A Universal Cassette-Based System for the Dissolution of Solid Targets
Abstract
:1. Introduction
2. Materials and Methods
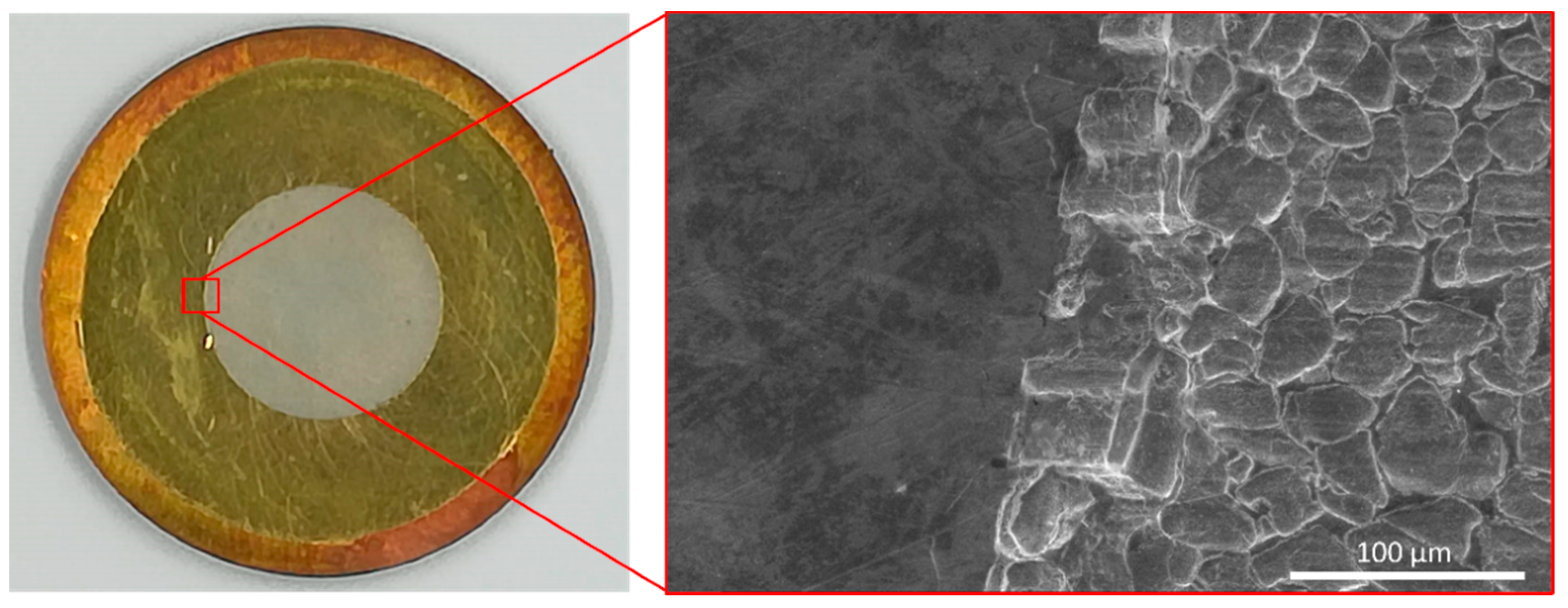
2.1. Irradiated Targets
2.2. Design of the Reactor Components
2.2.1. Reactor Dissolution Vial
- Borosilicate glass;
- Quartz;
- PEEK.
2.2.2. Vial-Holder and Mounting Rods
2.2.3. Target Holder
2.3. Dissolution Tests
3. Results
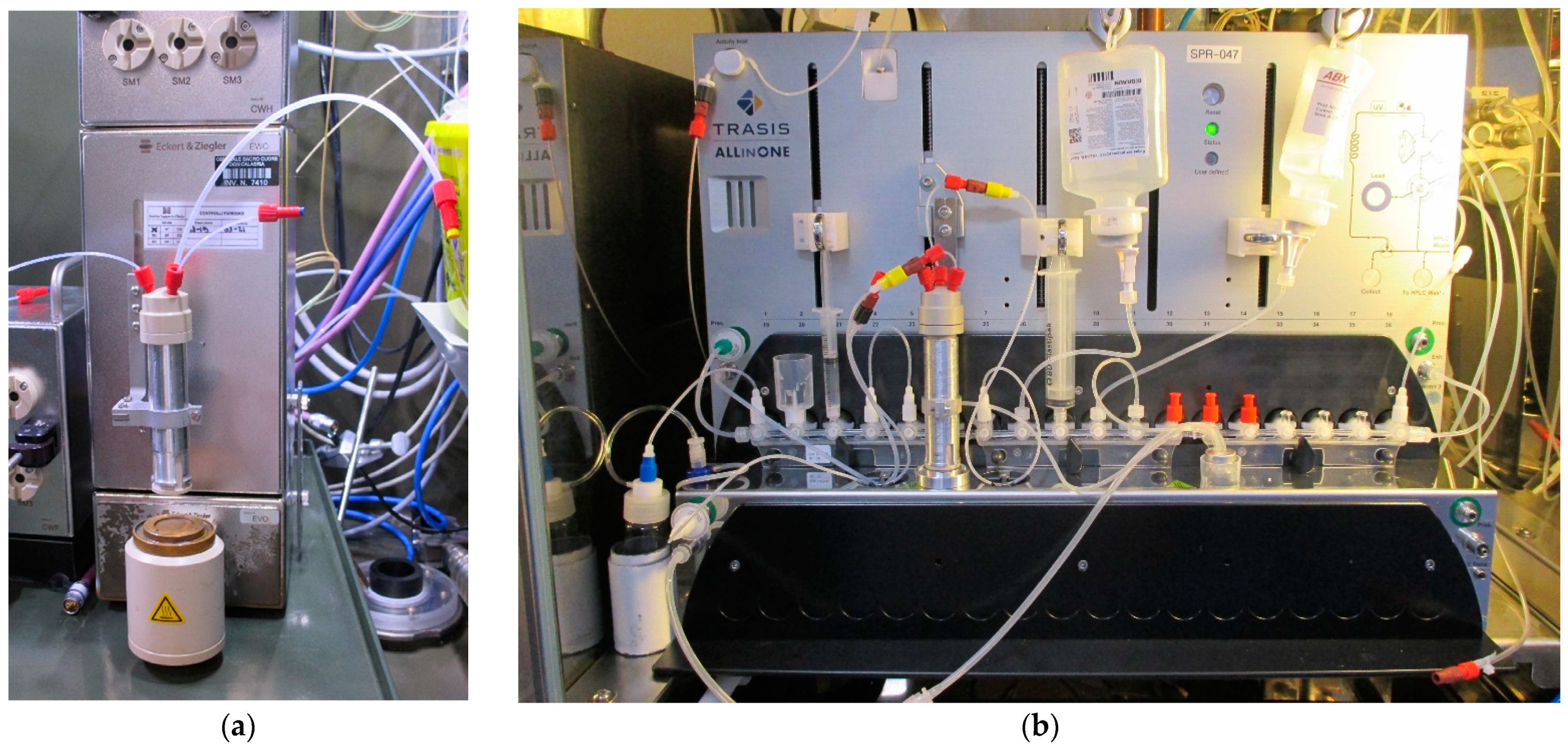
3.1. Dissolution Reactor Assembly
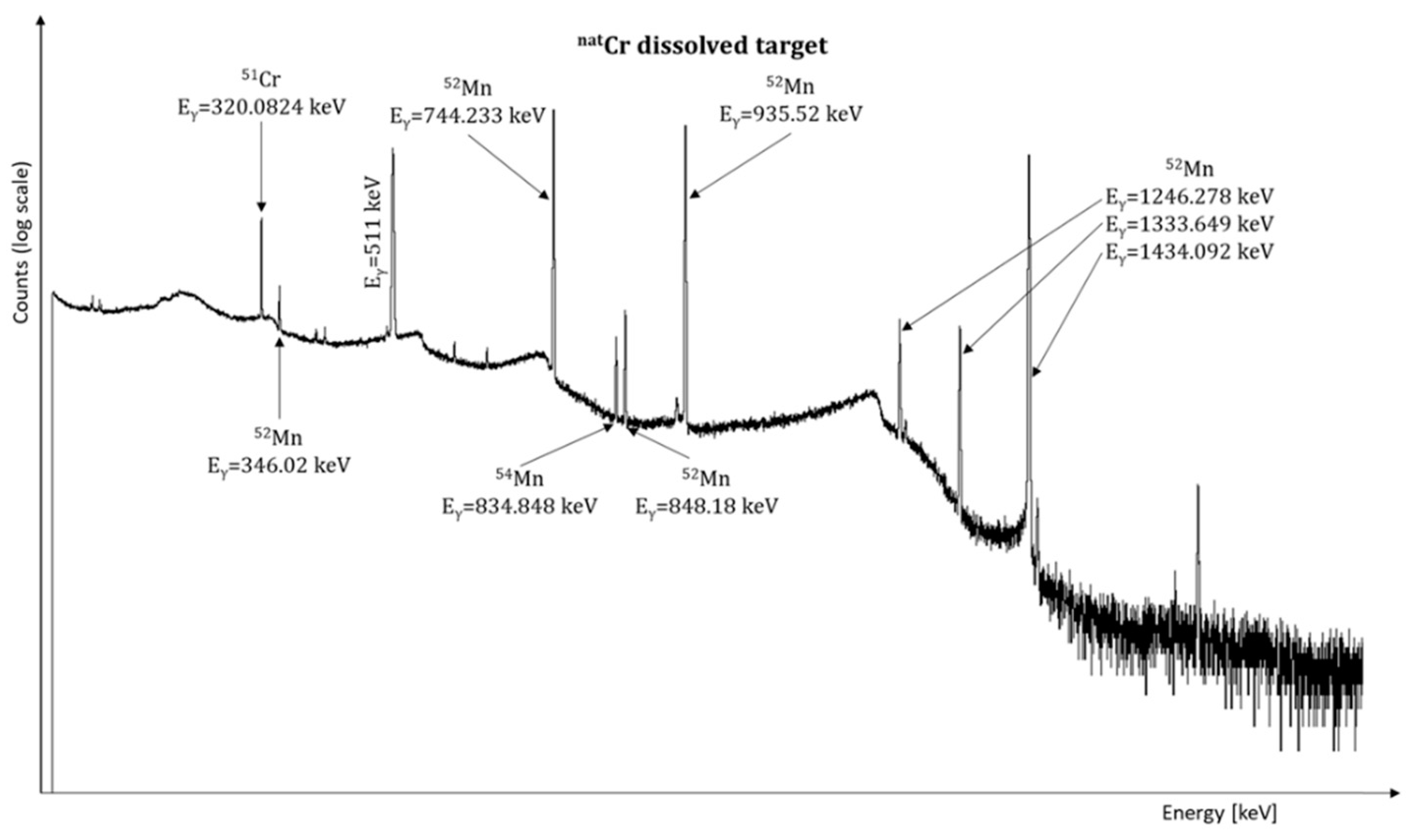
3.2. Operational Testing
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Cyclotron Produced Radionuclides: Principles and Practice; International Atomic Energy Agency: Vienna, Austria, 2008; ISBN 978-92-0-100208-2.
- McCarthy, D.W.; Shefer, R.E.; Klinkowstein, R.E.; Bass, L.A.; Margeneau, W.H.; Cutler, C.S.; Anderson, C.J.; Welch, M.J. Efficient Production of High Specific Activity 64Cu Using a Biomedical Cyclotron. Nucl. Med. Biol. 1997, 24, 35–43. [Google Scholar] [CrossRef]
- Obata, A.; Kasamatsu, S.; McCarthy, D.W.; Welch, M.J.; Saji, H.; Yonekura, Y.; Fujibayashi, Y. Production of Therapeutic Quantities of 64Cu Using a 12 MeV Cyclotron. Nucl. Med. Biol. 2003, 30, 535–539. [Google Scholar] [CrossRef]
- Fonslet, J.; Tietze, S.; Jensen, A.I.; Graves, S.A.; Severin, G.W. Optimized Procedures for Manganese-52: Production, Separation and Radiolabeling. Appl. Radiat. Isot. 2017, 121, 38–43. [Google Scholar] [CrossRef] [Green Version]
- Pyles, J.M.; Massicano, A.V.; Appiah, J.-P.; Bartels, J.L.; Alford, A.; Lapi, S.E. Production of 52Mn Using a Semi-Automated Module. Appl. Radiat. Isot. 2021, 174, 109741. [Google Scholar] [CrossRef]
