Modulation of Oxidative Stress and Hemostasis by Flavonoids from Lentil Aerial Parts
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Extract and Phenolic Fraction from Lentil Aerial Parts
2.4. Isolation of Flavonoids from Lentil Aerial Parts
2.5. Stock Solutions
2.6. Quantification of Flavonoids in the Tested Crude Extract and Phenolic Fraction of the Lentil Aerial Parts
2.7. Human Plasma Isolation
- The extract from the lentil aerial parts at the final concentrations of 1–50 μg/mL;
- The phenolic fraction from lentil aerial parts at the final concentrations of 1–50 μg/mL;
- Flavonoids (compounds 1–7) from the lentil aerial parts at the final concentrations of 1–50 μg/mL;
- Quercetin or kaempferol at the final concentrations of 1–50 μg/mL;
- Human plasma was also pre-incubated (5 min, at 37 °C; for parameters of oxidative stress) with:
- The extract from the lentil aerial parts at the final concentrations of 1–50 μg/mL;
- The phenolic fraction from lentil aerial parts at the final concentrations of 1–50 μg/mL
- Flavonoids (compounds 1–7) from the lentil aerial parts at the final concentrations of 1–50 μg/mL;
- Quercetin or kaempferol at the final concentrations of 1–50 μg/mL;
- And then treated with 4.7 mM H2O2/3.8 mM Fe2SO4/2.5 mM EDTA (25 min, at 37 °C).
2.8. Markers of Oxidative Stress
2.8.1. Lipid Peroxidation Measurement
2.8.2. Carbonyl Group Measurement
2.8.3. Thiol Group Determination
2.9. Parameters of Hemostasis
2.10. Data Analysis
3. Results
3.1. Quantitative Analysis of the Tested Extract and Phenolic Fraction of the Lentil Aerial Parts
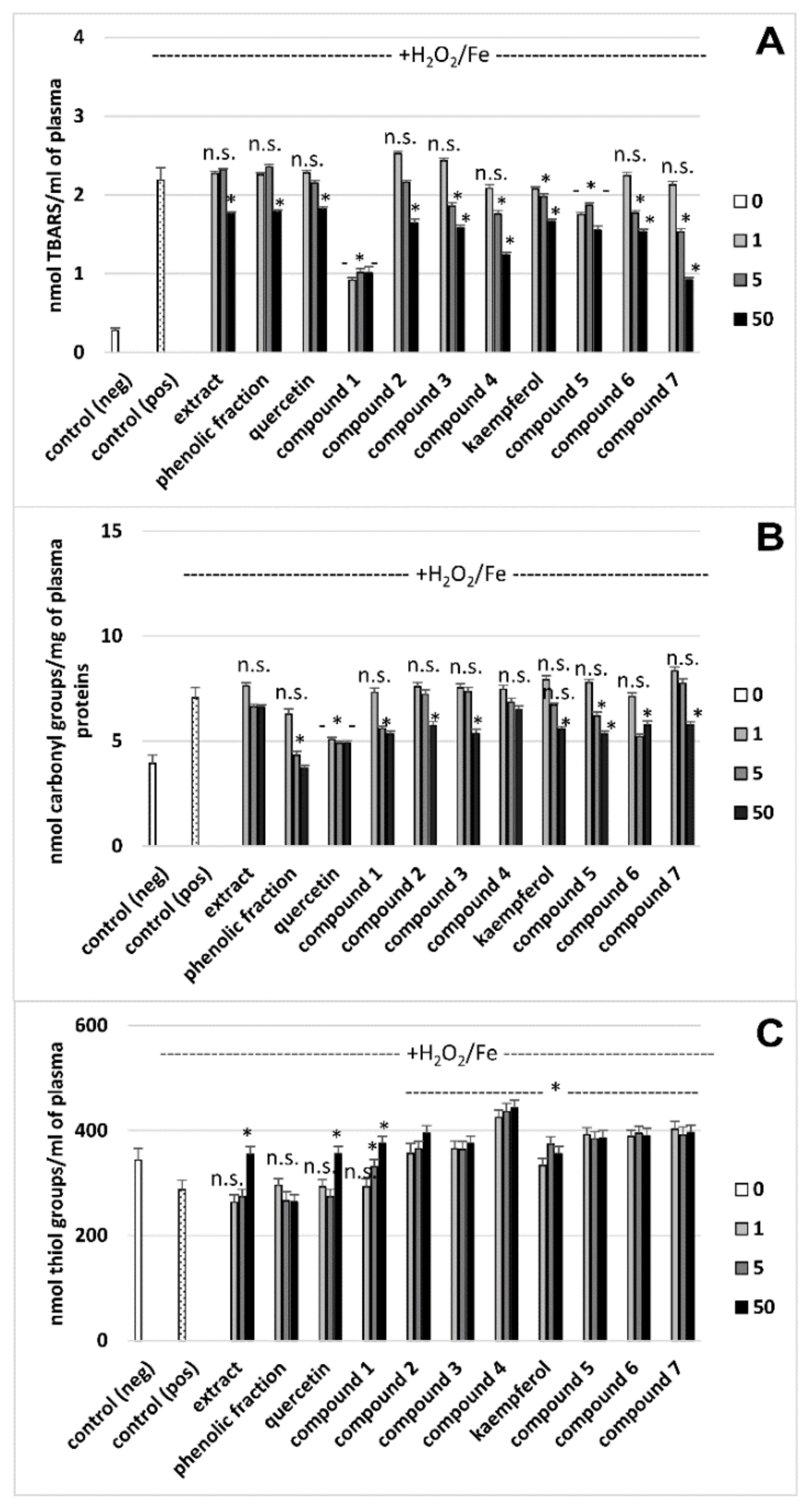
3.2. Effects on Oxidative Stress Biomarkers in Human Plasma In Vitro
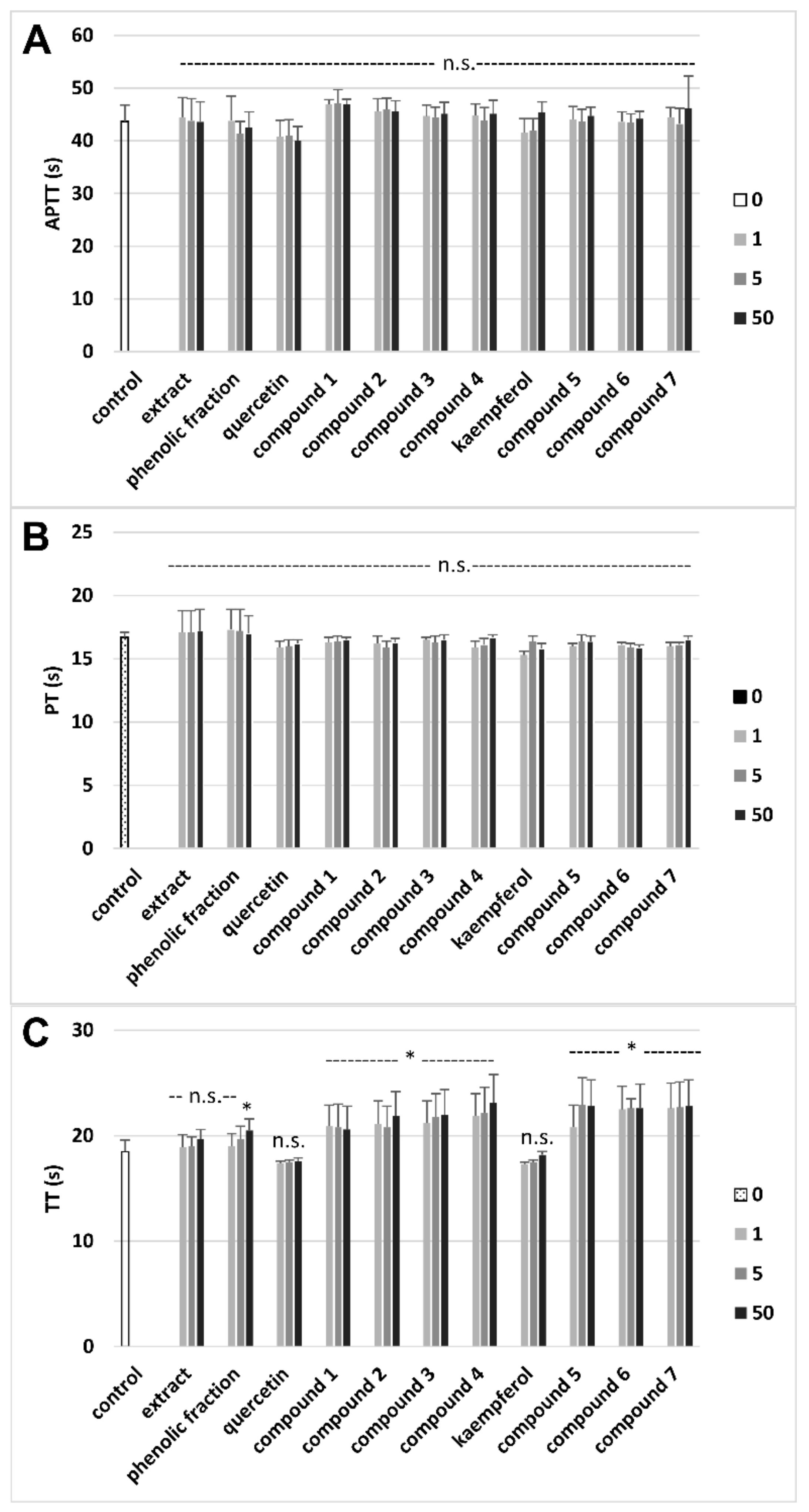
3.3. Effects on Hemostatic Parameters of Plasma
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| TBARS | thiobarbituric acid reactive substances |
