Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116
Abstract
1. Introduction
2. Results
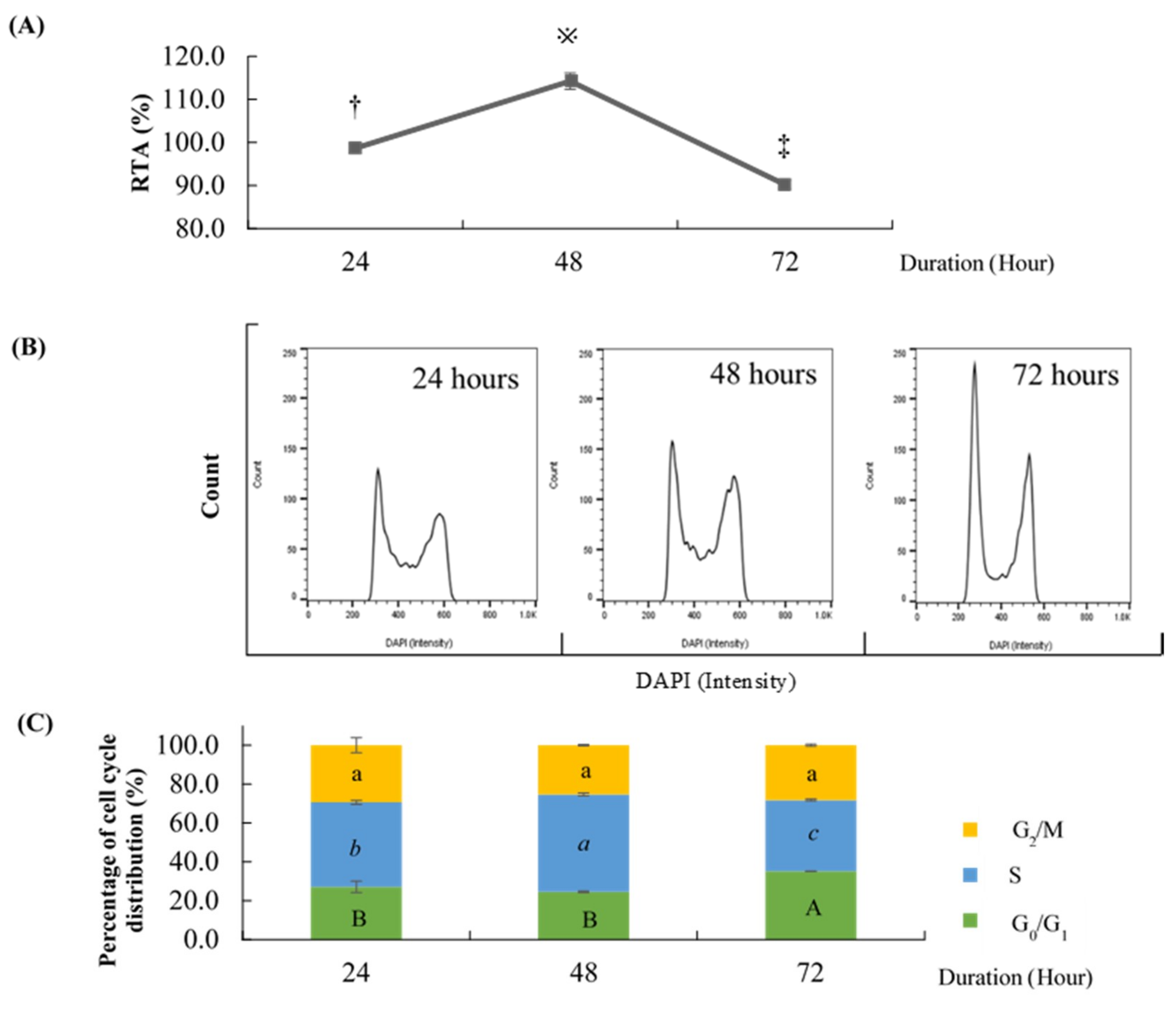
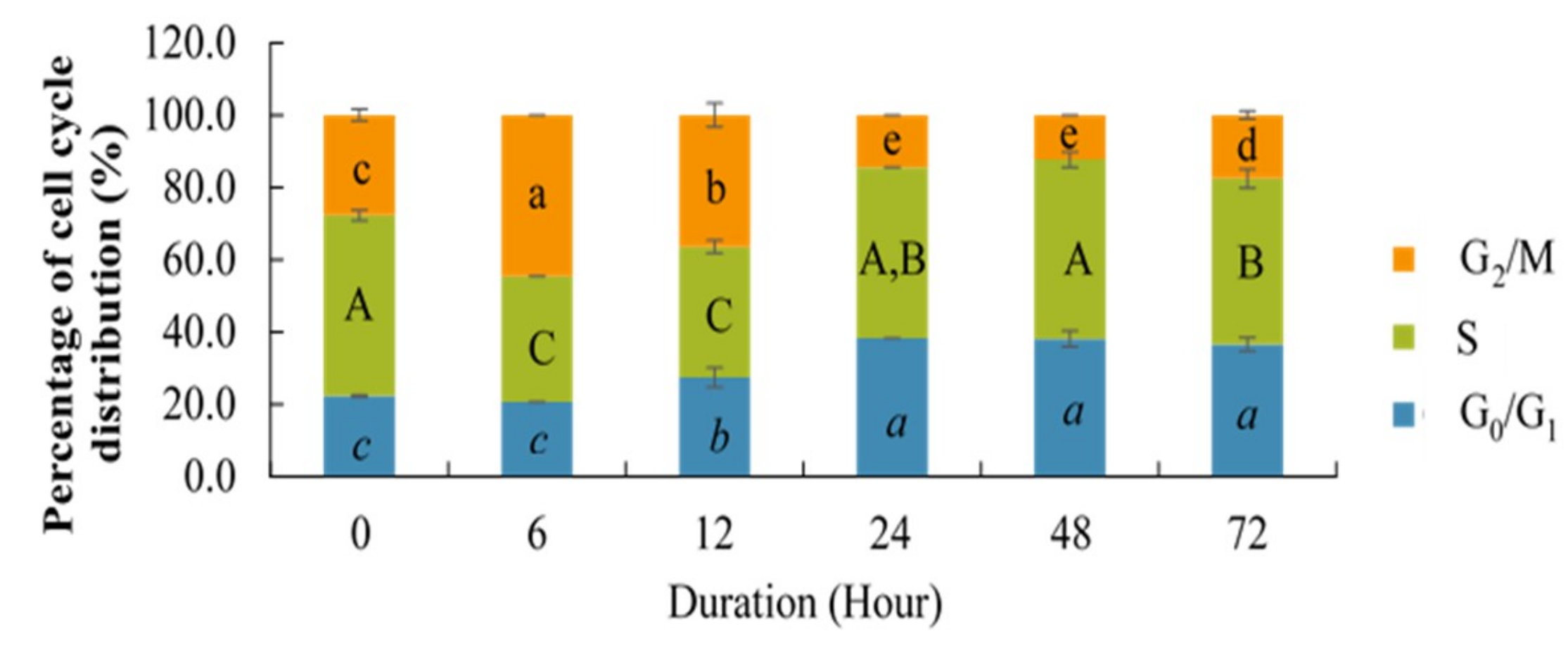
2.1. Effect of Different Time Points on the Percentage of Cell Cycle Distribution and Relative Telomerase Activity (RTA)
2.2. Effect of Different Telomerase Inhibitor Compounds on IC50
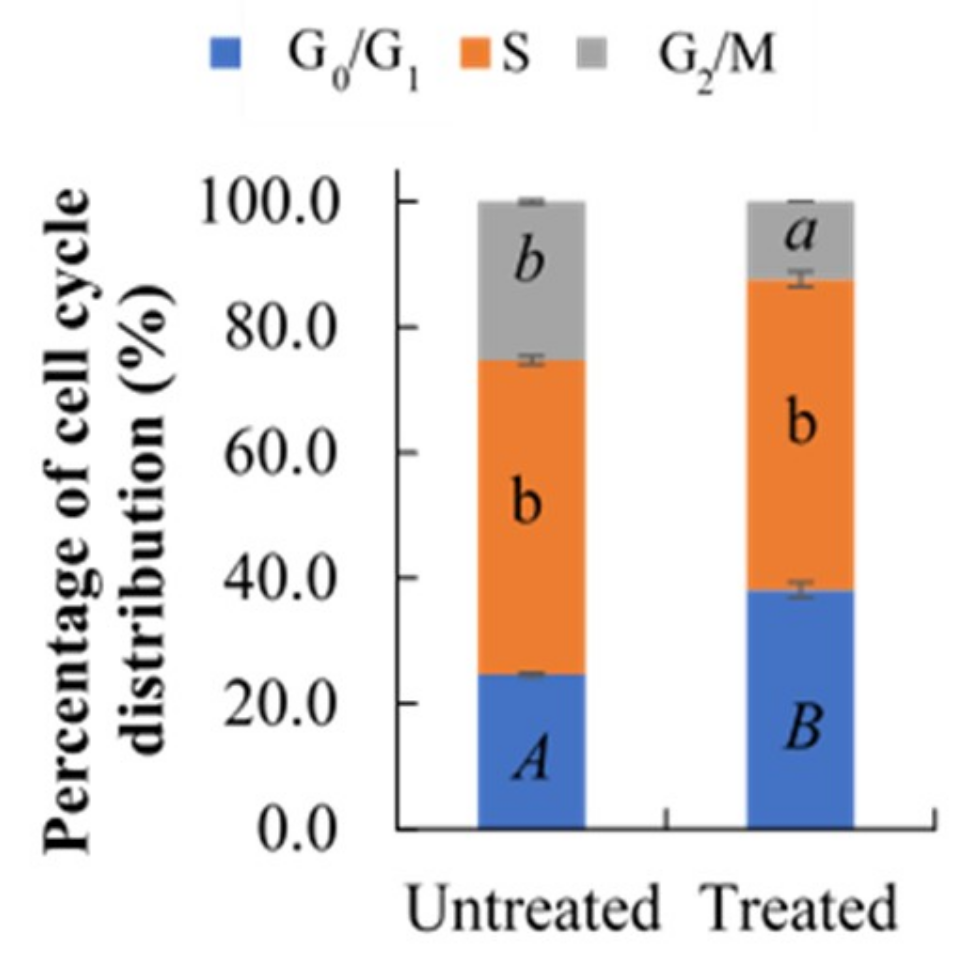
2.3. Effect of Berberine Treatment on Cell Cycle Distribution
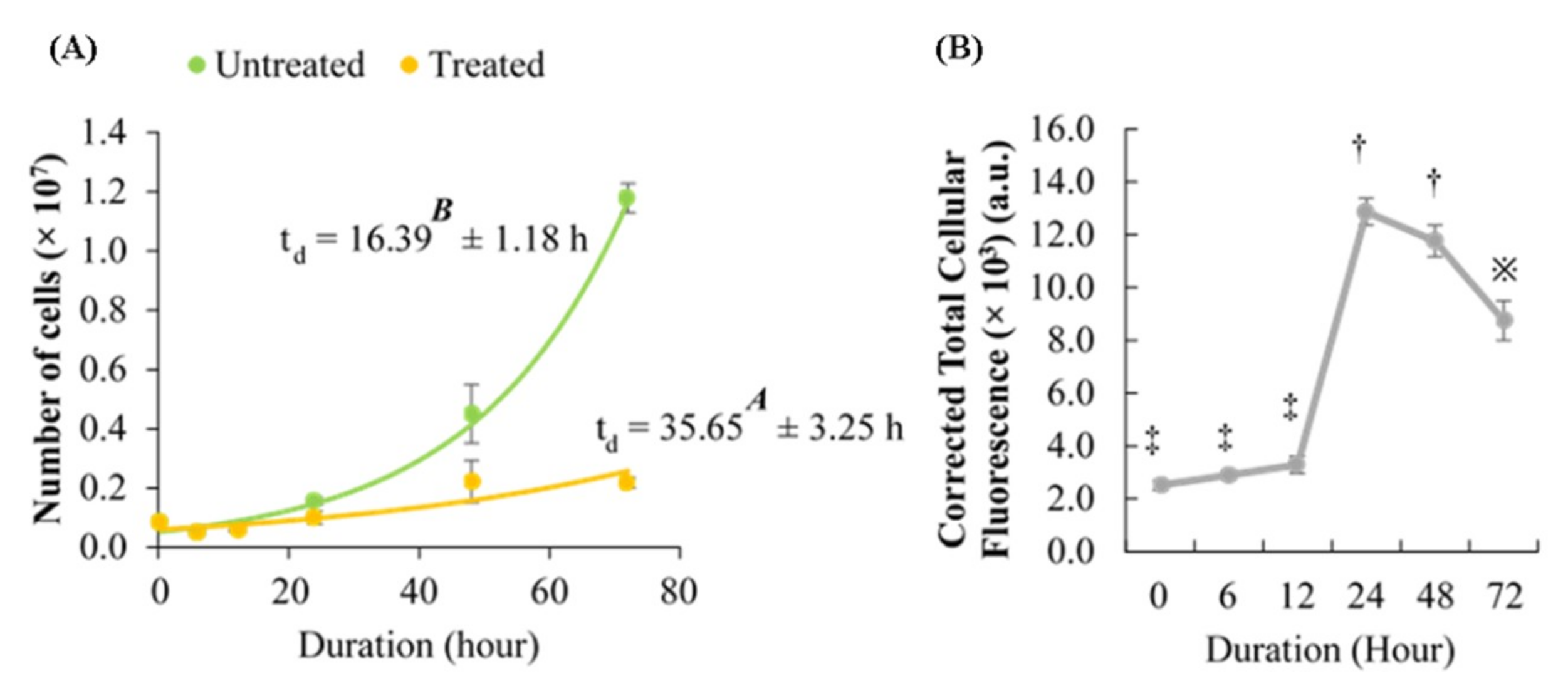
2.4. Effect of Berberine Treatment on Cell Growth and Berberine Cellular Localization
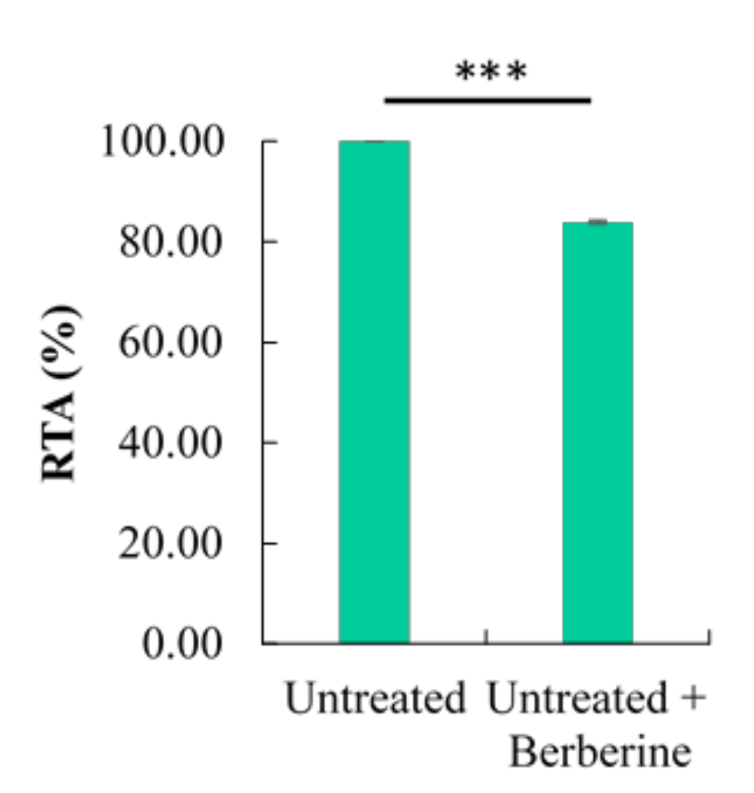
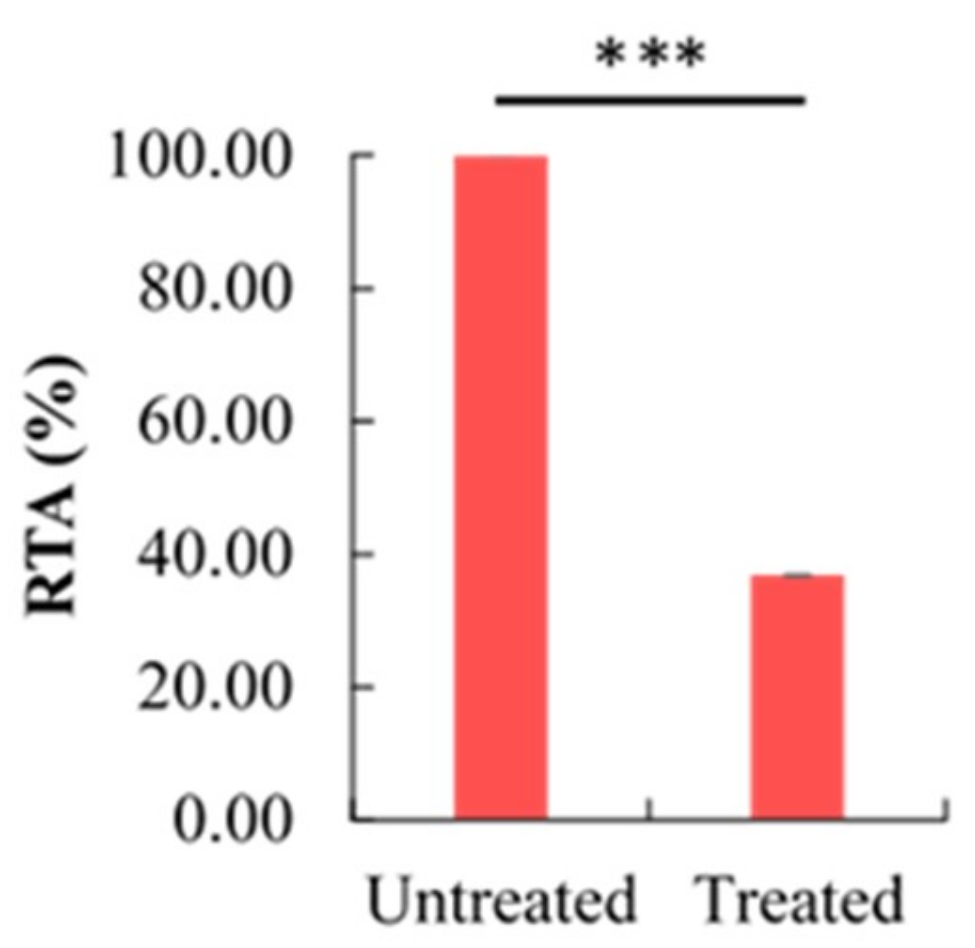
2.5. Effect of Berberine Treatment on Relative Telomerase Activity (RTA)
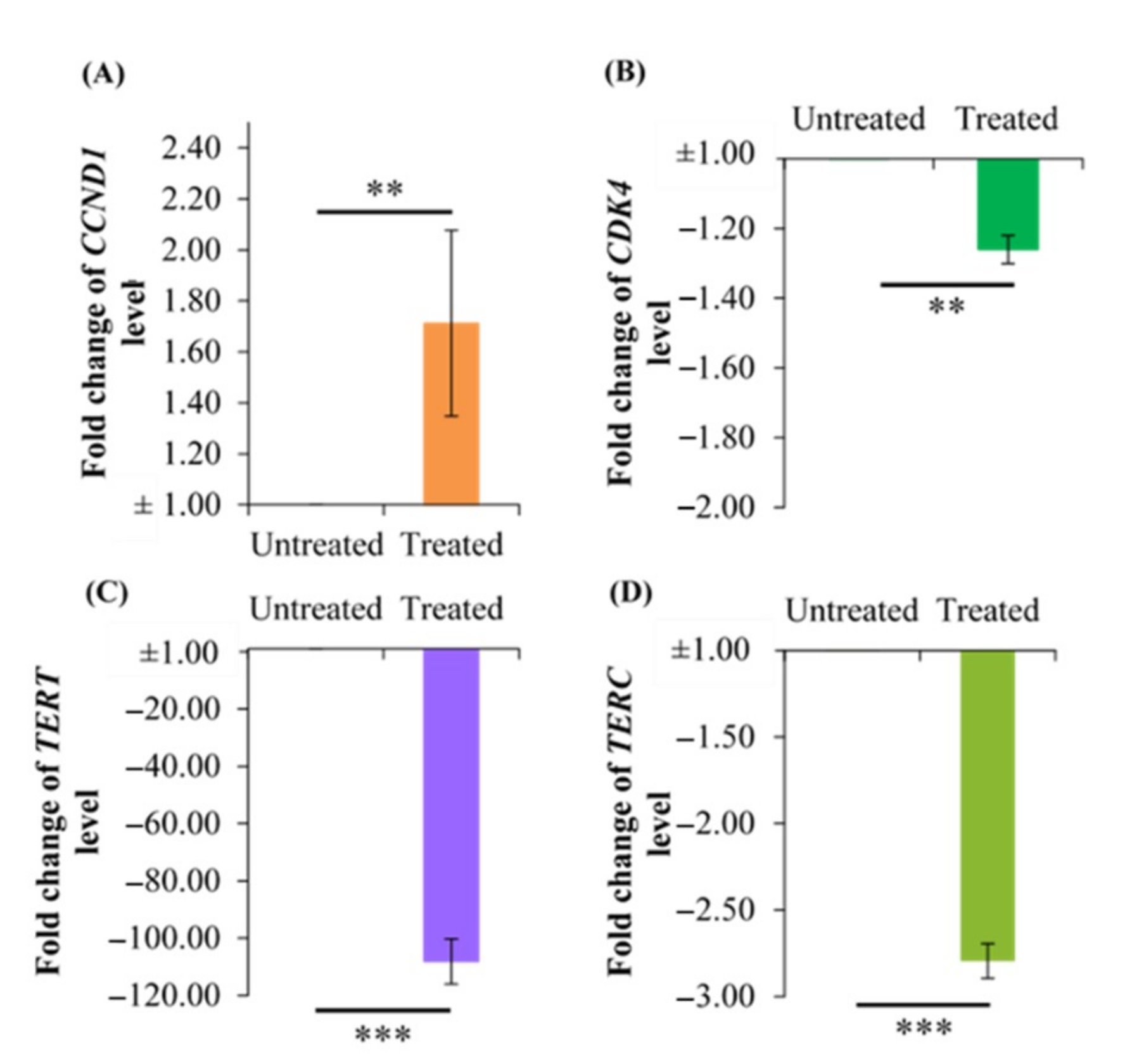
2.6. Effect of Berberine Treatment on CCDN1, CDK4, TERT, and TERC RNA Levels
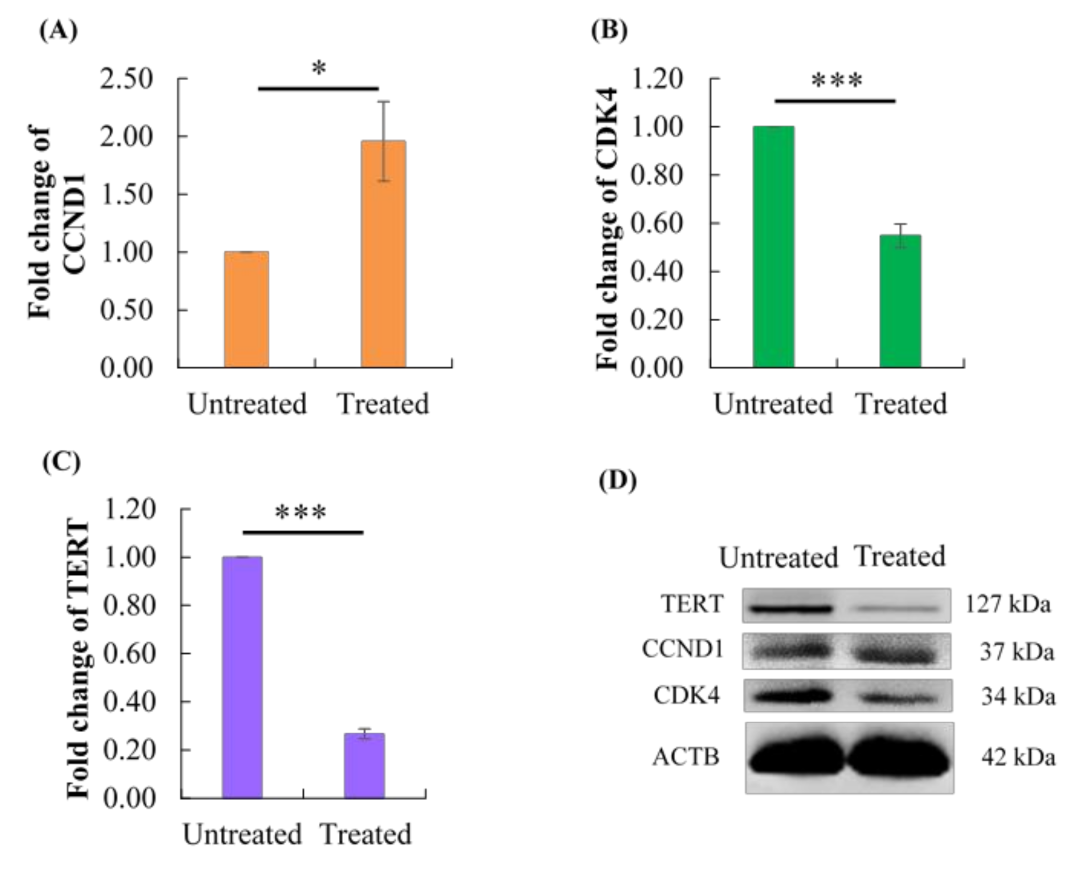
2.7. Effect of Berberine Treatment on CCND1, CDK4, and TERT Protein Levels
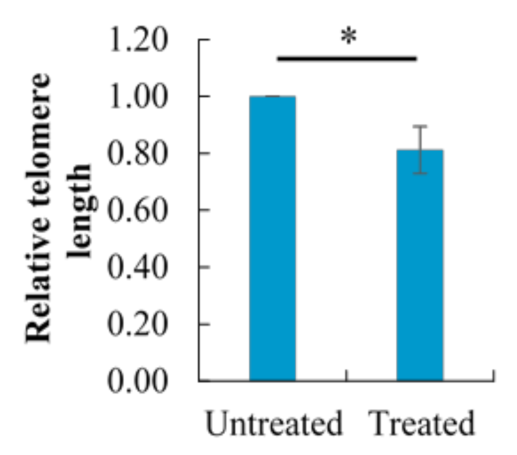
2.8. Effect of Berberine Treatment on Relative Telomere Length
3. Discussion
4. Materials and Methods
4.1. Reagents, Primers, and Antibodies
4.2. Cell Culture and Harvesting
4.3. Cell Cycle Analysis
4.4. Sulforhodamine B (SRB) Assay
4.5. Cell Count and Growth Curve Analysis
4.6. Berberine Cellular Localization
4.7. TELOTAGGG Telomerase PCR ELISA
4.8. RT-PCR
4.9. Relative Telomere Length Quantification
