Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens
Abstract
1. Introduction
2. Results and Discussions
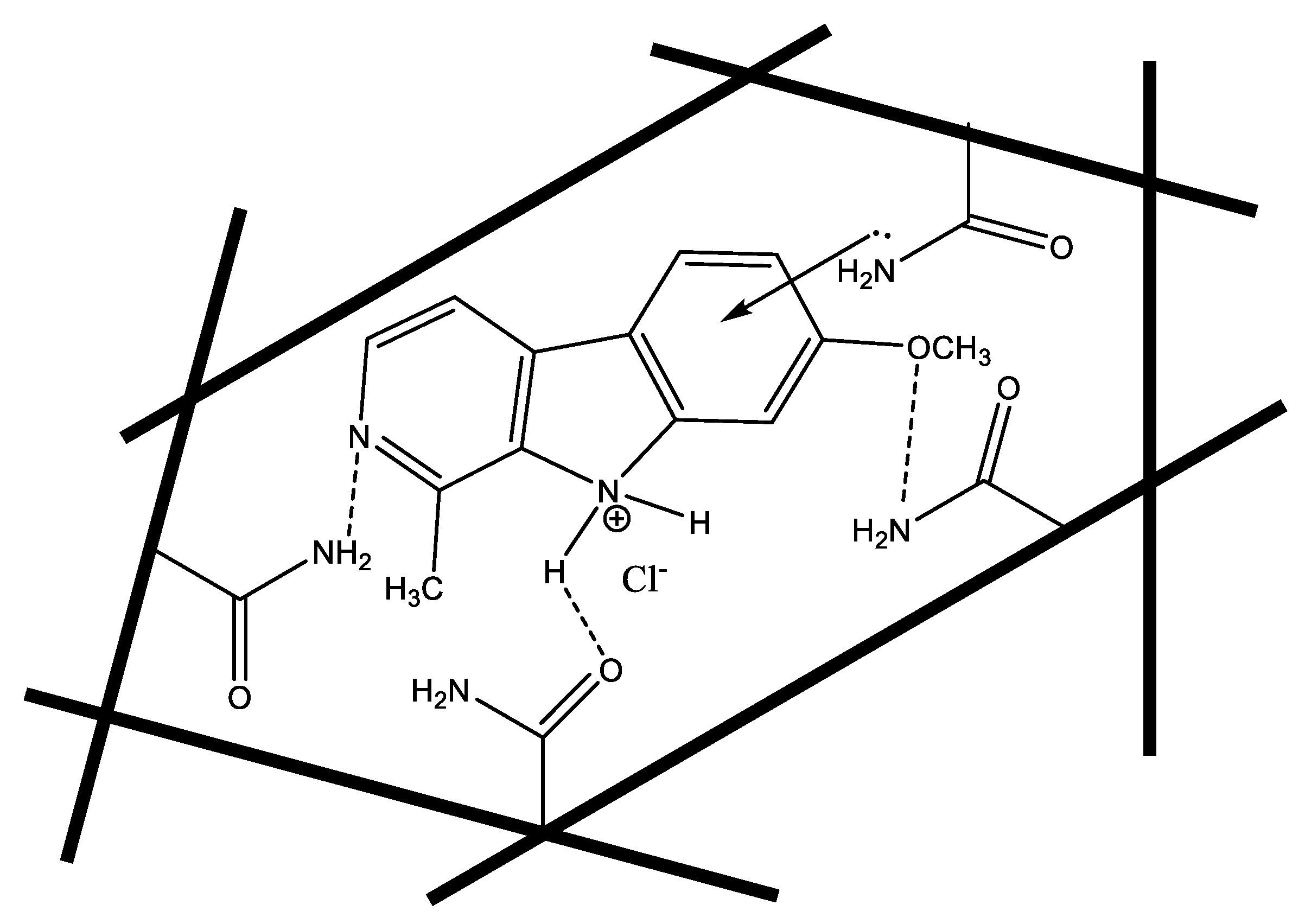
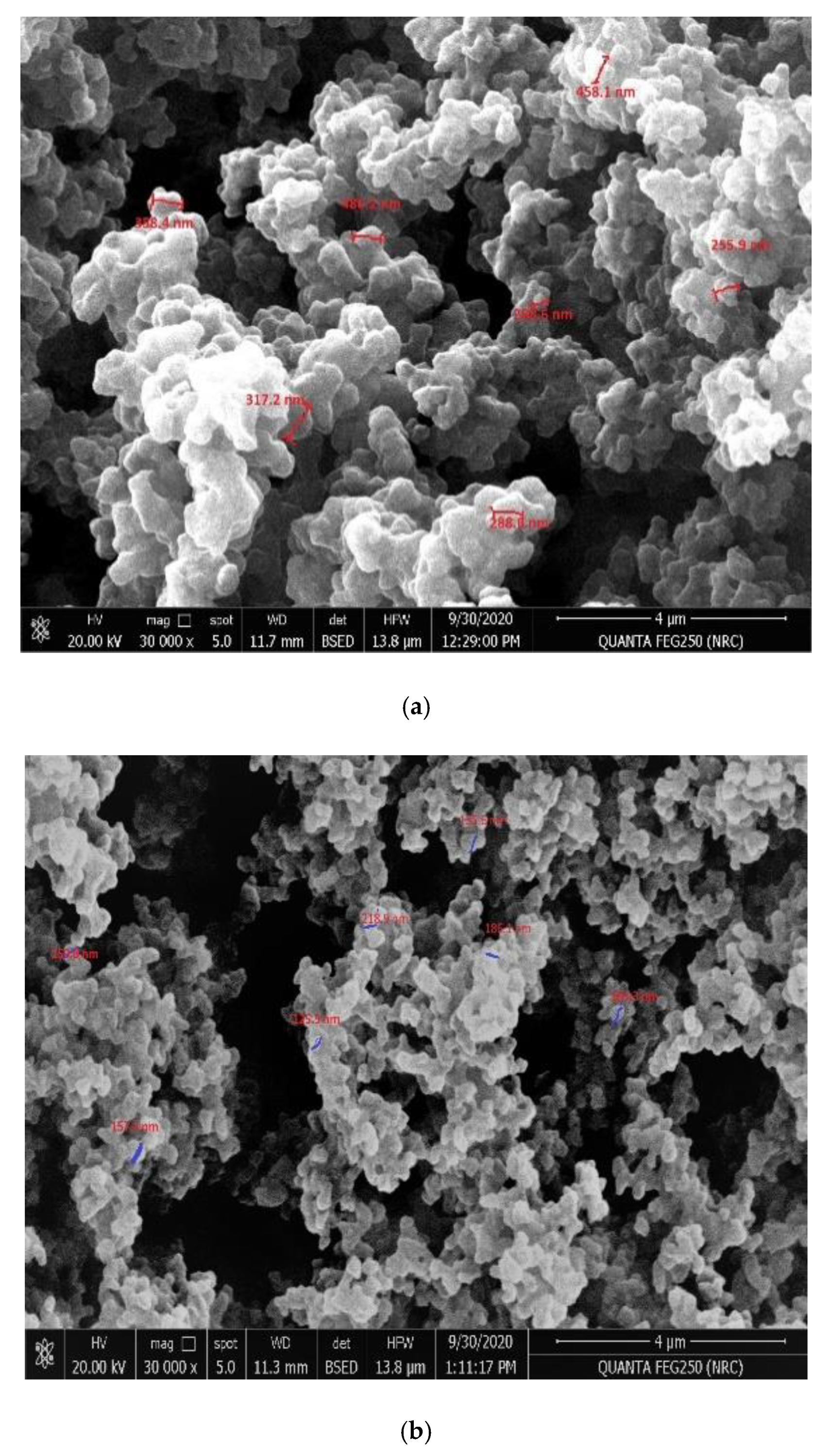
