The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extract Preparation
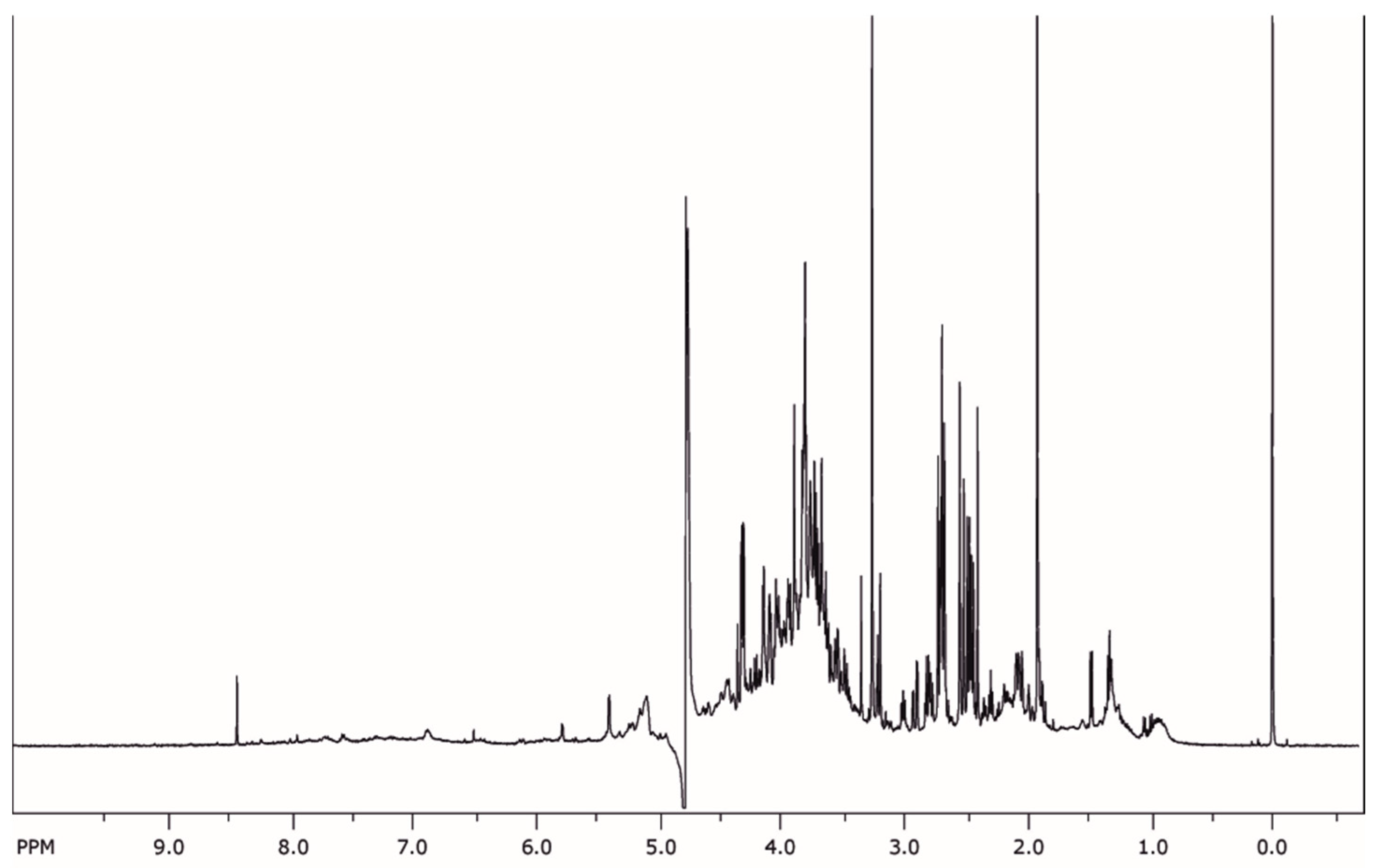
2.2. Phytochemical Analysis
2.3. Cell Viability
2.4. Cell Cycle Progression
2.5. Cell Migration
2.6. Anti-Inflammatory Activity
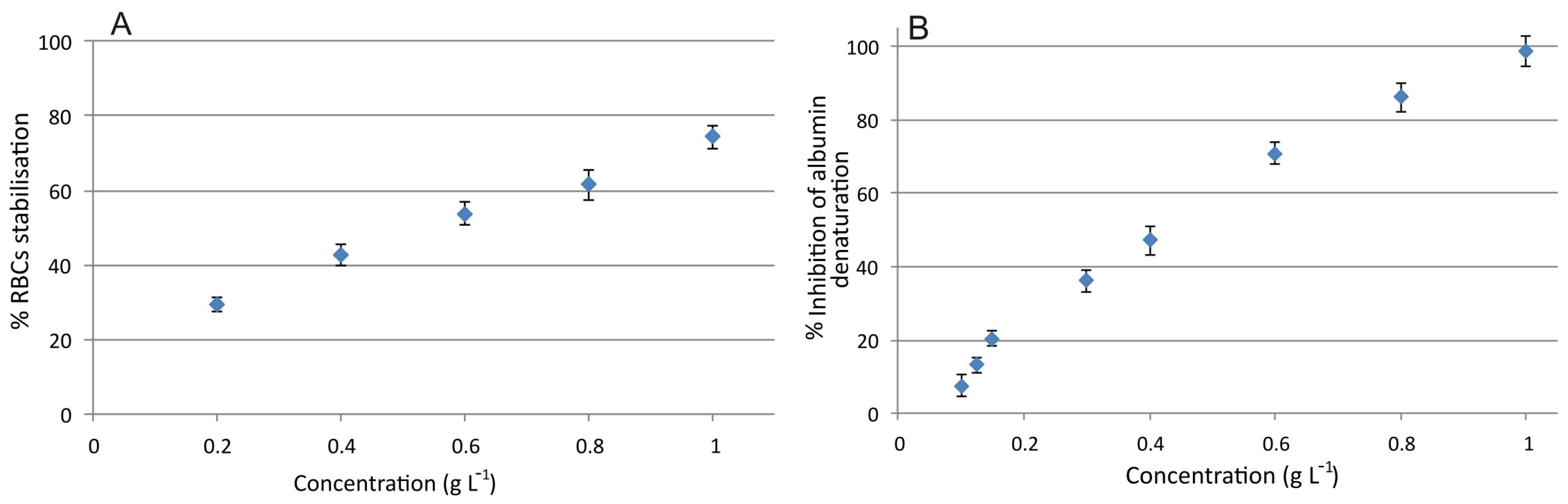
2.6.1. Red Blood Cell Membrane Stabilization
2.6.2. Protein Denaturation Inhibition
2.7. Antioxidant Activity of the Extract
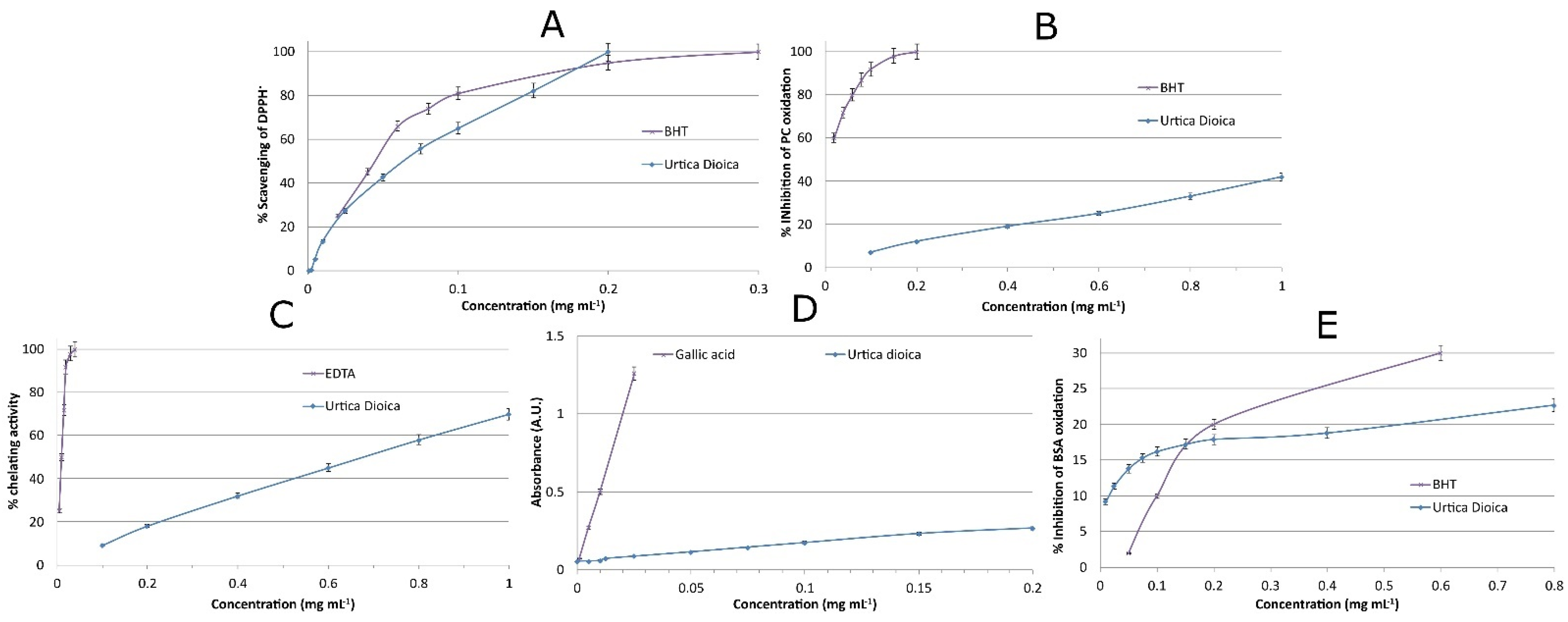
2.7.1. DPPH Assay
2.7.2. Inhibition of Lipid Oxidation—TBARS Assay
2.7.3. Metal Chelating Activity
2.7.4. Reducing Power
2.7.5. Prevention of Oxidative Damage to Proteins
2.8. Blood Compatibility
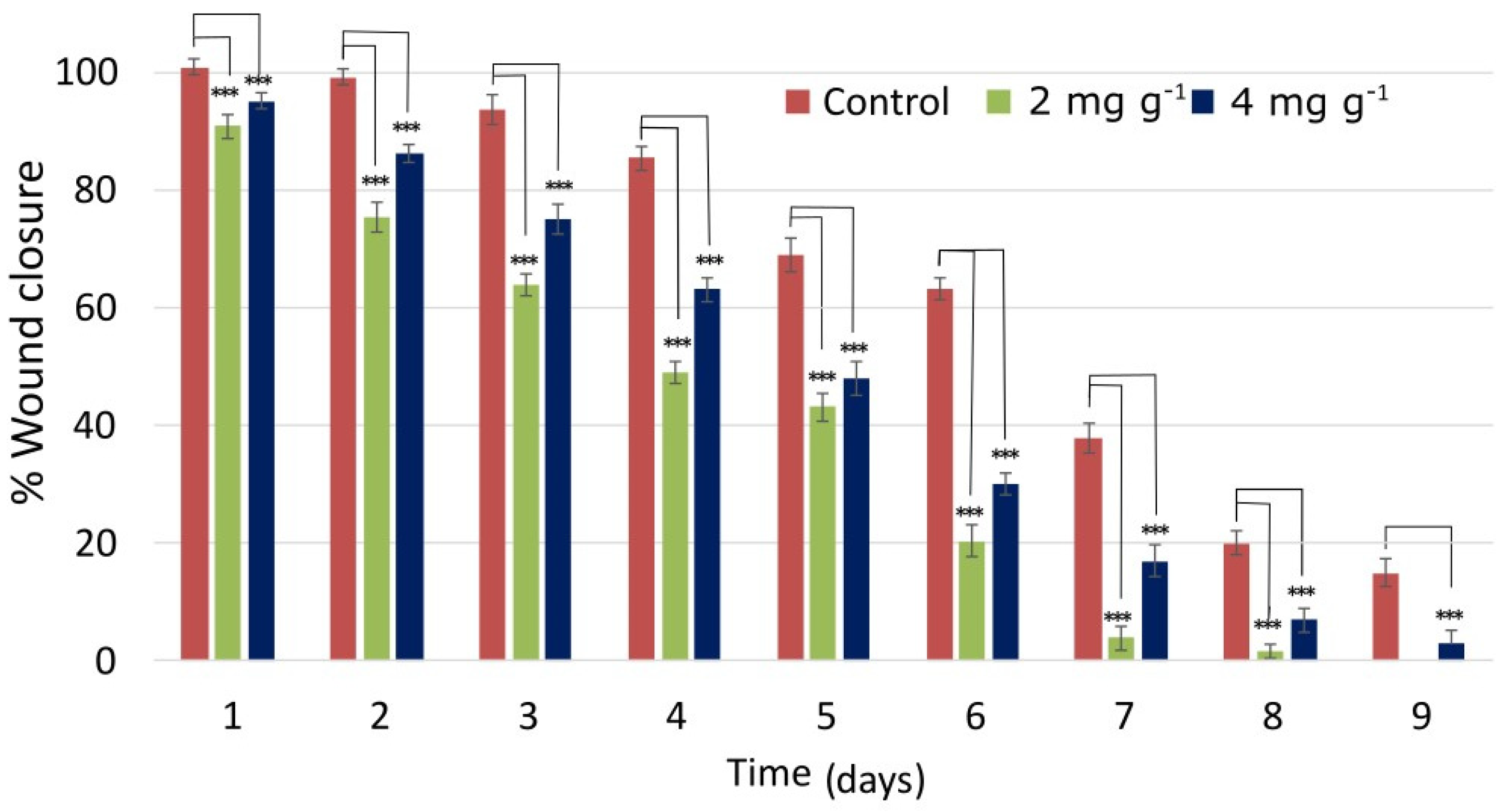
2.9. In Vivo Wound Healing Experiments
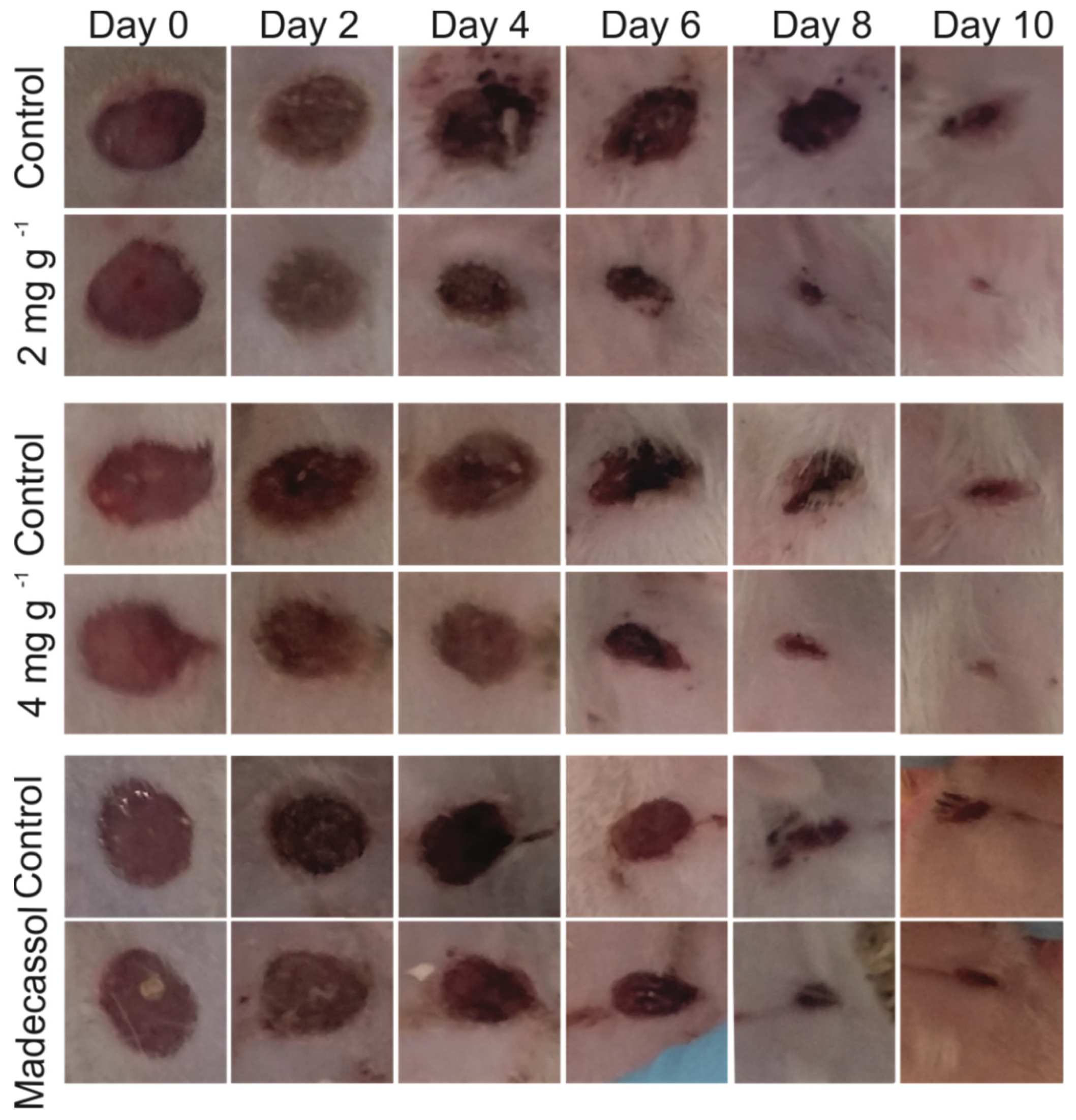
2.9.1. Wound Closure
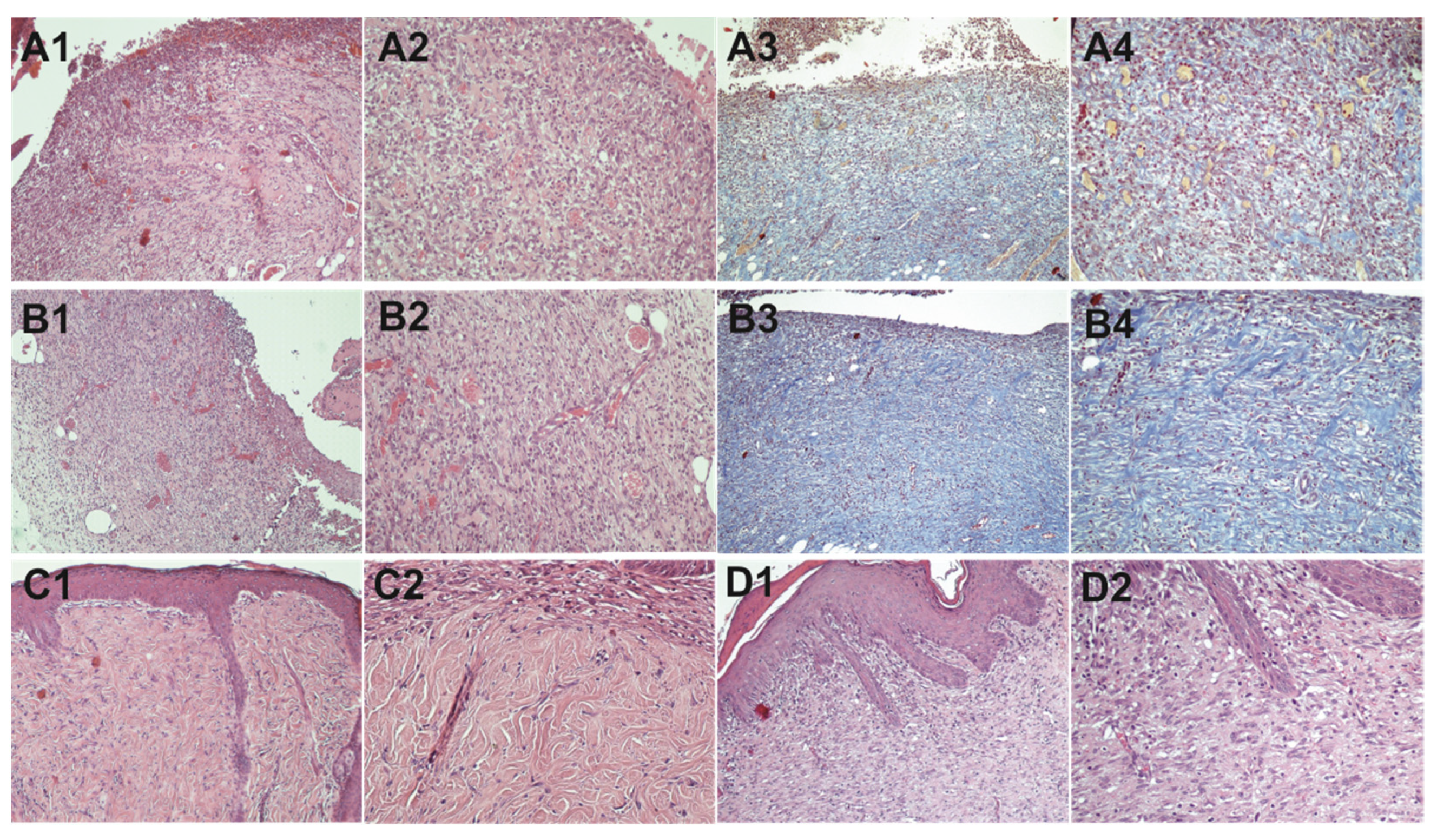
2.9.2. Histopathological Study
3. Materials and Methods
3.1. Cell Lines and Chemicals
3.2. Plant Material and Extract Preparation
3.3. Phytochemical Analysis and NMR Fingerprinting
3.3.1. Test for Amino Acids
3.3.2. Test for Xanthoproteins
3.3.3. Test for Quinones
3.3.4. Test for Coumarins
3.3.5. Test for Carboxylic Acids
3.3.6. Test for Tannins
3.3.7. Test for Glycosides
3.3.8. Test for Flavonoids
3.3.9. Test for Saponins
3.3.10. Test for Terpenoids
3.3.11. Test for Cardiac Glycosides
3.3.12. Test for Resins
3.3.13. Test for Steroids
3.3.14. Test for Phenols
3.3.15. Test for Reducing Sugars
3.3.16. Test for Anthraquinones
3.3.17. Test for Phlobatannins
3.3.18. Test for Proteins
3.3.19. Test for Carbohydrates
3.3.20. Test for Monosaccharides
3.3.21. Test for Aldoses/Ketoses
3.4. In Vitro Experiments
3.4.1. Cell Proliferation Assay
3.4.2. Cell Cycle Analysis
3.4.3. Cell Migration Assay
3.5. Anti-Inflammatory Properties
3.5.1. Inhibition of Albumin Denaturation
3.5.2. Human Red Blood Cell Membrane Stabilization
3.6. Antioxidant Assays
3.6.1. DPPH Assay
3.6.2. Inhibition of Lipid Oxidation—TBARS Assay
3.6.3. Metal Chelating Activity
3.6.4. Reducing Power
3.6.5. Prevention of Oxidative Damage to Proteins
