Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract
Abstract
1. Introduction
2. Results
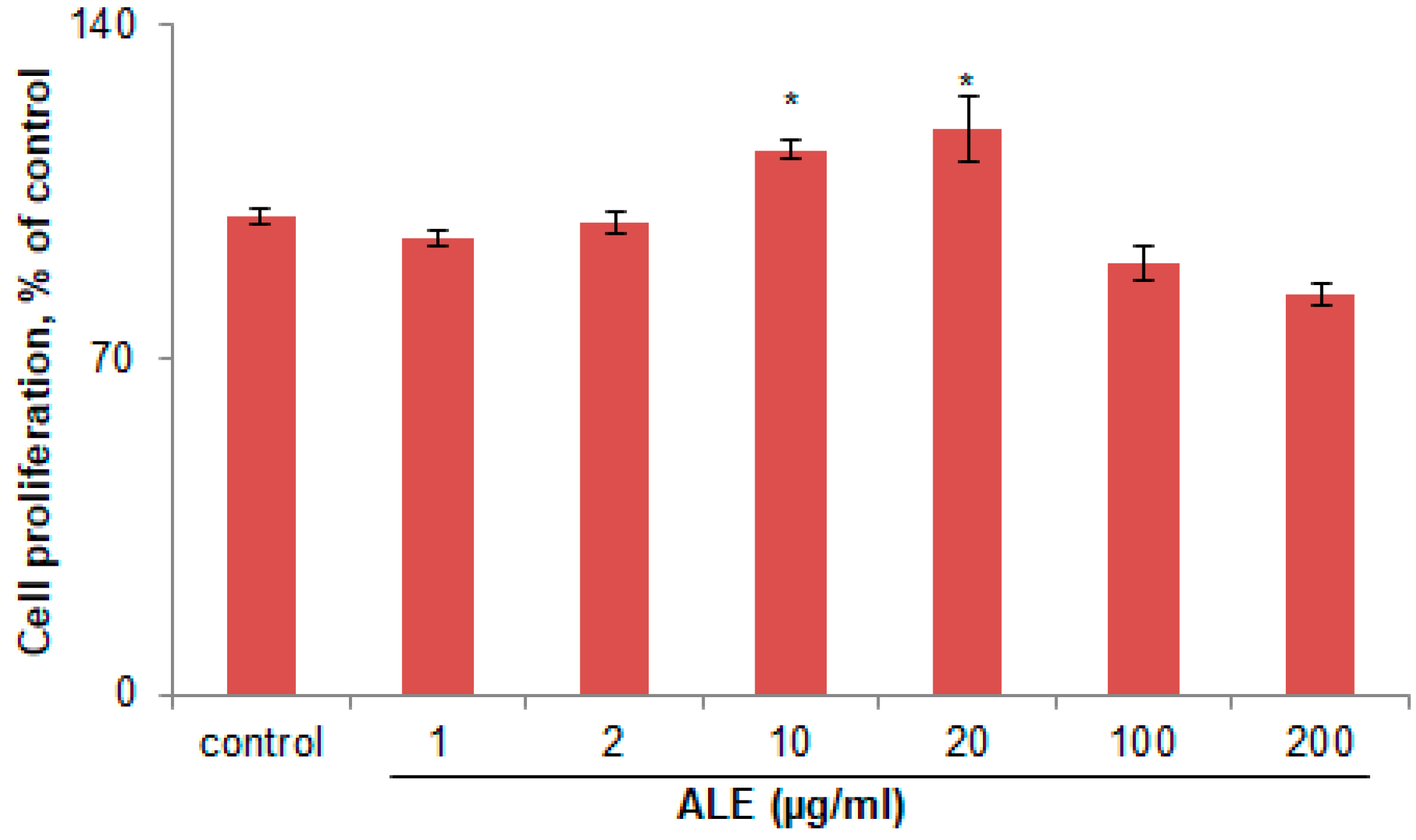
2.1. ALE Had No Cytotoxic Effect on B16 Melanoma Cells
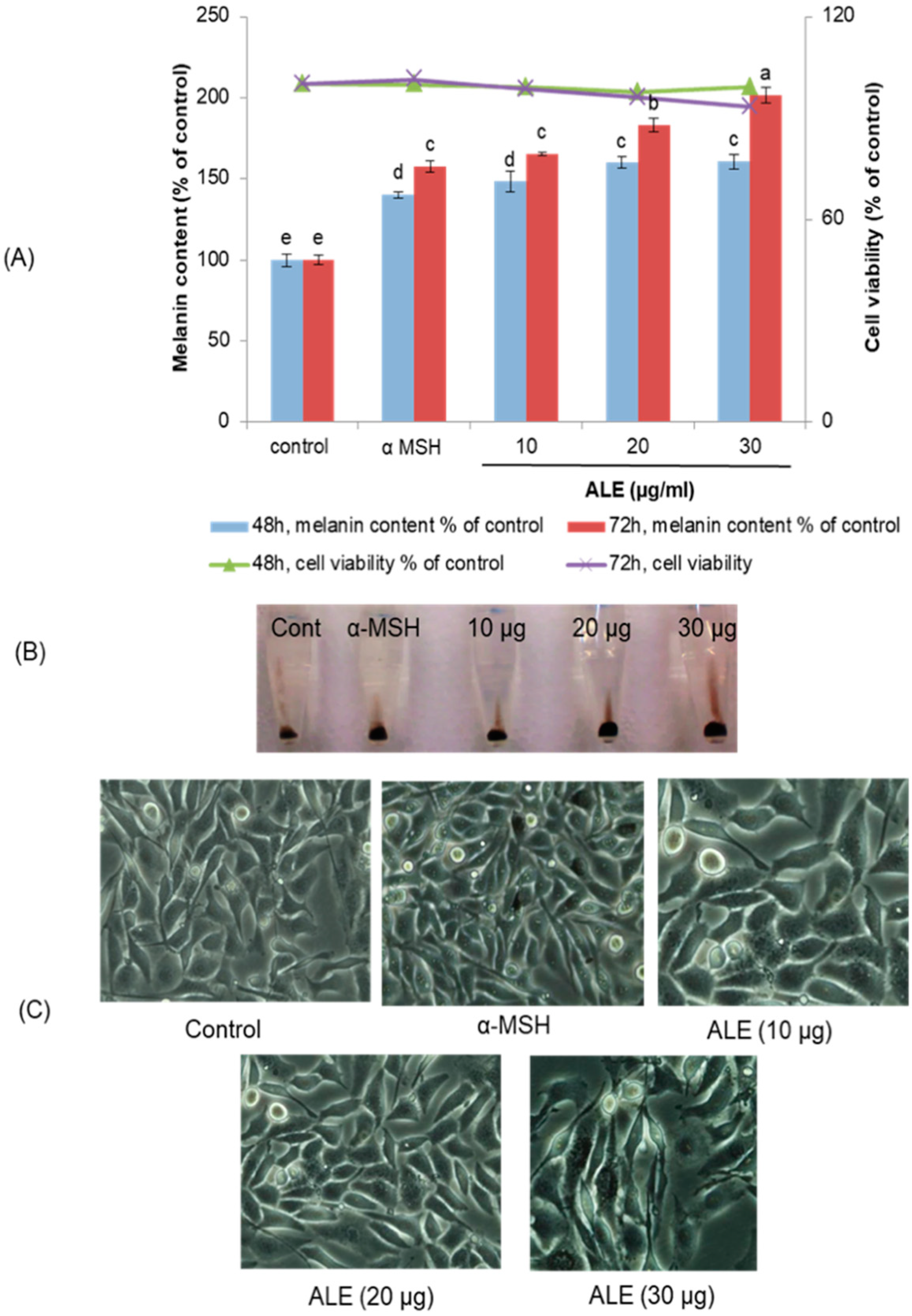
2.2. ALE Stimulated Melanogenesis in B16 Cells
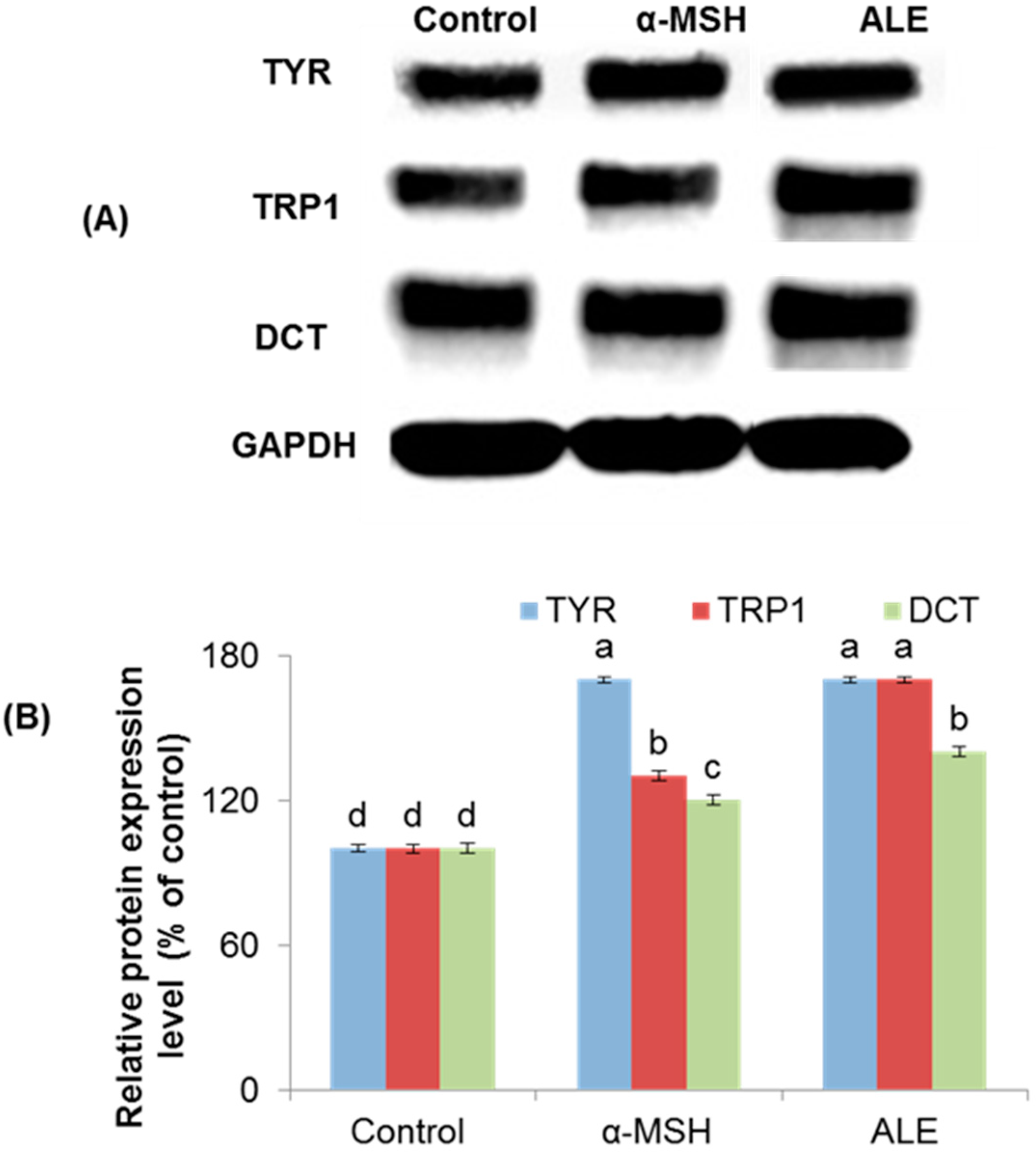
2.3. ALE Modulated the Melanogenic Enzymes Expression in B16 Cells
2.4. Antioxidant Activity of ALE
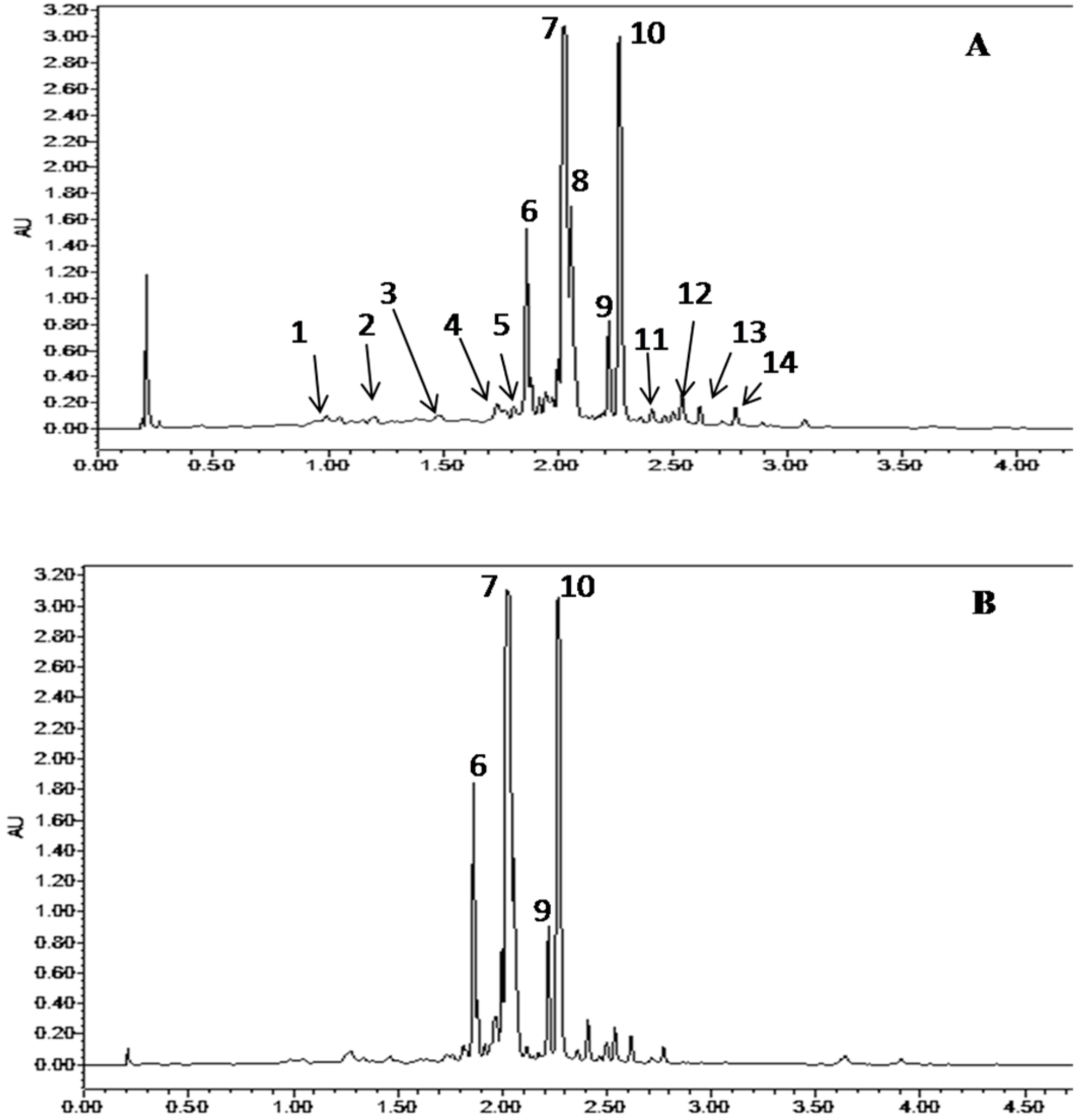
2.5. Quantification and Characterization of ALE Phenolic Compounds
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of ALE
4.3. Cell Culture
4.4. Cell Viability Assay
4.5. Measurement of Melanin Content
4.6. Western Blot
4.7. Evaluation of ALE Antioxidant Activity
4.7.1. DPPH Radical Scavenging Assay
4.7.2. Scavenging Activity on ABTS+ Radical
4.7.3. β-Carotene/Linoleic Acid Bleaching Assay
4.7.4. Reducing Power Activity
4.8. Polyphenols Quantification in ALE
4.8.1. Total Polyphenol Content (TPC)
4.8.2. Total Flavonoids Content
4.8.3. Flavanols Content
4.8.4. Proanthocyanidins Content
4.9. Phenols Characterization
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ALE | argan leaves extract |
| ABTS | 2,2’-azinobis-3-ethylbenzothiazoline- 6-sulphonate |
| BHT | butylated hydroxytoluene |
| CTE | catechin equivalents |
| CYE | cyanidin equivalents |
| DCT | dopachrome tautomerase |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl radical |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| DM | dry matter |
| GAE | gallic acid equivalents |
| MITF | microphthalmia-associated transcription factor |
| α-MSH | alpha-melanocyte stimulating hormone |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide |
| PVDF | polyvinylidene difluoride membrane |
| QE | quercetin equivalents |