- Kasbollah, A.; Eu, P.; Cowell, S.; Deb, P. Review on Production of 89Zr in a Medical Cyclotron for PET Radiopharmaceuticals. J. Nucl. Med. Technol. 2013, 41, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Solbach, C.; Bertram, J.; Scheel, W.; Baur, B.; Machulla, H.; Reske, S. Production of Zr-89 on a PETtrace Cyclotron 2013. J. Nucl. Med. 2013, 54, 1098. [Google Scholar]
- Martini, P.; Boschi, A.; Cicoria, G.; Zagni, F.; Corazza, A.; Uccelli, L.; Pasquali, M.; Pupillo, G.; Marengo, M.; Loriggiola, M. In-House Cyclotron Production of High-Purity Tc-99m and Tc-99m Radiopharmaceuticals. Appl. Radiat. Isot. 2018, 139, 325–331. [Google Scholar] [CrossRef]
- Martini, P.; Boschi, A.; Cicoria, G.; Uccelli, L.; Pasquali, M.; Duatti, A.; Pupillo, G.; Marengo, M.; Loriggiola, M.; Esposito, J. A Solvent-Extraction Module for Cyclotron Production of High-Purity Technetium-99m. Appl. Radiat. Isot. 2016, 118, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Bénard, F.; Buckley, K.R.; Ruth, T.J.; Zeisler, S.K.; Klug, J.; Hanemaayer, V.; Vuckovic, M.; Hou, X.; Celler, A.; Appiah, J.-P. Implementation of Multi-Curie Production of 99mTc by Conventional Medical Cyclotrons. J. Nucl. Med. 2014, 55, 1017–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Synowiecki, M.A.; Perk, L.R.; Nijsen, J.F.W. Production of Novel Diagnostic Radionuclides in Small Medical Cyclotrons. EJNMMI Radiopharm. Chem. 2018, 3, 1–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uccelli, L.; Martini, P.; Cittanti, C.; Carnevale, A.; Missiroli, L.; Giganti, M.; Bartolomei, M.; Boschi, A. Therapeutic Radiometals: Worldwide Scientific Literature Trend Analysis (2008–2018). Molecules 2019, 24, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pupillo, G.; Mou, L.; Boschi, A.; Calzaferri, S.; Canton, L.; Cisternino, S.; De Dominicis, L.; Duatti, A.; Fontana, A.; Haddad, F. Production of 47 Sc with Natural Vanadium Targets: Results of the PASTA Project. J. Radioanal. Nucl. Chem. 2019, 322, 1711–1718. [Google Scholar] [CrossRef]
