New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples
Abstract
:1. Introduction
2. Results and Discussion
2.1. Simultaneous Arsenic and Selenium Speciation Analysis
2.2. Speciation Analysis of Water-Soluble Arsenic and Selenium by HPLC–ICP–MS
3. Materials and Methods
3.1. Reagents and Standards
3.2. Analytical Instruments
3.3. Method Development for Simultaneous Speciation Analysis
Analytical Column and Mobile Phase Effect
- -
- PRP-X100 (250 mm × 4.1 mm, 10 μm), packing material type: PS-DVB/Trimethyl ammonium exchanger (Hamilton, Reno, NV, USA);
- -
- PRPX-200 PEEK (250 mm × 4.6 mm, 10 µm), packing material type: PSDVB/Sulfonic Acid (Hamilton, USA);
- -
- BioWAX (50 mm × 2.1 mm, 5 µm), packing material type: nonporous/dietyloamine (Agilent, Santa Clara, CA, USA);
- -
- Supelco SAX (250 mm × 4.6 mm, 5 µm), packing material type: silica gel, spherical particle platform/propyltrimethylammonium phase (Sigma–Aldrich, USA);
- -
- Waters SAX (250 mm × 4.6 mm, 5 µm), packing material type: silica-based quaternary ammonium bonded sorbent (Waters, Milford, MA, USA);
- -
- Dionex CS5A (250 mm × 4 mm, 9 µm), packing material type: latex, DVB/Cation: Sulfonic Acid/Anion: Quaternary Ammonium (Thermo, Waltham, MA, USA);
- -
- Dionex CG5A (50 mm × 4 mm, 9 µm), packing material type: latex, DVB/Cation: Sulfonic Acid/Anion: Quaternary Ammonium (Thermo, USA);
- -
- Dionex AS22 (250 mm × 4 mm, 6 µm), packing material type: DVB/Alkanol Quaternary Ammonium Ion (Thermo, USA);
- -
- Dionex AG22 (250 mm × 4 mm, 6 µm), packing material type: DVB/Alkanol Quaternary Ammonium Ion (Thermo, USA).
3.4. Oven Temperature Effect
3.5. Final Method
3.6. Samples and Sample Preparation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chiocchetti, G.; Jadan-Piedra, C.; Velez, D.; Devesa, V. Metal(loid) Contamination in Seafood Products. Crit. Rev. Food Sci. Nutr. 2017, 57, 3715–3728. [Google Scholar] [CrossRef] [PubMed]
- Baki, M.A.; Hossain, M.; Akter, J.; Quraishi, S.B.; Shojib, F.H.; Ullah, A.K.M.A.; Khan, F. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Pastorelli, A.; Baldini, M.; Stacchini, P.; Morelli, S.; Sagratella, E.; Zaza, S.; Ciardullo, S. Human exposure to lead, cadmium and mercury through fish and seafood product consumption in Italy: A pilot evaluation. Food Addit. Contam. Part A 2012, 29, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Maciel, J.V.; Knorr, C.L.; Flores, E.M.M.; Müller, E.I.; Mesko, M.F.; Primel, E.G.; Duarte, F.A. Feasibility of microwave-induced combustion for trace element determination in Engraulisanchoita by ICP-MS. Food Chem. 2014, 145, 927–931. [Google Scholar] [CrossRef]
- Costa, L.G. Contaminants in fish: Risk-benefit considerations. Arh. Hig. Rada Toksikol. 2007, 58, 367–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guérin, T.; Chekri, R.; Vastel, C.; Sirot, V.; Volatier, J.-L.; Leblanc, J.-C.; Noël, L. Determination of 20 trace elements in fish and other seafood from the French market. Food Chem. 2011, 127, 934–942. [Google Scholar] [CrossRef]
- Martí-Cid, R.; Bocio, A.; Llobet, J.M.; Domingo, J.L. Intake of chemical contaminants through fish and seafood consumption by children of Catalonia, Spain: Health risks. Food Chem. Toxicol. 2007, 45, 1968–1974. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, K.; Mamun, H.A.; Masunaga, S. Assessment of trace metals in fish species of urban rivers in Bangladesh and health implications. Environ. Toxicol. Pharmacol. 2015, 39, 347–357. [Google Scholar] [CrossRef]
- Kwoczek, M.; Szefer, P.; Hać, E.; Grembecka, M. Essential and Toxic Elements in Seafood Available in Poland from Different Geographical Regions. J. Agric. Food Chem. 2006, 54, 3015–3024. [Google Scholar] [CrossRef]
- Llobet, J.M.; Falcó, G.; Casas, C.; Teixidó, A.; Domingo, J.L. Concentrations of Arsenic, Cadmium, Mercury, and Lead in Common Foods and Estimated Daily Intake by Children, Adolescents, Adults, and Seniors of Catalonia, Spain. J. Agric. Food Chem. 2002, 51, 838–842. [Google Scholar] [CrossRef]
- Salvo, A.; Potortì, A.G.; Cicero, N.; Bruno, M.; Turco, V.L.; Bella, G.D.; Dugo, G. Statistical characterisation of heavy metal contents in Paracentrotus lividus from Mediterranean Sea. Nat. Prod. Res. 2014, 28, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Commission Regulation (EU) 2015/1006 of 25 June 2015 amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Inorganic Arsenic in Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2015/1006/oj (accessed on 13 February 2021).