| RIPA | radioimmunoprecipitation |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TPC | total phenols content |
| TFC | total flavonoids content |
| TRP1 | tyrosinase-related protein 1 |
| TYE | tyrosol equivalents |
| TYR | tyrosinase |
| UPLC-ESI-HRMS | ultra-performance liquid chromatography–electrospray-ionization-high resolution mass spectrometry |
References
- Lin, J.Y.; Fisher, D.E. Melanocyte biology and skin pigmentation. Nature 2007, 445, 843–850. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. Epidermal pigmentation in the human lineage is an adaptation to ultraviolet radiation. J. Hum. Evol. 2013, 65, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Kumarasinghe, P.; Uprety, S.; Sarkar, R. Hypopigmentary disorders in Asian patients. Pigment. Int. 2017, 4, 13. [Google Scholar] [CrossRef]
- Miazek, N.; Michalek, I.; Pawlowska-Kisiel, M.; Olszewska, M.; Rudnicka, L. Pityriasis alba—Common disease, enigmatic entity: Up to date review of the literature. Pediatr. Dermatol. 2015, 32, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Relyveld, G.N.; Dingemans, K.P.; Menke, H.E.; Bos, J.D.; Westerhof, W. Ultrastructural findings in progressive macular hypomelanosis indicate decreased melanin production. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 568–574. [Google Scholar] [CrossRef]
- Desai, S.R.; Owen, J.L. Progressive macular hypomelanosis: An update. Pigment Int. 2014, 1, 52. [Google Scholar] [CrossRef]
- Videira, I.F.D.S.; Moura, D.F.L.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef]
- Mallick, S.; Singh, S.K.; Sarkar, C.; Saha, B.; Bhadra, R. Human placental lipid induces melanogenesis by increasing the expression of tyrosinase and its related proteins in vitro. Pigment Cell. Res. 2005, 18, 25–33. [Google Scholar] [CrossRef]
