Xenides, D.; Denawi, H.; Mandado, M.; Rérat, M. From Pyridine Adduct of Borabenzene to (In)Finite Graphene Architectures Functionalized with N→B Dative Bonds. Prototype Systems of Strong One- and Two-Photon Quantum Transitions Triggering Large Nonlinear Optical Responses. J. Phys. Chem. C 2020, 124, 21063–21074. [Google Scholar] [CrossRef]
- Tanaka, H.; Oda, S.; Ricci, G.; Gotoh, H.; Tabata, K.; Kawasumi, R.; Beljonne, D.; Olivier, Y.; Hatakeyama, T. Hypsochromic Shift of Multiple-Resonance-Induced Thermally Activated Delayed Fluorescence by Oxygen Atom Incorporation. Angew. Chem. Int. Ed. 2021, 60, 17910–17914. [Google Scholar] [CrossRef]
- Kim, J.H.; Chung, W.J.; Kim, J.; Lee, J.Y. Concentration Quenching–Resistant Multiresonance Thermally Activated Delayed Fluorescence Emitters. Mater. Today Energy 2021, 21, 100792. [Google Scholar] [CrossRef]
- Sakamaki, T.; Nakamuro, T.; Yamashita, K.; Hirata, K.; Shang, R.; Nakamura, E. B2N2-Doped Dibenzo[a,m]Rubicene: Modular Synthesis, Properties, and Coordination-Induced Color Tunability. Chem. Mater. 2021, 33, 5337–5344. [Google Scholar] [CrossRef]
- Liu, G.; Sasabe, H.; Kumada, K.; Matsunaga, A.; Katagiri, H.; Kido, J. Facile Synthesis of Multi-Resonance Ultra-Pure-Green TADF Emitters Based on Bridged Diarylamine Derivatives for Efficient OLEDs with Narrow Emission. J. Mater. Chem. C 2021, 9, 8308–8313. [Google Scholar] [CrossRef]
- Oda, S.; Kumano, W.; Hama, T.; Kawasumi, R.; Yoshiura, K.; Hatakeyama, T. Carbazole-Based DABNA Analogues as Highly Efficient Thermally Activated Delayed Fluorescence Materials for Narrowband Organic Light-Emitting Diodes. Angew. Chem. Int. Ed. 2021, 60, 2882–2886. [Google Scholar] [CrossRef]
- Yang, H.; Cao, Y.; Gao, Y.; Fu, Y.; Huang, L.; Liu, J.; Feng, X.; Du, S.; Gao, H.-J. NBN-Doped Nanographene Embedded with Five- and Seven-Membered Rings on Au (111) Surface*. Chin. Phys. B 2021, 30, 056802. [Google Scholar] [CrossRef]
- Zhuang, F.; Yang, J.; Sun, Z.; Zhang, P.; Chen, Q.; Wang, J.; Pei, J. BN Fused Diazulenyl-Carbazole: Synthesis, Structure, and Properties. Chin. J. Chem. 2021, 39, 909–912. [Google Scholar] [CrossRef]
- Zhao, J.; Huang, H.; Zhou, W.; Wu, D.; Xia, J. Synthesis and Characterization of a BN-Embedded Nine-Ring Fused Heteroaromatics with Dual Channel Detection of Fluoride Anions. Dye. Pigment. 2021, 194, 109648. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Liu, X.; Xu, L.; Rao, B.; Yan, N.; He, G. Dithienoazaborine Derivatives with Selective π-Conjugated Extension via Late-Stage Functionalization. J. Mater. Chem. C 2021, 9, 4053–4061. [Google Scholar] [CrossRef]
- Min, Y.; Dou, C.; Tian, H.; Liu, J.; Wang, L. Isomers of B←N-Fused Dibenzo-azaacenes: How B←N Affects Opto-electronic Properties and Device Behaviors? Chem. Eur. J. 2021, 27, 4364–4372. [Google Scholar] [CrossRef]
- Crumbach, M.; Ayhan, O.; Fritze, L.; Sprenger, J.A.P.; Zapf, L.; Finze, M.; Helten, H. BNB-Doped Phenalenyls—Aromaticity Switch upon One-Electron Reduction. Chem. Commun. 2021, 57, 2408–2411. [Google Scholar] [CrossRef]
- Ito, M.; Sakai, M.; Ando, N.; Yamaguchi, S. Electron-Deficient Heteroacenes That Contain Two Boron Atoms: Near-Infrared Fluorescence Based on a Push–Pull Effect. Angew. Chem. Int. Ed. 2021, 60, 21853. [Google Scholar] [CrossRef]
- Jin, L.; Bilbao, N.; Lv, Y.; Wang, X.-Y.; Soltani, P.; Mali, K.S.; Narita, A.; De Feyter, S.; Müllen, K.; Chen, Z. 2D Self-Assembly and Electronic Characterization of Oxygen–Boron–Oxygen-Doped Chiral Graphene Nanoribbons. Chem. Commun. 2021, 57, 6031–6034. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhao, L.; Wang, X.; Yang, Q.; Li, W.; Tian, H.; Shao, S.; Wang, L.; Jing, X.; Wang, F. Novel Boron- and Sulfur-Doped Polycyclic Aromatic Hydrocarbon as Multiple Resonance Emitter for Ultrapure Blue Thermally Activated Delayed Fluorescence Polymers. Sci. China Chem. 2021, 64, 547–551. [Google Scholar] [CrossRef]
- Feofanov, M.; Akhmetov, V.; Takayama, R.; Amsharov, K. Catalyst-Free Synthesis of O-Heteroacenes by Ladderization of Fluorinated Oligophenylenes. Angew. Chem. Int. Ed. 2021, 60, 5199–5203. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhu, D.; Lin, G.; Du, M.; Shi, D.; Peng, Q.; Jiang, L.; Liu, Z.; Zhang, G.; Zhang, D. New Fused Conjugated Molecules with Fused Thiophene and Pyran Units for Organic Electronic Materials. RSC Adv. 2020, 10, 12378–12383. [Google Scholar] [CrossRef]
