Phytochemical and Biological Evaluation of a Newly Designed Nutraceutical Self-Nanoemulsifying Self-Nanosuspension for Protection and Treatment of Cisplatin Induced Testicular Toxicity in Male Rats
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Investigation of Spirulina Powder
2.1.1. GC/MS Analysis of Lipid Content
2.1.2. The Total Protein and Amino Acids Composition
2.1.3. Determination of Total Phenolics (TPC) and Total Flavonoids (TFC) Contents
2.1.4. HPLC Determination of Vitamin B12, Vitamin C, and Vitamin E
2.1.5. Determination of Mineral Content
2.2. Formulation of a New Nutraceutical SNESNS
2.2.1. Screening of SNEDDS Components
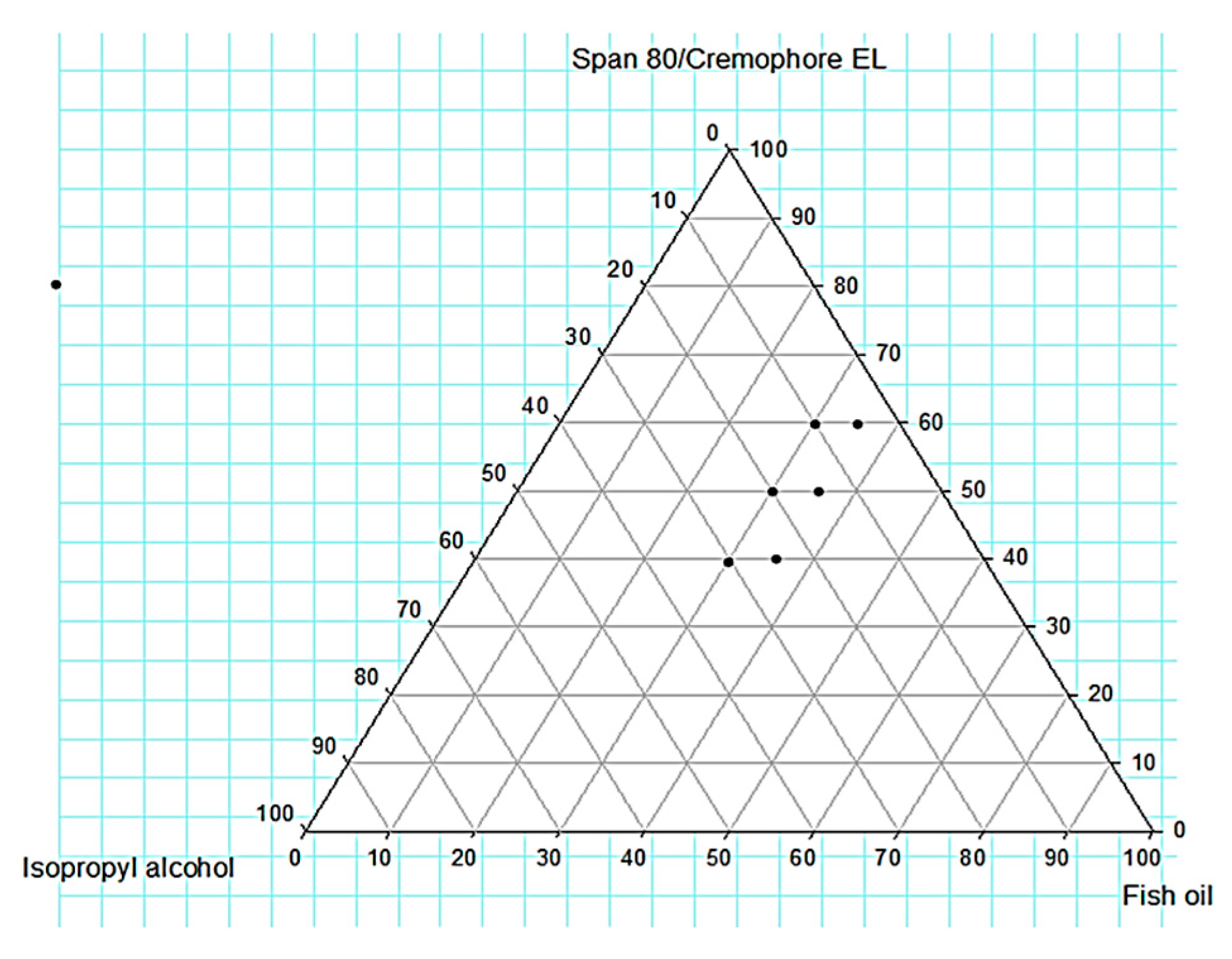
2.2.2. Construction of Ternary Phase Diagram
2.2.3. Characterization of SNEDDS