- Pupillo, G.; Mou, L.; Martini, P.; Pasquali, M.; Boschi, A.; Cicoria, G.; Duatti, A.; Haddad, F.; Esposito, J. Production of 67Cu by Enriched 70Zn Targets: First Measurements of Formation Cross Sections of 67Cu, 64Cu, 67Ga, 66Ga, 69mZn and 65Zn in Interactions of 70Zn with Protons above 45 MeV. Radiochim. Acta 2020, 108, 593–602. [Google Scholar] [CrossRef]
- Boschi, A.; Martini, P.; Costa, V.; Pagnoni, A.; Uccelli, L. Interdisciplinary Tasks in the Cyclotron Production of Radiometals for Medical Applications. The Case of 47Sc as Example. Molecules 2019, 24, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smilkov, K.; Janevik, E.; Guerrini, R.; Pasquali, M.; Boschi, A.; Uccelli, L.; Di Domenico, G.; Duatti, A. Preparation and First Biological Evaluation of Novel Re-188/Tc-99m Peptide Conjugates with Substance-P. Appl. Radiat. Isot. 2014, 92, 25–31. [Google Scholar] [CrossRef]
- Srivastava, S.C. A Bridge Not Too Far: Personalized Medicine with the Use of Theragnostic Radiopharmaceuticals. J. Postgrad. Med. Educ. Res. 2013, 47, 31–46. [Google Scholar] [CrossRef]
- Qaim, S.M. Medical Radionuclide Production; De Gruyter: Berlin, Germany, 2019. [Google Scholar]
- Gagnon, K.; Wilson, J.S.; Holt, C.M.B.; Abrams, D.N.; McEwan, A.J.B.; Mitlin, D.; McQuarrie, S.A. Cyclotron Production of 99mTc: Recycling of Enriched 100Mo Metal Targets. Appl. Radiat. Isot. 2012, 70, 1685–1690. [Google Scholar] [CrossRef]
- Skliarova, H.; Cisternino, S.; Cicoria, G.; Marengo, M.; Palmieri, V. Innovative Target for Production of Technetium-99m by Biomedical Cyclotron. Molecules 2019, 24, 25. [Google Scholar] [CrossRef] [Green Version]