3.7. Blood Compatibility
3.8. Study of the Wound Healing Efficacy
3.8.1. Animals
3.8.2. Full-Thickness Wound Creation
3.8.3. Treatment Schedule
3.8.4. Histopathological Examination
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kasote, D.; Ahmad, A.; Viljoen, A. Proangiogenic Potential of Medicinal Plants in Wound Healing. In Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; pp. 149–164. [Google Scholar]
- Ustuner, O.; Anlas, C.; Bakirel, T.; Ustun-Alkan, F.; Sigirci, B.D.; Ak, S.; Akpulat, H.A.; Donmez, C.; Koca-Caliskan, U. In vitro evaluation of antioxidant, anti-inflammatory, antimicrobial and wound healing potential of Thymus Sipyleus boiss. subsp. Rosulans (borbas) Jalas. Molecules 2019, 24, 3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.W.; Baek, S.R.; Lee, E.S.; Lee, S.H.; Moh, S.H.; Kim, S.Y.; Moh, J.H.; Kondo, C.; Cheon, Y.W. Wound healing effects of rose placenta in a mouse model of full-thickness wounds. Arch. Plast. Surg. 2015, 42, 686–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, S.; Lou, Q.; Huang, G.; Guo, J.; Wang, X.; Huang, T. Dispersive solid-phase extraction based on MoS2/carbon dot composite combined with HPLC to determine brominated flame retardants in water. Anal. Bioanal. Chem. 2018, 410, 7337–7346. [Google Scholar] [CrossRef]
- Dwita, L.P.; Hasanah, F.; Srirustami, R.; Repi; Purnomo, R.; Harsodjo, S. Wound healing properties of Epiphyllum oxypetalum (DC.) Haw. leaf extract in streptozotocin-induced diabetic mice by topical application. Wound Med. 2019, 26, 100160. [Google Scholar] [CrossRef]
- Süntar, I.; Akkol, E.K.; Nahar, L.; Sarker, S.D. Wound healing and antioxidant properties: Do they coexist in plants? Free Radic. Antioxid. 2012, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Rawat, S.; Singh, R.; Thakur, P.; Kaur, S.; Semwal, A. Wound healing Agents from Medicinal Plants: A Review. Asian Pac. J. Trop. Biomed. 2012, 2, S1910–S1917. [Google Scholar] [CrossRef]