Determination of Emulsification Time (ET), Dispersibility, and %Transmittance (%T)
Simulation of the Physiological Dilution
Globule Size(GS) Analysis, Polydispersity Index (PDI), and Zeta Potential
Preparation of the Extract Loaded SNESNS
2.2.4. Characterization of the Extract Loaded SNESNS
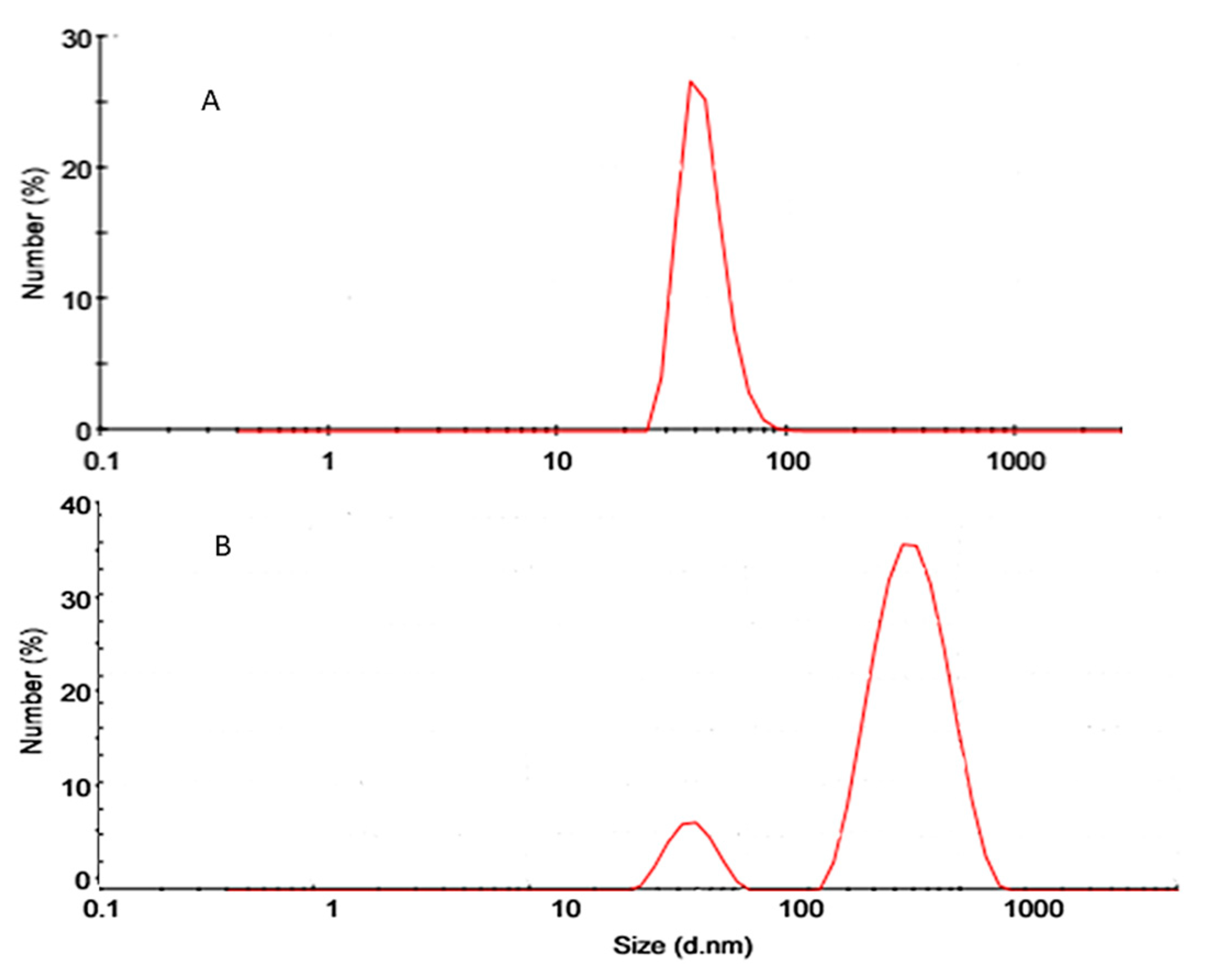
Particle Size Analysis (PS), Polydispersity Index (PDI), and Zeta Potential
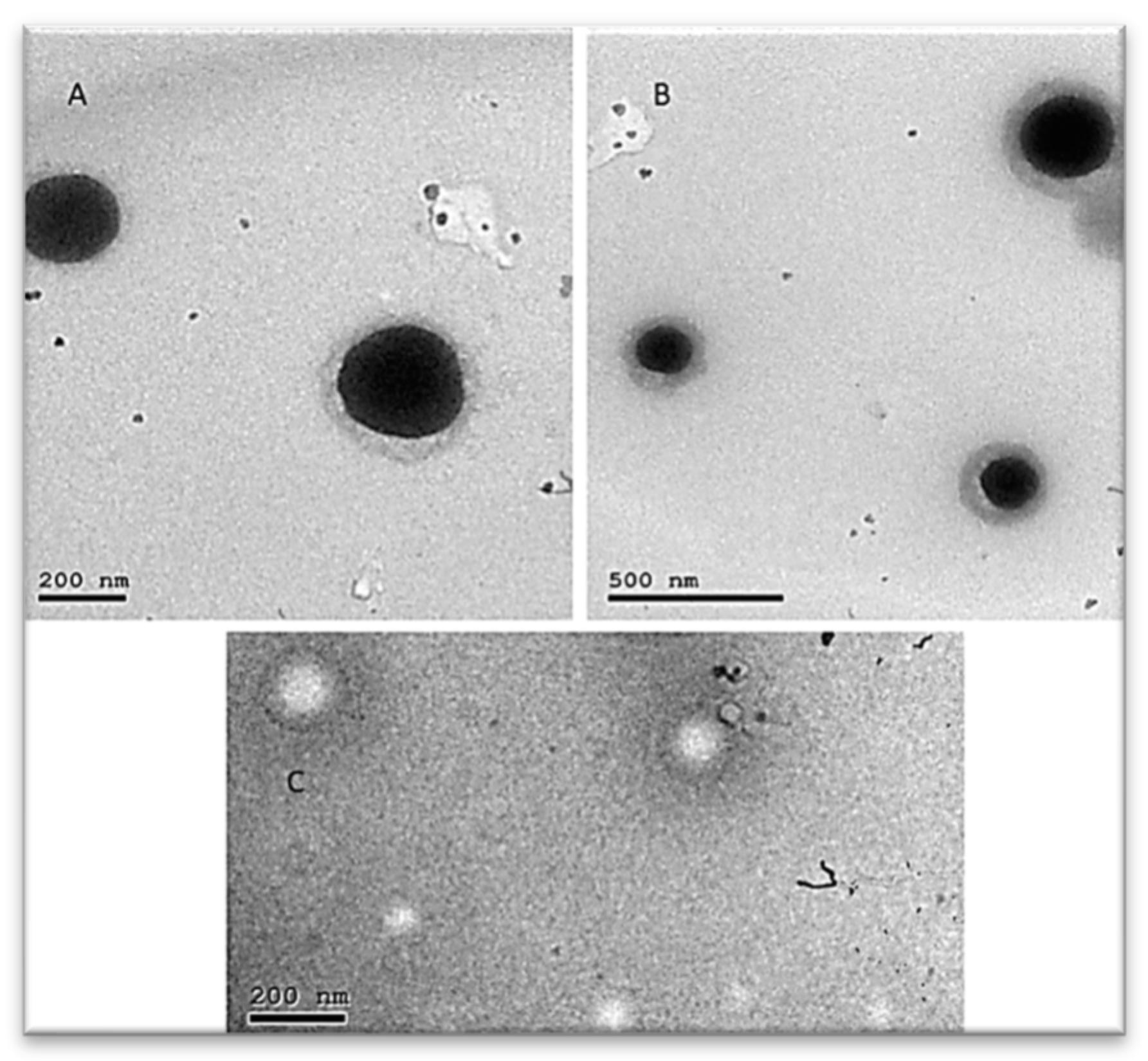
Transmission Electron Microscopy (TEM)
2.3. Methods Validation for NCF Quantitative Analysis
2.3.1. Optimization of Chromatographic Conditions
2.3.2. Method Validation for GC/FID Assay of FAME in NCF and Its Ingredients
Content of Palmitic Acid, GLA, LA, EPA, and DHA
Validation of the GC/FID Method