- General Veterinary Inspectorate, National Standard of Food Safety in Poland GB 2762-2012. Available online: https://www.wetgiw.gov.pl/english/general (accessed on 29 April 2021).
- Preventing Disease through Healthy Environments Exposure to Arsenic: A Major Public Health Concern Available, Public Health and Environment World Health Organization 2010. Available online: https://apps.who.int/iris/handle/10665/329482 (accessed on 28 April 2021).
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press (US): Washington, DC, USA, 2000. [Google Scholar]
- Da Silva, E.G.; Mataveli, L.R.V.; Arruda, M.A.Z. Speciation analysis of selenium in plankton, Brazil nut and human urine samples by HPLC–ICP-MS. Talanta 2013, 110, 53–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siepak, J. Analiza specjacyjna w badaniach środowiska. Rocznik Ochrona Środowiska 2015, 17, 526–539. [Google Scholar]
- Wolle, M.M.; Conklin, S.D.; Wittenberg, J. Matrix-induced transformation of arsenic species in seafoods. Anal. Chim. Acta 2019, 1060, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Wang, L.; Ma, L.; Yang, Z. Speciation analysis of six arsenic species in marketed shellfish: Extraction optimization and health risk assessment. Food Chem. 2018, 244, 311–316. [Google Scholar] [CrossRef]
- Sloth, J.J.; Larsen, E.H. Selective arsenic speciation analysis of human urine reference materials using gradient elution ion-exchange HPLC-ICP-MS. J. Anal. At. Spectrom. 2004, 19, 973–978. [Google Scholar] [CrossRef]
- Park, M.-K.; Choi, M.; Kim, L.; Choi, S.-D. An improved rapid analytical method for the arsenic speciation analysis of marine environmental samples using high-performance liquid chromatography/inductively coupled plasma mass spectrometry. Environ. Monit. Assess. 2019, 191, 525. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, L.; Li, S.; Cao, J.; Yang, Z. Species-specific bioaccumulation and correlated health risk of arsenic compounds in freshwater fish from a typical mine-impacted river. Sci. Total Environ. 2018, 625, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Bissen, M.; Frimmel, F.H. Speciation of As(III), As(V), MMA and DMA in contaminated soil extracts by HPLC-ICP/MS. Anal. Bioanal. Chem. 2000, 367, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Xie, S.; Liu, J.; Wei, C.; Zhang, H.; Chen, T.; Zhang, J. Arsenic concentrations and speciation in wild birds from an abandoned realgar mine in China. Chemosphere 2018, 193, 777–784. [Google Scholar] [CrossRef]
- Moreda-Pineiro, A.; Moreda-Pineiro, J.; Herbello-Hermelo, P.; Bermejo-Barrera, P.; López-Mahía, S.M.P.; Prada-Rodríguez, D. Application of fast ultrasound water-bath assisted enzymatic hydrolysis–High performance liquid chromatography–inductively coupled plasma-mass spectrometry procedures for arsenic speciation in seafood materials. J. Chromatogr. A 2011, 1218, 6970–6980. [Google Scholar] [CrossRef]
- Wang, M.; Feng, W.; Shi, J.; Zhang, F.; Wang, B.; Zhu, M.; Li, B.; Zhao, Y.; Chai, Z. Development of a mild mercaptoethanol extraction method for determination of mercury species in biological samples by HPLC–ICP-MS. Talanta 2007, 71, 2034–2039. [Google Scholar] [CrossRef]
- Moreno, P.; Quijano, M.A.; Gutiérrez, A.M.; Pérez-Conde, M.C.; Cámara, C. Stability of total selenium and selenium species in lyophilised oysters and in their enzymatic extracts. Anal. Bioanal. Chem. 2002, 374, 466–476. [Google Scholar] [PubMed]