- Premarathna, A.D.; Ranahewa, T.H.; Wijesekera, S.K.; Wijesundara, R.R.M.K.K.; Jayasooriya, A.P.; Wijewardana, V.; Rajapakse, R.P.V.J. Wound healing properties of aqueous extracts of Sargassum illicifolium: An in vitro assay. Wound Med. 2019, 24, 1–7. [Google Scholar] [CrossRef]
- Ghosh, D.; Mondal, S.; Ramakrishna, K. A topical ointment formulation containing leaves extract of Aegialitis rotundifolia Roxb., accelerates excision, incision and burn wound healing in rats. Wound Med. 2019, 26, 100168. [Google Scholar] [CrossRef]
- Sharma, S.; Vig, A.P. Evaluation of in vitro antioxidant properties of methanol and aqueous extracts of Parkinsonia aculeata L. leaves. Sci. World J. 2013, 2013, 604865. [Google Scholar] [CrossRef] [Green Version]
- Pilarski, R.; Zieliński, H.; Ciesiołka, D.; Gulewicz, K. Antioxidant activity of ethanolic and aqueous extracts of Uncaria tomentosa (Willd.) DC. J. Ethnopharmacol. 2006, 104, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nagappan, R. Evaluation of aqueous and ethanol extract of bioactive medicinal plant, Cassia didymobotrya (Fresenius) Irwin & Barneby against immature stages of filarial vector, Culex quinquefasciatus Say (Diptera: Culicidae). Asian Pac. J. Trop. Biomed. 2012, 2, 707–711. [Google Scholar] [CrossRef] [Green Version]
- Ayal, G.; Belay, A.; Kahaliw, W. Evaluation of wound healing and anti-inflammatory activity of the leaves of Calpurnia aurea (Ait.) Benth (fabaceae) in mice. Wound Med. 2019, 25, 100151. [Google Scholar] [CrossRef]
- Amri, B.; Martino, E.; Vitulo, F.; Corana, F.; Ben-Kaâb, L.B.; Rui, M.; Rossi, D.; Mori, M.; Rossi, S.; Collina, S. Marrubium vulgare L. leave extract: Phytochemical composition, antioxidant and wound healing properties. Molecules 2017, 22, 1851. [Google Scholar] [CrossRef] [Green Version]
- Joshi, B.C.; Mukhija, M.; Kalia, A.N. Pharmacognostical review of Urtica dioica L. Int. J. Green Pharm. 2014, 8, 201–209. [Google Scholar]
- Bisht, S.; Bhandari, S.; Bisht, N. Urtica dioica (L): An undervalued, economically important plant. Agric. Sci. Res. 2012, 2, 250–252. [Google Scholar]
