Optimization of Infrared Heating Conditions for Precooked Cowpea Production Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Moisture Conditioning and Infrared Heating Pre-Treatment of Cowpea Seeds
2.4. Physicochemical and Functional Analyses
2.4.1. Bulk Density
2.4.2. Water Absorption Capacity
2.4.3. Soluble Pectin
2.5. Total Phenolic, Total Flavonoid Content, and Antioxidant Capacity Assays
2.5.1. Extraction Procedure
2.5.2. UHPLC Identification and Quantification of Phenolic Compounds
2.5.3. Total Phenolic Content (TPC)
2.5.4. Total Flavonoid Content (TFC)
2.5.5. Ferric Reducing Antioxidant Power (FRAP) Assay
2.6. Antinutritional Factors (ANFs) Analyses
2.6.1. Total Phytic Acid Content
2.6.2. Oxalate Content
2.7. Statistical Analysis
3. Results and Discussion
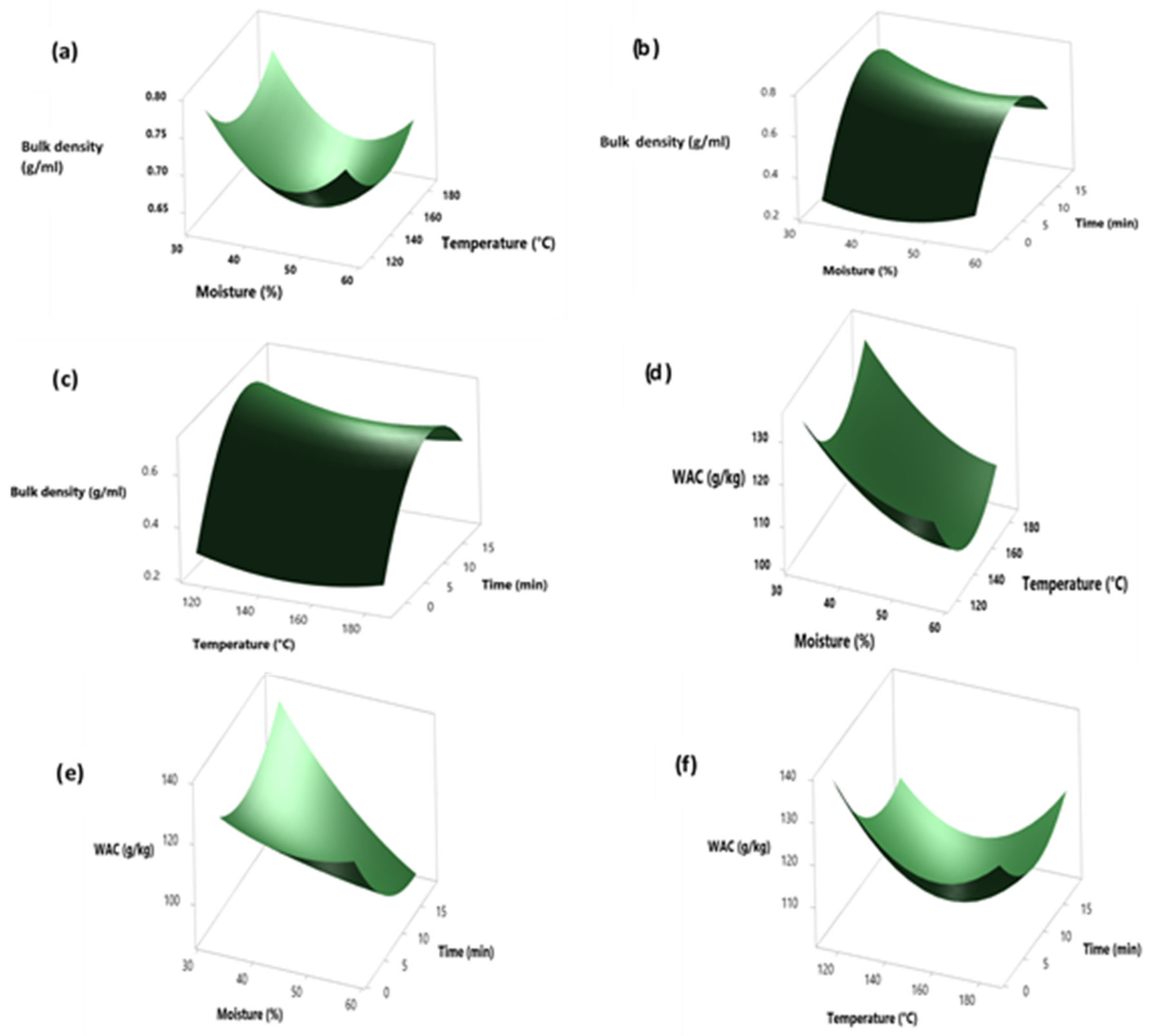
3.1. Modeling and Optimization of the Infrared-Induced Physicochemical Perturbations of Cowpea
3.2. Model Validation, Adequacy, and Factor Effects
3.3. Multi-Response Numerical Optimization
3.4. Pectin Solubility
3.5. Total Phenolic, Total Flavonoid Content, and Antioxidant Capacity
3.6. Level of Individual Phenolic Compounds
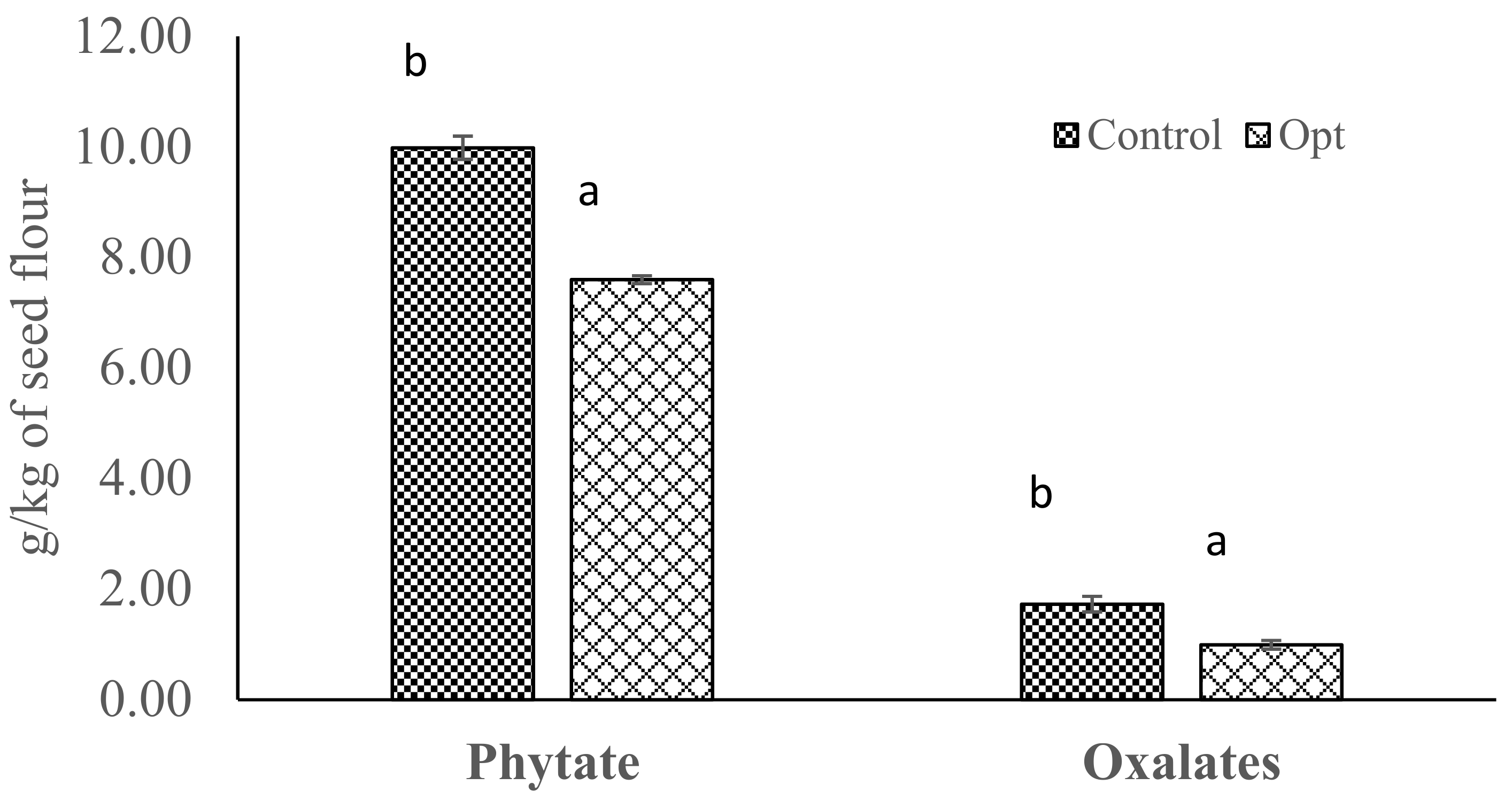
3.7. Phytate and Oxalates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Samples Availability
References
- Gomes, A.M.F.; Draper, D.; Nhantumbo, N.; Massinga, R.; Ramalho, J.; Marques, I.; Ribeiro-Barros, A. Diversity of Cowpea [Vigna unguiculata (L.) Walp] Landraces in Mozambique: New Opportunities for Crop Improvement and Future Breeding Programs. Agronomy 2021, 11, 991. [Google Scholar] [CrossRef]
- Moloto, M.R.; Phan, A.D.T.; Shai, J.L.; Sultanbawa, Y.; Sivakumar, D. Comparison of Phenolic Compounds, Carotenoids, Amino Acid Composition, In Vitro Antioxidant and Anti-Diabetic Activities in the Leaves of Seven Cowpea (Vigna unguiculata) Cultivars. Foods 2020, 9, 1285. [Google Scholar] [CrossRef] [PubMed]
- Dos Anjos, F.; Vazquez-Anon, M.; Dierenfeld, E.S.; Parsons, C.M.; Chimonyo, M. Chemical composition, amino acid digestibility, and true metabolizable energy of cowpeas as affected by roasting and extrusion processing treatments using the cecectomized rooster assay. J. Appl. Poult. Res. 2016, 25, 85–94. [Google Scholar] [CrossRef]