- Moukal, A. L’arganier, Argania spinosa L. Skeels, usage thérapeutique, cosmétique et alimentaire. Phytothérapie 2004, 5, 135–141. [Google Scholar] [CrossRef]
- Villareal, M.O.; Kume, S.; Bourhim, T.; Bakhtaoui, F.Z.; Kashiwagi, K.; Han, J.; Gadhi, C.; Isoda, H. Activation of MITF by argan oil leads to the inhibition of the tyrosinase and dopachrome tautomerase expressions in B16 murine melanoma cells. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef]
- Bourhim, T.; Villareal, M.O.; Hafidi, A.; Gadhi, C.; Isoda, H. Depigmenting effect of argan press-cake extract through the down-regulation of Mitf and melanogenic enzymes expression in B16 murine melanoma cells. Cytotechnology 2018, 70, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Akšić, M.M.F.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Use of RP-HPLC-ESI (−)-MS/MS to differentiate various proanthocyanidin isomers in lager beer extracts. J. Am. Soc. Brew. Chem. 2008, 66, 109–115. [Google Scholar] [CrossRef]
- Barbosa-Pereira, L.; Angulo, I.; Paseiro-Losada, P.; Cruz, J.M. Phenolic profile and antioxidant properties of a crude extract obtained from a brewery waste stream. Food Res. Int. 2013, 51, 663–669. [Google Scholar] [CrossRef]
- Spínola, V.; Pinto, J.; Castilho, P.C. Identification and quantification of phenolic compounds of selected fruits from Madeira Island by HPLC-DAD–ESI-MSn and screening for their antioxidant activity. Food Chem. 2015, 173, 14–30. [Google Scholar] [CrossRef]
- Joguet, N.; Maugard, T. Characterization and quantification of phenolic compounds of Argania spinosa leaves by HPLC-PDA-ESI-MS analyses and their antioxidant activity. Chem. Nat. Compd. 2013, 48, 1069–1071. [Google Scholar] [CrossRef]