| APTT | the activated partial thromboplastin time |
| PT | the prothrombin time |
| TT | the thrombin time |
| n.s. | statistically irrelevant |
References
- Tang, G.Y.; Meng, X.; Li, Y.; Zhao, C.N.; Liu, Q.; Li, H.B. Effects of Vegetables on Cardiovascular Diseases and Related Mechanisms. Nutrients 2017, 9, 857. [Google Scholar] [CrossRef]
- Olas, B. Anti-Aggregatory potential of selected vegetables—Promising dietary components for the prevention and treatment of cardiovascular disease. Adv. Nutr. 2019, 10, 280–290. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Crozier, A. Dietary flavonoids and phenolic compounds. In Plant Phenolics and Human Health: Biochemistry, Nutrition, and Pharmacology; Fraga, C.G., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–49. [Google Scholar]
- Faris, M.A.I.E.; Takruri, H.R.; Issa, A.Y. Role of lentils (Lens culinaris L.) in human health and nutrition: A review. Med. J. Nutrition Metab. 2013, 6, 3–16. [Google Scholar] [CrossRef]
- Cheng, Y.; Sheen, J.M.C.; Hu, W.L.; Hung, Y.C. Polyphenols and Oxidative Stress in Atherosclerosis-Related Ischemic Heart Disease and Stroke. Oxid. Med. Cell Longev. 2017, 26, 438. [Google Scholar] [CrossRef] [PubMed]
- Żuchowski, J.; Pecio, Ł.; Stochmal, A. Novel flavonol glycosides from the aerial parts of lentil (Lens culinaris). Molecules 2014, 19, 18152–18178. [Google Scholar] [CrossRef] [PubMed]
- Rolnik, A.; Żuchowski, J.; Stochmal, A.; Olas, B. Quercetin and kaempferol derivatives isolated from aerial parts of Lens culinaris Medik as modulators of blood platelet functions. Ind. Crops Prod. 2020, 152, 1–8. [Google Scholar] [CrossRef]
- Whitaker, J.R.; Granum, P.E. An absolute method for protein determination based on difference in absorbance at 235 and 280 nm. Anal. Biochem. 1980, 109, 156–159. [Google Scholar] [CrossRef]
