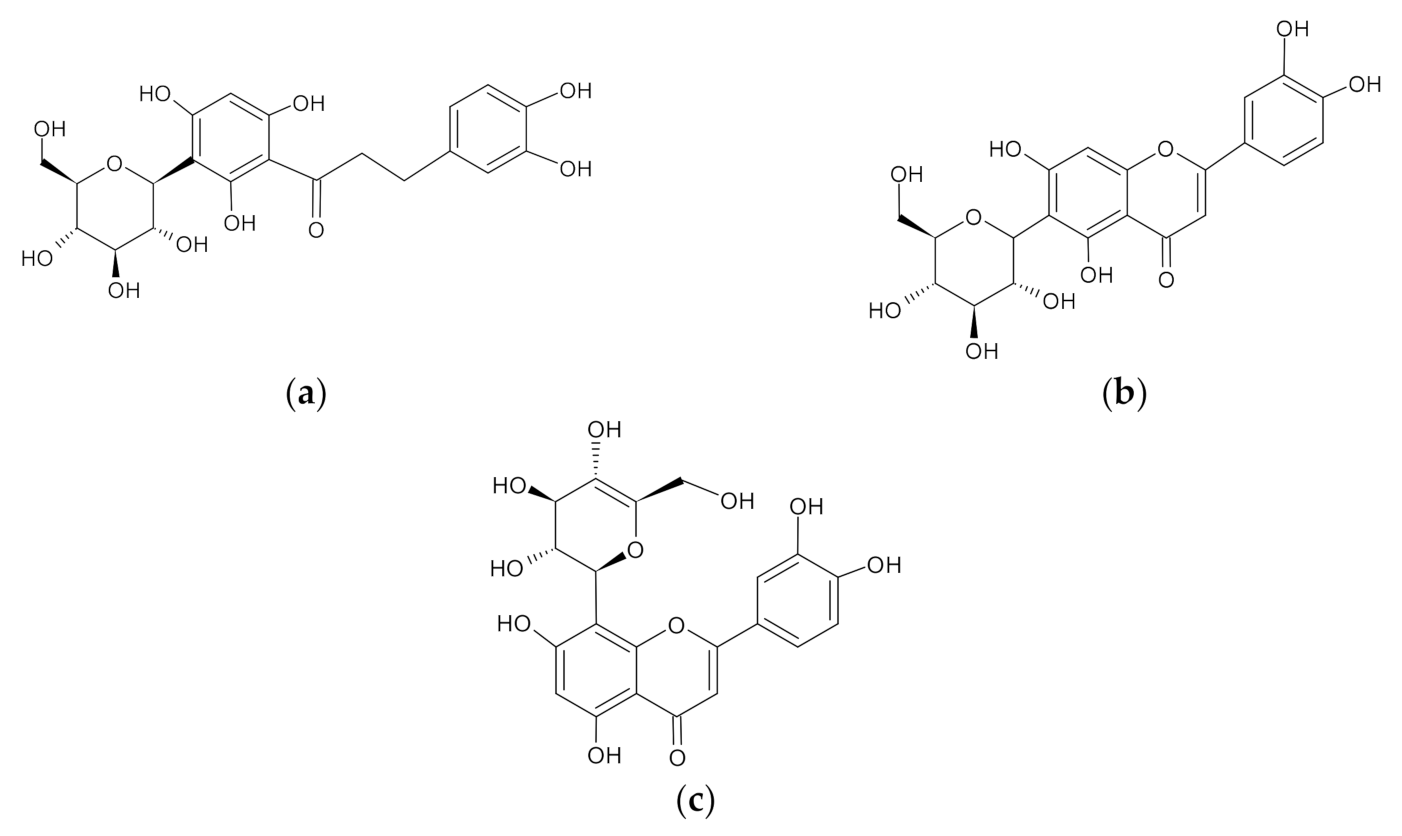
Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells
Abstract
:1. Introduction
2. Results
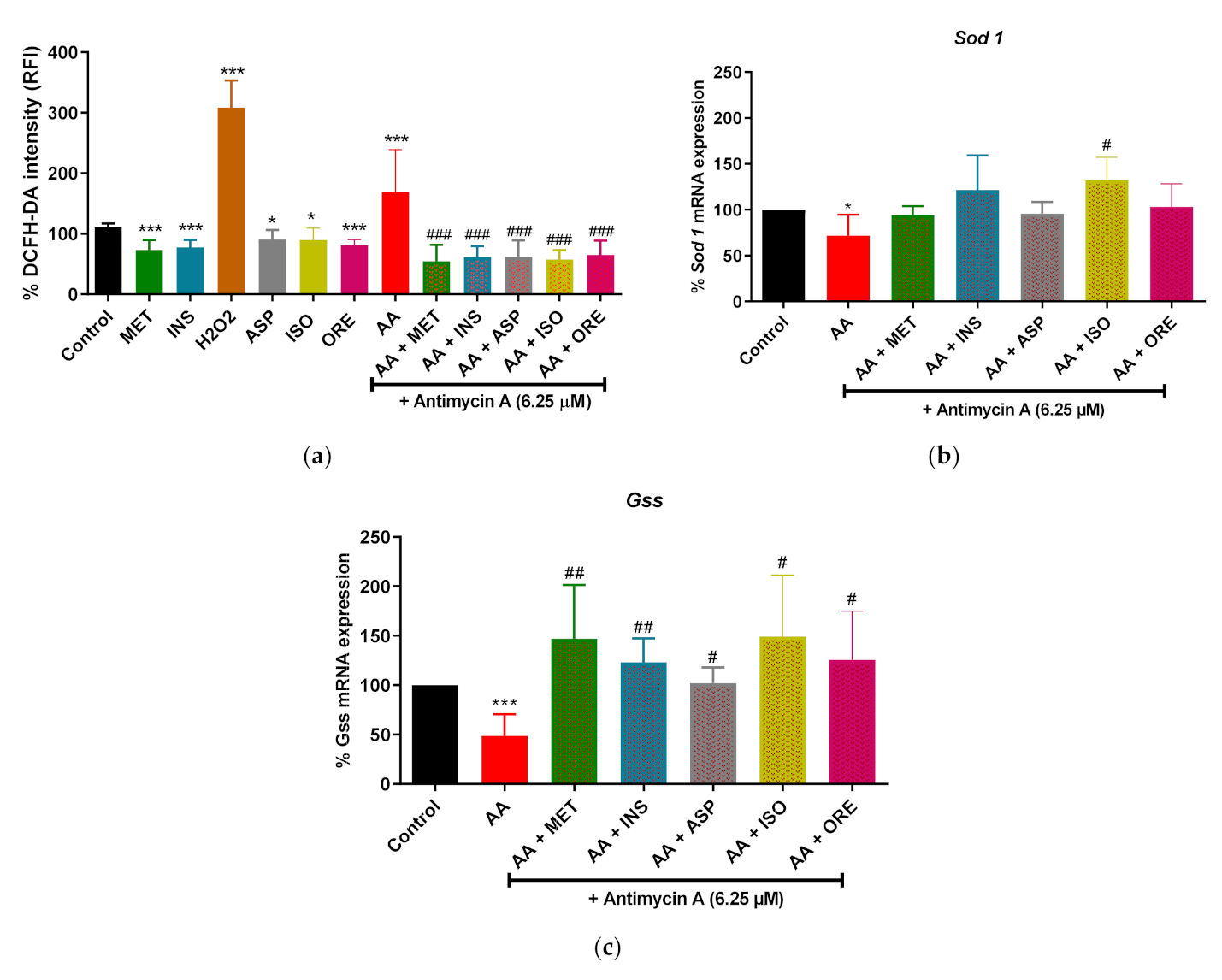
2.1. Aspalathin, Isoorientin, and Orientin Reduced ROS Production and Increased the Expression of Some Antioxidant Genes
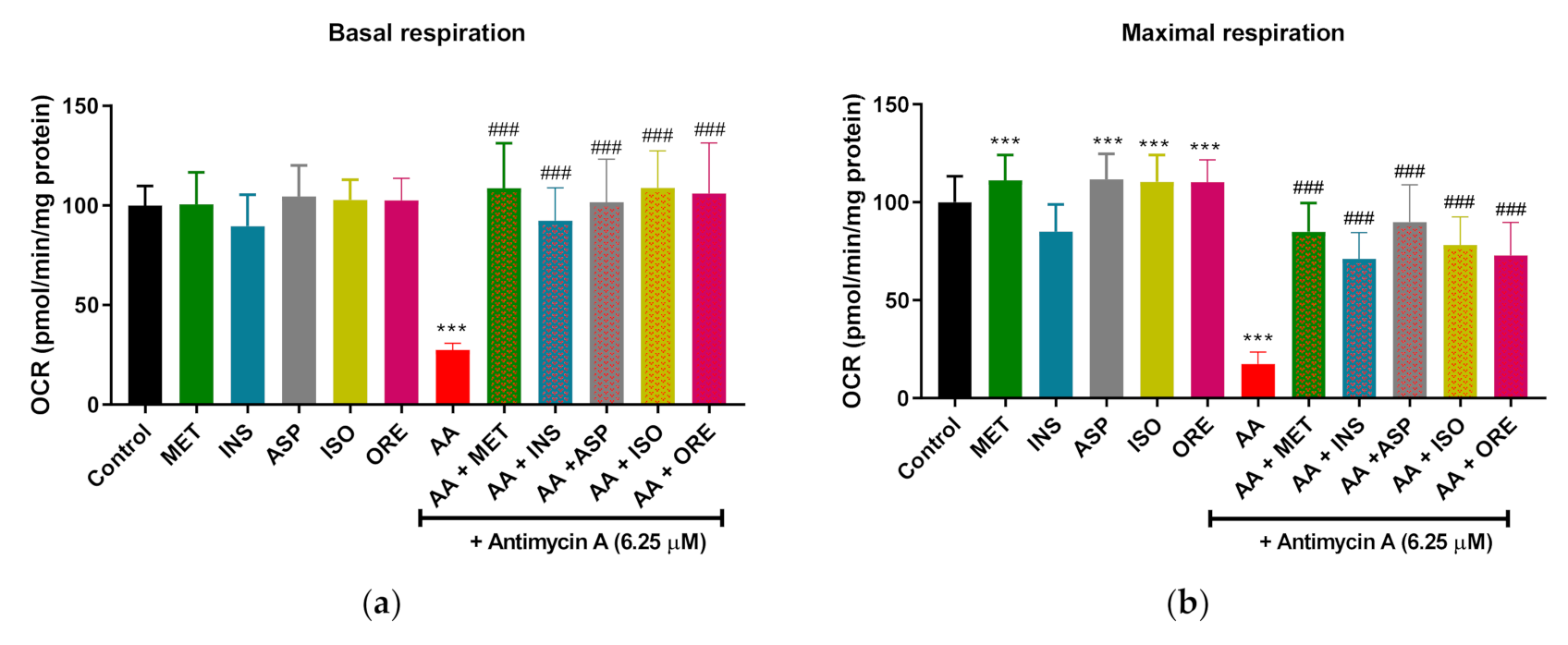
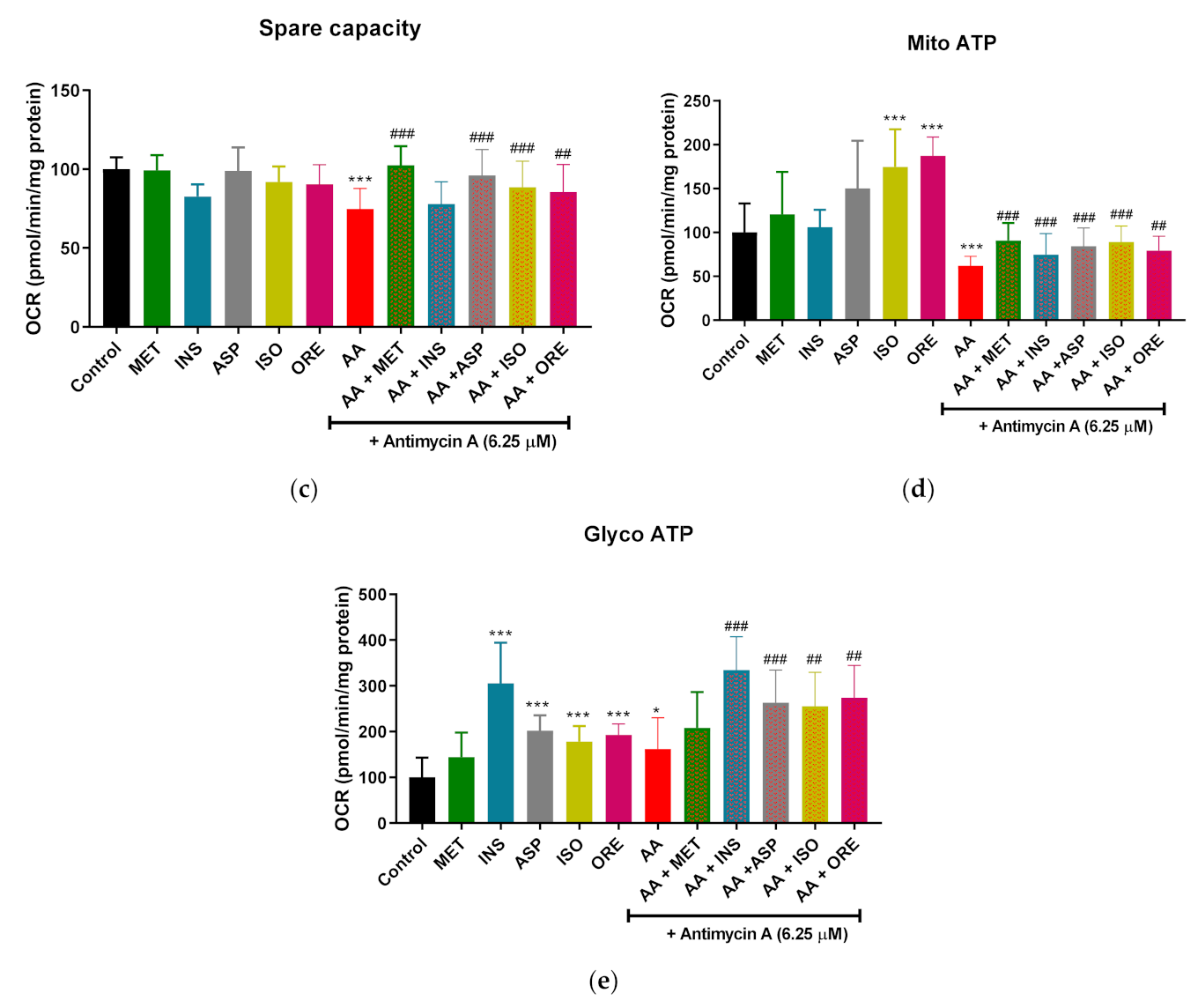
2.2. Aspalathin, Isoorientin, and Orientin Enhance the Parameters of Mitochondrial Respiration and Glycolysis following Exposure to Antimycin A in Cultured Skeletal Muscle Cells
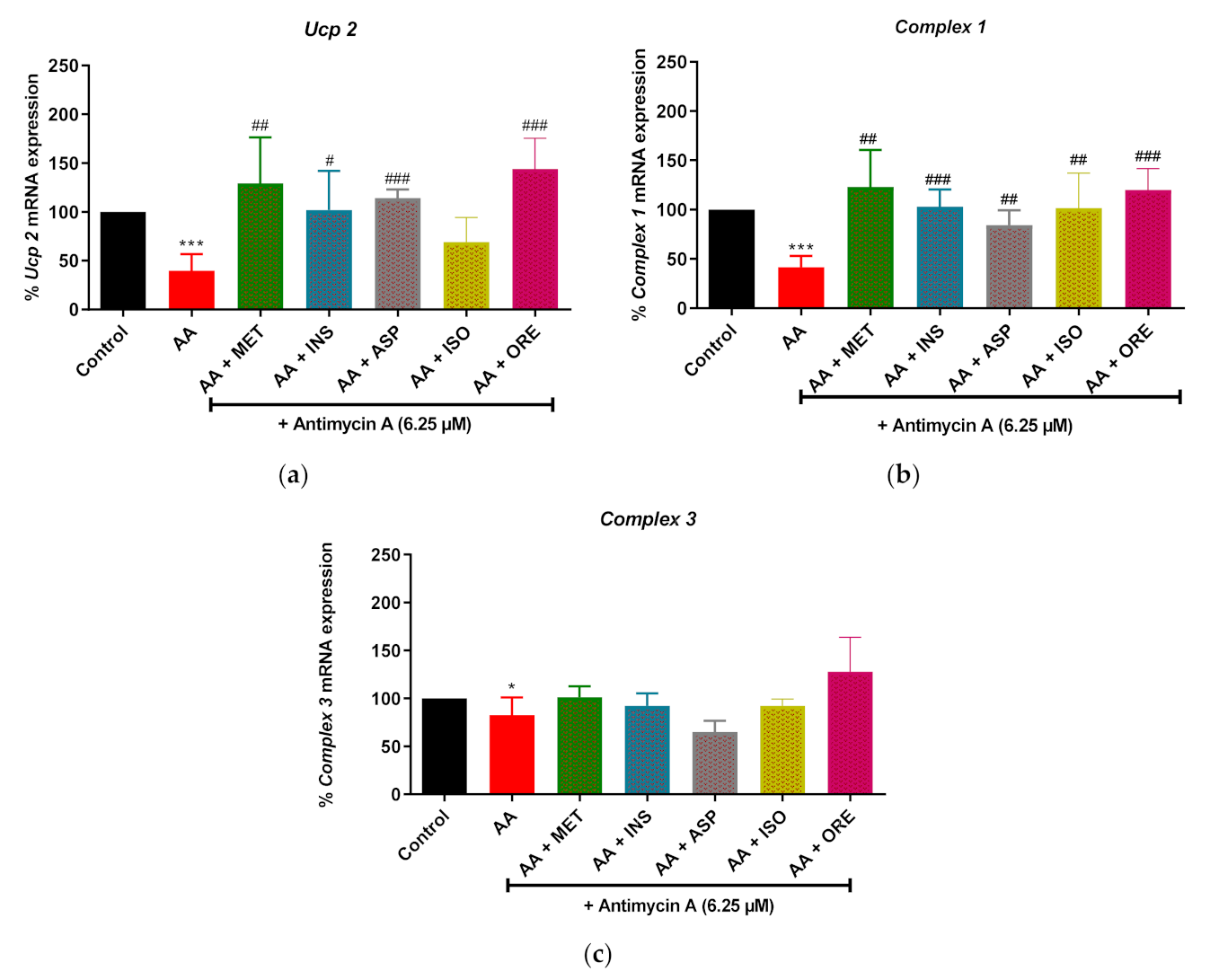
2.3. Aspalathin, Isoorientin and Orientin Modulates the mRNA Expression of Genes Involved in Mitochondrial Bioenergetics following Exposure to Antimycin A in Cultured Skeletal Muscle Cells
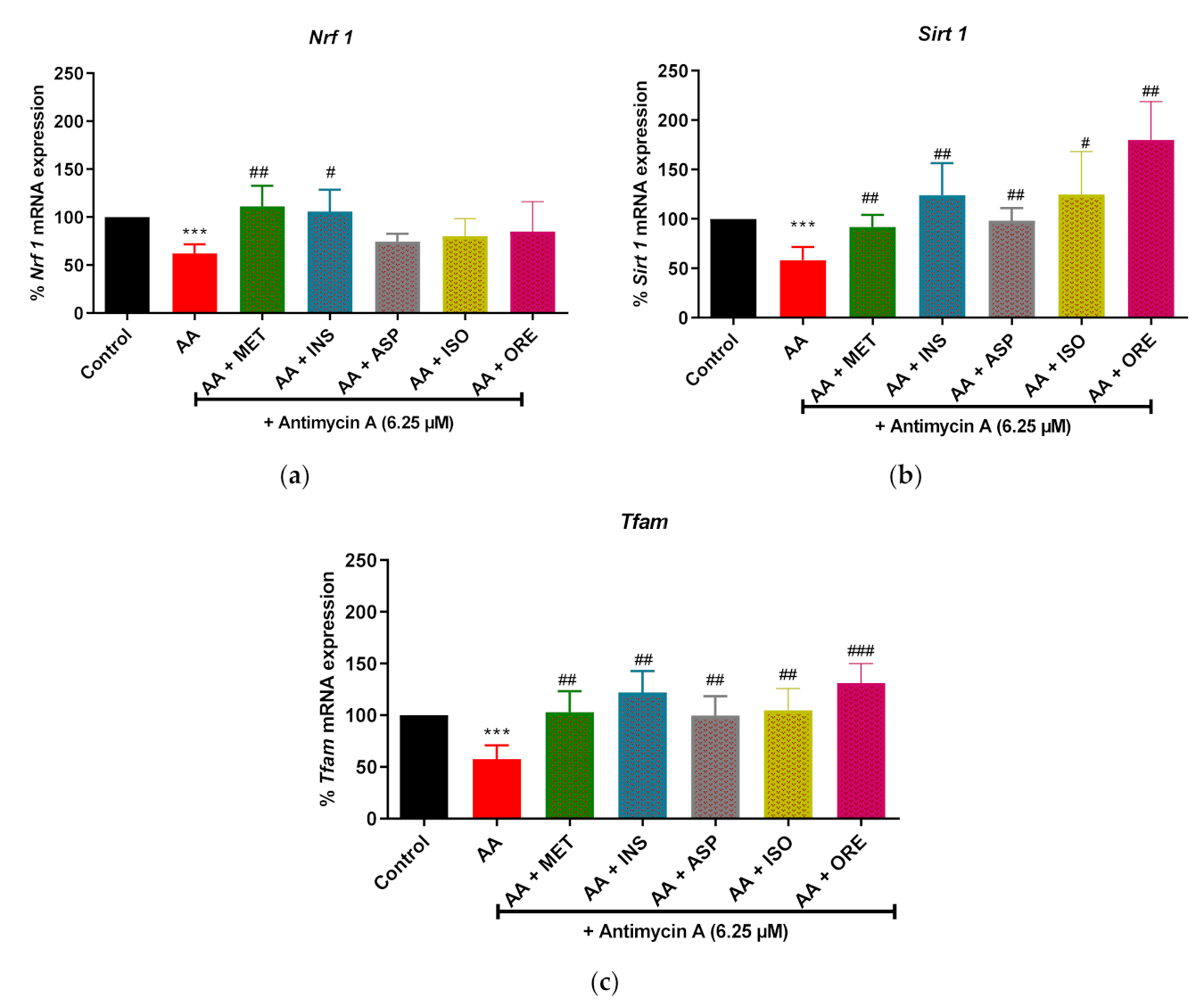
2.4. Aspalathin, Isoorientin, and Orientin Enhanced the mRNA Expression of Genes of the Markers of Mitochondrial Biogenesis in Cultured Skeletal Muscle Cells
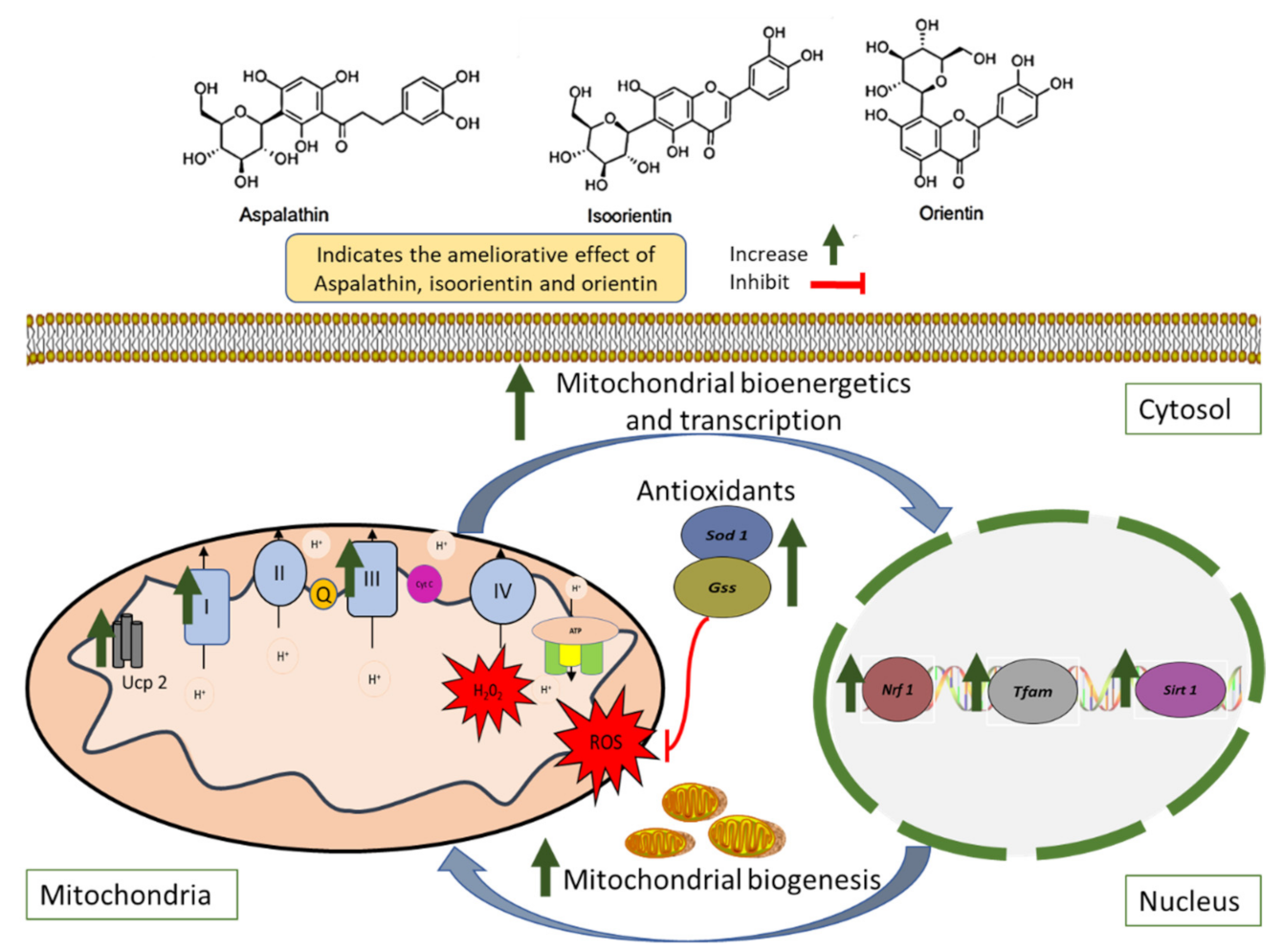
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture and Differentiation
4.3. Experimental Model of Mitochondrial Dysfunction and Preparation of Treatment Compounds
4.4. Assessing the Production of Reactive Oxygen Species
4.5. RT-PCR for mRNA Expression Analysis
4.6. Assessment of Mitochondrial Bioenergetics and Real-Time ATP Production
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. 2), S157–S163. [Google Scholar] [CrossRef] [Green Version]
- Garneau, L.; Aguer, C. Role of myokines in the development of skeletal muscle insulin resistance and related metabolic defects in type 2 diabetes. Diabetes Metab. 2019, 45, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Creo, A.L.; Cortes, T.M.; Dasari, S.; Nair, K.S. Altered mitochondrial function in insulin-deficient and insulin-resistant states. J. Clin. Investig. 2018, 128, 3671–3681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montgomery, M.K.; Turner, N. Mitochondrial dysfunction and insulin resistance: An update. Endocr. Connect. 2015, 4, R1–R15. [Google Scholar] [CrossRef] [Green Version]