- Akissoé, L.; Madodé, Y.; Hemery, Y.; Donadjè, B.; Icard-Vernière, C.; Hounhouigan, D.; Mouquet-Rivier, C. Impact of traditional processing on proximate composition, folate, mineral, phytate, and alpha-galacto-oligosaccharide contents of two West African cowpea (Vigna unguiculata L. Walp) based doughnuts. J. Food Compos. Anal. 2021, 96, 103753. [Google Scholar] [CrossRef]
- Sreerama, Y.; Sashikala, V.B.; Pratape, V.M. Phenolic compounds in cowpea and horse gram flours in comparison to chickpea flour: Evaluation of their antioxidant and enzyme inhibitory properties associated with hyperglycemia and hypertension. Food Chem. 2012, 133, 156–162. [Google Scholar] [CrossRef]
- Kalpanadevi, V.; Mohan, V. Effect of processing on antinutrients and in vitro protein digestibility of the underutilized legume, Vigna unguiculata (L.) Walp subsp. unguiculata. LWT-Food Sci. Technol. 2013, 51, 455–461. [Google Scholar] [CrossRef]
- Kayitesi, E. Micronisation of Cowpeas: The Effects on Sensory Quality, Phenolic Compounds and Bioactive Properties. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2013. [Google Scholar]
- Ogundele, O.M.; Kayitesi, E. Influence of infrared heating processing technology on the cooking characteristics and functionality of African legumes: A review. J. Food Sci. Technol. 2019, 56, 1669–1682. [Google Scholar] [CrossRef]
- Demirci, A.; Krishnamurthy, K.; Khurana, H.K.; Jun, S.; Irudayaraj, J. Infrared Radiation for Food Processing. Food Lipids 2008, 7, 113–142. [Google Scholar] [CrossRef]
- Ogundele, O.M.; Emmambux, M.N. Effect of infrared heating of pre-soaked whole and dehulled bambara groundnut (Vigna subter-ranea) seeds on their cooking characteristics and microstructure. LWT 2018, 97, 581–587. [Google Scholar] [CrossRef]
- Kayitesi, E.; Duodu, K.G.; Minnaar, A.; de Kock, H.L. Effect of micronisation of pre-conditioned cowpeas on cooking time and sen-sory properties of cooked cowpeas. J. Sci. Food Agric. 2013, 93, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Yeung, H.; Ehlers, J.; Waniska, R.; Alviola, J.; Rooney, L. Rapid screening methods to evaluate cowpea cooking characteristics. Field Crop. Res. 2009, 112, 245–252. [Google Scholar] [CrossRef]
- Mwangwela, A.M.; Waniska, R.D.; Minnaar, A. Hydrothermal treatments of two cowpea (Vigna unguiculata L. Walp) varieties: Effect of micronisation on physicochemical and structural characteristics. J. Sci. Food Agric. 2006, 86, 35–45. [Google Scholar] [CrossRef]