- Wachowicz, B. Adenine nucleotides in thrombocytes of birds. Cell Biochem. Funct. 1984, 2, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Olas, B.; Żuchowski, J.; Lis, B.; Skalski, B.; Kontek, B.; Grabarczyk, Ł.; Stochmal, A. Comparative chemical composition, antioxidant and anticoagulant properties of phenolic fraction (a rich in non-acylated and acylated flavonoids and non-polar compounds) and non-polar fraction from Elaeagnus rhamnoides (L.) A. Nelson fruits. Food Chem. 2018, 247, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Bartosz, G. Druga Twarz Tlenu: Wolne Rodniki w Przyrodzie; Wydaw Naukowe PWN: Warszava, Poland, 2008; pp. 35–50. [Google Scholar]
- Skalski, B.; Lis, B.; Pecio, Ł.; Kontek, B.; Olas, B.; Żuchowski, J.; Stochmal, A. Isorhamnetin and its new derivatives isolated from sea buckthorn berries prevent H2O2/Fe-Induced oxidative stress and changes in hemostasis. Food Chem. Toxicol. 2019, 125, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Ando, Y.; Steiner, M. Sulfhydryl and disulfide groups of platelet membranes. I. Determination of sulfhydryl groups. Biochim. Biophys. Acta 1973, 311, 26–37. [Google Scholar] [CrossRef]
- Ando, Y.; Steiner, M. Sulfhydryl and disulfide groups of platelet membranes. II. Determination of disulfide groups. Biochim. Biophys. Acta 1973, 311, 38–44. [Google Scholar] [CrossRef]
- Malinowska, J.; Kołodziejczyk-Czepas, J.; Moniuszko-Szajwaj, B.; Kowalska, I.; Oleszek, W.; Stochmal, A.; Olas, B. Phenolic fractions from Trifolium pallidum and Trifolium scabrum aerial parts in human plasma protect against changes induced by hyperhomocysteinemia in vitro. Food Chem. Toxicol. 2012, 50, 4023–4027. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 1–16. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, P.; Tuli, H.S.; Sharma, A.K. Phytochemical and Pharmacological Properties of Flavonols. In eLS; Wiley: Hoboken, NJ, USA, 2018; pp. 1–12. [Google Scholar]