2.1. Polymers’ Characterizations
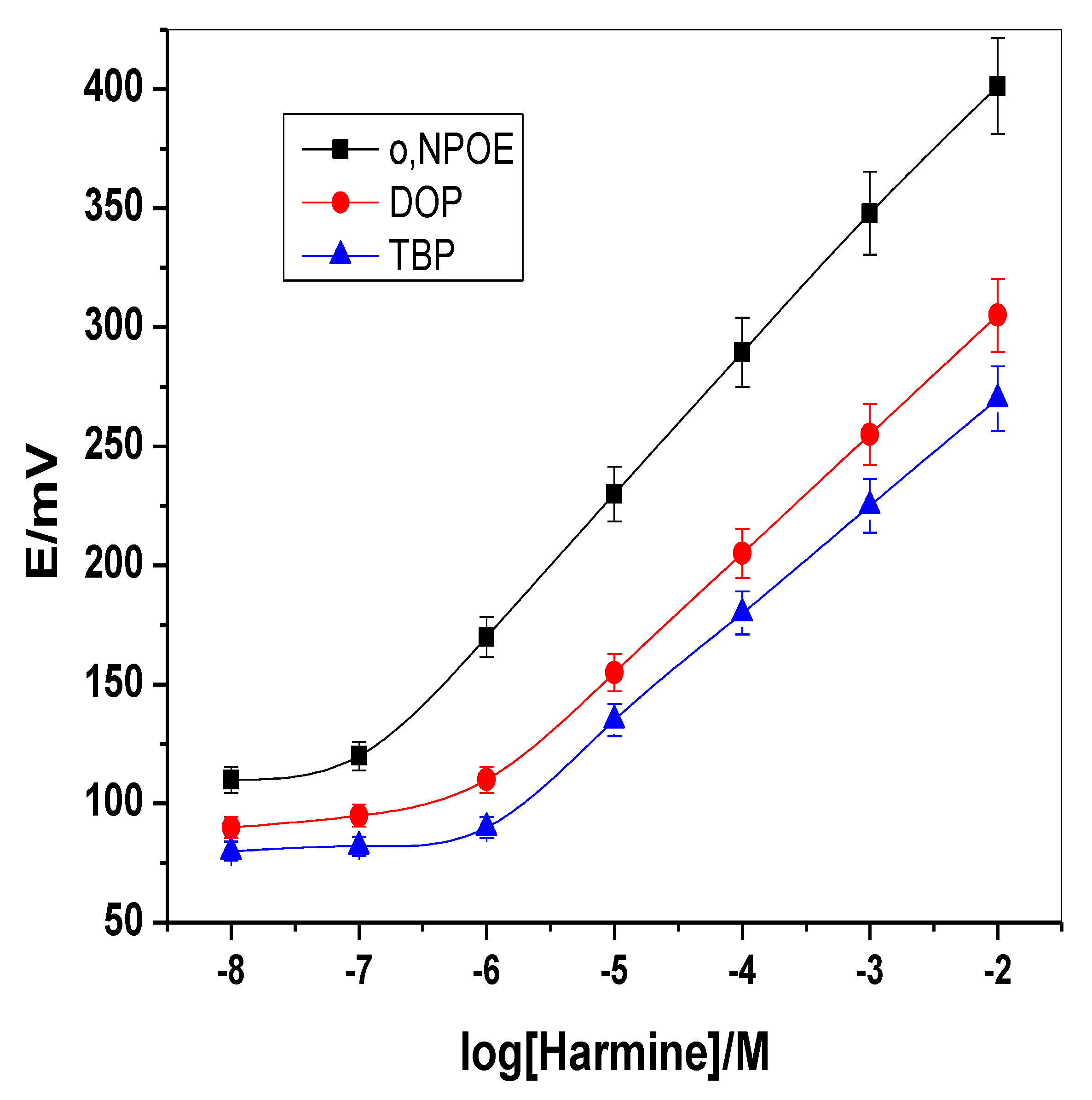
2.2. Membrane Optimization