- Kukrić, Z.Z.; Topalić-Trivunović, L.N.; Kukavica, B.M.; Matoš, S.B.; Pavičić, S.S.; Boroja, M.M.; Savić, A.V. Characterization of antioxidant and antimicrobial activities of nettle leaves (Urtica dioica L.). Acta Period. Technol. 2012, 43, 257–272. [Google Scholar] [CrossRef]
- Zouari Bouassida, K.; Bardaa, S.; Khimiri, M.; Rebaii, T.; Tounsi, S.; Jlaiel, L.; Trigui, M. Exploring the Urtica dioica Leaves Hemostatic and Wound-Healing Potential. BioMed Res. Int. 2017, 2017, 1047523. [Google Scholar] [CrossRef] [Green Version]
- Razika, L.; Thanina, A.C.; Nadjiba, C.M.; Narimen, B.; Mahdi, D.M.; Karim, A. Antioxidant and wound healing potential of saponins extracted from the leaves of Algerian Urtica dioica L. Pak. J. Pharm. Sci. 2017, 30, 1023–1029. [Google Scholar] [PubMed]
- Fattahi, S.; Zabihi, E.; Abedian, Z.; Pourbagher, R.; Motevalizadeh Ardekani, A.; Mostafazadeh, A.; Akhavan-Niaki, H. Total Phenolic and Flavonoid Contents of Aqueous Extract of Stinging Nettle and In Vitro Antiproliferative Effect on Hela and BT-474 Cell Lines. Int. J. Mol. Cell. Med. 2014, 3, 102–107. [Google Scholar]
- Budovsky, A.; Yarmolinsky, L.; Ben-Shabat, S. Effect of medicinal plants on wound healing. Wound Repair Regen. 2015, 23, 171–183. [Google Scholar] [CrossRef]
- Rai, V.; Kumar, A.; Das, V.; Ghosh, S. Evaluation of chemical constituents and in vitro antimicrobial, antioxidant and cytotoxicity potential of rhizome of Astilbe rivularis (Bodho-okhati), an indigenous medicinal plant from Eastern Himalayan region of India. BMC Complement. Altern. Med. 2019, 19, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordanian, B.; Behbahani, M.; Carapetian, J.; Fazilati, M. In vitro evaluation of cytotoxic activity of flower, leaf, stem and root extracts of five Artemisia species. Res. Pharm. Sci. 2014, 9, 91–96. [Google Scholar] [PubMed]
- Fouché, M.; Willers, C.; Hamman, S.; Malherbe, C.; Steenekamp, J. Wound healing effects of Aloe muth-muth: In vitro investigations using immortalized human keratinocytes (HaCaT). Biology 2020, 9, 350. [Google Scholar] [CrossRef]