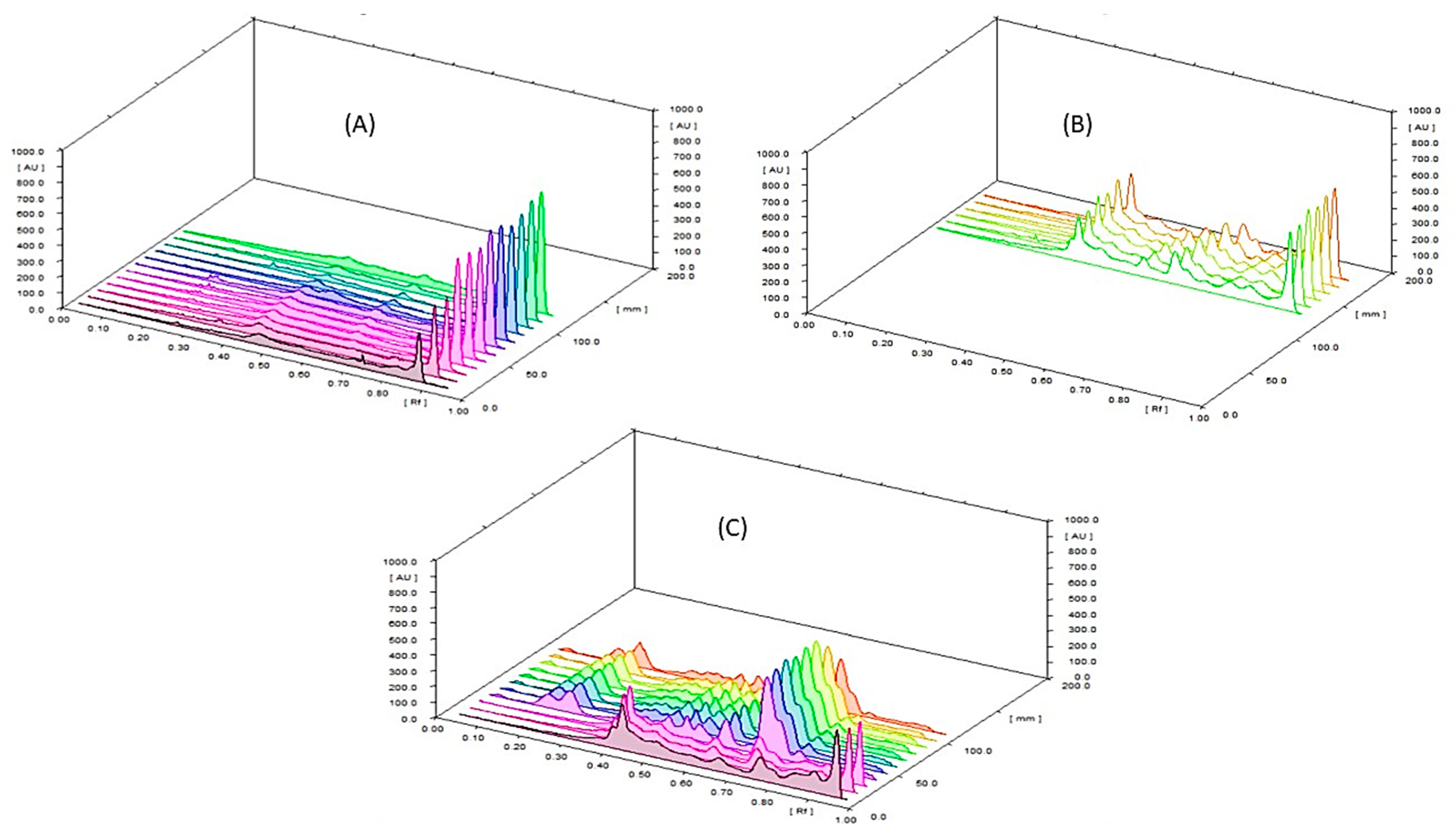
2.3.3. Method Validation for HPLC and HPTLC Assay of β-Carotene (Vitamin A) in SP and NCF
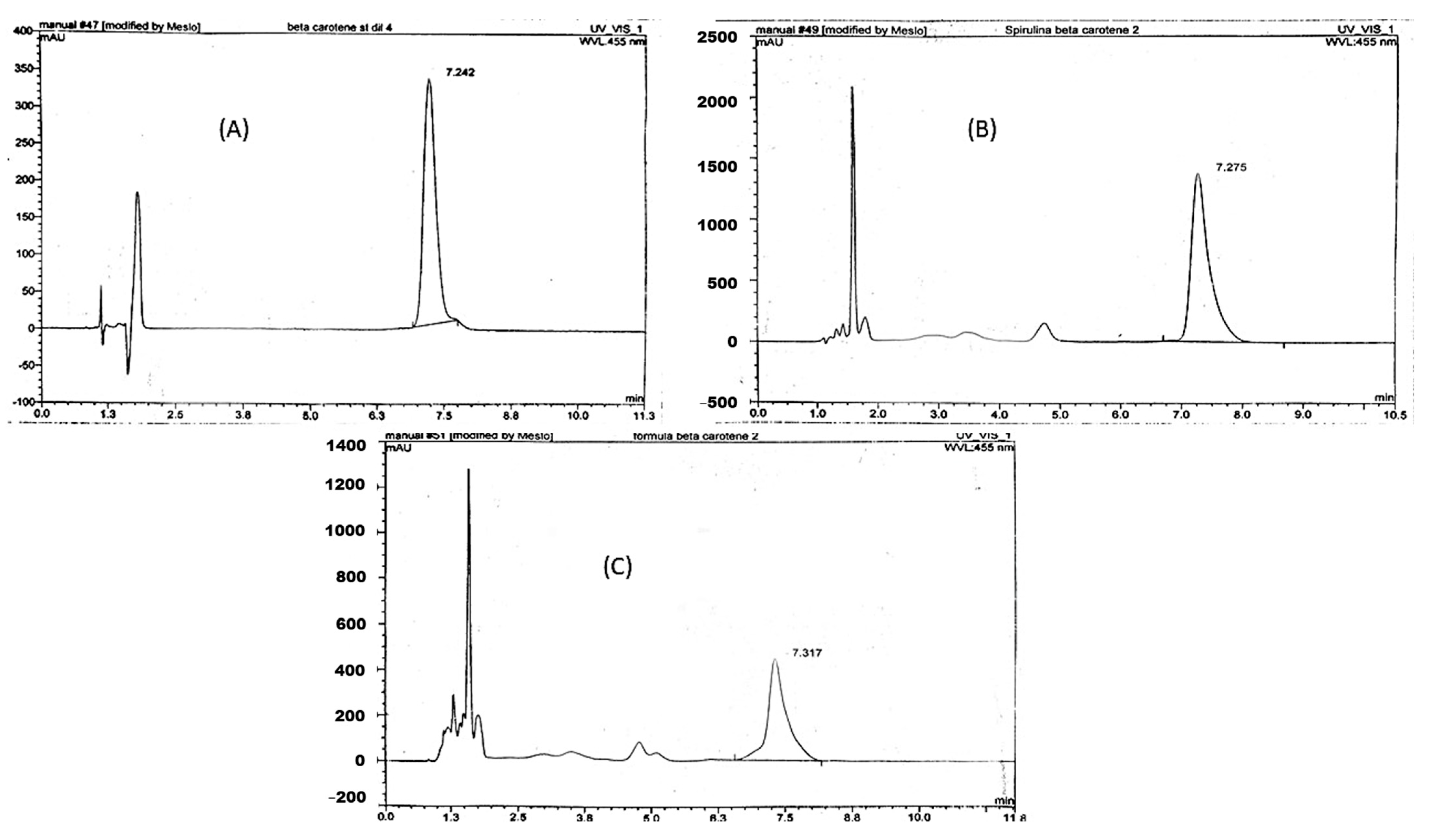
Assay of β-Carotene (Vitamin A) by HPLC and HPTLC
HPLC and HPTLC Method Validation for Assay of β-Carotene in SP and NCF
2.3.4. Method Validation for UV Colorimetric Assay of Total Steroidal Saponins in TT and NCF
Content of Total Steroidal Saponins by UV Colorimetric Method
Validation of UV Colorimetric Method for Assay of Total Steroidal Saponins in TT and NCF
2.4. Biological Evaluation of the NCF
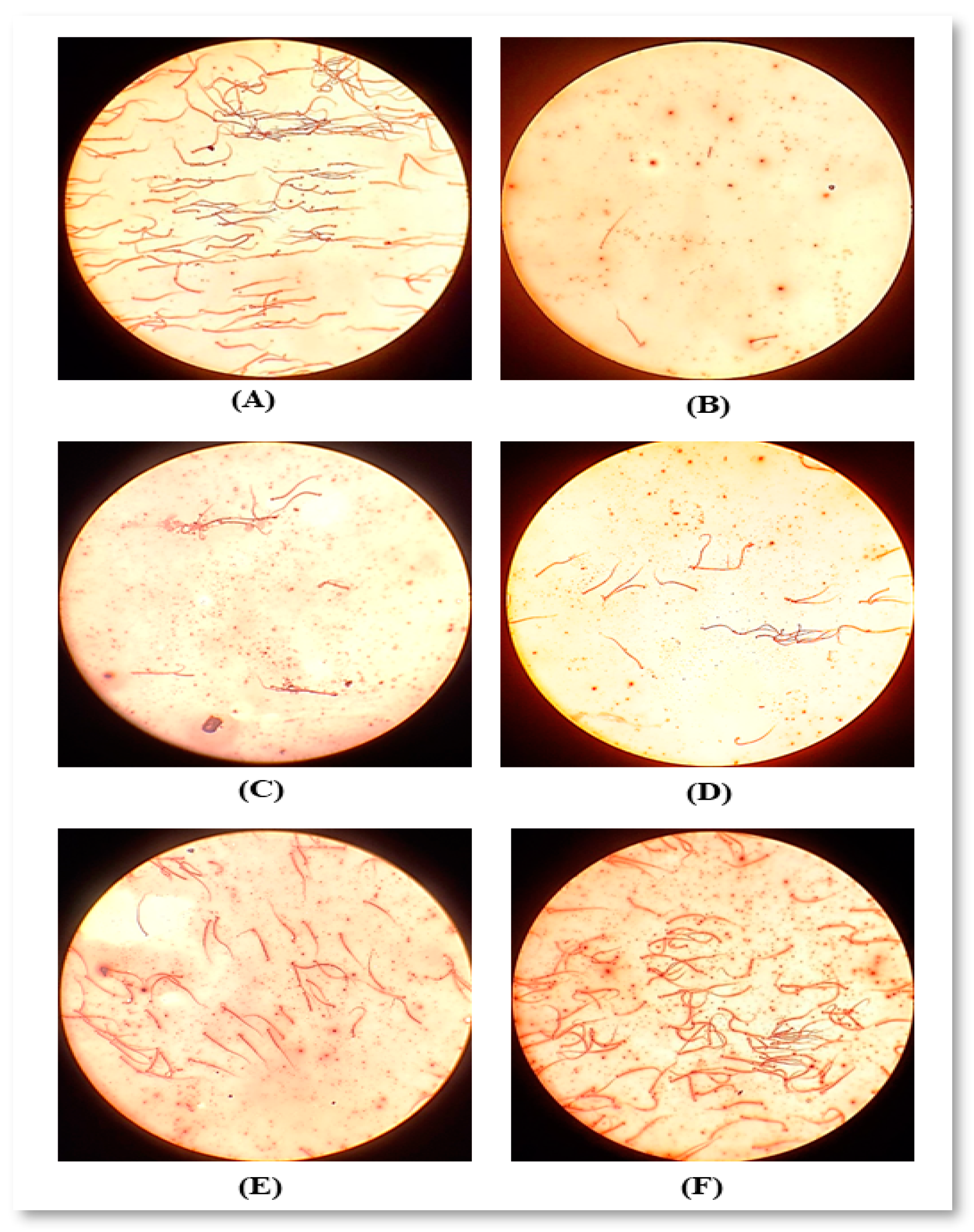
2.4.1. Sperm Count and Motility
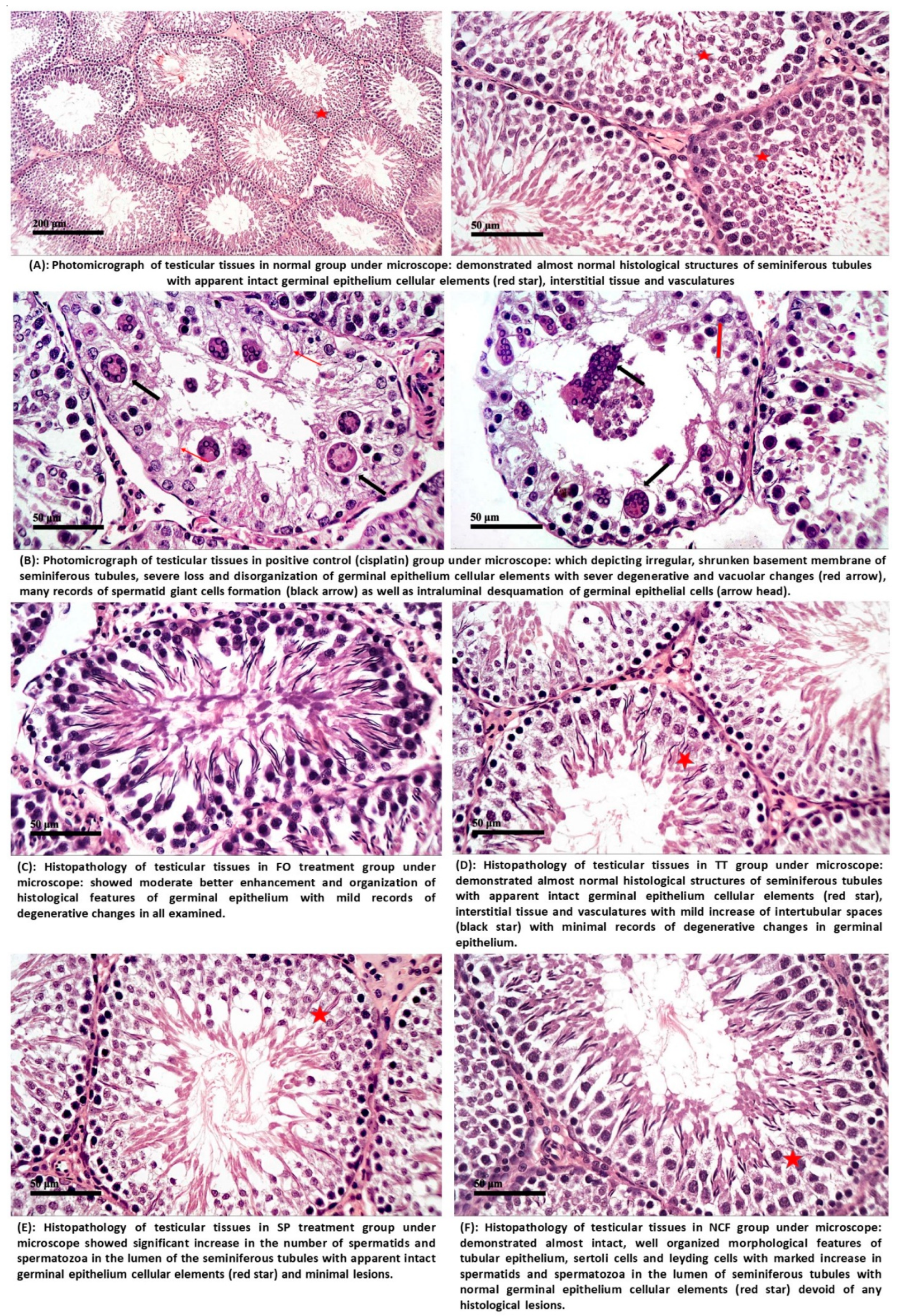
2.4.2. Histopathological Investigation
