Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line
Abstract
:1. Introduction
2. Results and Discussion
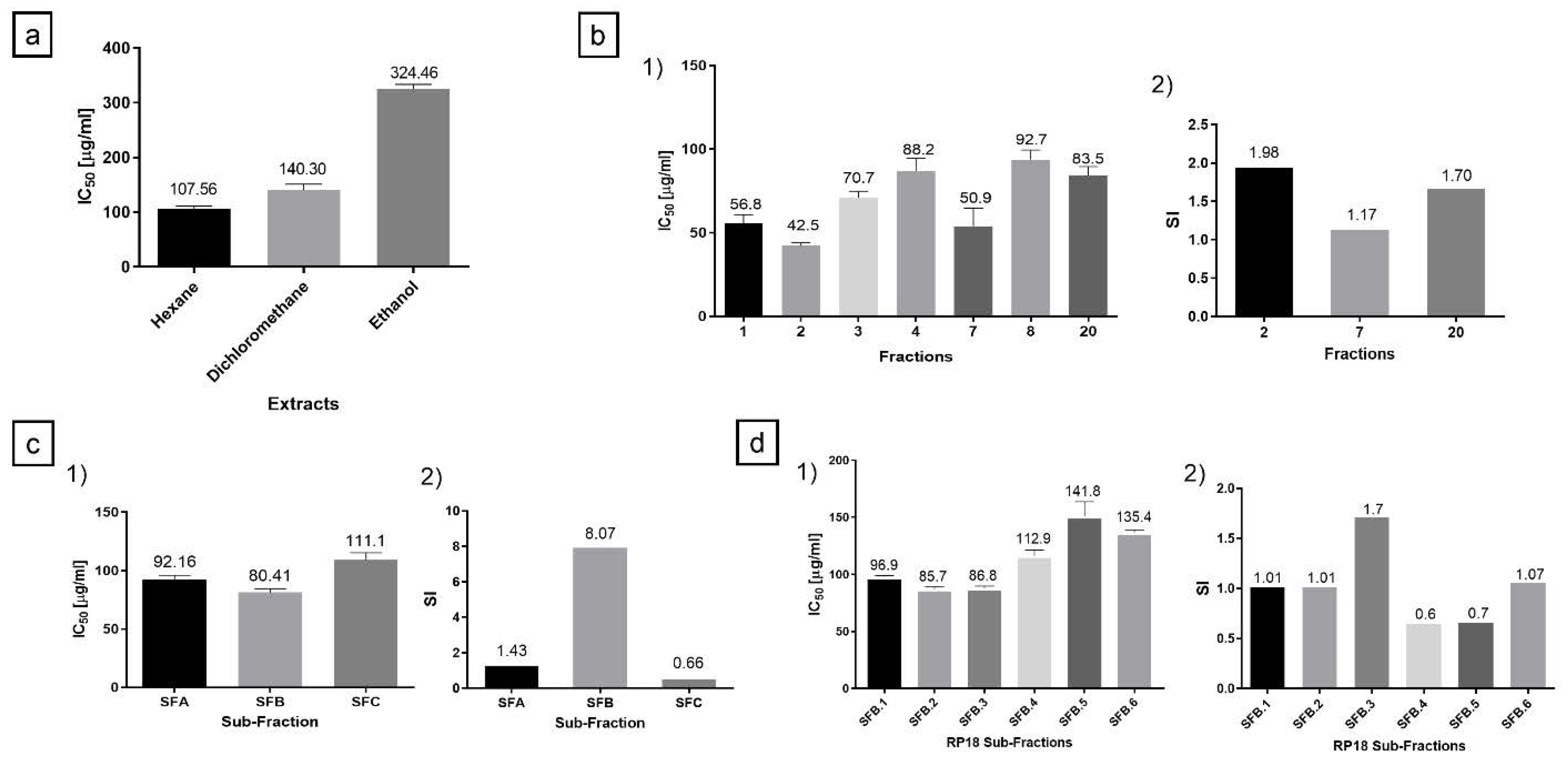
2.1. Hexane Extract Bio-Guided Fractionation Led to Active Fractions on PC-3 and MRC-5 Cells
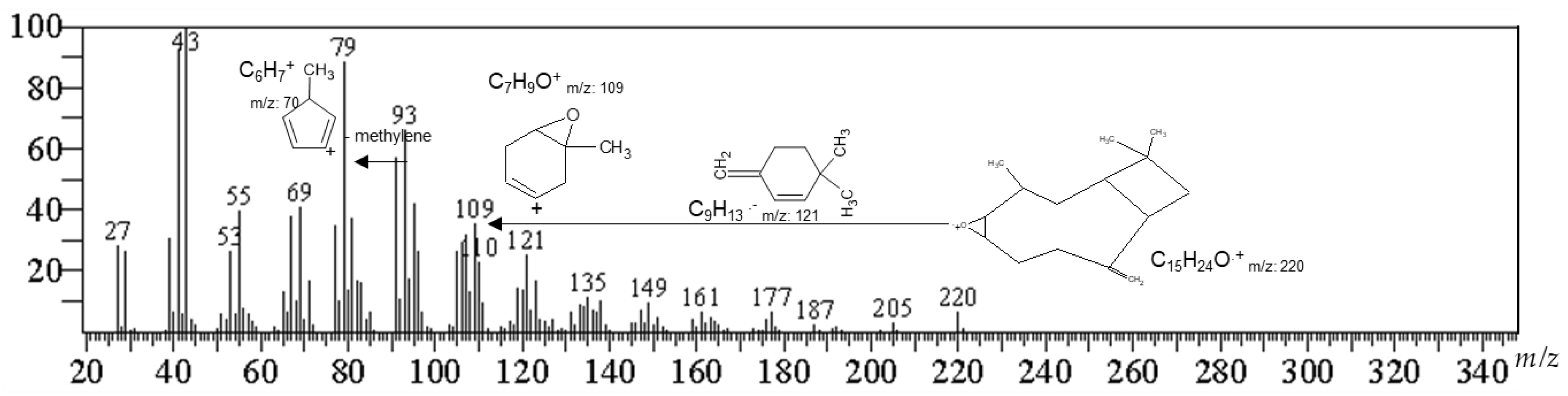
2.2. Identification of the Active Compound by Gas Chromatography-Mass Spectrometry (GC/MS)
2.3. Cytotoxicity and Selectivity Index for OXC
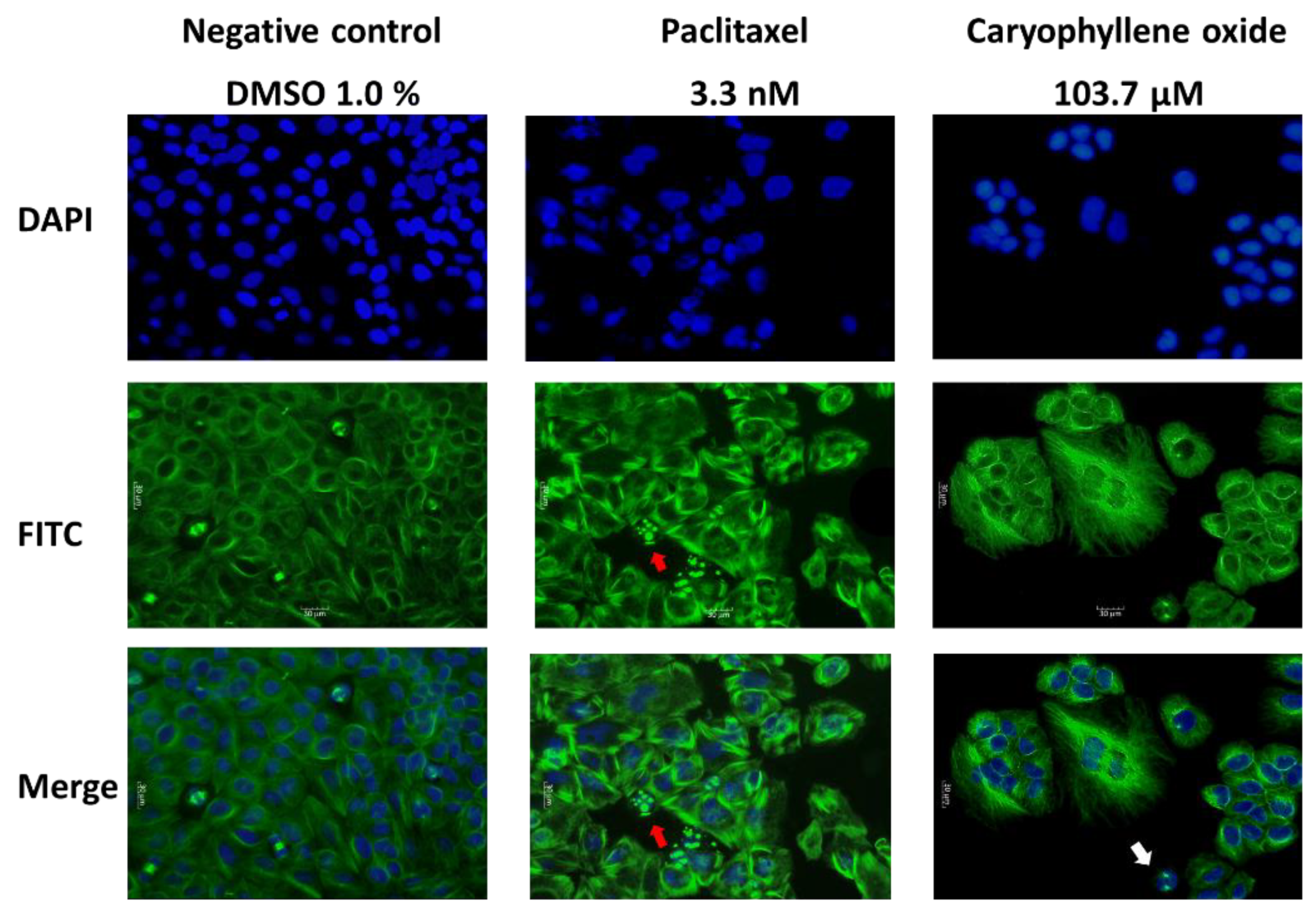
2.4. OXC Exert Morphological Changes Leading to the Emergence of Apoptotic Cells
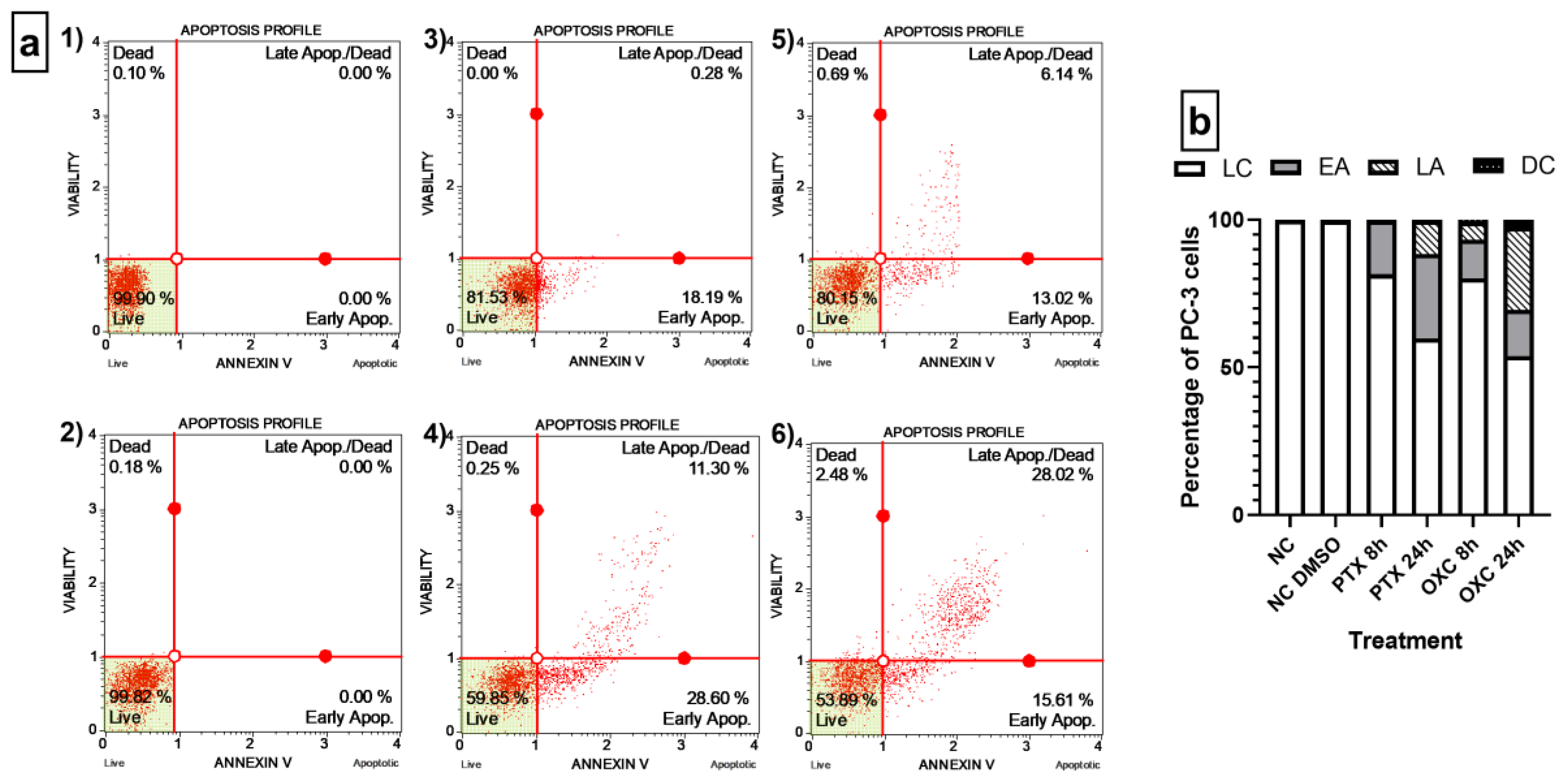
2.5. Caryophyllene Oxide Induces Early and Late Apoptosis
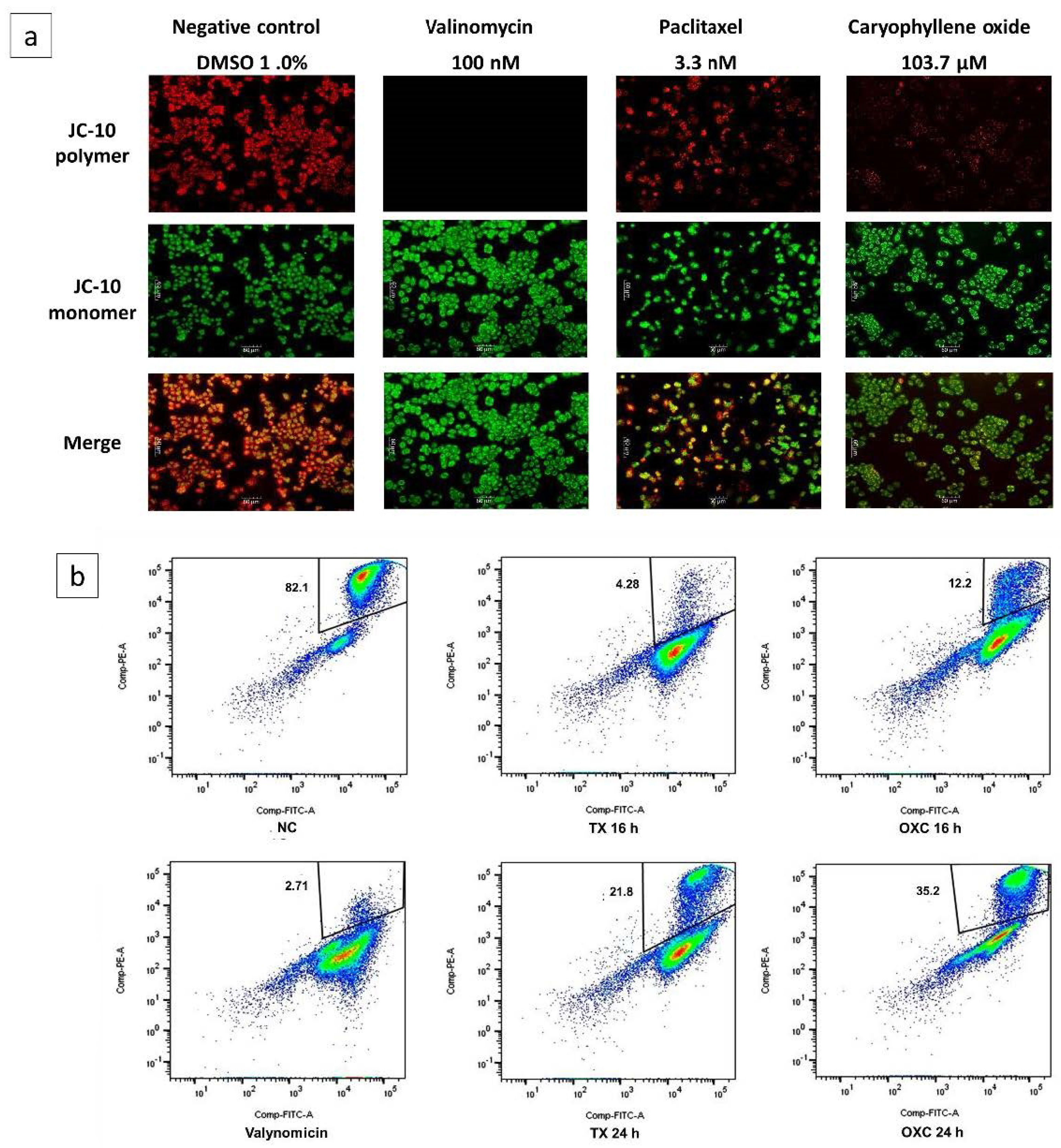
2.6. Loss of Mitochondrial Membrane Potential (Δψm) Induced by OXC in PC-3 Cells
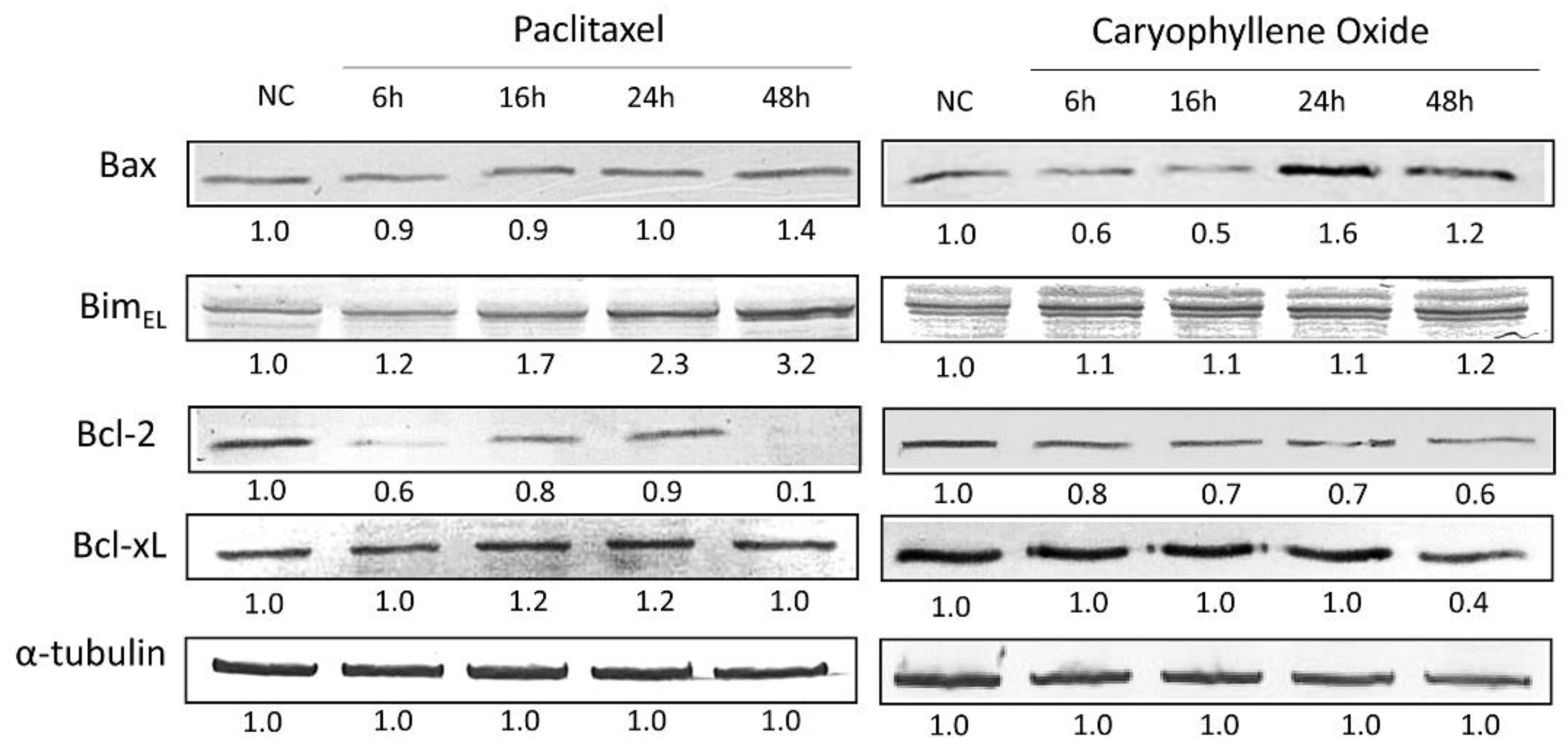
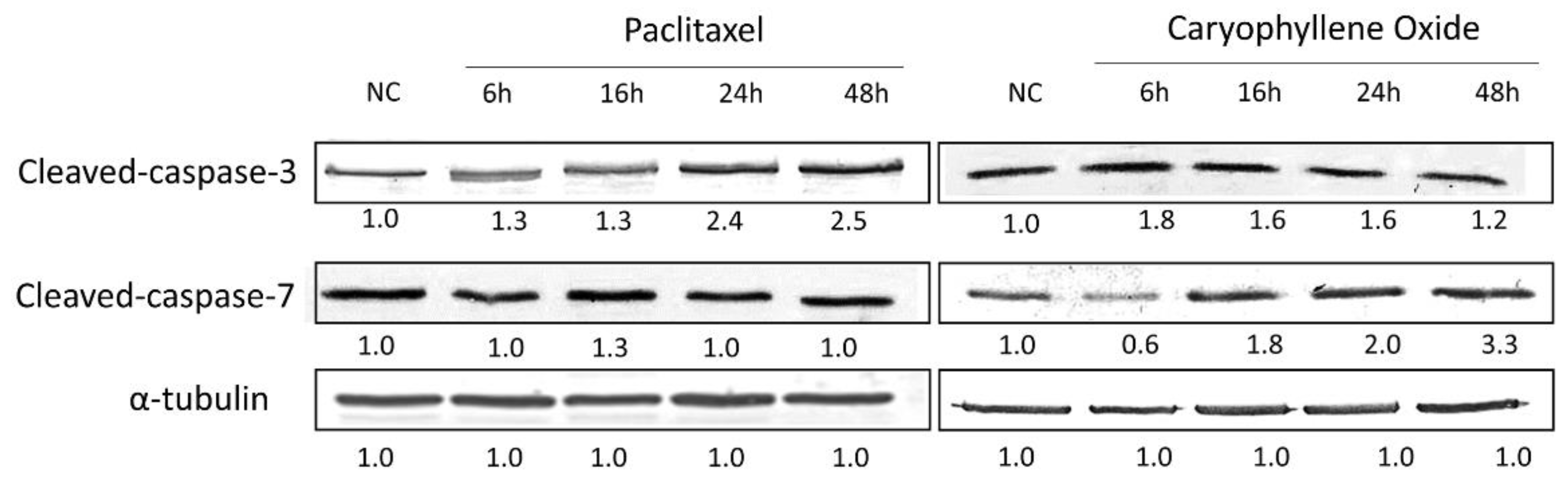
2.7. Apoptotic Proteins’ Expression in PC-3 Cells after Being Exposed for 6, 16, 24, and 48 h to OXC