- Ndungu, K.E.; Emmambux, M.N.; Minnaar, A. Micronisation and hot air roasting of cowpeas as pretreatments to control the de-velopment of hard-to-cook phenomenon. J. Sci. Food Agric. 2012, 92, 1194–1200. [Google Scholar] [CrossRef]
- Vilakati, N.; MacIntyre, U.; Oelofse, A.; Taylor, J. Influence of micronization (infrared treatment) on the protein and functional quality of a ready-to-eat sorghum-cowpea African porridge for young child-feeding. LWT-Food Sci. Technol. 2015, 63, 1191–1198. [Google Scholar] [CrossRef] [Green Version]
- Ogundele, O.M. Nutritional and Functional Properties of Soaked and Micronized Bambara Groundnut Seeds and Their Flours. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 2016. [Google Scholar]
- Adebo, O.A.; Njobeh, P.B.; Mulaba-Bafubiandi, A.F.; Adebiyi, J.A.; Desobgo, Z.S.C.; Kayitesi, E. Optimization of fermentation conditions for ting production using response surface methodology. J. Food Process. Preserv. 2018, 42, e13381. [Google Scholar] [CrossRef]
- Oyedeji, A.B.; Mellem, J.J.; Ijabadeniyi, O.A. Improvement of some quality attributes of soymilk through optimization of selected soybean sprouting parameters using response surface methodology. CyTA—J. Food 2018, 16, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Gbashi, S.; Madala, N.E.; De Saeger, S.; De Boevre, M.; Njobeh, P.B. Numerical optimization of temperature-time degradation of multiple mycotoxins. Food Chem. Toxicol. 2019, 125, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Bas, D.; Boyacı, I.H. Modeling and optimization I: Usability of response surface methodology. J. Food Eng. 2007, 78, 836–845. [Google Scholar] [CrossRef]
- Alves, J.L.F.; Da Silva, J.C.G.; Di Domenico, M.; Galdino, W.V.D.A.; Andersen, S.L.F.; Alves, R.F.; De Sena, R.F. Exploring Açaí Seed (Euterpe oleracea) Pyrolysis Using Multi-component Kinetics and Thermodynamics Assessment Towards Its Bioenergy Potential. BioEnergy Res. 2021, 14, 209–225. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Kalita, P.; Tapan, B.K.; Pal, T.K.; Kalita, R. Estimation of Total Flavonoids Content (Tfc) And Anti Oxidant Activities Of Methanolic Whole Plant Extract Of Biophytum Sensitivum Linn. J. Drug Deliv. Ther. 2013, 3, 33–37. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.O.; Afolayan, A.J.; Masika, P.J. Antioxidant Properties of the Methanol Extracts of the Leaves and Stems of Celtis africana. Rec. Nat. Prod. 2009, 3, 23–31. [Google Scholar]