- Fattahi, S.; Ardekani, A.M.; Zabihi, E.; Abedian, Z.; Mostafazadeh, A.; Pourbagher, R.; Akhavan-Niaki, H. Antioxidant and apoptotic effects of an aqueous extract of Urtica dioica on the MCF-7 human breast cancer cell line. Asian Pac. J. Cancer Prev. 2013, 14, 5317–5323. [Google Scholar] [CrossRef] [Green Version]
- Kaptaner İğci, B.; Aytaç, Z. An investigation on the in vitro wound healing activity and phytochemical composition of Hypericum pseudolaeve N. Robson growing in Turkey. Turk. J. Pharm. Sci. 2020, 17, 610–619. [Google Scholar] [CrossRef]
- Bolla, S.R.; Mohammed Al-Subaie, A.; Yousuf Al-Jindan, R.; Papayya Balakrishna, J.; Kanchi Ravi, P.; Veeraraghavan, V.P.; Arumugam Pillai, A.; Gollapalli, S.S.R.; Palpath Joseph, J.; Surapaneni, K.M. In vitro wound healing potency of methanolic leaf extract of Aristolochia saccata is possibly mediated by its stimulatory effect on collagen-1 expression. Heliyon 2019, 5, e01648. [Google Scholar] [CrossRef] [Green Version]
- Addis, R.; Cruciani, S.; Santaniello, S.; Bellu, E.; Sarais, G.; Ventura, C.; Maioli, M.; Pintore, G. Fibroblast proliferation and migration in wound healing by phytochemicals: Evidence for a novel synergic outcome. Int. J. Med. Sci. 2020, 17, 1030. [Google Scholar] [CrossRef] [Green Version]
- Li, L.J.; Wang, M.Z.; Yuan, T.J.; Xu, X.H.; Dad, H.A.; Yu, C.L.; Hou, J.; Peng, L.H. The crude ethanol extract of Periplaneta americana L. stimulates wound healing in vitro & in vivo. Chin. Med. 2019, 14, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhao, B.; Zhang, X.; Han, W.; Cheng, J.; Qin, Y. Wound healing effect of an Astragalus membranaceus polysaccharide and its mechanism. Mol. Med. Rep. 2017, 15, 4077–4083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Negahdari, S.; Galehdari, H.; Kesmati, M.; Rezaie, A.; Shariati, G. Wound healing activity of extracts and formulations of Aloe vera, henna, Adiantum capillus-veneris, and myrrh on mouse dermal fibroblast cells. Int. J. Prev. Med. 2017, 8, 15. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Mohd Esa, N.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In Vitro Wound Healing Potential of Stem Extract of Alternanthera sessilis. Evid.-Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anosike, C.A.; Obidoa, O.; Ezeanyika, L.U. Membrane stabilization as a mechanism of the anti-inflammatory activity of methanol extract of garden egg (Solanum aethiopicum). DARU J. Pharm. Sci. 2012, 20, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osman, N.I.; Sidik, N.J.; Awal, A.; Adam, N.A.M.; Rezali, N.I. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. And total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult. Ethnopharmacol. 2016, 5, 343–349. [Google Scholar] [CrossRef]
- Dar, S.A.; Ganai, F.A.; Yousuf, A.R.; Balkhi, M.U.H.; Bhat, T.M.; Sharma, P. Pharmacological and toxicological evaluation of Urtica dioica. Pharm. Biol. 2013, 51, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Azam, S.; Jainul, M.A.; Faruq, K.O.; Islam, A. Antibacterial activities and in vitro anti-inflammatory (Membrane Stability) properties of methanolic extracts of Gardenia coronaria leaves. Int. J. Microbiol. 2014, 2014, 410935. [Google Scholar] [CrossRef] [Green Version]
- Parvin, M.S.; Das, N.; Jahan, N.; Akhter, M.A.; Nahar, L.; Islam, M.E. Evaluation of in vitro anti-inflammatory and antibacterial potential of Crescentia cujete leaves and stem bark Pharmacology and Toxicology. BMC Res. Notes 2015, 8, 412. [Google Scholar] [CrossRef] [Green Version]
- Anosike, C.A.; Igboegwu, O.N.; Nwodo, O.F.C. Antioxidant properties and membrane stabilization effects of methanol extract of Mucuna pruriens leaves on normal and sickle erythrocytes. J. Tradit. Complement. Med. 2019, 9, 278–284. [Google Scholar] [CrossRef]
- Yang, D.J.; Moh, S.H.; Son, D.H.; You, S.; Kinyua, A.W.; Ko, C.M.; Song, M.; Yeo, J.; Choi, Y.H.; Kim, K.W. Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions. Molecules 2016, 21, 899. [Google Scholar] [CrossRef] [Green Version]