- Zhang, Q.; Yang, G. Selenium speciation in bay scallops by high performance liquid chromatography separation and inductively coupled plasma mass spectrometry detection after complete enzymatic extraction. J. Chromatogr. A 2014, 1325, 83–91. [Google Scholar] [CrossRef]
- Kohlmeyer, U.; Kuballa, J.; Jantzen, E. Simultaneous separation of 17 inorganic and organic arsenic compounds in marine biota by means of high-performance liquid chromatography/inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2002, 16, 965–974. [Google Scholar] [CrossRef]
- Sloth, J.J.; Larsen, E.H.; Julshamn, K. Determination of organoarsenic species in marine samples using gradient elution cation exchange HPLC-ICP-MS. J. Anal. At. Spectrom. 2003, 18, 452–459. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Kuehnelt, D. Determination of arsenic species: A critical review of methods and applications, 2000–2003. Analyst 2004, 129, 373–395. [Google Scholar] [CrossRef]
- Sakai, T.; Inoue, Y.; Date, Y.; Aoyama, T.; Yoshida, K.; Endo, G. Simultaneous determination of neutral, anionic and cationic compounds within one chromatographic run using an inductively coupled plasma mass spectrometer as element-specific detector. Appl. Organomet. Chem. 2001, 15, 285–290. [Google Scholar] [CrossRef]
- Luvonga, C.; Rimmer, C.A.; Yu, L.L.; Lee, S.B. Analytical Methodologies for the Determination of Organoarsenicals in Edible Marine Species: A Review. J. Agric. Food Chem. 2020, 7, 1910–1934. [Google Scholar] [CrossRef]
- Wolle, M.M.; Todorov, T.I.; Conklin, S.D. Arsenic Species in Seaweeds Commercially Available in the United States. ACS Food Sci. Technol. 2021, 1, 511–523. [Google Scholar] [CrossRef]
- Nakazato, T.; Taniguchi, T.; Tao, H.; Tominagaa, M.; Miyazakia, A. Ion-exclusion chromatography combined with ICP-MS and hydride generation-ICP-MS for the determination of arsenic species in biological matrices. J. Anal. At. Spectrom. 2000, 15, 1546–1552. [Google Scholar] [CrossRef]
- Milovanovic, I.; Lajin, B.; Braeuer, S.; Steiner, O.; Lisa, F.; Goessler, W. Simultaneous selenium and sulfur speciation analysis in cultivated Pleurotus pulmonarius mushroom. Food Chem. 2018, 279, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Ruttens, A.; Blanpain, A.C.; de Temmerman, L.; Waegeneers, N. Arsenic speciation analysis in food in Belgium Part1: Fish, molluscs and crustaceans. J. Geochem. Explor. 2012, 121, 55–61. [Google Scholar] [CrossRef]
- Schmidt, L.; Landero, J.A.; Novo, D.L.R.; Duarte, F.; Mesko, M.F.; Caruso, J.A.; Flores, E.M.M. A feasible method for As speciation in several types of seafood by LC-ICP-MS/MS. Food Chem. 2018, 255, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Kapolna, E.; Fedor, P. Speciation analysis of selenium enriched green onions (Allium fistulosum) by HPLC-ICP-MS. Microchem. J. 2006, 84, 56–62. [Google Scholar] [CrossRef]
- Yamashita, Y.; Yamashita, M.; Iida, H. Selenium Content in Seafood in Japan. Nutrients 2013, 5, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.; Juhasz, A.L.; Weber, J. Arsenic uptake and speciation in vegetables grown under greenhouse conditions. Environ. Geochem. Health 2009, 31, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; He, J.; Zhao, M.; Qiu, L.; Fan, L.; Meng, S.; Yang, G.; Li, T.; Li, Q.; et al. Occurrence, speciation analysis and health risk assessment of arsenic in Chinese mitten crabs (Eriocheir sinensis) collected from China. J. Food Compos. Anal. 2020, 94, 103647. [Google Scholar] [CrossRef]