- Gao, Y.; Shang, C.; Maroof, M.A.S.; Biyashev, R.M.; Grabau, E.A.; Kwanyuen, P.; Burton, J.W.; Buss, G.R. A Modified Colorimetric Method for Phytic Acid Analysis in Soybean. Crop. Sci. 2007, 47, 1797–1803. [Google Scholar] [CrossRef] [Green Version]
- Muchoki, C.; Lamuka, P.; Imungi, J. Reduction of nitrates, oxalates and phenols in fermented solar-dried stored cowpea (Vigna un-guiculata L.) leaf vegetables. Afr. J. Food Agric. Nutr. Dev. 2010, 10. [Google Scholar] [CrossRef] [Green Version]
- Gajic, I.S.; Savic, I.; Gajic, D.; Dosic, A. Ultrasound-Assisted Extraction of Carotenoids from Orange Peel Using Olive Oil and Its Encapsulation in Ca-Alginate Beads. Biomolecules 2021, 11, 225. [Google Scholar] [CrossRef]
- Aboud, S.A.; Altemimi, A.B.; Al-HiIphy, A.R.; Yi-Chen, L.; Cacciola, F. A comprehensive review on infrared heating applications in food processing. Molecules 2019, 24, 4125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.R.; Duodu, K.G. Effects of processing sorghum and millets on their phenolic phytochemicals and the implications of this to the health-enhancing properties of sorghum and millet food and beverage products. J. Sci. Food Agric. 2015, 95, 225–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savic, I.M.; Gajic, I.M.S. Optimization study on extraction of antioxidants from plum seeds (Prunus domestica L.). Optim. Eng. 2021, 22, 141–158. [Google Scholar] [CrossRef]
- Luthria, D.L.; Pastor-Corrales, M.A. Phenolic acids content of fifteen dry edible bean (Phaseolus vulgaris L.) varieties. J. Food Compos. Anal. 2006, 19, 205–211. [Google Scholar] [CrossRef]
- Bishnoi, S.; Khetarpaul, N.; Yadav, R.K. Effect of domestic processing and cooking methods on phytic acid and polyphenol contents of pea cultivars (Pisum sativum). Plant Foods Hum. Nutr. 1994, 45, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Rooney, L.; Marovatsanga, L.; Taylor, J. Effect of Chemical Treatments on Polyphenols and Malt Quality in Sorghum. J. Cereal Sci. 2000, 31, 295–302. [Google Scholar] [CrossRef]