- Zamora, M. Targeting mitochondrial biogenesis to treat insulin resistance. Curr. Pharm. Des. 2014, 20, 5527–5557. [Google Scholar] [CrossRef]
- Murphy, M.P. Mitochondrial dysfunction indirectly elevates ros production by the endoplasmic reticulum. Cell Metab. 2013, 18, 145–146. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, M.K. Mitochondrial dysfunction and diabetes: Is mitochondrial transfer a friend or foe? Biology 2019, 8, 33. [Google Scholar] [CrossRef] [Green Version]
- Affourtit, C. Mitochondrial involvement in skeletal muscle insulin resistance: A case of imbalanced bioenergetics. Biochim. Biophys. Acta 2016, 1857, 1678–1693. [Google Scholar] [CrossRef] [Green Version]
- Fazakerley, D.J.; Minard, A.Y.; Krycer, J.R.; Thomas, K.C.; Stöckli, J.; Harney, D.J.; Burchfield, J.G.; Maghzal, G.J.; Caldwell, S.; Hartley, R.; et al. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. J. Biol. Chem. 2018, 293, 7315–7328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konaté, K.; Yomalan, K.; Sytar, O.; Zerbo, P.; Brestic, M.; Patrick, V.D.; Gagniuc, P.; Barro, N. Free radicals scavenging capacity, antidiabetic and antihypertensive activities of flavonoid-rich fractions from leaves of Trichilia emetica and Opilia amentaceain an animal model of type 2 diabetes mellitus. Evid.-Based Complement. Altern. Med. 2014, 2014, 1–13. [Google Scholar] [CrossRef] [Green Version]