2.3. Time Response and pH Effect
2.4. Selectivity Behavior
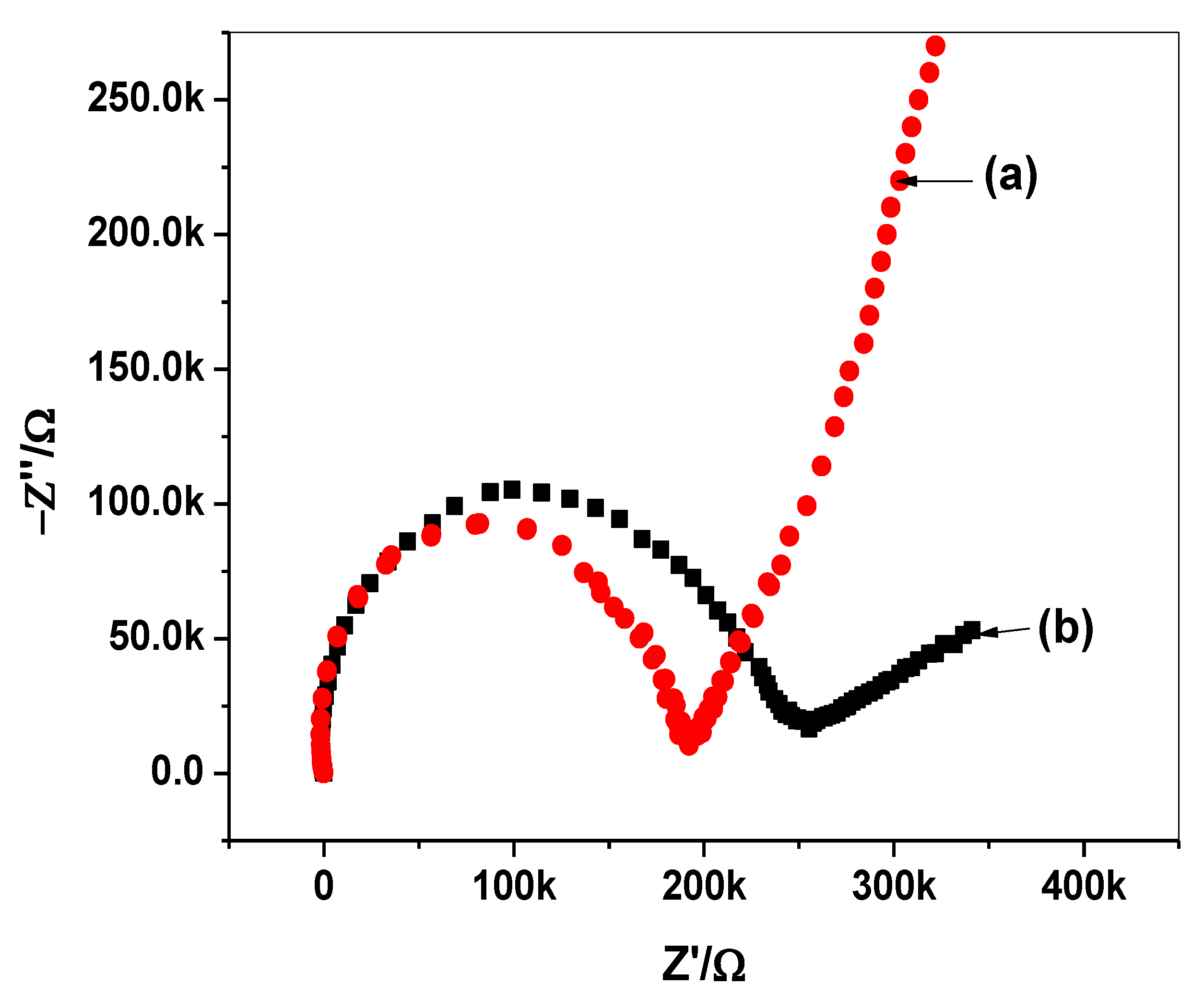