- Khattab, R.; Arntfield, S. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT-Food Sci. Technol. 2009, 42, 1113–1118. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, Y.; Zhang, C.; Kong, X.; Hua, Y. The Heat-Induced Protein Aggregate Correlated with Trypsin Inhibitor Inactivation in Soymilk Processing. J. Agric. Food Chem. 2012, 60, 8012–8019. [Google Scholar] [CrossRef]

| Levels | ||||||
|---|---|---|---|---|---|---|
| Factors | Codes | −α | −1 | 0 | 1 | +α |
| Moisture level (%) | X1 | 32.6 | 40 | 45 | 54 | 57.3 |
| Infrared temperature (°C) | X2 | 114.7 | 130 | 150 | 170 | 185.3 |
| Infrared time (min) | X3 | NA * | 2 | 8 | 14 | 18.5 |
| Experimental Runs | Moisture | Temperature | Time | Bulk Density (g/mL) | WAC (g/kg) |
|---|---|---|---|---|---|
| 1 | 40.00 | 130.00 | 2.00 | 0.67 | 125.54 |
| 2 | 40.00 | 130.00 | 14.00 | 0.64 | 114.66 |
| 3 | 40.00 | 170.00 | 2.00 | 0.66 | 126.83 |
| 4 | 40.00 | 170.00 | 14.00 | 0.62 | 126.78 |
| 5 | 54.00 | 130.00 | 2.00 | 0.62 | 126.78 |
| 6 | 54.00 | 130.00 | 14.00 | 0.63 | 99.96 |
| 7 | 54.00 | 170.00 | 2.00 | 0.62 | 125.85 |
| 8 | 54.00 | 170.00 | 14.00 | 0.60 | 102.39 |
| 9 (C) | 45.00 | 150.00 | 8.00 | 0.62 | 103.38 |
| 10 | 32.65 | 150.00 | 8.00 | 0.67 | 118.06 |
| 11 | 57.35 | 150.00 | 8.00 | 0.61 | 95.45 |
| 12 | 45.00 | 114.72 | 8.00 | 0.64 | 125.62 |
| 13 | 45.00 | 185.28 | 8.00 | 0.60 | 103.84 |
| 15 | 45.00 | 150.00 | 18.58 | 0.62 | 98.55 |
| 16 (C) | 45.00 | 150.00 | 8.00 | 0.62 | 108.60 |
| Coefficient | Bulk Density | WAC |
|---|---|---|
| β0 | 0.74 | 1248.00 |
| β1 | −0.00 | −18.88 |
| β2 | 0.00 | −9.08 |
| β3 | −0.01 | −4.17 |
| β11 | 0.00 | 0.17 |
| β22 | −0.00 | 0.03 |
| β33 | 0.00 | 0.36 |
| β12 | −0.00 | 0.02 |
| β13 | 0.00 | −0.02 |
| β23 | −0.004 | −0.02 |
| R2 (%) | 95.08% | 92.15% |
| R2adj (%) | 93.56% | 89.71% |
| Residual | 0.01 | 4.09 |
| Bulk Density | WAC | |||
|---|---|---|---|---|
| Term | Effect | p-Values * | Effect | p-Values * |
| Linear effects (L) | ||||
| Constant | - | 0.000 | - | 0.000 |
| Moisture | −0.029 | 0.000 | −2.258 | 0.186 |
| Temperature | −0.018 | 0.000 | −6.881 | 0.000 |
| Time | −0.019 | 0.000 | −24.249 | 0.000 |
| Quadratic effects (Q) | ||||
| Moisture × Moisture | 0.006 | 0.329 | 16.650 | 0.001 |
| Temperature × Temperature | −0.006 | 0.347 | 21.660 | 0.000 |