- Tamri, P.; Hemmati, A.; Boroujerdnia, M.G. Wound healing properties of quince seed mucilage: In vivo evaluation in rabbit full-thickness wound model. Int. J. Surg. 2014, 12, 843–847. [Google Scholar] [CrossRef] [Green Version]
- Mohammadpour, M.; Behjati, M.; Sadeghi, A.; Fassihi, A. Wound healing by topical application of antioxidant iron chelators: Kojic acid and deferiprone. Int. Wound J. 2013, 10, 260–264. [Google Scholar] [CrossRef]
- Gioti, E.M.; Fiamegos, Y.C.; Skalkos, D.C.; Stalikas, C.D. Antioxidant activity and bioactive components of the aerial parts of Hypericum perforatum L. from Epirus, Greece. Food Chem. 2009, 117, 398–404. [Google Scholar] [CrossRef]
- Irshad, M.; Zafaryab, M.; Singh, M.; Rizvi, M.M.A. Comparative Analysis of the Antioxidant Activity of Cassia fistula Extracts. Int. J. Med. Chem. 2012, 2012, 157125. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.C.; Lancel, S.; Boulanger, E.; Neviere, R. Targeting oxidative stress and mitochondrial dysfunction in the treatment of impaired wound healing: A systematic review. Antioxidants 2018, 7, 98. [Google Scholar] [CrossRef] [Green Version]
- Chatzimitakos, T.G.; Kasouni, A.I.; Troganis, A.N.; Stalikas, C.D. Carbonization of Human Fingernails: Toward the Sustainable Production of Multifunctional Nitrogen and Sulfur Codoped Carbon Nanodots with Highly Luminescent Probing and Cell Proliferative/Migration Properties. ACS Appl. Mater. Interfaces 2018, 10, 16024–16032. [Google Scholar] [CrossRef]
- Okur, M.E.; Ayla, Ş.; Batur, Ş.; Yoltaş, A.; Genç, E.; Pertek, S.; Üstündağ Okur, N. Evaluation of In Situ Gel Containing Pycnogenol for Cutaneous Wound Healing. Medeni. Med. J. 2019, 34, 67–75. [Google Scholar] [CrossRef]
- Yalcin, B.M.; Pirdal, H. The carbon monoxide measurements’ effect on smokers to give cessation decision in primary care. J. Exp. Clin. Med. 2017, 34, 53–57. [Google Scholar] [CrossRef]
- Boxi, M.; Rajesh, Y.; Raja Kumar, V.; Praveen, B.; Mangamma, K. Extraction, phytochemical screening and in-vitro evaluation of anti-oxidant properties of Commicarpus chinesis (Aqueous leaf extract). Int. J. Pharma Bio Sci. 2010, 1, 537–547. [Google Scholar]