- de Beer, D.; Malherbe, C.J.; Beelders, T.; Willenburg, E.L.; Brand, D.J.; Joubert, E. Isolation of aspalathin and nothofagin from rooibos (Aspalathus linearis) using high-performance countercurrent chromatography: Sample loading and compound stability considerations. J. Chromatogr. A 2015, 1381, 29–36. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jing, S.; Lin, H.; Sun, W.; Jiang, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Anti-fatigue effect of anwulignan via the NRF2 and PGC-1α signaling pathway in mice. Food Funct. 2019, 10, 7755–7766. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, N.; Pico, C.; Macarulla, M.T.; Oliver, P.; Miranda, J.; Palou, A.; Portillo, M.P. A combination of resveratrol and quercetin induces browning in white adipose tissue of rats fed an obesogenic diet. Obesity 2017, 25, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Mthembu, S.; Dludla, P.; Ziqubu, K.; Nyambuya, T.; Kappo, A.; Madoroba, E.; Nyawo, T.; Nkambule, B.; Silvestri, S.; Muller, C.; et al. The Potential role of polyphenols in modulating mitochondrial bioenergetics within the skeletal muscle: A systematic review of preclinical models. Molecules 2021, 26, 2791. [Google Scholar] [CrossRef]
- Ku, S.-K.; Kwak, S.; Kim, Y.; Bae, J.-S. Aspalathin and nothofagin from rooibos (Aspalathus linearis) inhibits high glucose-induced inflammation in vitro and in vivo. Inflammation 2014, 38, 445–455. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J.F. Aspalathin-enriched green rooibos extract reduces hepatic insulin resistance by modulating PI3K/AKT and AMPK Pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef] [Green Version]
- Moens, C.; Bensellam, M.; Himpe, E.; Muller, C.J.F.; Jonas, J.; Bouwens, L. Aspalathin protects insulin-producing β Cells against glucotoxicity and oxidative stress-induced cell death. Mol. Nutr. Food Res. 2020, 64, e1901009. [Google Scholar] [CrossRef]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-Tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Muller, C.J.F.; Malherbe, C.J.; Chellan, N.; Yagasaki, K.; Miura, Y.; Joubert, E. Potential of rooibos, its major