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: Globocan sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. Ualcan: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Tatsumoto, N.; Hiyama, E.; Murakami, Y.; Imamura, Y.; Shay, J.W.; Matsuura, Y.; Yokoyama, T. High telomerase activity is an independent prognostic indicator of poor outcome in colorectal cancer. Clin. Cancer Res. 2000, 6, 2696–2701. [Google Scholar] [PubMed]
- Zvereva, M.I.; Shcherbakova, D.M.; Dontsova, O.A. Telomerase: Structure, functions, and activity regulation. Biochem. Biokhim. 2010, 75, 1563–1583. [Google Scholar] [CrossRef] [PubMed]
- Bertorelle, R.; De Rossi, A. Telomerase as biomarker in colorectal cancer. In Biomarkers in Cancer; Preedy, V.R., Patel, V.B., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 659–683. [Google Scholar]
- Chang, J.T.C.; Chen, Y.L.; Yang, H.T.; Chen, C.Y.; Cheng, A.J. Differential regulation of telomerase activity by six telomerase subunits. Eur. J. Biochem. 2002, 269, 3442–3450. [Google Scholar] [CrossRef] [PubMed]
- MacNeil, D.; Bensoussan, H.; Autexier, C. Telomerase regulation from beginning to the end. Genes 2016, 7, 64. [Google Scholar] [CrossRef]
- Wyatt, H.D.M.; West, S.C.; Beattie, T.L. Intertpreting telomerase structure and function. Nucleic Acids Res. 2010, 38, 5609–5622. [Google Scholar] [CrossRef]