- Skliarova, H.; Cisternino, S.; Cicoria, G.; Cazzola, E.; Gorgoni, G.; Marengo, M.; Esposito, J. Cyclotron Solid Targets Preparation for Medical Radionuclides Production in the Framework of LARAMED Project. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020; Volume 1548, p. 012022. [Google Scholar]
- Pandey, M.K.; DeGrado, T.R. Cyclotron Production of PET Radiometals in Liquid Targets: Aspects and Prospects. Curr. Radiopharm. 2020, 14, 325–339. [Google Scholar] [CrossRef]
- Riga, S.; Cicoria, G.; Pancaldi, D.; Zagni, F.; Vichi, S.; Dassenno, M.; Mora, L.; Lodi, F.; Morigi, M.P.; Marengo, M. Production of Ga-68 with a General Electric PETtrace Cyclotron by Liquid Target. Phys. Med. 2018, 55, 116–126. [Google Scholar] [CrossRef]
- Oehlke, E.; Hoehr, C.; Hou, X.; Hanemaayer, V.; Zeisler, S.; Adam, M.J.; Ruth, T.J.; Celler, A.; Buckley, K.; Benard, F. Production of Y-86 and Other Radiometals for Research Purposes Using a Solution Target System. Nucl. Med. Biol. 2015, 42, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Pandey, M.K.; Engelbrecht, H.P.; Byrne, J.F.; Packard, A.B.; DeGrado, T.R. Production of 89Zr via the 89Y (p, n) 89Zr Reaction in Aqueous Solution: Effect of Solution Composition on in-Target Chemistry. Nucl. Med. Biol. 2014, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Boschi, A.; Martini, P.; Pasquali, M.; Uccelli, L. Recent Achievements in Tc-99m Radiopharmaceutical Direct Production by Medical Cyclotrons. Drug Dev. Ind. Pharm. 2017, 43, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- IBA Pinctada Metal. Available online: https://www.iba-radiopharmasolutions.com/more-chemistry (accessed on 15 September 2021).
- ARTMS QIS. Available online: http://artms.ca/hardware-and-consumables (accessed on 15 September 2021).
- Comecer ALCEO. Available online: https://www.comecer.com/it/scopri-le-nuove-caratteristiche-alceo/ (accessed on 15 September 2021).