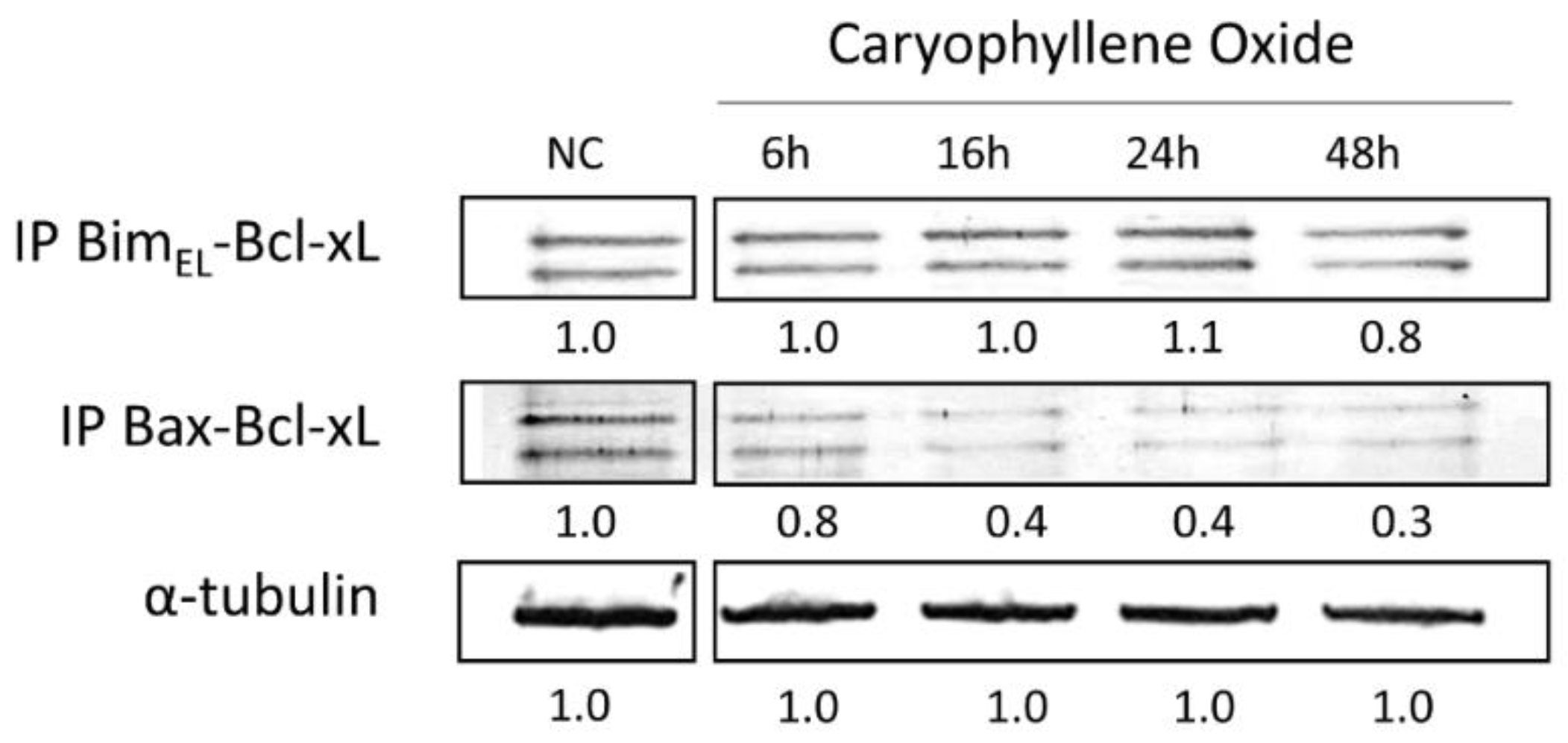
2.8. Protein–Protein Interactions after Treatment with OXC
3. Materials and Methods
3.1. General Conditions
3.2. Cell Viability Assay
3.3. Detection of Morphological Changes by Immunofluorescence
3.4. Secondary Metabolites’ Identification
3.5. The Muse Annexin V and Dead Cell Assay
3.6. Analysis of the Mitochondrial Membrane Potential (Δψm)
3.7. Detection of Apoptotic Proteins by SDS-PAGE and Western Blot
3.8. Bcl-xL Protein Complex Formation by Co-Immunoprecipitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, H.; Sun, Y.; Wu, C.; Magyar, C.E.; Li, X.; Cheng, L.; Yao, J.L.; Shen, S.; Osunkoya, A.O.; Liang, C.; et al. Pathogenesis of prostatic small cell carcinoma involves the inactivation of the P53 pathway. Endocr. Relat. Cancer 2012, 19, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.