- Nguyen, T.H.D.; Tam, J.; Wu, R.A.; Greber, B.J.; Toso, D.; Nogales, E.; Collins, K. Cryo-em structure of substrate-bound human telomerase holoenzyme. Nature 2018, 557, 190–195. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase inhibitors from natural products and their anticancer potential. Int. J. Mol. Sci. 2018, 19, 13. [Google Scholar] [CrossRef]
- O’Brien, P.; Carrasco-Pozo, C.; Speisky, H. Boldine and its antioxidant or health-promoting properties. Chem. Biol. Interact. 2006, 159, 1–17. [Google Scholar] [CrossRef]
- Brantley, S.J.; Oberlies, N.H.; Kroll, D.J.; Paine, M.F. Two flavonolignans from milk thistle (silybum marianum) inhibit cyp2c9-mediated warfarin metabolism at clinically achievable concentrations. J. Pharmacol. Exp. Ther. 2010, 332, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Davis-Searles, P.R.; Nakanishi, Y.; Kim, N.-C.; Graf, T.N.; Oberlies, N.H.; Wani, M.C.; Wall, M.E.; Agarwal, R.; Kroll, D.J. Milk thistle and prostate cancer: Differential effects of pure flavonolignans from silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res. 2005, 65, 4448–4457. [Google Scholar] [CrossRef] [PubMed]
- Surai, P. Silymarin as a natural antioxidant: An overview of the current evidence and perspectives. Antioxidants 2015, 4, 204–247. [Google Scholar] [CrossRef]
- Mahata, S.; Bharti, A.C.; Shukla, S.; Tyagi, A.; Husain, S.A.; Das, B.C. Berberine modulates ap-1 activity to suppress hpv transcription and downstream signaling to induce growth arrest and apoptosis in cervical cancer cells. Mol. Cancer 2011, 10, 39. [Google Scholar] [CrossRef]