- Tahrouch, S.; Andary, C.; Rapior, S.; Mondolot, L.; Gargadennec, A.; Fruchier, A. Polyphenol investigation of Argania spinosa (Sapotaceae) endemic tree from Morocco. Acta Bot. Gallica 2000, 147, 225–232. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.M.; Arráez-Román, D.; Segura-Carretero, A. HPLC–DAD–ESI-MS/MS screening of bioactive components from Rhus coriaria L. (Sumac) fruits. Food Chem. 2015, 166, 179–191. [Google Scholar] [CrossRef]
- Sawada, H.; Fukuchi, T.; Abe, H. Oxidative stress markers in aqueous humor of patients with senile cataracts. Curr. Eye Res. 2009, 34, 36–41. [Google Scholar] [CrossRef]
- Van Staden, A.B.; Lall, N. Medicinal plants as alternative treatments for progressive macular hypomelanosis. In Medicinal Plants for Holistic Health and Well-Being; Lall, N., Ed.; Academic Press: Cambridge, MA, USA, 2018; Chapter 5; pp. 145–182. [Google Scholar] [CrossRef]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef]
- Mercolini, L.; Bedgood, D.R.; Prenzler, P.D.; Robards, K. Analytical profiling of bioactive phenolic compounds in argan (Argania spinosa) leaves by combined microextraction by packed sorbent (MEPS) and LC-DAD-MS/MS. Phytochem. Anal. 2016, 27, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, F.; Tahrouch, S.; Hatimi, A. Geoclimatic influences on flavonoids contents of the leaves of the argan tree. J. Mater. Environ. Sci. 2013, 4, 881–886. [Google Scholar]
- Djidel, S.; Chater, C.F.; Khennouf, S.; Baghiani, A.; Harzallah, D. Evaluation of phenolic compounds, flavonoids and antioxidant properties of Argania spinosa (L.) Skeels leaf extracts. Glob. J. Res. Med. Plants Indig. Med. 2014, 3, 416–426. [Google Scholar]
- El Adib, S.; Aissi, O.; Charrouf, Z.; Ben Jeddi, F.; Mess aoud, C. Argania spinosa var. mutica and var. apiculata: Variation of fatty-acid composition, phenolic content, and antioxidant and α-amylase inhibitory activities among varieties, organs, and development stages. Chem. Biodivers. 2015, 12, 1322–1338. [Google Scholar] [CrossRef] [PubMed]