-H.; Zhang, Y.-Q.; Huang, J.-T. Neuroendocrine cells of prostate cancer: Biologic functions and molecular mechanisms. Asian J. Androl. 2019, 21, 291–295. [Google Scholar] [CrossRef]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of Prostate Cancer Models for Preclinical Study: Advantages and Limitations of Cell Lines, Patient-Derived Xenografts, and Three-Dimensional Culture of Patient-Derived Cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgado, C.; Méndez, G. Topological properties and in vitro identification of essential nodes of the Paclitaxel and Vincristine interactomes in PC-3 cells. Biomed. J. 2019, 42, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Scher, H.I.; Scardino, P.T.; Zelefsky, M.J. Cancer of the prostate. In Prostate and Other Genitourinary Cancers: From Cancer: Principles & Practice of Oncology, 10th ed.; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2016; pp. 1009–1107. [Google Scholar]
- Carroll, A.G.; Voeller, H.J.; Sugars, L.; Gelmann, E.P. p53 oncogene mutations in three human prostate cancer cell lines. Prostate 1993, 23, 123–134. [Google Scholar] [CrossRef]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Boniface, P.K.; Ferreira, S.B.; Kaiser, C.R. Current state of knowledge on the traditional uses, phytochemistry, and pharmacology of the genus Hymenaea. J. Ethnopharmacol. 2017, 206, 193–223. [Google Scholar] [CrossRef]
- Da Silva, F.G.; De Souza, C.; Rolim, L.A.; Barbosa-Filho, J.M.; Da Silva, J.R.G. The genus Hymenaea (Fabaceae): A Chemical and Pharmacological Review. In Atta-ur-Rahman: Studies in Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; Volume 58, pp. 339–388. [Google Scholar]
- Schwartz, G. Jatoba-Hymenaea courbaril. In Exotic Fruits; Rodrigues, S., De Oliveira Sousa, E.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 257–261. [Google Scholar]