- Gelbart, W.Z.; Johnson, R.R. Solid Target System with In-Situ Target Dissolution. Instruments 2019, 3, 14. [Google Scholar] [CrossRef] [Green Version]
- Beaudoin, J.-F.; Tremblay, S.; Alnahwi, A.; Guerin, B. Target Carrier and Dissolution System for Facilitating End-to-End in-Vault Cyclotron Production of Radiometals in Large Quantities. J. Nucl. Med. 2019, 60, 1162. [Google Scholar]
- Esposito, J.; Bettoni, D.; Boschi, A.; Calderolla, M.; Cisternino, S.; Fiorentini, G.; Keppel, G.; Martini, P.; Maggiore, M.; Mou, L.; et al. LARAMED: A Laboratory for Radioisotopes of Medical Interest. Molecules 2018, 24, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmieri, V.; Skliarova, H.; Cisternino, S.; Marengo, M.; Cicoria, G. Method for Obtaining a Solid Target for Radiopharmaceuticals Production. International Patent Application PCT/IB2018/056826, 7 September 2018. [Google Scholar]
- Cicoria, G.; Pancaldi, D.; Lodi, F.; Malizia, C.; Costa, S.; Lucconi, G.; Lnfantino, A.; Zagni, F.; Fanti, S.; Boschi, S. A Complete Compact Automatic Modular System for 64CuCl2 Production and Labelling of 64Cu-Tracers. In European Journal of Nuclear Medicine and Molecular Imaging; 233 Spring St: New York, NY, USA, 2014; Volume 41, p. 348. [Google Scholar]