- Bergqvist, C.; Herbert, R.; Persson, I.; Greger, M. Plants influence on arsenic availability and speciation in the rhizosphere, roots and shoots of three different vegetables. Environ. Pollut. 2014, 184, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Hackethal, C.; Kopp, J.F.; Sarvan, I.; Schwerdtle, T.; Lindtner, O. Total arsenic and water-soluble arsenic species in foods of the first German total diet study (BfR MEAL Study). Food Chem. 2021, 346, 128913. [Google Scholar] [CrossRef]
- Francesconi, K.; Visoottiviseth, P.; Sridokchan, W.; Goessler, W. Arsenic species in an arsenic hyperaccumulating fern, Pityrogramma calomelanos: A potential phytoremediator of arsenic-contaminated soils. Sci. Total Environ. 2002, 284, 27–35. [Google Scholar] [CrossRef]
- Pinter, I.F.; Salomon, M.V.; Gil, R.; Mastrantonio, L.; Bottini, R.; Piccoli, P. Arsenic and trace elements in soil, water, grapevine and onion in Jáchal, Argentina. Sci. Total Environ. 2018, 615, 1485–1498. [Google Scholar] [CrossRef]
- European Food Safety Authority. Dietary exposure to inorganic arsenic in the European population. EFSA J. 2014, 12, 68. [Google Scholar]
- Wróbel, K.; Wróbel, K.; Kannamkumarath, S.S.; Caruso, J.; Wysocka, I.A.; Bulska, E.; Swiątek, J.; Wierzbicka, M. HPLC–ICP-MS speciation of selenium in enriched onion leaves—A potential dietary source of Se-methylselenocysteine. Food Chem. 2004, 86, 617–623. [Google Scholar] [CrossRef]
- Shah, M.; Kannamkumarath, S.S.; Wuilloud, J.C.; Wuilloud, R.G.; Caruso, J.A. Identification and characterization of selenium species in enriched green onion (Allium fistulosum) by HPLC-ICP-MS and ESI-ITMSJ. J. Anal. At. Spectrom. 2004, 19, 381–386. [Google Scholar] [CrossRef]
- Bakırdere, S.; Volkan, M.; Ataman, O.Y. Selenium speciation in chicken breast samples from inorganic and organic selenium fed chickens using high performance liquid chromatography inductively coupled plasma-mass spectrometry. J. Food Compos. Anal. 2018, 71, 28–35. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Sentkowska, A. Selenium in plant foods: Speciation analysis, bioavailability, and factors affecting omposition. Crit. Rev. Food Sci. Nutr. 2021, 61, 1340–1352. [Google Scholar] [CrossRef] [PubMed]
- Kapolna, E.; Shah, M.; Caruso, J.; Fodor, P. Selenium speciation studies in Se-enriched chives (Allium schoenoprasum) by HPLC-ICP–MS. Food Chem. 2007, 101, 1398–1406. [Google Scholar] [CrossRef]
- Jagtap, R.; Maher, W.; Krikowa, F.; Ellwood, M.J.; Foster, S. Measurement of selenomethionine and selenocysteine in fish tissues using HPLC-ICP-MS. Microchem. J. 2016, 128, 248–257. [Google Scholar] [CrossRef]
- Sele, V.; Ørnsrud, R.; Sloth, J.J.; Berntssen, M.H.G.; Amlund, H. Selenium and selenium species in feeds and muscle tissue of Atlantic salmon. J. Trace Elem. Med. Biol. 2018, 47, 124–133. [Google Scholar] [CrossRef]
- Francesconi, K.A.; Tanggaar, R.; McKenzie, C.J.; Goessler, W. Arsenic Metabolites in Human Urine after Ingestion of an Arsenosugar. Clin. Chem. 2002, 1, 92–101. [Google Scholar] [CrossRef]
- Certificate of Tuna Fish Tissue BCR-627, Institute for Reference Materials and Measurements (IRMM), Geel, Belgium. Available online: https://crm.jrc.ec.europa.eu/p/40456/40494/By-analyte-group/Extractable-element-species/BCR-627-TUNA-FISH-TISSUE-As-species/BCR-627 (accessed on 1 July 2021).