2.4.3. In Vitro Antioxidant (DPPH Radical Scavenging Activity) for SP and NCF
2.4.4. Biochemical Analysis
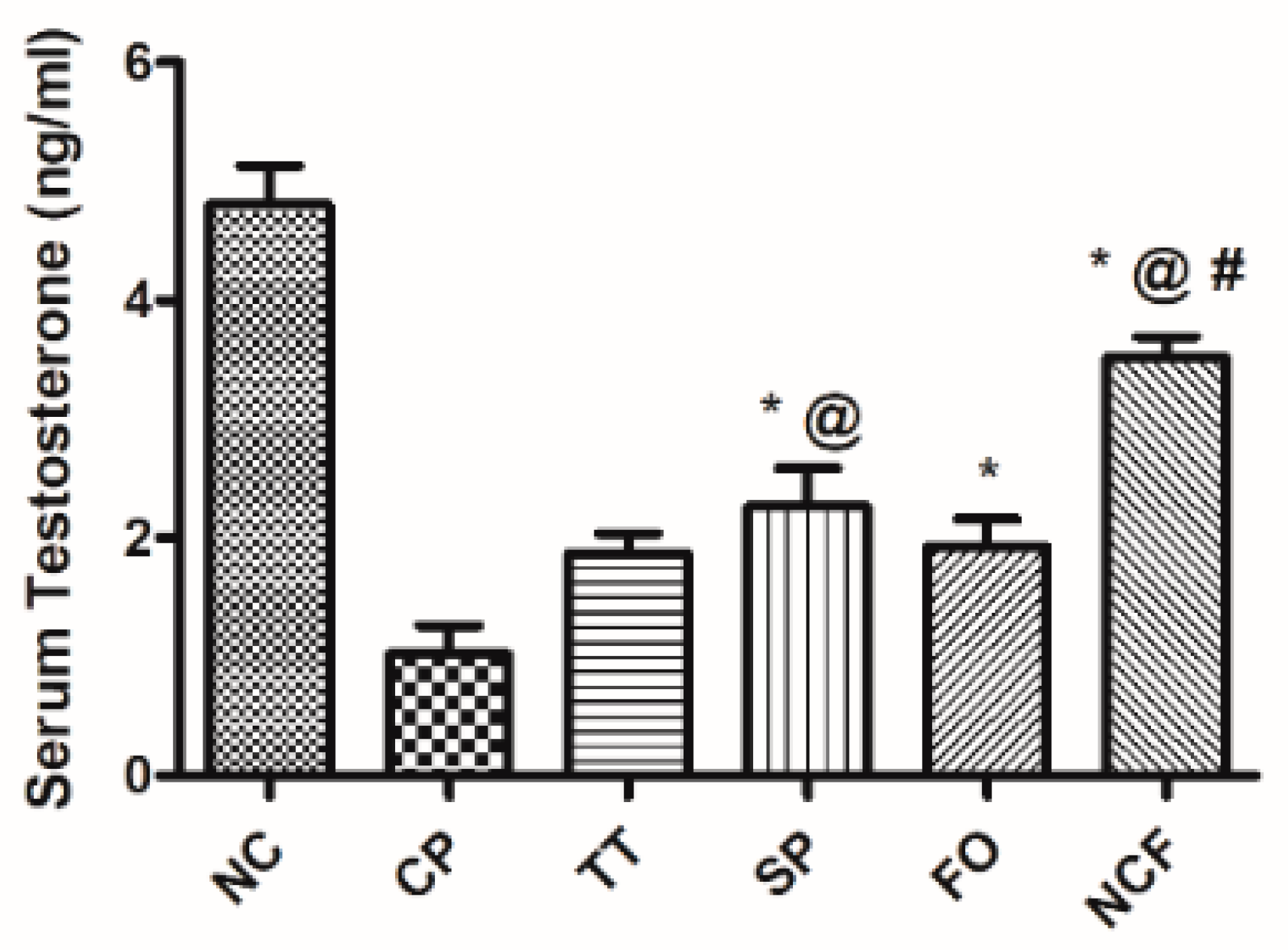
Effect of Cisplatin-Induced Alterations in Serum Testosterone
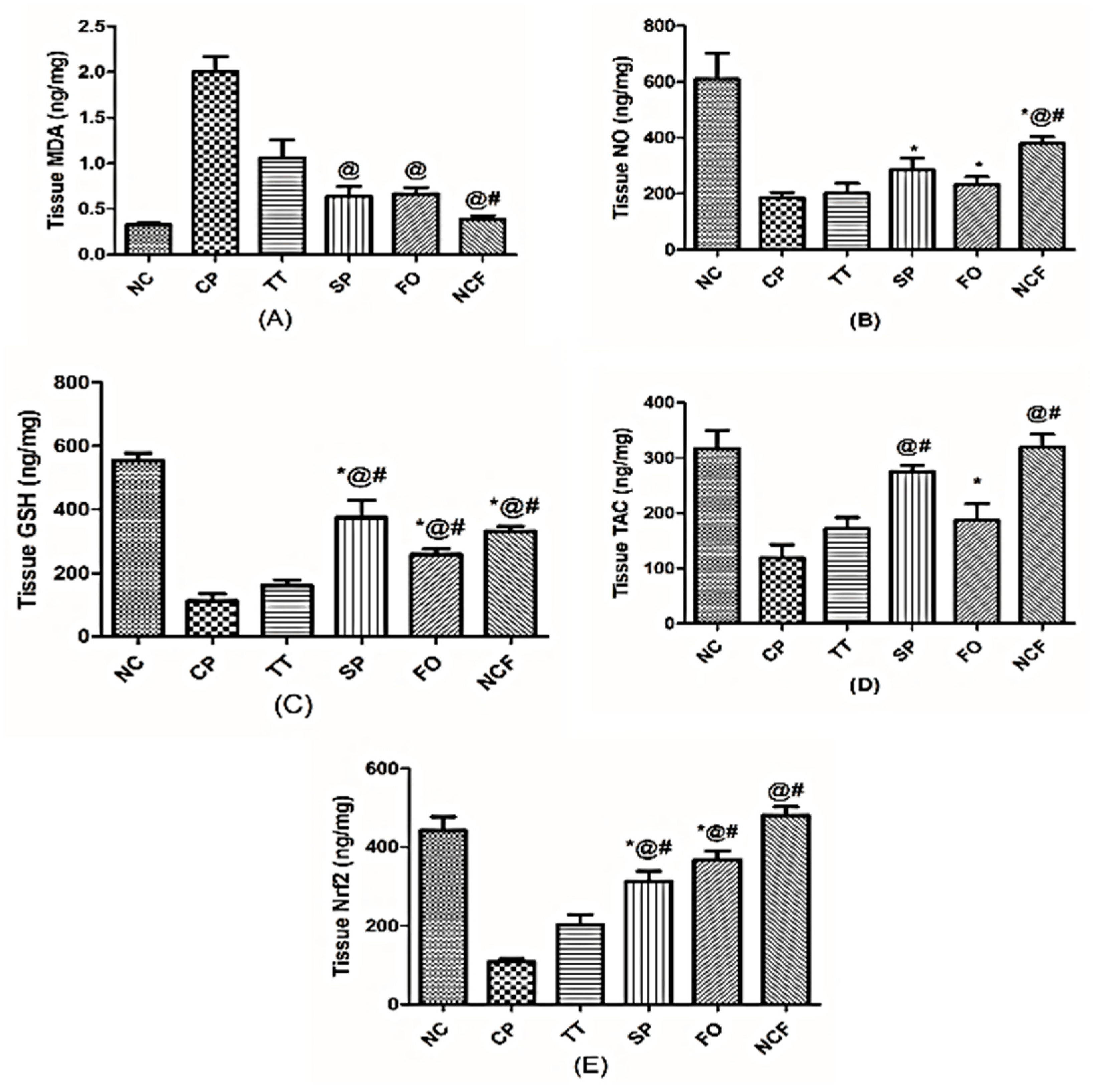
Changes in Testicular Oxidative Stress and Antioxidant Markers
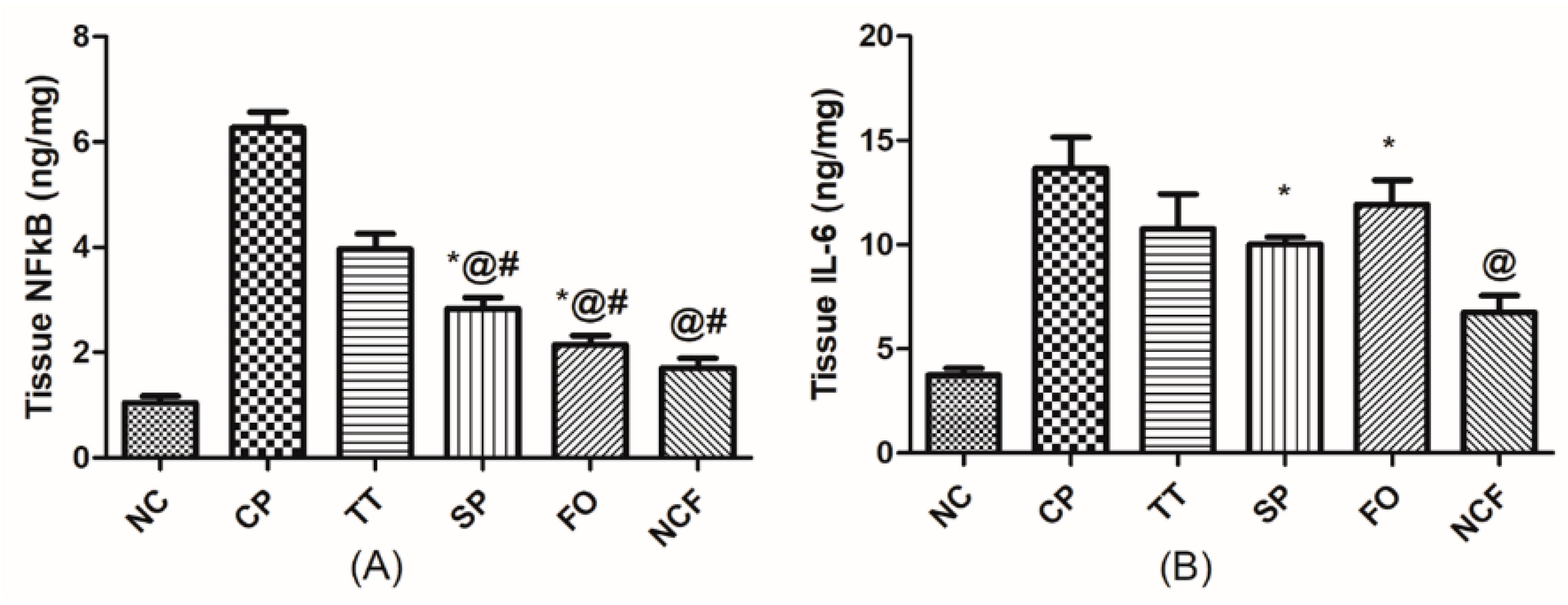
Changes in Testicular Inflammatory Status
Effect on Testicular Caspase 3
2.5. Discussion
3. Material and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Drugs
3.4. Reference Standards
3.5. Animals
3.6. Ethics Statement
3.7. Phytochemical Investigation of SP
3.7.1. Determination of Lipid Content by GC/MS
3.7.2. Investigation of Amino Acid Profile
3.7.3. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.7.4. Determination of Vitamin B12, Vitamin C, and Vitamin E by HPLC
3.7.5. Determination of Mineral Content
3.8. Formulation of a Novel Herbal SNESNS
3.8.1. Preparation of SNEDDS
Screening of Surfactants and Co-Surfactants for SNEDDS
Construction of Ternary Phase Diagram
3.8.2. Characterization of SNEDDS
Assessment of Emulsification Time (ET), Dispersibility, and %Transmittance (%T)
- Grade A: a transparent or bluish nanoemulsion formed within 1 min of mixing.
- Grade B: translucent or bluish-white nanoemulsion formed within 1 min of mixing.
- Grade C: milky emulsion formed within 2 min.
- Grade D: greyish white or slightly oily emulsion developed in more than 2 min.
- Grade E: a system that does not display satisfactory self-emulsification resulting in large visible oil droplets.