- Dakiche, H.; Khali, M.; Abu-el-haija, A.K.; Al-Maaytah, A.; Al-balas, Q.A. Biological activities and phenolic contents of Argania spinosa L. (Sapotaceae) leaf extract. Trop. J. Pharm. Res. 2016, 15, 2563–2570. [Google Scholar] [CrossRef]
- Kechebar, M.S.A.; Karoune, S.; Falleh, H.; Belhamra, M.; Rahmoune, C.; Ksouri, R. LC-MS phenolic composition changes and antioxidant capacities of the Saharan tree Argania spinosa leaves under salinity. Courr. Savoir 2017, 23, 29–38. [Google Scholar]
- Lfitat, A.; Zejli, H.; Bousselham, A.; El Atki, Y.; Lyoussi, B.; Gourch, A.; Abdellaoui, A. Comparative Evaluation of Argania spinosa and Olea europaea Leaf Phenolic Compounds and their Antioxidant Activity. Botanica 2020, 26, 76–87. [Google Scholar] [CrossRef]
- Cooper-Driver, G.A.; Bhattacharya, M. Role of phenolics in plant evolution. Phytochemistry 1998, 49, 1165–1174. [Google Scholar]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Al-rimawi, F.; Odeh, I.; Bisher, A.; Abb adi, J.; Qabb ajeh, M. Effect of geographical region and harvesting date on antioxidant activity, phenolic and flavonoid content of olive leaves. J. Food Nutr. Res. 2014, 2, 925–930. [Google Scholar] [CrossRef]
- Herrera, M.C.; de Castro, M.D. Ultrasound-assisted extraction of phenolic compounds from strawberries prior to liquid chromatographic separation and photodiode array ultraviolet detection. J. Chromatogr. A 2005, 1100, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72, S159–S166. [Google Scholar] [CrossRef] [PubMed]
- Takekoshi, S.S.; Nagata, H.; Kitatani, K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai. J. Exp. Clin. Med. 2014, 39, 116–121. [Google Scholar]