- Shanley, P.; Schulze, M. Jatobá, Hymenaea courbaril L. In Frutíferas e Plantas Úteis na Vida Amazônica; Shanley, P., Medina, G., Eds.; CIFOR: Belém, Brazil, 2005; pp. 109–118. [Google Scholar]
- Sajjadi, S.E.; Ghanadian, M.; Haghighi, M.; Mouhebat, L. Cytotoxic effect of Cousinia verbascifolia Bunge against OVCAR-3 and HT-29 cancer cells. J. Herb. Med. Pharm. 2015, 4, 15–19. [Google Scholar]
- Srisawat, T.; Chumkaew, P.; Heed-Chim, W.; Sukpondma, Y.; Kanokwiroon, K. Phytochemical Screening and Cytotoxicity of Crude Extracts of Vatica diospyroides Symington Type LS. Trop. J. Pharm. Res. 2013, 12, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Artun, F.T.; Karagoz, A.; Ozcan, G.; Melikoglu, G.; Anil, S.; Kultur, S.; Sutlupinar, N. In vitro anticancer and cytotoxic activities of some plant extracts on HeLa and Vero cell lines. J. BUON 2016, 21, 1019. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, M.P.; Bozinis, M.C.V.; Andrade, W.M.; Costa, C.R.; da Silva, A.L.; de Oliveira, C.M.A.; Kato, L.; Fernandes, O.D.F.L.; Souza, L.K.H.; Silva, M.D.R.R. Antifungal and cytotoxicity activities of the fresh xylem sap of Hymenaea courbaril L. and its major constituent fisetin. BMC Complement. Altern. Med. 2014, 14, 245. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Natural products isolation. In Methods in Biotechnology: Natural Products Isolation, 2nd ed.; Sarker, S.D., Latif, Z., Gray, A.I., Eds.; Humana Press: Totowa, NJ, USA, 2006; pp. 1–25. [Google Scholar]
- Coté, H.; Boucher, M.-A.; Pichette, A.; Legault, J. Anti-Inflammatory, Antioxidant, Antibiotic, and Cytotoxic Activities of Tanacetum vulgare L. Essential Oil and Its Constituents. Medicines 2017, 4, 34. [Google Scholar] [CrossRef] [PubMed]
- Fidyt, K.; Fiedorowicz, A.; Strządałam, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 5, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Hanušová, V.; Caltovám, K.; Svobodovám, H.; Ambrožm, M.; Skarka, A.; Murínová, N.; Králová, V.; Tomšík, P.; Skálová, L. The effects of β-caryophyllene oxide and trans-nerolidol on the efficacy of doxorubicin in breast cancer cells and breast tu-mor-bearing mice. Biomed. Pharm. 2017, 95, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Huang, S.-B.; Yang, M.-C.; Lin, Y.-T.; Chu, I.-H.; Shen, Y.-N.; Chiu, Y.-H.; Hung, S.-H.; Kang, L.; Hong, Y.-R.; et al. Combining Paclitaxel with ABT-263 Has a Synergistic Effect on Paclitaxel Resistant Prostate Cancer Cells. PLoS ONE 2015, 10, e0120913. [Google Scholar] [CrossRef]
- Luminex Corpotation. MuseTM Annexin V & Dead Cell Kit User’s Guide. Available online: https://www.luminexcorp.com/download/__trashed-6-5/ (accessed on 22 April 2020).