- Choi, J.-H.; Park, S.-E.; Kim, S.-J.; Kim, S. Kaempferol inhibits thrombosis and platelet activation. Biochimie 2015, 115, 177–186. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Dueñas, M.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Antioxidant activity of a red lentil extract and its fractions. Int. J. Mol. Sci. 2009, 10, 5513–5527. [Google Scholar] [CrossRef]
- Amarowicz, R.; Estrella, I.; Hernández, T.; Robredo, S.; Troszyńska, A.; Kosińska, A.; Pegg, R.B. Free radical-scavenging capacity, antioxidant activity, and phenolic composition of green lentil (Lens culinaris). Food Chem. 2010, 121, 705–711. [Google Scholar] [CrossRef]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant activity and phenolic compositions of Lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011, 59, 2268–2276. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.; Vandenberg, A.; Smits, J. Treating chronic arsenic toxicity with high selenium lentil diets. Toxicol. Appl. Pharmacol. 2013, 272, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Alshikh, N.; de Camargo, A.C.; Shahidi, F. Phenolics of selected lentil cultivars: Antioxidant activities and inhibition of low-density lipoprotein and DNA damage. J. Funct. Foods 2015, 18, 1022–1038. [Google Scholar] [CrossRef]
- Zhang, B.; Deng, Z.; Ramdath, D.D.; Tang, Y.; Chen, P.X.; Liu, R.; Liu, Q.; Tsao, R. Phenolic profiles of 20 Canadian lentil cultivars and their contribution to antioxidant activity and inhibitory effects on α-glucosidase and pancreatic lipase. Food Chem. 2015, 172, 862–872. [Google Scholar] [CrossRef]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Aslam Gondal, T.; Saeed, F.; Imran, A.; Shahbaz, M.; Tsouh Fokou, P.V.; Umair Arshad, M.; Khan, H.; et al. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef]