Simulation of the Physiological Dilution Process after Oral Administration
Globule Size(GS) Analysis, Polydispersity Index (PDI), and Zeta Potential (ZP)
3.8.3. Preparation of the Extract Loaded SNESNS
3.8.4. Characterization of the Extract Loaded SNESNS
Particle Size (PS), Polydispersity Index (PDI), and Zeta Potential (ZP)
Transmission Electron Microscopy (TEM)
3.9. Quantitative Analysis and Method Validation of NCF
3.9.1. Quantitative Analysis of GC/FID Assay of FAME in NCF and its Ingredients
Standard Preparation
Sample Preparation
3.9.2. Quantitative Analysis of β-Carotene in SP and NCF by HPLC and HPTLC
Standard Preparation
Sample Preparation
3.9.3. UV Colorimetric Investigation of Total Steroidal Saponins in TT and NCF
Standard Preparation
Sample Preparation
4. Biological Evaluation of the NCF
4.1. Experimental Design
4.2. Sperm Count and Motility
4.3. Histopathology
4.4. In Vitro Antioxidant Activity (DPPH Radical Scavenging Activity)
4.5. Biochemical Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Huyghe, E.; Plante, P.; Thonneau, P.F. Testicular cancer variations in time and space in Europe. Eur. Urol. 2007, 51, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Jahan, S.; Munawar, A.; Razak, S.; Anam, S.; Ain, Q.U.; Ullah, H.; Afsar, T.; Abulmeaty, M.; Almajwal, A. Ameliorative effects of rutin against cisplatin-induced reproductive toxicity in male rats. BMC Urol. 2018, 18, 107. [Google Scholar] [CrossRef]
- Fallahzadeh, A.R.; Rezaei, Z.; Rahimi, H.R.; Barmak, M.J.; Sadeghi, H.; Mehrabi, S.; Rabani, S.M.; Kashani, I.R.; Barati, V.; Mahmoudi, R. Evaluation of the effect of pentoxifylline on cisplatin-induced testicular toxicity in rats. Toxicol. Res. 2017, 33, 255–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seyidoglu, N.; Inan, S.; Aydin, C. A prominent superfood: Spirulina platensis. In Superfood and Functional Food: The Development of Superfoods and Their Roles as Medicine; BoD—Books on Demand: Norderstedt, Germany, 2017; pp. 1–27. [Google Scholar]
- Chamorro, G.; Salazar, M.; Favila, L.; Bourges, H. Pharmacology and toxicology of Spirulina alga. Rev. Investig. Clin. Organo Hosp. Enferm. Nutr. 1996, 48, 389–399. [Google Scholar]
- Kim, M.Y.; Cheong, S.H.; Lee, J.H.; Kim, M.J.; Sok, D.-E.; Kim, M.R. Spirulina improves antioxidant status by reducing oxidative stress in rabbits fed a high-cholesterol diet. J. Med. Food 2010, 13, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Salgado, R.; Marques-Silva, M.; Gonçalves, E.; Mathias, A.; Aguiar, J.; Wolff, P. Effect of oral administration of Tribulus terrestris extract on semen quality and body fat index of infertile men. Andrologia 2017, 49, e12655. [Google Scholar] [CrossRef]
- Gauthaman, K.; Ganesan, A.P. The hormonal effects of Tribulus terrestris and its role in the management of male erectile dysfunction–an evaluation using primates, rabbit and rat. Phytomedicine 2008, 15, 44–54. [Google Scholar] [CrossRef]
- Rajendar, B.; Bharavi, K.; Rao, G.; Kishore, P.; Kumar, P.R.; Kumar, C.S.; Patel, T.P. Protective effect of an aphrodisiac herb Tribulus terrestris Linn on cadmium-induced testicular damage. Indian J. Pharm. 2011, 43, 568. [Google Scholar]
- Pan, H.-C.; Kao, T.-K.; Ou, Y.-C.; Yang, D.-Y.; Yen, Y.-J.; Wang, C.-C.; Chuang, Y.-H.; Liao, S.-L.; Raung, S.-L.; Wu, C.-W. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J. Nutr. Biochem. 2009, 20, 715–725. [Google Scholar] [CrossRef]
- Uygur, R.; Aktas, C.; Tulubas, F.; Uygur, E.; Kanter, M.; Erboga, M.; Caglar, V.; Topcu, B.; Ozen, O. Protective effects of fish omega-3 fatty acids on doxorubicin-induced testicular apoptosis and oxidative damage in rats. Andrologia 2014, 46, 917–926. [Google Scholar] [CrossRef]
- Lind, M.L.; Jacobsen, J.; Holm, R.; Müllertz, A. Intestinal lymphatic transport of halofantrine in rats assessed using a chylomicron flow blocking approach: The influence of polysorbate 60 and 80. Eur. J. Pharm. Sci. 2008, 35, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Basalious, E.B.; Shawky, N.; Badr-Eldin, S.M. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine. I: Development and optimization. Int. J. Pharm. 2010, 391, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, J.; Wang, Y.; Liu, X.; Liu, Y.; Fu, Q.; Meng, P.; He, Z. Solid self-emulsifying nitrendipine pellets: Preparation and in vitro/in vivo evaluation. Int. J. Pharm. 2010, 383, 1–6. [Google Scholar] [CrossRef]
- Elsayed, I.; Abdelbary, A.A.; Elshafeey, A.H. Nanosizing of a poorly soluble drug: Technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int. J. Nanomed. 2014, 9, 2943. [Google Scholar]
- Choopani, A.; Poorsoltan, M.; Fazilati, M.; Latifi, A.M.; Salavati, H. Spirulina: A source of gamma-linoleic acid and its applications. Appl. Biotechnol. Rep. 2016, 3, 483–488. [Google Scholar]
- Liestianty, D.; Rodianawati, I.; Arfah, R.A.; Assa, A. Nutritional analysis of spirulina sp to promote as superfood candidate. In IOP Conference Series: Materials Science and Engineering; IOP: Bristol, UK, 2019; p. 012031. [Google Scholar]
- El-Moataaz, S.; Ismael, H.; Aborhyem, S. Assessment of chemical composition of spirulina platensis and its effect on fasting blood glucose and lipid profile in diabetic rats. J. High. Inst. Public Health 2019, 49, 199–211. [Google Scholar] [CrossRef]
- Sharoba, A.M. Nutritional value of spirulina and its use in the preparation of some complementary baby food formulas. J. Food Dairy Sci. 2014, 5, 517–538. [Google Scholar] [CrossRef]
- Machu, L.; Misurcova, L.; Vavra Ambrozova, J.; Orsavova, J.; Mlcek, J.; Sochor, J.; Jurikova, T. Phenolic content and antioxidant capacity in algal food products. Molecules 2015, 20, 1118–1133. [Google Scholar] [CrossRef] [Green Version]
- Ali, I.H.; Doumandji, A. Comparative phytochemical analysis and in vitro antimicrobial activities of the cyanobacterium Spirulina platensis and the green alga Chlorella pyrenoidosa: Potential application of bioactive components as an alternative to infectious diseases. Bull. Inst. Sci. Rabat Sect. Sci. Vie 2017, 39, 41–49. [Google Scholar]
- Seghiri, R.; Kharbach, M.; Essamri, A. Functional composition, nutritional properties, and biological activities of Moroccan Spirulina microalga. J. Food Qual. 2019, 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Salmeán, G.G.; Castillo, L.H.F.; Chamorro-Cevallos, G. Nutritional and toxicological aspects of Spirulina (Arthrospira). Nutr. Hosp. Organo Of. Soc. Española Nutr. Parenter. Enter. 2015, 32, 34–40. [Google Scholar]
- Beg, S.; Swain, S.; Singh, H.P.; Patra, C.N.; Rao, M.B. Development, optimization, and characterization of solid self-nanoemulsifying drug delivery systems of valsartan using porous carriers. AAPS Pharmscitech 2012, 13, 1416–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kommuru, T.; Gurley, B.; Khan, M.; Reddy, I. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: Formulation development and bioavailability assessment. Int. J. Pharm. 2001, 212, 233–246. [Google Scholar] [CrossRef]
- Pouton, C.W.; Porter, C.J. Formulation of lipid-based delivery systems for oral administration: Materials, methods and strategies. Adv. Drug Deliv. Rev. 2008, 60, 625–637. [Google Scholar] [CrossRef]
- Parmar, N.; Singla, N.; Amin, S.; Kohli, K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloids Surf. B 2011, 86, 327–338. [Google Scholar] [CrossRef]
- El-Laithy, H.M.; Basalious, E.B.; El-Hoseiny, B.M.; Adel, M.M. Novel self-nanoemulsifying self-nanosuspension (SNESNS) for enhancing oral bioavailability of diacerein: Simultaneous portal blood absorption and lymphatic delivery. Int. J. Pharm. 2015, 490, 146–154. [Google Scholar] [CrossRef]