- Srinivasan, G.V.; Unnikrishnan, K.P.; Rema Shree, A.B.; Balachandran, I. Hplc estimation of berberine in tinospora cordifolia and tinospora sinensis. Indian J. Pharm. Sci. 2008, 70, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Eskandari-Nasab, E.; Dahmardeh, F.; Rezaeifar, A.; Dahmardeh, T. Telomere and telomerase: From discovery to cancer treatment. Gene Cell Tissue 2015, 2, e28084. [Google Scholar] [CrossRef]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Noureini, S.K.; Wink, M. Dose-dependent cytotoxic effects of boldine in hepg-2 cells-telomerase inhibition and apoptosis induction. Molecules 2015, 20, 3730–3743. [Google Scholar] [CrossRef]
- Zhu, X.; Kumar, R.; Mandal, M.; Sharma, N.; Sharma, H.W.; Dhingra, U.; Sokoloski, J.A.; Hsiao, R.; Narayanan, R. Cell cycle-dependent modulation of telomerase activity in tumor cells. Proc. Natl. Acad. Sci. USA 1996, 93, 6091–6095. [Google Scholar] [CrossRef]
- Klein, E.A.; Assoian, R.K. Transcriptional regulation of the cyclin d1 gene at a glance. J. Cell Sci. 2008, 121, 3853–3857. [Google Scholar] [CrossRef]
- Ioset, J.-R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug screening for kinetoplastids diseases. In A Training Manual for Screening in Neglected Diseases; Department for International Development: London, UK, 2009. [Google Scholar]
- National Cancer Institute. Nci-60 Screening Methodology. Available online: https://dtp.cancer.gov/discovery_development/nci-60/methodology.htm (accessed on 19 May 2019).