- Atala, E.; Fuentes, J.; Wehrhahn, M.J.; Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 2017, 234, 479–485. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, H.; Han, J.; Wang, W.; Zheng, Q.; Wang, D. Protective effects of kaempferol against myocardial ischemia/reperfusion injury in isolated rat heart via antioxidant activity and inhibition of glycogen synthase kinase-3 oxidative. Oxid. Med. Cell Longev. 2015, 8, 1405. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, K.J.; Kim, S. Comparative effect of quercetin and quercetin-3-O-β-d-glucoside on fibrin polymers, blood clots, and in rodent models. J. Biochem. Mol. Toxicol. 2016, 30, 548–558. [Google Scholar] [CrossRef]
- Liu, L.; Ma, H.; Yang, N.; Tang, Y.; Guo, J.; Tao, W.; Duan, J. A Series of natural flavonoids as thrombin inhibitors: Structure-activity relationships. Thromb. Res. 2010, 126, 365–378. [Google Scholar] [CrossRef]
- Burak, C.; Brüll, V.; Langguth, P.; Zimmermann, B.F.; Stoffel-Wagner, B.; Sausen, U.; Stehle, P.; Wolffram, S.; Egert, S. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur. J. Nutr. 2017, 56, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Baba, S.; Osakabe, N.; Natsume, M.; Muto, Y.; Takizawa, T.; Terao, J. In vivo comparison of the bioavailability of (+)-catechin, (−)-epicatechin and their mixture in orally administered ats. J. Nutr. 2001, 131, 2885–2891. [Google Scholar] [CrossRef] [PubMed]
- Stainer, A.R.; Sasikumar, P.; Bye, A.P.; Unsworth, A.J.; Holbrook, L.M.; Tindall, M.; Lovegrove, J.A.; Gibbins, J.M. The metabolites of the dietary flavonoid quercetin possess potent antithrombotic activity and interact with aspirin to enhance antiplatelet effects. TH Open 2019, 3, 244–258. [Google Scholar] [CrossRef] [PubMed]
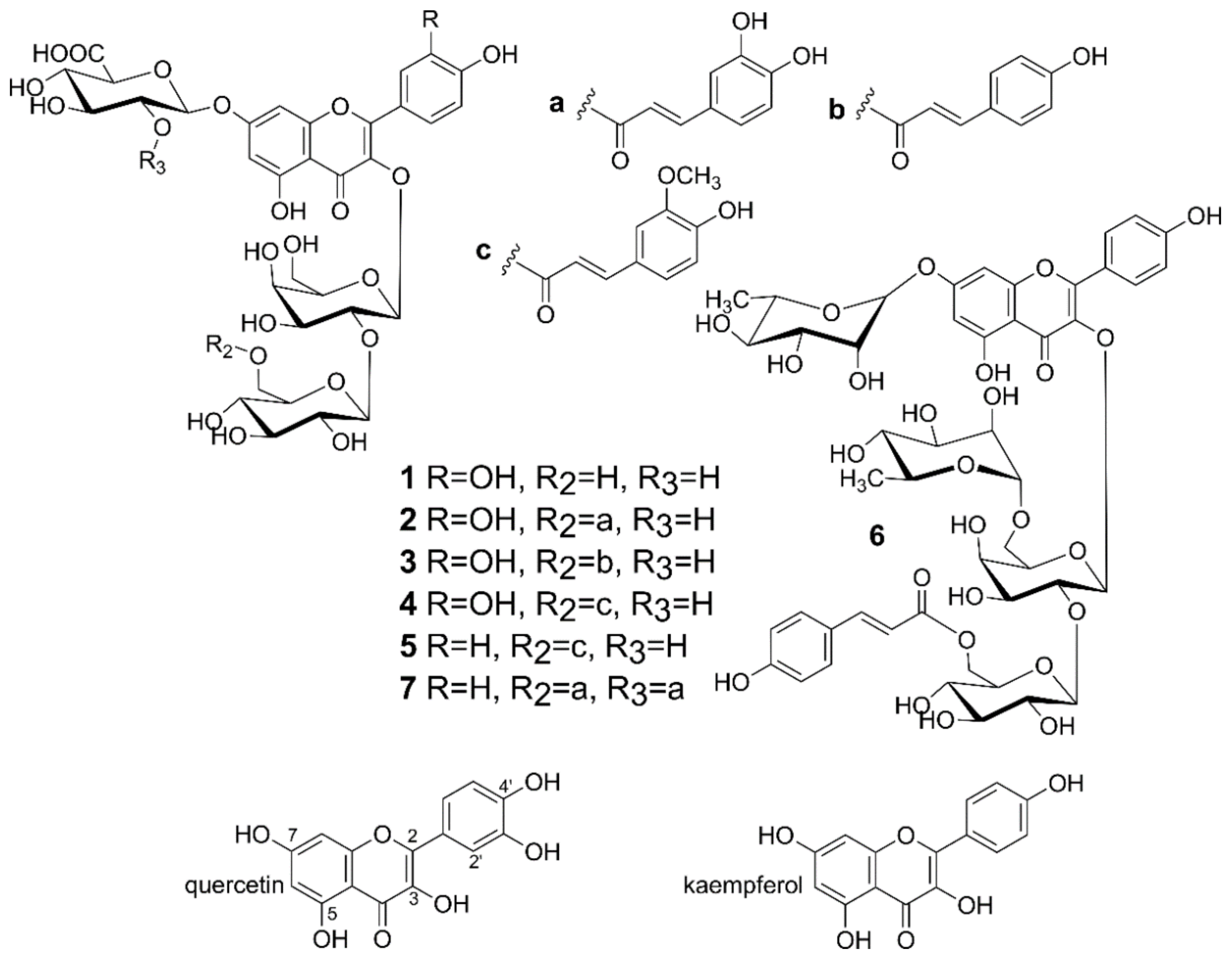
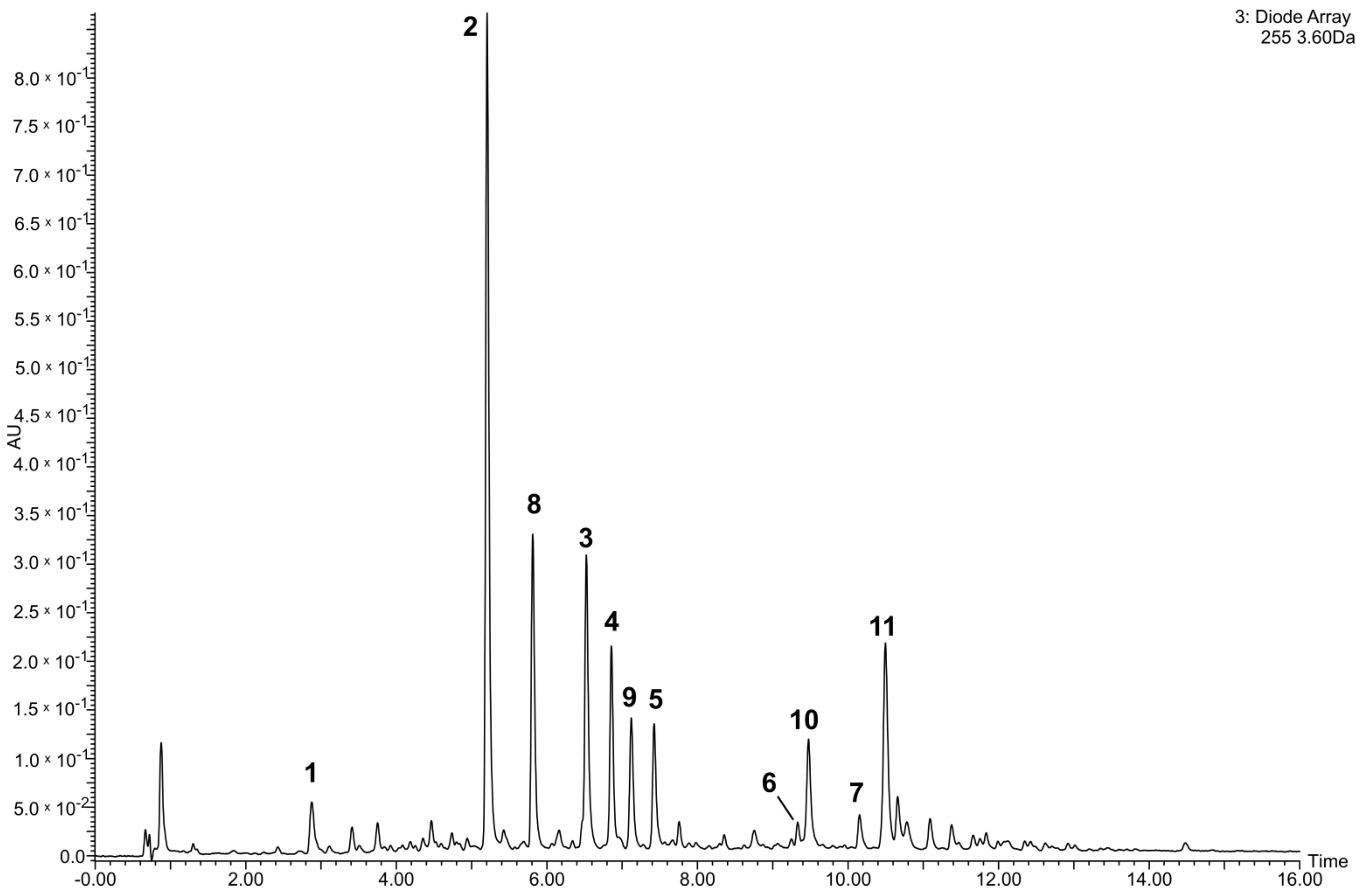
| Compound | Preparations | |
|---|---|---|
| CE | PF | |
| 1 | 3.4 ± 0.2 | 16.5 ± 0.1 |