- Ye, Y.; Chou, G.X.; Wang, H.; Chu, J.H.; Yu, Z.L. Flavonoids, apigenin and icariin exert potent melanogenic activities in murine B16 melanoma cells. Phytomedicine 2010, 18, 32–35. [Google Scholar] [CrossRef]
- Villareal, M.O.; Kume, S.; Neffati, M.; Isoda, H. Upregulation of Mitf by phenolic compounds-rich Cymbopogon schoenanthus treatment promotes melanogenesis in B16 melanoma cells and human epidermal melanocytes. Biomed. Res. Int. 2017, 2017, 8303671. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Hayakawa, H.; Taketomi, A.; Sakumi, K.; Kuwano, M.; Sekiguchi, M. Generation and elimination of 8-oxo-7, 8-dihydro-2’-deoxyguanosine 5’-triphosphate, a mutagenic substrate for DNA synthesis, in human cells. Biochemistry 1995, 34, 89–95. [Google Scholar] [CrossRef]
- Davies, K.J. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life 2000, 50, 279–289. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Eller, M.S.; Geller, A.C.; Yaar, M. The pathogenesis of melanoma induced by ultraviolet radiation. N. Engl. J. Med. 1999, 340, 1341–1348. [Google Scholar] [CrossRef]
- Jablonski, N.G.; Chaplin, G. The evolution of human skin coloration. J. Hum. Evol. 2000, 39, 57–106. [Google Scholar] [CrossRef]
- Von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Li, H.B.; Wong, C.C.; Cheng, K.W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Miraliakbari, H.; Shahidi, F. Antioxidant activity of minor components of tree nut oils. Food Chem. 2008, 111, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Bounatirou, S.; Smiti, S.; Miguel, M.G.; Faleiro, L.; Rejeb, M.N.; Neffati, M.; Pedro, L.G. Chemical composition, antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff. et Link. Food Chem. 2007, 105, 146–155. [Google Scholar] [CrossRef]