| Time × Time | 0.006 | 0.404 | 25.710 | 0.000 |
| Interactive effects (I) | ||||
| Moisture × Temperature | −0.002 | 0.368 | 5.466 | 0.002 |
| Moisture × Time | 0.014 | 0.000 | −1.871 | 0.269 |
| Temperature × Time | −0.009 | 0.001 | −4.026 | 0.022 |
| Samples | ||
|---|---|---|
| Control | Treated (Opt) | |
| TPC (mg CE/g) | 1.10 b (0.02) | 0.47 a (0.04) |
| TFC (mg CE/g) | 4.23 b (0.08) 1 | 1.66 a (0.07) |
| Antioxidant properties | ||
| FRAP (mg GAE/g) | 8.21 b (0.44) | 2.32 a (0.01) |
| No | Phenolic Compounds | Control Sample (µg/g) | Treated (opt) (µg/g) |
|---|---|---|---|
| 1 | Kaempferol | 6.60 a (0.09) | 2.80 a (0.31) 1 |
| 2 | Luteolin | 6.52 a (0.01) | 11.32 b (0.08) |
| 3 | Ferulic acid | 5.08 a (0.54) | 22.96 b (1.23) |
| 4 | Taxifolin | 979.12 b (18.67) | 494.24 a (1.96) |
| 5 | Apigenin | 2.16 a (0.13) | 3.80 a (0.49) |
| 6 | Quercetin | 1.20 a (0.11) | 0.68 a (0.03) |
| 7 | p-Coumaric acid | 83.84 a (4.95) | 52.00 a (1.46) |
| 8 | Sinapic acid | 92.96 b (0.52) | 26.72 a (0.88) |
| 9 | Caffeic acid | 289.76 b (0.42) | 130.80 a (1.04) |
| 10 | Gallic acid | 169.64 a (0.23) | 328.48 b (1.75) |
| 11 | Vanillic acid | 349.68 a (1.04) | 684.44 a (4.18) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogundele, O.M.; Gbashi, S.; Oyeyinka, S.A.; Kayitesi, E.; Adebo, O.A. Optimization of Infrared Heating Conditions for Precooked Cowpea Production Using Response Surface Methodology. Molecules 2021, 26, 6137. https://doi.org/10.3390/molecules26206137
Ogundele OM, Gbashi S, Oyeyinka SA, Kayitesi E, Adebo OA. Optimization of Infrared Heating Conditions for Precooked Cowpea Production Using Response Surface Methodology. Molecules. 2021; 26(20):6137. https://doi.org/10.3390/molecules26206137
Chicago/Turabian StyleOgundele, Opeolu M., Sefater Gbashi, Samson A. Oyeyinka, Eugenie Kayitesi, and Oluwafemi A. Adebo. 2021. "Optimization of Infrared Heating Conditions for Precooked Cowpea Production Using Response Surface Methodology" Molecules 26, no. 20: 6137. https://doi.org/10.3390/molecules26206137
APA StyleOgundele, O. M., Gbashi, S., Oyeyinka, S. A., Kayitesi, E., & Adebo, O. A. (2021). Optimization of Infrared Heating Conditions for Precooked Cowpea Production Using Response Surface Methodology. Molecules, 26(20), 6137. https://doi.org/10.3390/molecules26206137