- Anselmi-Tamburini, U. Spark Plasma Sintering. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Hu, Z.-Y.; Zhang, Z.-H.; Cheng, X.-W.; Wang, F.-C.; Zhang, Y.-F.; Li, S.-L. A Review of Multi-Physical Fields Induced Phenomena and Effects in Spark Plasma Sintering: Fundamentals and Applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Skliarova, H.; Cisternino, S.; Cicoria, G.; Marengo, M.; Cazzola, E.; Gorgoni, G.; Palmieri, V. Medical Cyclotron Solid Target Preparation by Ultrathick Film Magnetron Sputtering Deposition. Instruments 2019, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- IAEA ISOTOPIA. Available online: https://www-nds.iaea.org/relnsd/isotopia/isotopia.html (accessed on 10 October 2020).
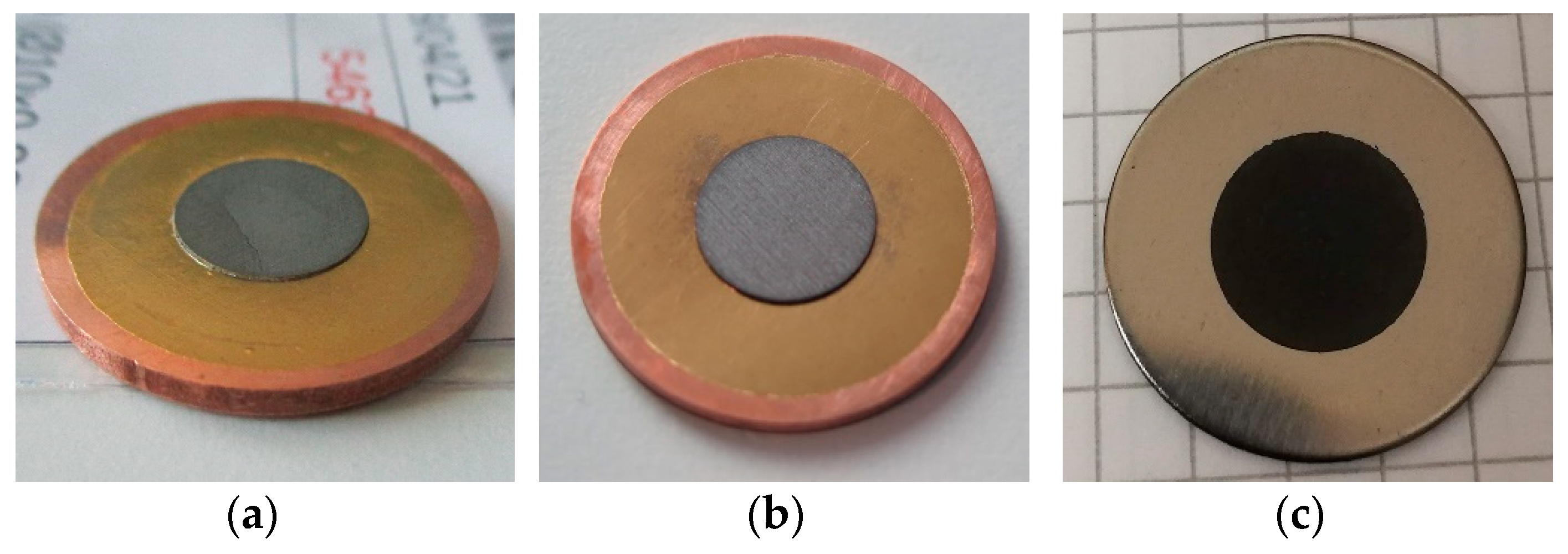
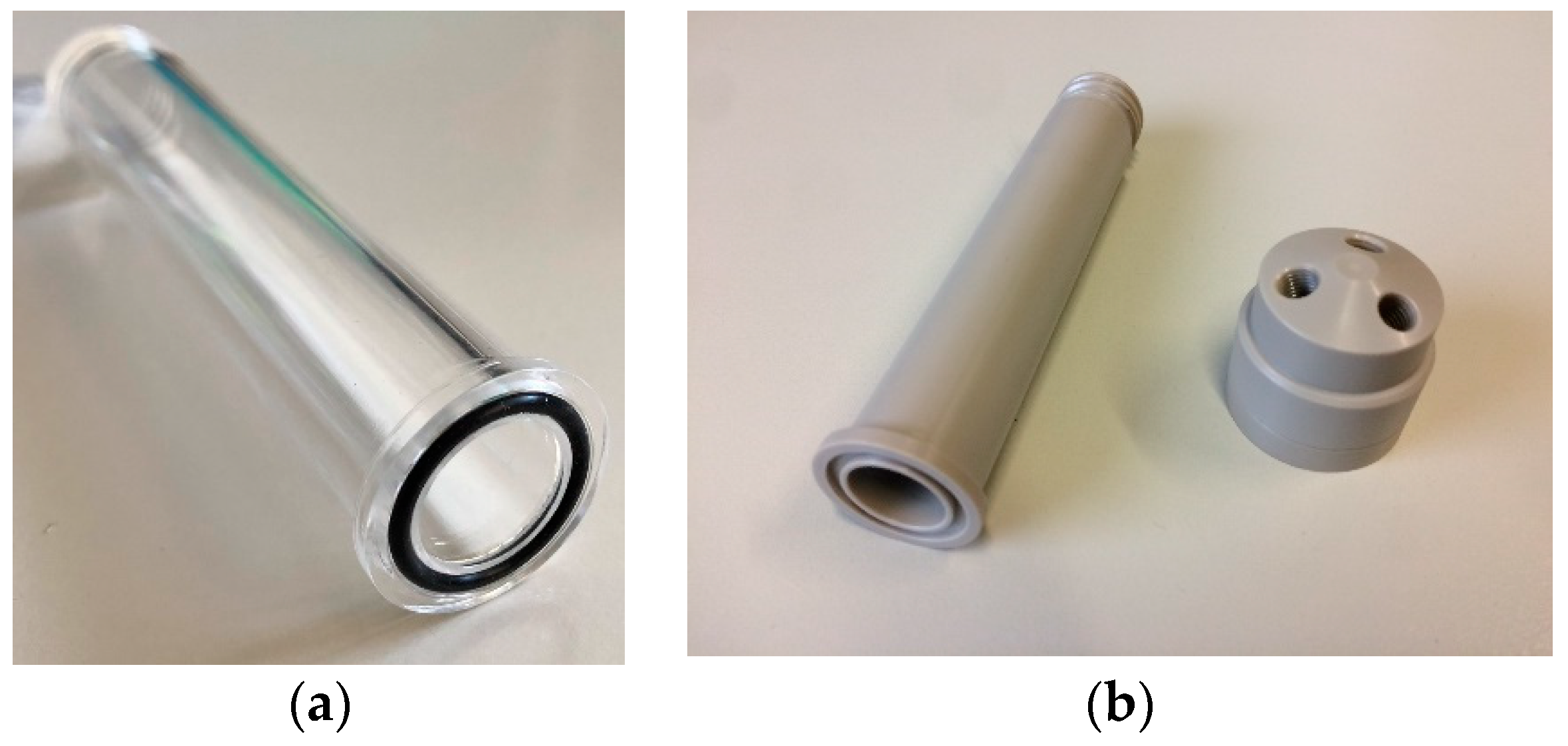
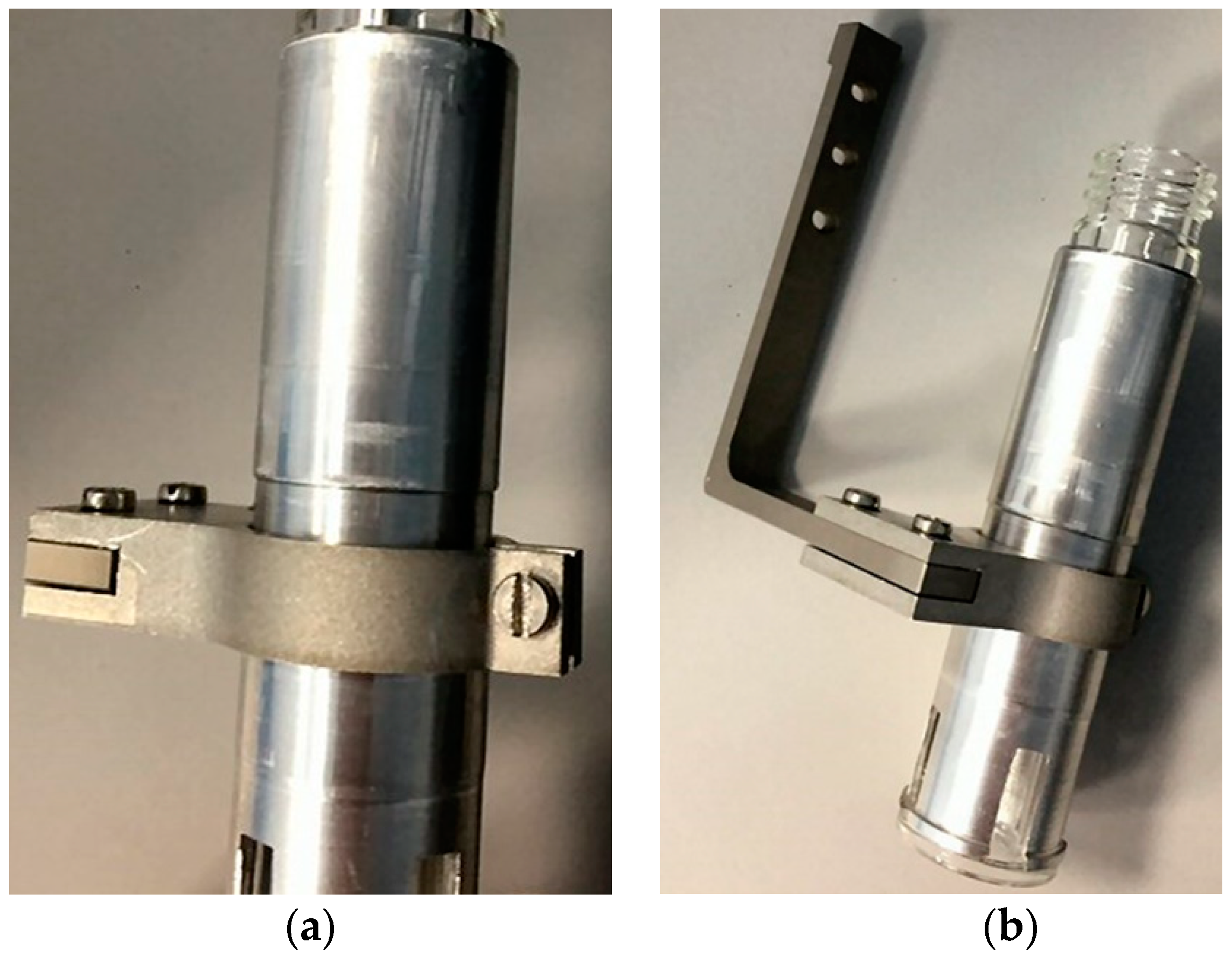
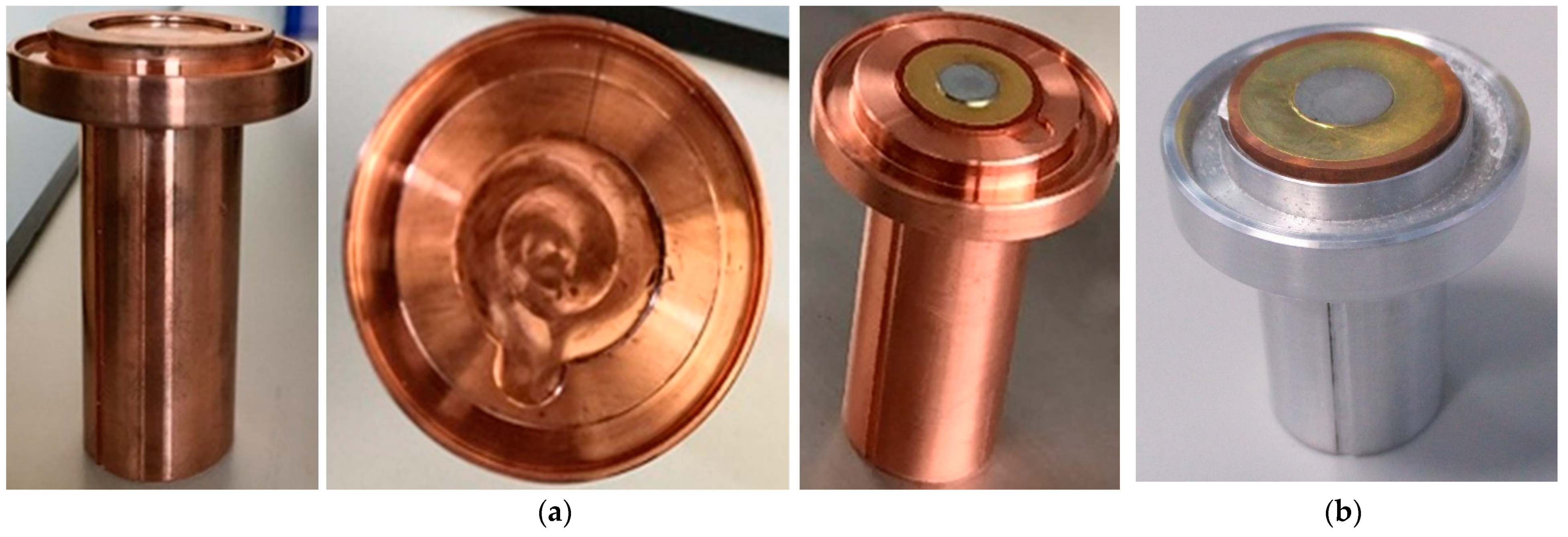
| Component | Material |
|---|---|
| Dissolution Vial | quartz; borosilicate glass; PEEK 1 |
| Vial-Holder and mounting rods | aluminium alloy 6082; stainless steel |
| Target holder | aluminium; copper |
| O-ring | NBR 2 70 |
| Target | Pellet Mass/Thickness | Irradiation Parameters | Module | Vial Material | Process Parameters |
|---|---|---|---|---|---|