C-glucosyl flavonoids, andZ-2-(β-D-glucopyranosyloxy)-3-phenylpropenoic acid in prevention of metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2018, 58, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; De Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomedicine 2014, 21, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.; Muller, C.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomedicine 2014, 21, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-H.; Kao, Y.-H.; Muller, C.J.; Joubert, E.; Chuu, C.-P. Aspalathin-rich green Aspalathus linearis extract suppresses migration and invasion of human castration-resistant prostate cancer cells via inhibition of YAP signaling. Phytomedicine 2020, 69, 153210. [Google Scholar] [CrossRef]
- Pringle, N.A.; Koekemoer, T.C.; Holzer, A.; Young, C.; Venables, L.; Van De Venter, M. Potential therapeutic benefits of green and fermented rooibos (Aspalathus linearis) in dermal wound healing. Planta Med. 2018, 84, 645–652. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; De Beer, D.; Dludla, P.V.; Ferreira, D.; Muller, C.J.F.; Joubert, E. Aspalathin from rooibos (Aspalathus linearis): A bioactive c-glucosyl dihydrochalcone with potential to target the metabolic syndrome. Planta Med. 2018, 84, 568–583. [Google Scholar] [CrossRef] [Green Version]
- Muller, C.; Joubert, E.; de Beer, D.; Sanderson, M.; Malherbe, C.; Fey, S.; Louw, J. Acute assessment of an aspalathin-enriched green rooibos (Aspalathus linearis) extract with hypoglycemic potential. Phytomedicine 2012, 20, 32–39. [Google Scholar] [CrossRef]
- Joubert, E.; De Beer, D. Rooibos (Aspalathus linearis) beyond the farm gate: From herbal tea to potential phytopharmaceutical. S. Afr. J. Bot. 2011, 77, 869–886. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, M.; Luo, H.; Li, H. Isoorientin alleviates UVB-induced skin injury by regulating mitochondrial ROS and cellular autophagy. Biochem. Biophys. Res. Commun. 