- Catalano, L.; Franco, I.; De Nobili, M.; Leita, L. Polyphenols in olive mill wastewaters and their depuration plant effluents: A comparison of the Folin-Ciocalteau and HPLC methods. Agrochimica 1999, 43, 193–205. [Google Scholar]
- Kim, D.O.; Chun, O.K.; Kim, Y.J.; Moon, H.Y.; Lee, C.Y. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 2003, 51, 6509–6515. [Google Scholar] [CrossRef]
- Nigel, C.W.; Glories, Y. Use of a modified dimethylamino-cinnamaldehyde reagent for analysis of flavanols. Am. J. Enol. Vitic. 1991, 42, 364–366. [Google Scholar]
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 1994. [Google Scholar]
| Parameters | ALE | BHT | Ascorbic Acid | |
|---|---|---|---|---|
| Phenol content | TPC (TYE mg/g) | 198.61 ± 0.20 | - | - |
| Flavonoids (CAE mg/g) | 128.41 ± 1.58 | - | - | |
| Flavanols (CAE mg/g) | 59.59 ± 1.55 | - | - | |
| Proanthocyanidins (CYE mg/g) | 6.85 ± 0.13 | - | - | |
| Antioxidant activities (IC50 mg/mL) | DPPH | 0.508 ± 0.016 c | 0.147 ± 0.001 b | 0.071 ± 0.003 a |
| Reducing power | 0.316 ± 0.011 b | 0.354 ± 0.011 c | 0.070 ± 0.011 a | |
| β-carotene | 1.679 ± 0.070 b | 0.050 ± 0.001 a | - | |
| ABTS | 0.373 ± 0.001 c | 0.340 ± 0.001 b | 0.078 ± 0.001 a |
| Peak Number | Retention Time, min (Area%) | Compound Identity | Molecular Formula | UPLC UV/Vis λmax (nm) | Experimental m/z (M − H)− | Theoretical m/z (M − H)− | References |
|---|---|---|---|---|---|---|---|
| 1 | 0.992 (1.36) | Gallic acid | C7H6O5 | - | 169.014 | 169.0135 | [12] |
| 2 | 1.204 (2.19) | (+)-Gallocatechin | C15H14O7 | - | 305.0654 | 305.0657 | [12,13] |
| 3 | 1.49 (3.10) | (−)-Epigallocatechin | C15H14O7 | - | 305.0657 | 305.0657 | [12,13] |
| 4 | 1.736 (3.18) | (+)-Catechin | C15H14O6 | - | 289.0707 | 289.0708 | [14] |
| 5 | 1.801 (2.95) | (−)-Epicatechin | C15H14O6 | - | 289.0707 | 289.0708 | [14] |