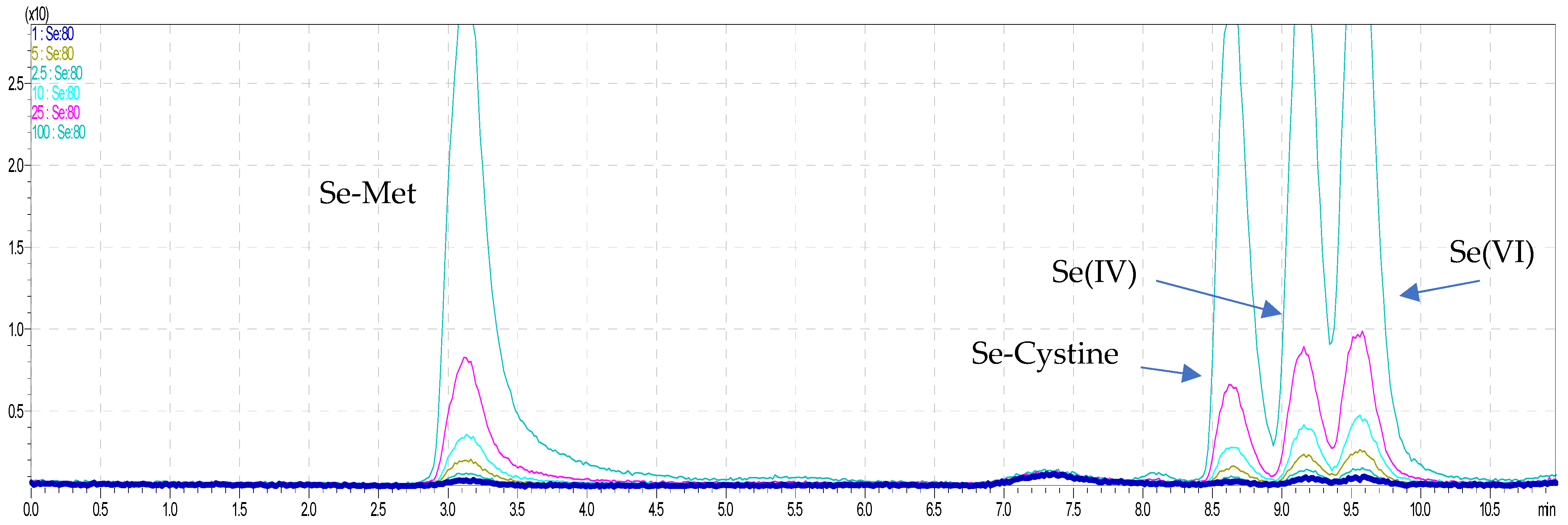
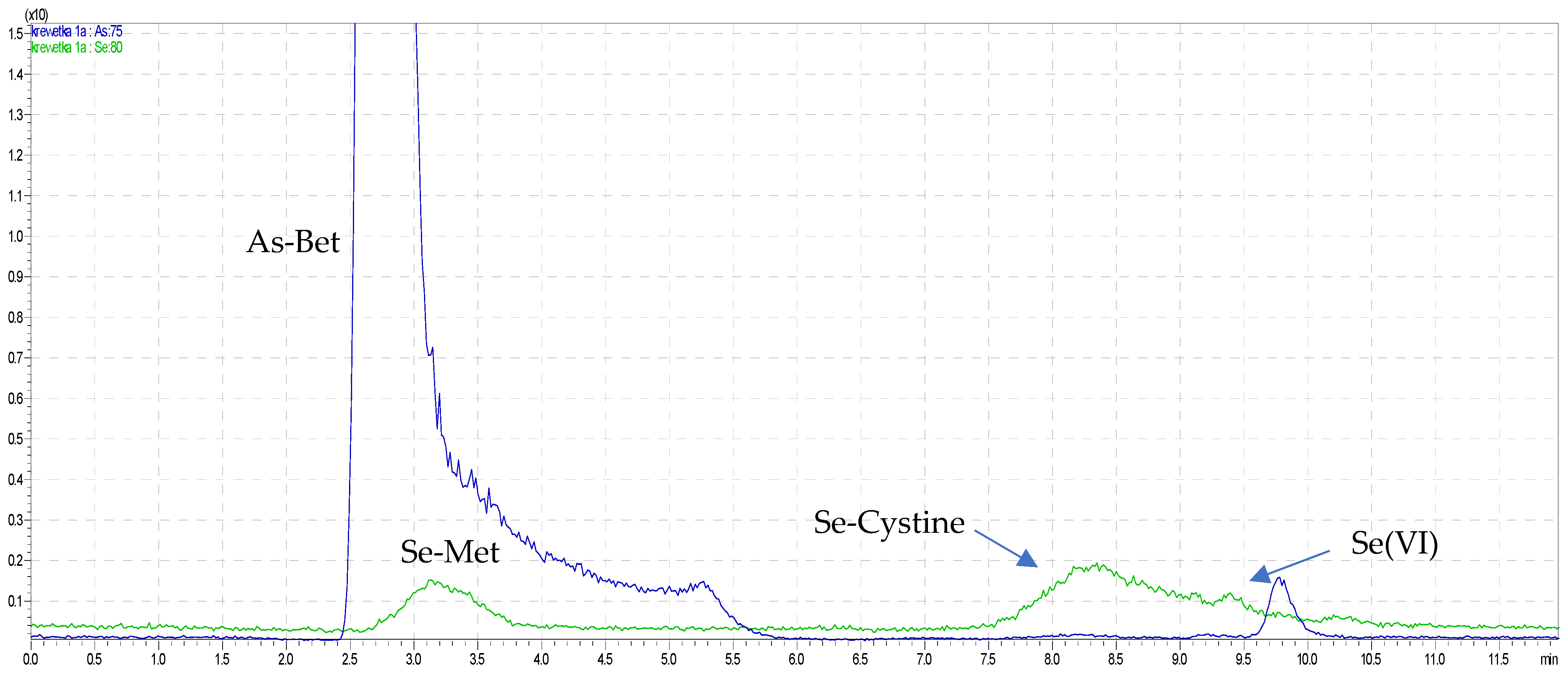
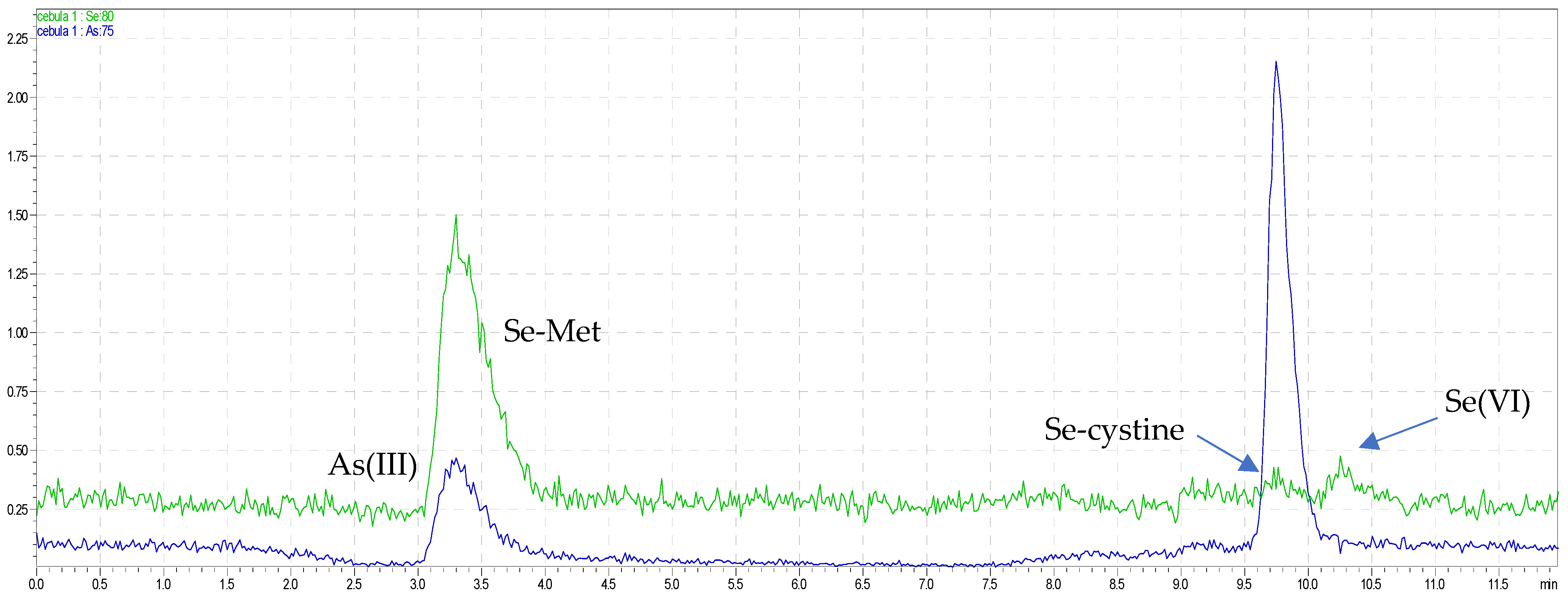
| Sample/Determined Form | As(III) | As(V) | MMA | DMA | As-Bet * | Water-Soluble as by ICP–MS | Column Recovery * |
|---|---|---|---|---|---|---|---|
| bio white onion | 166.1 ± 7.5 | <LOD | <LOD | <LOD | <LOD | 0.171 ± 0.002 | 97.7 ± 3.7 |
| pink onion | 139.2 ± 5.6 | <LOD | <LOD | <LOD | <LOD | 0.153 ± 0.003 | 92.8 ± 4.7 |
| sweet onion | 149.3 ± 5.9 | <LOD | <LOD | <LOD | <LOD | 0.164 ± 0.002 | 93.3 ± 4.8 |
| bio red onion | 189.3 ± 7.9 | <LOD | <LOD | <LOD | <LOD | 0.206 ± 0.005 | 94.6 ± 4.7 |
| red shrimp | <LOD | <LOD | <LOD | <LOD | 15.48 ± 0.62 | 16.50 ± 0.09 | 93.9 ± 3.9 |
| clam | <LOD | <LOD | 336.9 ± 12.4 | <LOD | 13.32 ± 0.58 | 13.91 ± 0.17 | 98.2 ± 4.8 |
| octopus | <LOD | <LOD | <LOD | <LOD | 2.419 ± 0.112 | 2.511 ± 0.032 | 96.4 ± 3.8 |
| squid | <LOD | <LOD | <LOD | <LOD | 4.691 ± 0.221 | 5.011 ± 0.061 | 93.7 ± 4.4 |
| red big shrimp | <LOD | <LOD | <LOD | 119.8 ± 5.5 | 20.69 ± 0.782 | 21.30 ± 0.51 | 97.7 ± 3.7 |
| white blanched shrimp Vannamei | <LOD | <LOD | <LOD | <LOD | 2.507 ± 0.100 | 2.652 ± 0.032 | 94.6 ± 2.7 |
| white shrimp Vannamei | <LOD | <LOD | <LOD | <LOD | 2.221 ± 0.931 | 2.304 ± 0.027 | 96.6 ± 4.8 |
| sample/determined form | Se(IV) | Se(VI) | Se-Met | Se-cystine | water-soluble Se by ICP–MS | Column recovery * | |