2019, 514, 1133–1139. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of anti-inflammatory properties of isoorientin isolated from tubers of pueraria tuberosa. Oxid. Med. Cell. Longev. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mazibuko-Mbeje, S.E.; Ziqubu, K.; Dludla, P.V.; Tiano, L.; Silvestri, S.; Orlando, P.; Nyawo, T.A.; Louw, J.; Kappo, A.P.; Muller, C.J. Isoorientin ameliorates lipid accumulation by regulating fat browning in palmitate-exposed 3T3-L1 adipocytes. Metab. Open 2020, 6, 100037. [Google Scholar] [CrossRef]
- Sun, A.; Ren, G.; Deng, C.; Zhang, J.; Luo, X.; Wu, X.; Mani, S.; Dou, W.; Wang, Z. C-glycosyl flavonoid orientin improves chemically induced inflammatory bowel disease in mice. J. Funct. Foods 2016, 21, 418–430. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Cheng, K.-K.; Sarmidi, M.R.; Yaakob, H.; Huri, H.Z. Celastrol protects against Antimycin A-induced insulin Resistance in Human Skeletal Muscle Cells. Molecules 2015, 20, 8242–8269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazibuko-Mbeje, S.E.; Mthembu, S.X.; Dludla, P.V.; Madoroba, E.; Chellan, N.; Kappo, A.P.; Muller, C.J. Antimycin A-induced mitochondrial dysfunction is consistent with impaired insulin signaling in cultured skeletal muscle cells. Toxicol. In Vitro 2021, 76, 105224. [Google Scholar] [CrossRef]
- Sylow, L.; Tokarz, V.L.; Richter, E.A.; Klip, A. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021, 33, 758–780. [Google Scholar] [CrossRef]
- Musi, N.; Hirshman, M.F.; Nygren, J.; Svanfeldt, M.; Bavenholm, P.; Rooyackers, O.; Zhou, G.; Williamson, J.M.; Ljunqvist, O.; Efendic, S.; et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 2002, 51, 2074–2081. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative stress increased respiration and generation of reactive oxygen species, resulting in ATP depletion, opening of mitochondrial permeability transition, and programmed cell death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Joubert, E.; Muller, C.J.; Louw, J.; Johnson, R. Hyperglycemia-induced oxidative stress and heart disease-cardioprotective effects of rooibos flavonoids and phenylpyruvic acid-2-O-β-D-glucoside. Nutr. Metab. 2017, 14, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.O.; Oluyede, D.M.; Adebimpe, M.O.; Olumegbon, L.T.; Awolaja, O.O.; Elekofehinti, O.O.; Crown, O.O. The cardiovascular protective effects of rooibos (Aspalathus linearis) extract on diesel exhaust particles induced inflammation and oxidative stress involve NF-κB- and Nrf2-dependent pathways modulation. Heliyon 2019, 5, e01426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marnewick, J.L.; Rautenbach, F.; Venter, I.; Neethling, H.; Blackhurst, D.M.; Wolmarans, P.; Macharia, M. Effects of rooibos (Aspalathus linearis) on oxidative stress and biochemical parameters in adults at risk for cardiovascular disease. J. Ethnopharmacol. 2011, 133, 46–52. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziqubu, K.; Muller, C.J.F.; Dludla, P.V.; Mthembu, S.X.H.; Obonye, N.; Louw, J.; Kappo, A.P.; Silvestri, S.; Orlando, P.; Tiano, L.; et al. Impact of isoorientin on metabolic activity and lipid accumulation in differentiated adipocytes. Molecules 2020, 25, 1773. [Google Scholar] [CrossRef] [Green Version]
- Ye, X.; Shen, Y.; Ni, C.; Ye, J.; Xin, Y.; Zhang, W.; Ren, Y. Irisin reverses insulin resistance in C2C12 cells via the p38-MAPK-PGC-1α pathway. Peptides 2019, 119, 170120. [Google Scholar] [CrossRef] [PubMed]