- Pandey, A.; Tripathi, S. Concept of Standarization Extraction and Prephytochemical Screening for Herbal Drug. J. Pharmacogn. Phytochem. 2014, 2, 115–119. [Google Scholar]
- Leelaprakash, G.; Mohan Dass, S. Invitro anti-inflammatory activity of methanol extract of Enicostemma axillare. Int. J. Drug Dev. Res. 2011, 3, 189–196. [Google Scholar]
- Chatzimitakos, T.; Kallimanis, A.; Avgeropoulos, A.; Stalikas, C.D. Antibacterial, Anti-Biofouling, and Antioxidant Prospects of Metal-Based Nanomaterials. Clean—Soil Air Water 2016, 44, 794–802. [Google Scholar] [CrossRef]
- Kasouni, A.; Chatzimitakos, T.G.; Troganis, A.N.; Stalikas, C.D. Citric acid-based carbon dots: From revealing new insights into their biological properties to demonstrating their enhanced wound healing potential by in vitro and in vivo experiments. Mater. Today Commun. 2021, 26, 102019. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasouni, A.I.; Chatzimitakos, T.G.; Stalikas, C.D.; Trangas, T.; Papoudou-Bai, A.; Troganis, A.N. The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study. Molecules 2021, 26, 6248. https://doi.org/10.3390/molecules26206248
Kasouni AI, Chatzimitakos TG, Stalikas CD, Trangas T, Papoudou-Bai A, Troganis AN. The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study. Molecules. 2021; 26(20):6248. https://doi.org/10.3390/molecules26206248
Chicago/Turabian StyleKasouni, Athanasia I., Theodoros G. Chatzimitakos, Constantine D. Stalikas, Theoni Trangas, Alexandra Papoudou-Bai, and Anastassios N. Troganis. 2021. "The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study" Molecules 26, no. 20: 6248. https://doi.org/10.3390/molecules26206248
APA StyleKasouni, A. I., Chatzimitakos, T. G., Stalikas, C. D., Trangas, T., Papoudou-Bai, A., & Troganis, A. N. (2021). The Unexplored Wound Healing Activity of Urtica dioica L. Extract: An In Vitro and In Vivo Study. Molecules, 26(20), 6248. https://doi.org/10.3390/molecules26206248