| bio white onion | <LOD | 9.101 ± 0.302 | 609.7 ± 12.1 | <LOD | 0.671 ± 0.009 | 92.36 ± 4.41 | |
| pink onion | 959.2 ± 45.8 | <LOD | <LOD | <LOD | 0.994 ± 0.021 | 96.89 ± 4.75 | |
| sweet onion | <LOD | <LOD | 8788 ± 431.5 | <LOD | 8.921 ± 0.107 | 98.74 ± 3.85 | |
| bio red onion | <LOD | <LOD | 5065 ± 217.8 | <LOD | 5.302 ± 0.153 | 95.57 ± 4.31 | |
| red shrimp | <LOD | 47.53 ± 1.91 | 417.1 ± 16.9 | 987.2 ± 37.4 | 1.611 ± 0.032 | 90.17 ± 5.23 | |
| clam | <LOD | 182.6 ± 7.6 | <LOD | <LOD | 0.203 ± 0.003 | 91.32 ± 4.75 | |
| octopus | <LOD | 19.21 ± 0.92 | 435.5 ± 17.4 | <LOD | 0.502 ± 0.009 | 91.01 ± 4.55 | |
| squid | <LOD | 67.41 ± 2.73 | <LOD | 202.2 ± 8.1 | 0.287 ± 0.006 | 96.31 ± 4.29 | |
| red big shrimp | <LOD | 137.6 ± 5.7 | <LOD | <LOD | 0.152 ± 0.003 | 91.74 ± 3.89 | |
| white blanched shrimp Vannamei | 94.5 ± 4.6 | <LOD | 760.3 ± 32.3 | <LOD | 0.879 ± 0.017 | 97.15 ± 3.79 | |
| white shrimp Vannamei | <LOD | <LOD | 776.9 ± 36.4 | <LOD | 0.854 ± 0.011 | 91.41 ± 4.29 |
| Column | Mobile Phase | As-Bet | As (III) | DMA | MMA | As (V) | Cr (III) | Cr (VI) | Se-Cystine | Se-Met | Se (IV) | Se (VI) | Sb (III) | Sb (V) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRPX-100 | HNO3 + CH3OH | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| PRPX-200 | HNO3 + CH3OH | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| NH4NO3 + CH3OH | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | |
| NH4NO3 + CH3OH + EDTA | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | |
| BIOWAX | NH4NO3 + CH3OH | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| SUPELCO | NH4NO3 + CH3OH | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| WATERS | NH4NO3 + CH3OH | ✕ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| DIONEX CG5A | NH4NO3 + CH3OH | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ |
| DIONEX CS5A | NH4NO3 + CH3OH | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ | ✕ |
| DIONEX AG22 + AS22 | NH4NO3 + CH3OH | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ |
| DIONEX AS22 + CG5A | NH4NO3 + CH3OH alkaline pH | ✓ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ |
| NH4NO3 + CH3OH acidic pH | ✕ | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | |
| (NH4)2CO3 alkaline pH | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✕ | ✕ | ✓ | ✓ | ✕ | ✕ | |
| NH4NO3 + NH4HCO3 | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | |
| NH4HCO3alkaline pH | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ | |
| (NH4)2CO3 + NH4HCO3 | ✕ | ✕ | ✓ | ✓ | ✓ | ✕ | ✕ | ✓ | ✓ | ✓ | ✓ | ✕ | ✕ |
| As(III) | As(V) | MMA | DMA | AsB | Se(IV) | Se(VI) | SeMet | Secystine | |
|---|---|---|---|---|---|---|---|---|---|
| Conc. Range | Up to 100 µg·L−1 * | ||||||||
| Slope | 9.004 | 21.08 | 8.863 | 0.495 | 7.749 | 3.407 | 6.405 | 4.087 | 4.086 |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9995 | 0.9993 | 0.9999 | 0.9999 | 0.9997 | 0.9993 |