| 2 | 20.5 ± 1.3 | 103.4 ± 1.7 |
| 3 | 10.1 ± 0.8 | 50.7 ± 0.8 |
| 4 | 6.9 ± 0.5 | 35.2 ± 1.2 |
| 5 | 4.2 ± 0.2 | 22.0 ± 0.4 |
| 6 | 1.0 ± 0.1 a | 5.6 ± 0.1 a |
| 7 | 1.1 ± 0.1 a | 5.5 ± 0.3 a |
| 8 | 9.6 ± 0.6 a | 48.8 ± 0.9 a |
| 9 | 5.5 ± 0.4 a | 28.2 ± 0.5 a |
| 10 | 6.4 ± 0.4 b | 33.3 ± 1.0 b |
| 11 | 12.2 ± 0.8 b | 63.2 ± 1.0 b |
| Tested Extract/Fraction/Phenolic Compound | Parameters of Oxidative Stress | Parameters of Hemostasis | ||
|---|---|---|---|---|
| Inhibition of Lipid Peroxidation Induced by H2O2/Fe (%) | Inhibition of Protein Carbonylation Induced by H2O2/Fe (%) | The Level of Protein Thiol Groups (nmol/mL of Plasma) in Plasma Treated with H2O2/Fe | Prolongation of TT (the Thrombin Time) (%) | |
| Extract (a) | 19.4 ± 4.8 Antioxidant action vs. control (plasma treated with H2O2/Fe) | 6.2 ± 1.9 (p > 0.05) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 355.2 ± 13.7 Antioxidant action vs. control (plasma treated with H2O2/Fe) | No effect vs. control |
| Phenolic fraction (b) | 18.2 ± 3.8 (p > 0.05; b vs. a) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 47.3 ± 7.9 (p < 0.05; b vs. a) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 263.8 ± 15.2 (p < 0.05; b vs. a) No effect vs. control (plasma treated with H2O2/Fe) | 10.8 ± 2.4 Anticoagulant action vs. control |
| Quercetin (c) | 16.5 ± 3.5 (p > 0.05; c vs. a, b)Antioxidant action vs. control (plasma treated with H2O2/Fe) | 30.4 ± 10.9 (p < 0.05; c vs. a; p > 0.05; c vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 355.7 ± 13.7 (p > 0.05; c vs. a; p < 0.05; c vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | No effect vs. control |
| Compound 1 (d) | 53.8 ± 12.7 (p < 0.05; d vs. a, b, c, e, f; p > 0.05; d vs. g) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 21.4 ± 8.7 (p > 0.05; d vs. c; p < 0.05; d vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 375.1 ± 11.6 ( p > 0.05; d vs. a, c; p < 0.05; d vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 11.1 ± 2.90 (p < 0.05; d vs. c) Anticoagulant action vs. control |