- Dickerson, R.; Banerjee, J.; Rauckhorst, A.; Pfeiffer, U.R.; Gordillo, G.M.; Khanna, S.; Osei, K.; Roy, S. Does oral supplementation of a fermented papaya preparation correct respiratory burst function of innate immune cells in type 2 diabetes mellitus patients? Antioxidants Redox Signal. 2015, 22, 339–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strübing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Muller, C.J.F.; Louw, J.; Mazibuko-Mbeje, S.E.; Tiano, L.; Silvestri, S.; Orlando, P.; Marcheggiani, F.; Cirilli, I.; Chellan, N.; et al. The combination effect of aspalathin and phenylpyruvic acid-2-O-β-d-glucoside from rooibos against hyperglycemia-induced cardiac damage: An in vitro study. Nutrients 2020, 12, 1151. [Google Scholar] [CrossRef] [Green Version]
- Mazibuko, S.E. In Vitro and In Vivo Effect of Aspalathus Linearis and Its Major Polyphenols on Carbohydrate and Lipid Metabolism in Insulin Resistant Models. 2014. Available online: http://hdl.handle.net/10530/1319 (accessed on 10 August 2021).
- Ziqubu, K.; Dludla, P.V.; Joubert, E.; Muller, C.J.; Louw, J.; Tiano, L.; Nkambule, B.B.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Isoorientin: A dietary flavone with the potential to ameliorate diverse metabolic complications. Pharmacol. Res. 2020, 158, 104867. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.Y.; Ling, A.P.K.; Koh, R.Y.; Wong, Y.P.; Say, Y.-H. A review on medicinal properties of orientin. Adv. Pharmacol. Sci. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Achilonu, M.C.; Kendrekar, P.S.; Joubert, E.; Ferreira, D.; Bonnet, S.L.; Van Der Westhuizen, J.H. Concise and scalable synthesis of aspalathin, a powerful plasma sugar-lowering natural product. J. Nat. Prod. 2013, 77, 583–588. [Google Scholar] [CrossRef]
- Dludla, P.V.; Jack, B.; Viraragavan, A.; Pheiffer, C.; Johnson, R.; Louw, J.; Muller, C.J. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicol. Rep. 2018, 5, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
| Probe | Function | Assay ID |
|---|---|---|