| Limit of detection | 1.257 | 1.027 | 0.229 | 1.133 | 1.659 | 0.359 | 1.116 | 1.987 | 2.307 |
| Generator power [W] | 1200 |
| Argon flow—plasma [L/min] | 8.0 |
| Argon flow—nebulizer [L/min] | 1.1 |
| Argon flow—auxiliary [L/min] | 0.7 |
| Nebulizer | Concentric type “micro” |
| Torch | Concentric type “mini” |
| Spray chamber temperature [°C] | 5.0 |
| Collision gas—He [mL/min] | 6.0 |
| Voltage on octapole rods [V] | −21 |
| Energy filter [V] | 7.0 |
| Sampling deep [mm] | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karaś, K.; Zioła-Frankowska, A.; Frankowski, M. New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples. Molecules 2021, 26, 6223. https://doi.org/10.3390/molecules26206223
Karaś K, Zioła-Frankowska A, Frankowski M. New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples. Molecules. 2021; 26(20):6223. https://doi.org/10.3390/molecules26206223
Chicago/Turabian StyleKaraś, Katarzyna, Anetta Zioła-Frankowska, and Marcin Frankowski. 2021. "New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples" Molecules 26, no. 20: 6223. https://doi.org/10.3390/molecules26206223
APA StyleKaraś, K., Zioła-Frankowska, A., & Frankowski, M. (2021). New Method for Simultaneous Arsenic and Selenium Speciation Analysis in Seafood and Onion Samples. Molecules, 26(20), 6223. https://doi.org/10.3390/molecules26206223