| NatY on Nb | 0.0758 g 150 μm | 12.5 MeV | Trasis | PEEK | 2 mL HCl 2 M |
| 10 µA | RT 1 | ||||
| 5 min | Time 1 h | ||||
| NatCr on Au/Cu | 0.02 g 400 μm | 16 MeV | E&Z | Borosilicate glass | 3 mL HCl 8 M |
| 10 µA | Heating up to 70 °C | ||||
| 15 min | Time 1 h | ||||
| NatMo on Au/Cu | 0.02 g 280 μm | 19 MeV | E&Z | Borosilicate glass | 1.5 mL (×3 times) H2O2 30% |
| 1 µA | Heating up to 90 °C | ||||
| 2 min | Time 30 min |
| Target | N Test | Target Weight | Difference (g) | Pellet’s Initial Weight (g) | |
|---|---|---|---|---|---|
| Before Dissolution (g) | After Dissolution (g) | ||||
| NatY on Nb | 6 | 6.50 ± 0.04 | 6.43 ± 0.04 | 0.0758 ± 0.0007 | 0.0758 |
| NatCr on Au/Cu | 5 | 6.90 ± 0.02 | 6.70 ± 0.02 | 0.200 ± 0.002 | 0.2 |
| NatMo on Au/Cu | 3 | 6.86 ± 0.02 | 6.66 ± 0.02 | 0.200 ± 0.002 | 0.2 |
| Isotope | Activity @ EOD (MBq) | Activity @ EOB (MBq) | |
|---|---|---|---|
| Measured | Rescaled | Predicted | |
| 89Zr | 8.8 | 8.9 | 9.7 |
| 52Mn | 21 | 22 | 23 |
| 99mTc | 0.87 | 0.88 | 0.92 |
| Backing | Pellet | Desired Isotope | Isotope Produced in the Backing | Half-Life (h) | E (keV) | MDA (Bq) |
|---|---|---|---|---|---|---|
| Nb | Y | 89Zr | 93mMo | 6.85 | 684.693 | 17.6 |
| Cu/Au | Cr | 52Mn | 197mHg | 23.8 | 133.98 | 23.4 |