| 6 | 1.86 (8.37) | Quercetin-3-O-Glucuronide | C21H18O13 | 231.9–253.3–347.1 | 477.1035 | 477.0663 | [15] |
| 7 | 2.03 (35.67) | Myricetin-3-O-Galactoside | C21H20O13 | 250.9–266.2–326.6 | 479.0825 | 479.0819 | [16,17] |
| 8 | 2.201 (4.35) | Unknown | - | - | 479.082 | - | - |
| 9 | 2.266 (19.39) | Myricitrin | C21H20O14 | - | 463.0877 | 463.087 | [16,17] |
| 10 | 2.408 (1.23) | Quercitrin | C21H20O11 | 250.3–266.2–326.6 | 447.093 | 447.0921 | [16,17] |
| 11 | 2.538 (3.96) | Quercetin-7-O-rhamnoside | C21H20O11 | - | 447.0929 | 447.0921 | [18] |
| 12 | 2.663 (0.33) | Myricetin | C15H10O8 | 222.2–304.3 | 317.029 | 317.0294 | [17] |
| 13 | 2.774 (2.30) | Rutin (Quercetin-3-O-rutinoside) | C30H26O14 | 257.0–313.0 | 609.1234 | 609.1236 | [15] |
| 14 | 3.073 (0.95) | Quercetin | C15H10O7 | 254.5–370.1 | 301.0345 | 301.0345 | [19] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourhim, T.; Villareal, M.O.; Couderc, F.; Hafidi, A.; Isoda, H.; Gadhi, C. Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract. Molecules 2021, 26, 371. https://doi.org/10.3390/molecules26020371
Bourhim T, Villareal MO, Couderc F, Hafidi A, Isoda H, Gadhi C. Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract. Molecules. 2021; 26(2):371. https://doi.org/10.3390/molecules26020371
Chicago/Turabian StyleBourhim, Thouria, Myra O. Villareal, François Couderc, Abdellatif Hafidi, Hiroko Isoda, and Chemseddoha Gadhi. 2021. "Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract" Molecules 26, no. 2: 371. https://doi.org/10.3390/molecules26020371
APA StyleBourhim, T., Villareal, M. O., Couderc, F., Hafidi, A., Isoda, H., & Gadhi, C. (2021). Melanogenesis Promoting Effect, Antioxidant Activity, and UPLC-ESI-HRMS Characterization of Phenolic Compounds of Argan Leaves Extract. Molecules, 26(2), 371. https://doi.org/10.3390/molecules26020371