| Uncoupling Protein 2 (Ucp 2) | Mitochondrial Bioenergetics | Mm00627599_mL |
| Complex 1; Ubiquinol-Cytochrome c Reductase Complex Assembly Factor 1 (Uqqc 1) | Mitochondrial Bioenergetics | Mm00479775_mL |
| Complex III; Ubiquinol-Cytochrome c Reductase Complex Assembly Factor 3 (Uqqc 3) | Mitochondrial Bioenergetics | Mm01231041_gL |
| Mitochondrial Transcription Factor A (Tfam) | Mitochondrial Biogenesis | Mm00447485_mL |
| Sirtuin (Sirt 1) | Mitochondrial Biogenesis | Mm01168521_mL |
| Nuclear Respiratory Factor 1 (Nrf 1) | Mitochondrial Biogenesis | Mm01135606_mL |
| Superoxide Dismutase 1 (Sod 1) | Antioxidant | Mm01344233_gL |
| Glutathione Synthase (Gss) | Antioxidant | Mm00515065_mL |
| Beta-2-Microglobulin (B2m) | Housekeeping | Mm00437762_mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mthembu, S.X.H.; Muller, C.J.F.; Dludla, P.V.; Madoroba, E.; Kappo, A.P.; Mazibuko-Mbeje, S.E. Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules 2021, 26, 6289. https://doi.org/10.3390/molecules26206289
Mthembu SXH, Muller CJF, Dludla PV, Madoroba E, Kappo AP, Mazibuko-Mbeje SE. Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules. 2021; 26(20):6289. https://doi.org/10.3390/molecules26206289
Chicago/Turabian StyleMthembu, Sinenhlanhla X. H., Christo J. F. Muller, Phiwayinkosi V. Dludla, Evelyn Madoroba, Abidemi P. Kappo, and Sithandiwe E. Mazibuko-Mbeje. 2021. "Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells" Molecules 26, no. 20: 6289. https://doi.org/10.3390/molecules26206289
APA StyleMthembu, S. X. H., Muller, C. J. F., Dludla, P. V., Madoroba, E., Kappo, A. P., & Mazibuko-Mbeje, S. E. (2021). Rooibos Flavonoids, Aspalathin, Isoorientin, and Orientin Ameliorate Antimycin A-Induced Mitochondrial Dysfunction by Improving Mitochondrial Bioenergetics in Cultured Skeletal Muscle Cells. Molecules, 26(20), 6289. https://doi.org/10.3390/molecules26206289