| 197gHg | 64.14 | 191.364 | 1843.8 | |||
| Cu/Au | Mo | 99mTc | 197mHg | 23.8 | 133.98 | 10.4 |
| 197gHg | 64.14 | 191.364 | 785.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sciacca, G.; Martini, P.; Cisternino, S.; Mou, L.; Amico, J.; Esposito, J.; Gorgoni, G.; Cazzola, E. A Universal Cassette-Based System for the Dissolution of Solid Targets. Molecules 2021, 26, 6255. https://doi.org/10.3390/molecules26206255
Sciacca G, Martini P, Cisternino S, Mou L, Amico J, Esposito J, Gorgoni G, Cazzola E. A Universal Cassette-Based System for the Dissolution of Solid Targets. Molecules. 2021; 26(20):6255. https://doi.org/10.3390/molecules26206255
Chicago/Turabian StyleSciacca, Gabriele, Petra Martini, Sara Cisternino, Liliana Mou, Jonathan Amico, Juan Esposito, Giancarlo Gorgoni, and Emiliano Cazzola. 2021. "A Universal Cassette-Based System for the Dissolution of Solid Targets" Molecules 26, no. 20: 6255. https://doi.org/10.3390/molecules26206255
APA StyleSciacca, G., Martini, P., Cisternino, S., Mou, L., Amico, J., Esposito, J., Gorgoni, G., & Cazzola, E. (2021). A Universal Cassette-Based System for the Dissolution of Solid Targets. Molecules, 26(20), 6255. https://doi.org/10.3390/molecules26206255