- Peng, C.C.; Chen, C.Y.; Chen, C.R.; Chen, C.J.; Shen, K.H.; Chen, K.C.; Peng, R.Y. Renal Damaging effect elicited by bicalu-tamide therapy uncovered multiple action mechanisms as evidenced by the cell model. Sci. Rep. 2019, 9, 1–16. [Google Scholar] [CrossRef]
- Méndez-Callejas, G.M.; Leone, S.; Tanzarella, C.; Antoccia, A. Combretastatin A-4 induces p53 mitochondrial-relocalisation independent-apoptosis in non-small lung cancer cells. Cell Biol. Int. 2014, 38, 296–308. [Google Scholar] [CrossRef]
- Acuña, U.; Mo, S.; Zi, J.; Orjala, J.; Carcache de blanco, E.J. Hapalindole H induces apoptosis as an inhibitor of NF-ĸB and affects the intrinsic mitochondrial pathway in PC-3 androgen-insensitive prostate cancer cells. Anticancer Res. 2018, 38, 3299–3307. [Google Scholar] [CrossRef] [Green Version]
- Datta, S.; Choudhury, D.; Das, A.; Das Mukherjee, D.; Das, N.; Roy, S.S.; Chakrabarti, G. Paclitaxel resistance development is associated with biphasic changes in reactive oxygen species, mitochondrial membrane potential and autophagy with elevated energy production capacity in lung cancer cells: A chronological study. Tumor Biol. 2017, 39, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, E.N.; Patnaik, J.; Mullins, M.R.; Lemasters, J.J. Free Tubulin Modulates Mitochondrial Membrane Potential in Cancer Cells. Cancer Res. 2010, 70, 10192–10201. [Google Scholar] [CrossRef] [Green Version]
- Neophytou, C.; Trougakos, I.; Erin, N.; Papageorgis, P. Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance. Cancers 2021, 13, 4363. [Google Scholar] [CrossRef]
- Dai, H.; Meng, X.W.; Kaufmann, S.H. BCL2 family, mitocondrial apoptosis, and beyond. Cancer Transl. Med. 2016, 2, 7–20. [Google Scholar] [CrossRef]
- Kutuk, O.; Letai, A. Displacement of Bim by Bmf and Puma rather than increase in Bim level mediates paclitaxel-induced apoptosis in breast cancer cells. Cell Death Differ. 2010, 17, 1624–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, M.-C.; Lin, R.-W.; Huang, S.-B.; Huang, S.-Y.; Chen, W.-J.; Wang, S.; Hong, Y.-R.; Wang, C. Bim directly antagonizes Bcl-xl in doxorubicin-induced prostate cancer cell apoptosis independently of p53. Cell Cycle 2016, 15, 394–402. [Google Scholar] [CrossRef] [Green Version]
- Logue, S.; Martin, S.J. Caspase activation cascades in apoptosis. Biochem. Soc. Trans. 2008, 36, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaseva, A.V.; Moll, U.M. The mitochondrial p53 pathway. Biochim. Biophys. Acta (BBA) Bioenerg. 2009, 1787, 414–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castilla, C.; Congregado, B.; Chinchón, D.; Torrubia, F.J.; Japón, M.A.; Sáez, C. Bcl-xL is overexpressed in hormone-resistant prostate cancer and promotes survival of lncap cells via interaction with proapoptotic bak. Endocrinology 2006, 147, 4960–4967. [Google Scholar] [CrossRef] [PubMed]
- Waksmundzka-Hajnos, M.; Sherma, J. High Performance Liquid Chromatography in Phytochemical Analysis, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010; p. 996. [Google Scholar]
- Méndez-Callejas, G.; Rodríguez-Mayusa, J.; Riveros-Quirogam, M.; Mahete-Pinilla, K.; Torrenegra-Guerrero, R. Antiprolifer-ative activity of chloroformic fractions from leaves and inflorescences of Ageratina gracilis. Emir. J. Food Agric. 2017, 29, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective cytotoxic activities of two novel synthetic drugs on human breast carcinoma MCF-7 cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar] [PubMed]

| Compound | tR (Min) | Base Peak | Diagnostic Ions m/z (%) | Area % (Abundance) |
|---|---|---|---|---|
| (1R,7S E)-7-Isopropyl-4,10-DiMethylenecyClodec-5-Enol | 27.544 | 105.05 (100) | 91.05 (93.75), 131.1 (91.68), 93.05 (69.45), 159.1 (63.17), 55.05 (58.92) | 12.14 |
| 29.346 | 91.05 (100) | 93.05 (81.21), 79.05 (80.58), 105.1 (67.12), 107.1 (65.48), 81.1 (59.08) | ||
| 29.976 | 159.1 (100) | 91.05 (68.01), 93.05 (62.62), 105.1 (62.43), 81.1 (49.6), 131.1 (48.79) | ||
| Caryophyllene Oxide | 30.949 | 93.1 (100) | 55.05 (76.72), 91.1 (68.76), 107.1 (67.28), 67.05 (64.59), 79.05 (64.22) | 24.95 |
| 31.719 | 79.05 (100) | 91.05 (80.93), 93.05 (71.69), 95.05 (67.83), 67.05 (67.8), 107.05 (66.27) | ||
| 33.313 | 79.1 (100) | 55.05 (98.23), 81.05 (97.82), 91.05 (97.07), 109.05 (96.05), 95.1 (95.3) | ||
| γ-Sitosterol | 63.317 | 105.1 (100) | 81.1 (92.99), 107.1 (91.59), 55.05 (90.65), 95.1 (86.66), 145.1 (83.99) | 23.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, C.; Mendez-Callejas, G.; Celis, C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules 2021, 26, 6142. https://doi.org/10.3390/molecules26206142
Delgado C, Mendez-Callejas G, Celis C. Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules. 2021; 26(20):6142. https://doi.org/10.3390/molecules26206142
Chicago/Turabian StyleDelgado, Claudia, Gina Mendez-Callejas, and Crispin Celis. 2021. "Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line" Molecules 26, no. 20: 6142. https://doi.org/10.3390/molecules26206142
APA StyleDelgado, C., Mendez-Callejas, G., & Celis, C. (2021). Caryophyllene Oxide, the Active Compound Isolated from Leaves of Hymenaea courbaril L. (Fabaceae) with Antiproliferative and Apoptotic Effects on PC-3 Androgen-Independent Prostate Cancer Cell Line. Molecules, 26(20), 6142. https://doi.org/10.3390/molecules26206142