- Barzegar, E.; Fouladdel, S.; Movahhed, T.K.; Atashpour, S.; Ghahremani, M.H.; Ostad, S.N.; Azizi, E. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer t47d and mcf7 cell lines. Iran. J. Basic Med. Sci. 2015, 18, 334. [Google Scholar] [PubMed]
- Li, F.; Dong, X.; Lin, P.; Jiang, J. Regulation of akt/foxo3a/skp2 axis is critically involved in berberine-induced cell cycle arrest in hepatocellular carcinoma cells. Int. J. Mol. Sci. 2018, 19, 327. [Google Scholar]
- Chen, Q.; Qin, R.; Fang, Y.; Li, H. Berberine sensitizes human ovarian cancer cells to cisplatin through mir-93/pten/akt signaling pathway. Cell. Physiol. Biochem. 2015, 36, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Jirawatnotai, S.; Hu, Y.; Livingston, D.M.; Sicinski, P. Proteomic identification of a direct role for cyclin d1 in DNA damage repair. Cancer Res. 2012, 72, 4289–4293. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Sun, G.; Luo, H.; Wang, X.F.; Lan, F.M.; Yue, X.; Fu, L.S.; Pu, P.Y.; Kang, C.S.; Liu, N. Mir-21 modulates h tert through a stat3-dependent manner on glioblastoma cell growth. CNS Neurosci. Ther 2012, 18, 722–728. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsieh, P.-L.; Liao, Y.-W.; Peng, C.-Y.; Lu, M.-Y.; Yang, C.-H.; Yu, C.-C.; Liu, C.-M. Correction: Berberine-targeted mir-21 chemosensitizes oral carcinomas stem cells. Oncotarget 2018, 9, 24870. [Google Scholar] [CrossRef]