- Dordević, L.; Milano, D.; Demitri, N.; Bonifazi, D. O-Annulation to Polycyclic Aromatic Hydrocarbons: A Tale of Optoelectronic Properties from Five- To Seven-Membered Rings. Org. Lett. 2020, 22, 4283–4288. [Google Scholar] [CrossRef] [PubMed]
- Ðorđević, L.; Valentini, C.; Demitri, N.; Mézière, C.; Allain, M.; Sallé, M.; Folli, A.; Murphy, D.; Mañas-Valero, S.; Coronado, E.; et al. O-Doped Nanographenes: A Pyrano/Pyrylium Route Towards Semiconducting Cationic Mixed-Valence Complexes. Angew. Chem. Int. Ed. 2020, 59, 4106–4114. [Google Scholar] [CrossRef]
- Fujimoto, K.; Izawa, S.; Takahashi, A.; Inuzuka, T.; Sanada, K.; Sakamoto, M.; Nakayama, Y.; Hiramoto, M.; Takahashi, M. Curved Perylene Diimides Fused with Seven-Membered Rings. Chem. Asian J. 2021, 16, 690–695. [Google Scholar] [CrossRef]
- Delouche, T.; Roisnel, T.; Dorcet, V.; Hissler, M.; Bouit, P.-A. Mixing Polyaromatic Scaffolds and Main Group Elements: Synthesis, Coordination and Optical Properties of Naphthyl-Fused Heteropines. Eur. J. Inorg. Chem. 2021, 2021, 1082–1089. [Google Scholar] [CrossRef]
- Mokrai, R.; Mocanu, A.; Duffy, M.P.; Vives, T.; Caytan, E.; Dorcet, V.; Roisnel, T.; Nyulászi, L.; Benkő, Z.; Bouit, P.-A.; et al. Stereospecific Synthesis of Chiral P-Containing Polyaromatics Based on 7-Membered P-Rings. Chem. Commun. 2021, 57, 7256–7259. [Google Scholar] [CrossRef] [PubMed]
- Hindenberg, P.; Busch, M.; Paul, A.; Bernhardt, M.; Gemessy, P.; Rominger, F.; Romero-Nieto, C. Diphosphahexaarenes as Highly Fluorescent and Stable Materials. Angew. Chem. Int. Ed. 2018, 57, 15157–15161. [Google Scholar] [CrossRef] [PubMed]
- Si, E.; Zhao, P.; Wang, L.; Duan, Z.; Mathey, F. New Access to Six-Membered Phosphacycle Annulated Polyaromatic Ring System. Eur. J. Org. Chem. 2020, 2020, 697–701. [Google Scholar] [CrossRef]
- Hertz, V.M.; Bolte, M.; Lerner, H.-W.; Wagner, M. Boron-Containing Polycyclic Aromatic Hydrocarbons: Facile Synthesis of Stable, Redox-Active Luminophores. Angew. Chem. 2015, 127, 8924–8928. [Google Scholar] [CrossRef]
- Hertz, V.M.; Massoth, J.G.; Bolte, M.; Lerner, H.-W.; Wagner, M. En Route to Stimuli-Responsive Boron-, Nitrogen-, and Sulfur-Doped Polycyclic Aromatic Hydrocarbons. Chem. Eur. J. 2016, 22, 13181–13188. [Google Scholar] [CrossRef]
- Ascherl, J.D.R.; Neiß, C.; Vogel, A.; Graf, J.; Rominger, F.; Oeser, T.; Hampel, F.; Görling, A.; Kivala, M. Phosphorus-Containing Dibenzonaphthanthrenes: Electronic Fine Tuning of Polycyclic Aromatic Hydrocarbons through Organophosphorus Chemistry. Chem. Eur. J. 2020, 26, 13157–13162. [Google Scholar] [CrossRef]
- Delouche, T.; Vacher, A.; Caytan, E.; Roisnel, T.; Le Guennic, B.; Jacquemin, D.; Hissler, M.; Bouit, P.-A. Multi-Stage Redox Systems Based on Dicationic P-Containing Polycyclic Aromatic Hydrocarbons. Chem. A Eur. J. 2020, 26, 8226–8229. [Google Scholar] [CrossRef]
- Niu, W.; Fu, Y.; Komber, H.; Ma, J.; Feng, X.; Mai, Y.; Liu, J. Sulfur-Doped Nanographenes Containing Multiple Subhelicenes. Org. Lett. 2021, 23, 2069–2073. [Google Scholar] [CrossRef]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biagiotti, G.; Perini, I.; Richichi, B.; Cicchi, S. Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes. Molecules 2021, 26, 6306. https://doi.org/10.3390/molecules26206306
Biagiotti G, Perini I, Richichi B, Cicchi S. Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes. Molecules. 2021; 26(20):6306. https://doi.org/10.3390/molecules26206306
Chicago/Turabian StyleBiagiotti, Giacomo, Ilaria Perini, Barbara Richichi, and Stefano Cicchi. 2021. "Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes" Molecules 26, no. 20: 6306. https://doi.org/10.3390/molecules26206306
APA StyleBiagiotti, G., Perini, I., Richichi, B., & Cicchi, S. (2021). Novel Synthetic Approach to Heteroatom Doped Polycyclic Aromatic Hydrocarbons: Optimizing the Bottom-Up Approach to Atomically Precise Doped Nanographenes. Molecules, 26(20), 6306. https://doi.org/10.3390/molecules26206306