- Bello, V.; Mattei, G.; Mazzoldi, P.; Vivenza, N.; Gasco, P.; Idee, J.M.; Robic, C.; Borsella, E. Transmission electron microscopy of lipid vesicles for drug delivery: Comparison between positive and negative staining. Microsc. Microanal. 2010, 16, 456. [Google Scholar] [CrossRef]
- Croom, E.; Pace, R.; Paletti, A.; Sardone, N.; Gray, D. Single-laboratory validation for the determination of terpene lactones in Ginkgo biloba dietary supplement crude materials and finished products by high-performance liquid chromatography with evaporative light-scattering detection. J. AOAC Int. 2007, 90, 647–658. [Google Scholar] [CrossRef]
- Kayesh, R.; Sultan, M.; Rahman, A.; Uddin, M.; Aktar, F.; Rashid, M. Development and validation of a RP-HPLC method for the quantification of omeprazole in pharmaceutical dosage form. J. Sci. Res. 2013, 5, 335–342. [Google Scholar] [CrossRef] [Green Version]
- Bhadra, S.; Das, S.C.; Roy, S.; Arefeen, S.; Rouf, A.S.S. Development and validation of RP-HPLC method for quantitative estimation of vinpocetine in pure and pharmaceutical dosage forms. Chromatogr. Res. Int. 2011, 2011, 801656. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Anbarasan, V.; Kumar, V.K.; Kumar, P.S.; Venkatachalam, T. In vitro evaluation of antioxidant activity of blue green algae Spirulina platensis. Int. J. Pharm. Sci. Res. 2011, 2, 2616. [Google Scholar]
- Afsar, T.; Razak, S.; Almajwal, A. Acacia hydaspica ethyl acetate extract protects against cisplatin-induced DNA damage, oxidative stress and testicular injuries in adult male rats. BMC Cancer 2017, 17, 883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; Von Knethen, A. Nrf2, the master regulator of anti-oxidative responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharpe, R.; Maddocks, S.; Millar, M.; Kerr, J.; Saunders, P.; McKinnell, C. Testosterone and spermatogenesis identification of stage-specific, androgen-regulated proteins secreted by adult rat seminiferous tubules. J. Androl. 1992, 13, 172–184. [Google Scholar]
- Kaya, K.; Ciftci, O.; Cetin, A.; Doğan, H.; Başak, N. Hesperidin protects testicular and spermatological damages induced by cisplatin in rats. Andrologia 2015, 47, 793–800. [Google Scholar] [CrossRef]
- Tousson, E.; Hafez, E.; Masoud, A.; Hassan, A.A. Abrogation by curcumin on testicular toxicity induced by Cisplatin in rats. J. Cancer Res. Treat. 2014, 2, 64–68. [Google Scholar]
- Mercantepe, T.; Unal, D.; Tümkaya, L.; Yazici, Z.A. Protective effects of amifostine, curcumin and caffeic acid phenethyl ester against cisplatin-induced testis tissue damage in rats. Exp. Ther. Med. 2018, 15, 3404–3412. [Google Scholar] [CrossRef] [Green Version]
- Ghanbari, A.; Moradi, M.; Raoofi, A.; Falahi, M.; Seydi, S. Tribulus terrestris hydroalcoholic extract administration effects on reproductive parameters and serum level of glucose in diabetic male rats. Int. J. Morphol. 2016, 34, 796–803. [Google Scholar] [CrossRef] [Green Version]
- Bashandy, S.A.; El Awdan, S.A.; Ebaid, H.; Alhazza, I.M. Antioxidant potential of Spirulina platensis mitigates oxidative stress and reprotoxicity induced by sodium arsenite in male rats. Oxid. Med. Cell. Longev. 2016, 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Zaima, N.; Kinoshita, S.; Hieda, N.; Kugo, H.; Narisawa, K.; Yamamoto, A.; Yanagimoto, K.; Moriyama, T. Effect of dietary fish oil on mouse testosterone level and the distribution of eicosapentaenoic acid-containing phosphatidylcholine in testicular interstitium. Biochem. Biophys. Rep. 2016, 7, 259–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strzezek, J.; Fraser, L.; Kuklinska, M.; Dziekonska, A.; Lecewicz, M. Effects of dietary supplementation with polyunsaturated fatty acids and antioxidants on biochemical characteristics of boar semen. Reprod. Biol. 2004, 4, 271–287. [Google Scholar] [PubMed]
- Yerra, V.G.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saral, S.; Ozcelik, E.; Cetin, A.; Saral, O.; Basak, N.; Aydın, M.; Ciftci, O. Protective role of Diospyros lotus on cisplatin-induced changes in sperm characteristics, testicular damage and oxidative stress in rats. Andrologia 2016, 48, 308–317. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Abdel-Wahab, B.A.; Alkahtani, S.A.; Elagab, E.A. Tadalafil alleviates cisplatin-induced reproductive toxicity through the activation of the Nrf2/HO-1 pathway and the inhibition of oxidative stress and apoptosis in male rats. Reprod. Toxicol. 2020, 96, 165–174. [Google Scholar] [CrossRef]
- Sahin, K.; Orhan, C.; Akdemir, F.; Tuzcu, M.; Gencoglu, H.; Sahin, N.; Turk, G.; Yilmaz, I.; Ozercan, I.H.; Juturu, V. Comparative evaluation of the sexual functions and NF-κB and Nrf2 pathways of some aphrodisiac herbal extracts in male rats. BMC Complement. Altern. Med. 2016, 16, 318. [Google Scholar] [CrossRef] [Green Version]
- El-Desoky, G.E.; Bashandy, S.A.; Alhazza, I.M.; Al-Othman, Z.A.; Aboul-Soud, M.A.; Yusuf, K. Improvement of mercuric chloride-induced testis injuries and sperm quality deteriorations by Spirulina platensis in rats. PLoS ONE 2013, 8, e59177. [Google Scholar] [CrossRef]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.-S.; Yu, S.; Kong, A.-N. Activation of Nrf2-antioxidant signaling attenuates NFκB-inflammatory response and elicits apoptosis. Biochem. Pharm. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [Green Version]
- Hamza, A.; Elwy, H.; Badawi, A. Fenugreek seed extract attenuates cisplatin-induced testicular damage in W istar rats. Andrologia 2016, 48, 211–221. [Google Scholar] [CrossRef]
- Aglan, H.S.; Gebremedhn, S.; Salilew-Wondim, D.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Regulation of Nrf2 and NF-κB during lead toxicity in bovine granulosa cells. Cell Tissue Res. 2020, 380, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Shaha, C.; Tripathi, R.; Mishra, D.P. Male germ cell apoptosis: Regulation and biology. Philos. Trans. Rolayl Soc. B Biol. Sci. 2010, 365, 1501–1515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahra, K.; Keshtmand, Z. Protective effects of tribulus terrestris hydroalcoholic extract against cisplatin-induced germ cell apoptosis in male mice. Int. J. Morphol. 2018, 36, 140–144. [Google Scholar]
- Lee, J.; Park, A.; Kim, M.J.; Lim, H.-J.; Rha, Y.-A.; Kang, H.-G. Spirulina extract enhanced a protective effect in type 1 diabetes by anti-apoptosis and anti-ROS production. Nutrients 2017, 9, 1363. [Google Scholar] [CrossRef] [Green Version]
- El-Tantawy, W.H. Antioxidant effects of Spirulina supplement against lead acetate-induced hepatic injury in rats. J. Tradit. Complement. Med. 2016, 6, 327–331. [Google Scholar] [CrossRef] [Green Version]
- Maged, A.; Mahmoud, A.; Ghorab, M. Hydroxypropyl-beta-cyclodextrin as cryoprotectant in nanoparticles prepared by nano-spray drying technique. J. Pharm. Sci. Emerg. Drugs 5 2017, 1, 2. [Google Scholar] [CrossRef]
- El-Said, M.; Amer, M. Oils, fats, waxes and surfactants. Anglo Egypt. BookShop Cairo 1965, 130–132. [Google Scholar]
- Vogel, A. Practical Organic Chemistry, 1961; Longmans Private LTD: Calcutta, India; Bombay, India; Madras, India, 1970. [Google Scholar]
- Eaton, D.C. Instructor’s Manual to Accompany Laboratory Investigations in Organic Chemistry; McGraw-Hill: New York, NY, USA, 1989. [Google Scholar]
- Pellet, P.; Young, V. Nutrition Evaluation of Food Proteins; Tokyo United Nations University: Tokio, Japan, 1980; pp. 103–133. [Google Scholar]
- Kumazawa, S.; Taniguchi, M.; Suzuki, Y.; Shimura, M.; Kwon, M.-S.; Nakayama, T. Antioxidant activity of polyphenols in carob pods. J. Agric. Food Chem. 2002, 50, 373–377. [Google Scholar] [CrossRef]