- Xin, X.; Senthilkumar, P.; Schnoor, J.L.; Ludewig, G. Effects of pcb126 and pcb153 on telomerase activity and telomere length in undifferentiated and differentiated hl-60 cells. Environ. Sci. Pollut. Res. 2016, 23, 2173–2185. [Google Scholar] [CrossRef]
- Wu, K.; Yang, Q.; Mu, Y.; Zhou, L.; Liu, Y.; Zhou, Q.; He, B. Berberine inhibits the proliferation of colon cancer cells by inactivating wnt/β-catenin signaling. Int. J. Oncol. 2012, 41, 292–298. [Google Scholar]
- Brattain, M.G.; Fine, W.D.; Khaled, F.M.; Thompson, J.; Brattain, D.E. Heterogeneity of malignant cells from a human colonic carcinoma. Cancer Res. 1981, 41, 1751–1756. [Google Scholar]
- Owa, T.; Ozawa, Y.; Semba, T. Joint Use of Sulfonamide Based Compound with Angiogenesis Inhibitor. EP Patent 2,364,699,A1, 14 September 2011. [Google Scholar]
- Jain, M.; Nilsson, R.; Sharma, S.; Madhusudhan, N.; Kitami, T.; Souza, A.L.; Kafri, R.; Kirschner, M.W.; Clish, C.B.; Mootha, V.K. Metabolite profiling identifies a key role for glycine in rapid cancer cell proliferation. Science 2012, 336, 1040–1044. [Google Scholar] [CrossRef]
- Pereira, P.D.; Serra-Caetano, A.; Cabrita, M.; Bekman, E.; Braga, J.; Rino, J.; Santus, R.; Filipe, P.L.; Sousa, A.E.; Ferreira, J.A. Quantification of cell cycle kinetics by edu (5-ethynyl-2′-deoxyuridine)-coupled-fluorescence-intensity analysis. Oncotarget 2017, 8, 40514. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. Cell Lines in the in vitro screen. Available online: https://dtp.cancer.gov/discovery_development/nci-60/cell_list.htm (accessed on 10 April 2019).