| Compound 2 (e) | 25.0 ± 6.9 (p < 0.05; e vs. c; p > 0.05; e vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 18.5 ± 6.0 (p > 0.05; e vs. c; p < 0.05; e vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 395.1 ± 20.2 (p > 0.05; e vs. a, c; p < 0.05; e vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 18.3 ± 3.6 (p < 0.05; e vs. c) Anticoagulant action vs. control |
| Compound 3 (f) | 27.7 ± 7.8 (p < 0.05; f vs. c; (p > 0.05; f vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 24.0 ± 7.0 (p > 0.05; f vs. c; p < 0.05; f vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 375.4 ± 16.4 (p > 0.05; f vs. a, c; p < 0.05; f vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 18.9 ± 5.4 (p < 0.05; f vs. c) Anticoagulant action vs. control |
| Compound 4 (g) | 43.3 ± 10.5 (p < 0.05; g vs. a, b, c) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 7.8 ± 10.5 (p > 0.05; g vs. a, c; p < 0.05; g vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 443.6 ± 17.6 (p < 0.05; g vs. a, b, c. d, f) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 24.9 ± 6.8 (p < 0.05; g vs. b, c, d) Anticoagulant action vs. control |
| Kaempferol (h) | 23.9 ± 4.9 (p > 0.05; h vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 20.8 ± 3.9 (p < 0.05; h vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 356.7 ± 14.2 (p > 0.05; h vs. a; p < 0.05; h vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | No effect vs. control |
| Compound 5 (i) | 29.0 ± 5.0 (p > 0.05; i vs. a, b, h) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 24.5 ± 4.8 (p > 0.05; i vs. h; p < 0.05; i vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 358.8 ± 14.8 (p > 0.05; i vs. a, h; p < 0.05; i vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 23.3 ± 6.7 (p < 0.05; i vs. b, h) Anticoagulant action vs. control |