- Bahorun, T.; Gressier, B.; Trotin, F.; Brunet, C.; Dine, T.; Luyckx, M.; Vasseur, J.; Cazin, M.; Cazin, J.-C.; Pinkas, M. Oxygen species scavenging activity of phenolic extracts from hawthorn fresh plant organs and pharmaceutical preparations. Arzneimittelforschung 1996, 46, 1086. [Google Scholar]
- Abushita, A.A.; Hebshi, E.A.; Daood, H.G.; Biacs, P.A. Determination of antioxidant vitamins in tomatoes. Food Chem. 1997, 60, 207–212. [Google Scholar] [CrossRef]
- Chapman, H.; Pratt, R. Methods of Analysis for Soil, Plants and Water; Dept. of Soil, Plant Nutrition; University of California: Oakland, CA, USA, 1961. [Google Scholar]
- Shakeel, F.; Ramadan, W. Transdermal delivery of anticancer drug caffeine from water-in-oil nanoemulsions. Colloids Surf. B 2010, 75, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Abouhussein, D.M.; Bahaa El Din Mahmoud, D.; Mohammad, F.E. Design of a liquid nano-sized drug delivery system with enhanced solubility of rivaroxaban for venous thromboembolism management in paediatric patients and emergency cases. J. Liposome Res. 2019, 29, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Liu, Y.; Feng, N.; Xu, J. Preparation and evaluation of self-microemulsifying drug delivery system of oridonin. Int. J. Pharm. 2008, 355, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.A.; Mohsen, A.M.; Ahmed, R.S.; Essam, T.M. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J. Mol. Liq. 2016, 218, 219–232. [Google Scholar] [CrossRef]
- Mahmoud, D.B.; Shukr, M.H.; Bendas, E.R. In vitro and in vivo evaluation of self-nanoemulsifying drug delivery systems of cilostazol for oral and parenteral administration. Int. J. Pharm. 2014, 476, 60–69. [Google Scholar] [CrossRef]
- Shafiq, S.; Shakeel, F.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K.; Ali, M. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur. J. Pharm. Biopharm. 2007, 66, 227–243. [Google Scholar] [CrossRef]
- Balakumar, K.; Raghavan, C.V.; Abdu, S. Self nanoemulsifying drug delivery system (SNEDDS) of rosuvastatin calcium: Design, formulation, bioavailability and pharmacokinetic evaluation. Colloids Surf. B 2013, 112, 337–343. [Google Scholar] [CrossRef]
- Khattab, A.; Hassanin, L.; Zaki, N. Self-nanoemulsifying drug delivery system of coenzyme (Q10) with improved dissolution, bioavailability, and protective efficiency on liver fibrosis. AAPS Pharmscitech 2017, 18, 1657–1672. [Google Scholar] [CrossRef]
- El Shagea, H.N.; El Kasabgy, N.A.; Fahmy, R.H.; Basalious, E.B. Freeze-dried self-nanoemulsifying self-nanosuspension (snesns): A new approach for the preparation of a highly drug-loaded dosage form. AAPS Pharmscitech 2019, 20, 258. [Google Scholar] [CrossRef]
- Abouhussein, D.M. Enhanced transdermal permeation of BCS class IV aprepitant using binary ethosome: Optimization, characterization and ex vivo permeation. J. Drug Deliv. Sci. Tec. 2020, 102185. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef] [Green Version]
- Hynstova, V.; Sterbova, D.; Klejdus, B.; Hedbavny, J.; Huska, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using high performance thin layer chromatography. J. Pharm. Biomed. Anal. 2018, 148, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Gjulemetowa, R.; Tomowa, M.; Simowa, M.; Pangarowa, T.; Peewa, S. Determination of furostanol saponins in the preparation Tribestan. Die Pharm. 1982, 37, 296. [Google Scholar]
- Ciftci, O.; Cetin, A.; Aydin, M.; Kaya, K.; Oguz, F. Fish oil, contained in eicosapentaenoic acid and docosahexaenoic acid, attenuates testicular and spermatological damage induced by cisplatin in rats. Andrologia 2014, 46, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- Saied, N.; Darwish, S. A possible ameliorating effects of Tribulus terrestris on testicular dysfunction induced by xenoestrogens exposure in adult rats. Curr. Sci. Int 2015, 4, 73–89. [Google Scholar]
- Afkhami-Ardakani, M.; Hasanzadeh, S.; Shahrooz, R.; Delirezh, N.; Malekinejad, H. Antioxidant effects of Spirulina platensis (Arthrospira platensis) on cyclophosphamide-induced testicular injury in rats. In Veterinary Research Forum; Urmia University: Urmia, Iran, 2018; p. 35. [Google Scholar]
- Bearden, H.; Fuquay, J. Applied Animal Reproduction; Reston Publishing Company. Inc. Prentice-Hall: Reston, VA, USA, 1980. [Google Scholar]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]




| Chemical/Element | Content in SP | Chemical/Element | Content in SP |
|---|---|---|---|
| Essential amino acids (mg/g): | Total protein content (%) | 68.77 | |
| Leucine | 63.94 | Total flavonoid content (mg RE/g DW) | 0.864 |
| Valine | 52.56 | Total polyphenol content (mg GAE/g DW) | 58.64 |
| Isoleucine | 45.62 | Total antioxidant activity (%) | 25.4 |
| Phenyl Alanine | 41.71 | Vitamins (mg/100 g): | |
| Threonine | 33.87 | Cyanocobalamin (Vit. B12) | 3.2 |
| Lysine | 27.71 | Ascorbic acid (Vit. C) | 191.906 |
| Histidine | 12 | α-Tocopherol (Vit. E) | undetectable |
| Methionine | 10.14 | β-Carotene(Vit. A) | 44 |
| Non-essential amino acids (mg/g): | Minerals (mg/100 g): | ||
| Glutamate | 141.58 | Iron (Fe) | 170 |
| Aspartic Acid | 85.23 | Zinc (Zn) | 160 |
| Arginine | 48.91 | Copper (Cu) | 20 |
| Glycine | 38.59 | Fatty acid methyl esters (FAME) (mg/g): | |
| Alanine | 27.07 | Palmitic acid | 132 |
| Tyrosine | 22.99 | Gamma-linolenic acid (GLA) | 58.74 |
| Serine | 21.58 | Linoleic acid | 27.7 |
| Proline | 14.2 | Total fatty acid content (%) | 22 |
| Chemical/Element | Content in the New Formula |
|---|---|
| Each 1 g new formula consisting of: | |
| Spirulina powder | 250 mg |
| Tribulus terrestris extract (standardized as 45% steroidal saponin) | 50 mg |
| Omega-3-fish oil (standardized as 50% EPA and DHA) | 100 mg |
| Total antioxidant activity (%) | 35.3 |
| β-Carotene (Vit. A) (mg/g) | 0.17 |
| Total fatty acid content (%) | 27.23 |
| Fatty acid content (mg/g): | |
| Palmitic acid | 110.68 |
| Gamma-linloenic acid (GLA) | 16.87 |
| Linoleic acid | 104.12 |
| Eicosapentaenoic acid (EPA) | 22.04 |
| Docosahexaenoic acid (DHA) | 18.58 |
| Content of steroidal saponins of Tribulus terrestris extract in new formula (mg steroidal saponin/g formula) | 36.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel-All, S.R.; Shakour, Z.T.A.; Abouhussein, D.M.N.; Reda, E.; Sallam, T.F.; El-Hefnawy, H.M.; Abdel-Monem, A.R. Phytochemical and Biological Evaluation of a Newly Designed Nutraceutical Self-Nanoemulsifying Self-Nanosuspension for Protection and Treatment of Cisplatin Induced Testicular Toxicity in Male Rats. Molecules 2021, 26, 408. https://doi.org/10.3390/molecules26020408
Abdel-All SR, Shakour ZTA, Abouhussein DMN, Reda E, Sallam TF, El-Hefnawy HM, Abdel-Monem AR. Phytochemical and Biological Evaluation of a Newly Designed Nutraceutical Self-Nanoemulsifying Self-Nanosuspension for Protection and Treatment of Cisplatin Induced Testicular Toxicity in Male Rats. Molecules. 2021; 26(2):408. https://doi.org/10.3390/molecules26020408
Chicago/Turabian StyleAbdel-All, Sherif R., Zeinab T. Abdel Shakour, Dalia M. N. Abouhussein, Enji Reda, Thoraya F. Sallam, Hala M. El-Hefnawy, and Azza R. Abdel-Monem. 2021. "Phytochemical and Biological Evaluation of a Newly Designed Nutraceutical Self-Nanoemulsifying Self-Nanosuspension for Protection and Treatment of Cisplatin Induced Testicular Toxicity in Male Rats" Molecules 26, no. 2: 408. https://doi.org/10.3390/molecules26020408
APA StyleAbdel-All, S. R., Shakour, Z. T. A., Abouhussein, D. M. N., Reda, E., Sallam, T. F., El-Hefnawy, H. M., & Abdel-Monem, A. R. (2021). Phytochemical and Biological Evaluation of a Newly Designed Nutraceutical Self-Nanoemulsifying Self-Nanosuspension for Protection and Treatment of Cisplatin Induced Testicular Toxicity in Male Rats. Molecules, 26(2), 408. https://doi.org/10.3390/molecules26020408