- Nguyen, D.; Grenier St-Sauveur, V.; Bergeron, D.; Dupuis-Sandoval, F.; Scott, M.S.; Bachand, F. A polyadenylation-dependent 3’ end maturation pathway is required for the synthesis of the human telomerase rna. Cell Rep. 2015, 13, 2244–2257. [Google Scholar] [CrossRef] [PubMed]
- Serafim, T.L.; Oliveira, P.J.; Sardao, V.A.; Perkins, E.; Parke, D.; Holy, J. Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemther. Pharmacol. 2008, 61, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Guaman Ortiz, L.M.; Croce, A.L.; Aredia, F.; Sapienza, S.; Fiorillo, G.; Syeda, T.M.; Buzzetti, F.; Lombardi, P.; Scovassi, A.I. Effect of new berberine derivatives on colon cancer cells. Acta Biochimica Biophysica Sinica 2015, 47, 824–833. [Google Scholar] [CrossRef]
- Li, W.; Hua, B.; Saud, S.M.; Lin, H.; Hou, W.; Matter, M.S.; Jia, L.; Colburn, N.H.; Young, M.R. Berberine regulates amp-activated protein kinase signaling pathways and inhibits colon tumorigenesis in mice. Mol. Carcinogenes. 2015, 54, 1096–1109. [Google Scholar] [CrossRef]
- Noureini, S.K.; Tanavar, F. Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations. Chemico-Biol. Interact. 2015, 231, 27–34. [Google Scholar] [CrossRef]
- Ko, E.; Seo, H.W.; Jung, G. Telomere length and reactive oxygen species levels are positively associated with a high risk of mortality and recurrence in hepatocellular carcinoma. Hepatology 2018, 67, 1378–1391. [Google Scholar] [CrossRef]
- Moraca, F.; Amato, J.; Ortuso, F.; Artese, A.; Pagano, B.; Novellino, E.; Alcaro, S.; Parrinello, M.; Limongelli, V. Ligand binding to telomeric g-quadruplex DNA investigated by funnel-metadynamics simulations. Proc. Natl. Acad. Sci. USA 2017, 114, E2136–E2145. [Google Scholar] [CrossRef]
- Bhadra, K.; Kumar, G.S. Interaction of berberine, palmatine, coralyne, and sanguinarine to quadruplex DNA: A comparative spectroscopic and calorimetric study. Biochimica Biophysica Acta 2011, 1810, 485–496. [Google Scholar] [CrossRef]
- Neidle, S. Quadruplex nucleic acids as novel therapeutic targets. J. Med. Chem. 2016, 59, 5987–6011. [Google Scholar] [CrossRef]
- Wang, Y.; Kheir, M.M.; Chai, Y.; Hu, J.; Xing, D.; Lei, F.; Du, L. Comprehensive study in the inhibitory effect of berberine on gene transcription, including tata box. PLoS ONE 2011, 6, e23495. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-Y.; Lu, X.; Lei, F.; Chai, Y.-S.; Wang, Y.-G.; Jiang, J.-F.; Feng, T.-S.; Wang, X.-P.; Yu, X.; Yan, X.-J. Tata boxes in gene transcription and poly (a) tails in mrna stability: New perspective on the effects of berberine. Sci. Rep. 2015, 5, 18326. [Google Scholar] [CrossRef]
- Fu, L.; Chen, W.; Guo, W.; Wang, J.; Tian, Y.; Shi, D.; Zhang, X.; Qiu, H.; Xiao, X.; Kang, T. Berberine targets ap-2/htert, nf-κb/cox-2, hif-1α/vegf and cytochrome-c/caspase signaling to suppress human cancer cell growth. PLoS ONE 2013, 8, e69240. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.X.; Su, H.F.; Lv, P.; Ma, Y.; Wang, S.K.; Miao, H.; Liu, H.Y.; Tan, J.H.; Ou, T.M.; Gu, L.Q.; et al. A newly identified berberine derivative induces cancer cell senescence by stabilizing endogenous g-quadruplexes and sparking a DNA damage response at the telomere region. Oncotarget 2015, 6, 35625–35635. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Rodrigues, F.; Santana-Lemos, B.A.; Scheucher, P.S.; Alves-Paiva, R.M.; Calado, R.T. Direct comparison of flow-fish and qpcr as diagnostic tests for telomere length measurement in humans. PLoS ONE 2014, 9, e113747. [Google Scholar] [CrossRef] [PubMed]
- Arora, A.; Balasubramanian, C.; Kumar, N.; Agrawal, S.; Ojha, R.P.; Maiti, S. Binding of berberine to human telomeric quadruplex - spectroscopic, calorimetric and molecular modeling studies. FEBS J. 2008, 275, 3971–3983. [Google Scholar] [CrossRef] [PubMed]
- Bazzicalupi, C.; Ferraroni, M.; Bilia, A.R.; Scheggi, F.; Gratteri, P. The crystal structure of human telomeric DNA complexed with berberine: An interesting case of stacked ligand to g-tetrad ratio higher than 1:1. Nucleic Acids Res. 2013, 41, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Ou, T.M.; Lu, Y.J.; Huang, Y.Y.; Wu, W.B.; Huang, Z.S.; Zhou, J.L.; Wong, K.Y.; Gu, L.Q. 9-substituted berberine derivatives as g-quadruplex stabilizing ligands in telomeric DNA. Bioorg. Med. Chem. 2007, 15, 5493–5501. [Google Scholar] [CrossRef]