| Compound 6 (j) | 29.8 ± 4.9 (p < 0.05; j vs. h; p > 0.05; j vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 18.2 ± 3.7 (p > 0.05; j vs. h; p < 0.05; j vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 390.3 ±14.2 (p > 0.05; j vs. a, h; p < 0.05; j vs. b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 22.2 ± 5.9 (p < 0.05; j vs. b, h) Anticoagulant action vs. control |
| Compound 7 (k) | 57.8 ± 14.9 (p < 0.05; k vs. a, b, h, i, j) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 18.1 ± 3.9 (p > 0.05; k vs. h; p < 0.05; k vs. a, b) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 396.1 ± 14.3 (p > 0.05; k vs. a, b, h) Antioxidant action vs. control (plasma treated with H2O2/Fe) | 23.2 ± 7.2 (p < 0.05; k vs. b, h) Anticoagulant action vs. control |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żuchowski, J.; Rolnik, A.; Adach, W.; Stochmal, A.; Olas, B. Modulation of Oxidative Stress and Hemostasis by Flavonoids from Lentil Aerial Parts. Molecules 2021, 26, 497. https://doi.org/10.3390/molecules26020497
Żuchowski J, Rolnik A, Adach W, Stochmal A, Olas B. Modulation of Oxidative Stress and Hemostasis by Flavonoids from Lentil Aerial Parts. Molecules. 2021; 26(2):497. https://doi.org/10.3390/molecules26020497
Chicago/Turabian StyleŻuchowski, Jerzy, Agata Rolnik, Weronika Adach, Anna Stochmal, and Beata Olas. 2021. "Modulation of Oxidative Stress and Hemostasis by Flavonoids from Lentil Aerial Parts" Molecules 26, no. 2: 497. https://doi.org/10.3390/molecules26020497
APA StyleŻuchowski, J., Rolnik, A., Adach, W., Stochmal, A., & Olas, B. (2021). Modulation of Oxidative Stress and Hemostasis by Flavonoids from Lentil Aerial Parts. Molecules, 26(2), 497. https://doi.org/10.3390/molecules26020497