- Xu, J.; Long, Y.; Ni, L.; Yuan, X.; Yu, N.; Wu, R.; Tao, J.; Zhang, Y. Anticancer effect of berberine based on experimental animal models of various cancers: A systematic review and meta-analysis. BMC Cancer 2019, 19, 589. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, H.; Zhang, B.; Cao, H.; Xu, X.; Ruan, H.; Yi, T.; Tan, L.; Qu, R.; Song, G. Berberine potently attenuates intestinal polyps growth in apcmin mice and familial adenomatous polyposis patients through inhibition of wnt signalling. J. Cell. Mol. Med. 2013, 17, 1484–1493. [Google Scholar] [CrossRef]
- Assefa, A.T.; Vandesompele, J.; Thas, O. On the utility of rna sample pooling to optimize cost and statistical power in rna sequencing experiments. BMC Genom. 2020, 21, 1–14. [Google Scholar] [CrossRef]
- Metzger, K.; Tuchscherer, A.; Palin, M.-F.; Ponsuksili, S.; Kalbe, C. Establishment and validation of cell pools using primary muscle cells derived from satellite cells of pig skeletal muscle. In Vitro Cell. Dev. Biol. Anim. 2019, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Diz, A.P.; Truebano, M.; Skibinski, D.O. The consequences of sample pooling in proteomics: An empirical study. Electrophoresis 2009, 30, 2967–2975. [Google Scholar] [CrossRef] [PubMed]
- Phang, C.-W.; Karsani, S.A.; Sethi, G.; Malek, S.N.A. Flavokawain c inhibits cell cycle and promotes apoptosis, associated with endoplasmic reticulum stress and regulation of mapks and akt signaling pathways in hct 116 human colon carcinoma cells. PLoS ONE 2016, 11, e0148775. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J. Guidelines for accurate ec50/ic50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nature Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Vasilishina, A.; Kropotov, A.; Spivak, I.; Bernadotte, A. Relative human telomere length quantification by real-time pcr. In Cellular Senescence; Springer: Berlin/Heidelberg, Germany, 2019; pp. 39–44. [Google Scholar]
| Compound | Boldine | Silymarin | Berberine |
|---|---|---|---|
| IC50 (µg/mL) | 37.87 ± 3.12 b | >200 c | 10.30 ± 0.89 a |
| Duration (Hour) | Cell Growth | Berberine Cellular Localization | |||
|---|---|---|---|---|---|
| Untreated | Treated | Phase Contrast | UV Channel | Merged | |
| 0 |  |  | 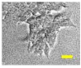 | 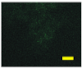 | 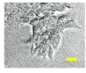 |
| 6 |  |  |  | 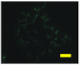 | 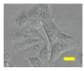 |
| 12 |  |  |  | 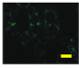 | 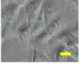 |
| 24 | 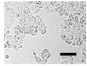 | 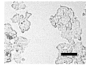 | 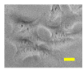 | 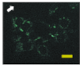 | 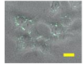 |
| 48 | 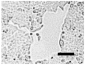 | 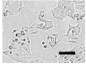 |  | 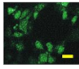 |  |
| 72 |  |  |  | 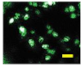 |  |
| NCBI ID | Gene | Forward (5′ to 3′) | Reverse (5′ to 3′) | Amplicon Size (bp) |
|---|---|---|---|---|
| 7015 | TERT | ACTGCGTGCGTCGGTATGC | CGGCTGGAGGTCTGTCAAGGTA | 97 |
| 7012 | TERC | AGAGGAACGGAGCGAGTC | GCATGTGTGAGCCGAGTC | 80 |
| 595 | CCND1 | AACACGGCTCACGCTTACC | GCCCCATCACGACAGACAAAG | 94 |
| 1019 | CDK4 | ATGTGGAGTGTTGGCTGTATC | CTGGTCGGCTTCAGAGTTTC | 78 |
| 2597 | GAPDH | TTGGTATCGTGGAAGGACTCA | CCAGTAGAGGCAGGGATGAT | 133 |
| 60 | ACTB | CGTCTTCCCCTCCATCGT | GCCTCGTCGCCCACATAG | 87 |
| Step | Cycles | Temperature (°C) | Duration |
|---|---|---|---|
| GoTaq® Hot Start Polymerase activation | 1 | 95 | 2 min |
| Denaturation | 40 | 95 | 15 s |
| Annealing | 53.5 | 30 s | |
| Extension | 60 | 30 s | |
| Dissociation | 1 | 60 to 95 | 5 min |
| Hold | 10 | ∞ |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samad, M.A.; Saiman, M.Z.; Abdul Majid, N.; Karsani, S.A.; Yaacob, J.S. Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules 2021, 26, 376. https://doi.org/10.3390/molecules26020376
Samad MA, Saiman MZ, Abdul Majid N, Karsani SA, Yaacob JS. Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules. 2021; 26(2):376. https://doi.org/10.3390/molecules26020376
Chicago/Turabian StyleSamad, Muhammad Azizan, Mohd Zuwairi Saiman, Nazia Abdul Majid, Saiful Anuar Karsani, and Jamilah Syafawati Yaacob. 2021. "Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116" Molecules 26, no. 2: 376. https://doi.org/10.3390/molecules26020376
APA StyleSamad, M. A., Saiman, M. Z., Abdul Majid, N., Karsani, S. A., & Yaacob, J. S. (2021). Berberine Inhibits Telomerase Activity and Induces Cell Cycle Arrest and Telomere Erosion in Colorectal Cancer Cell Line, HCT 116. Molecules, 26(2), 376. https://doi.org/10.3390/molecules26020376