2.5. Impedance Spectroscopy and Chronpotentiometry Measurements
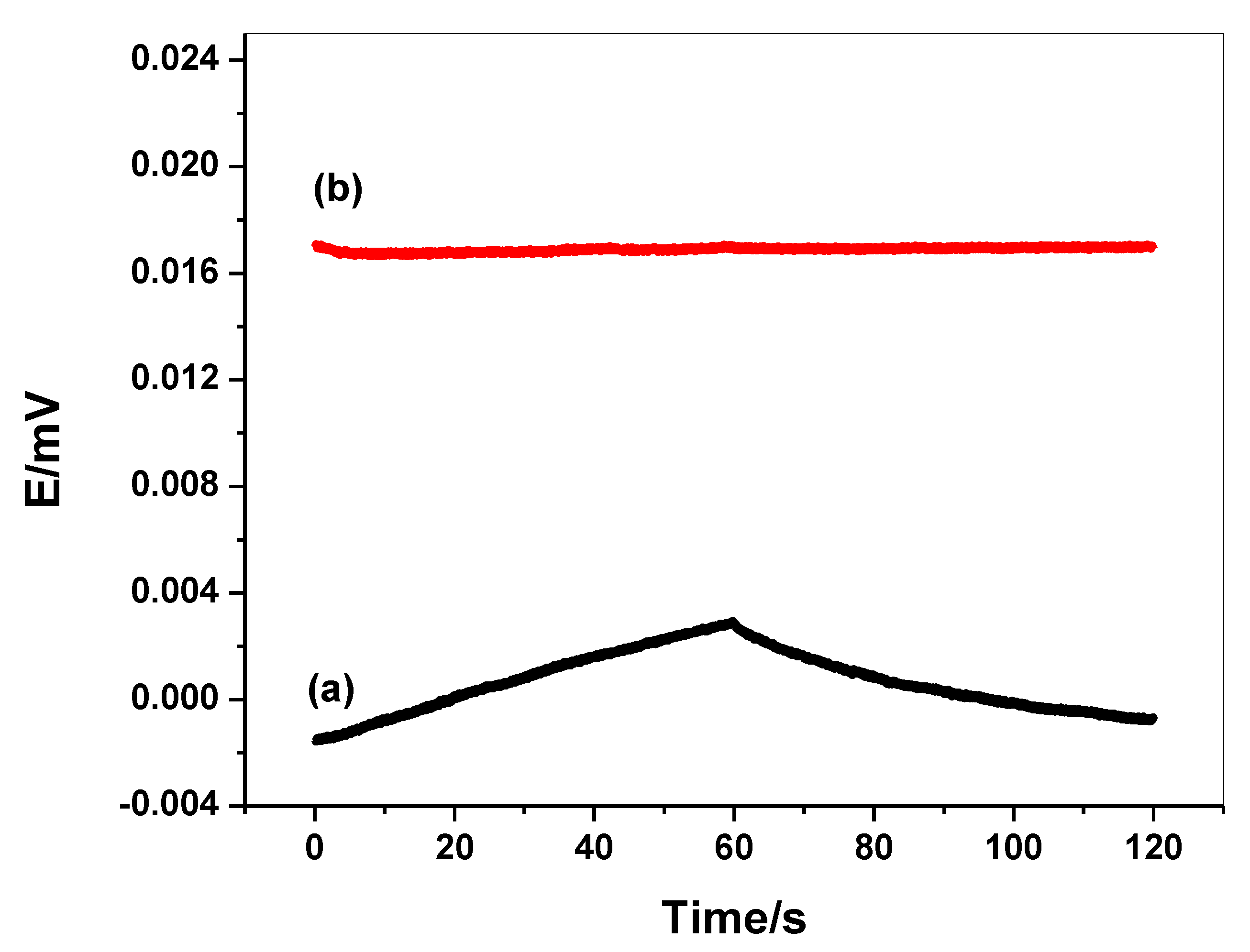
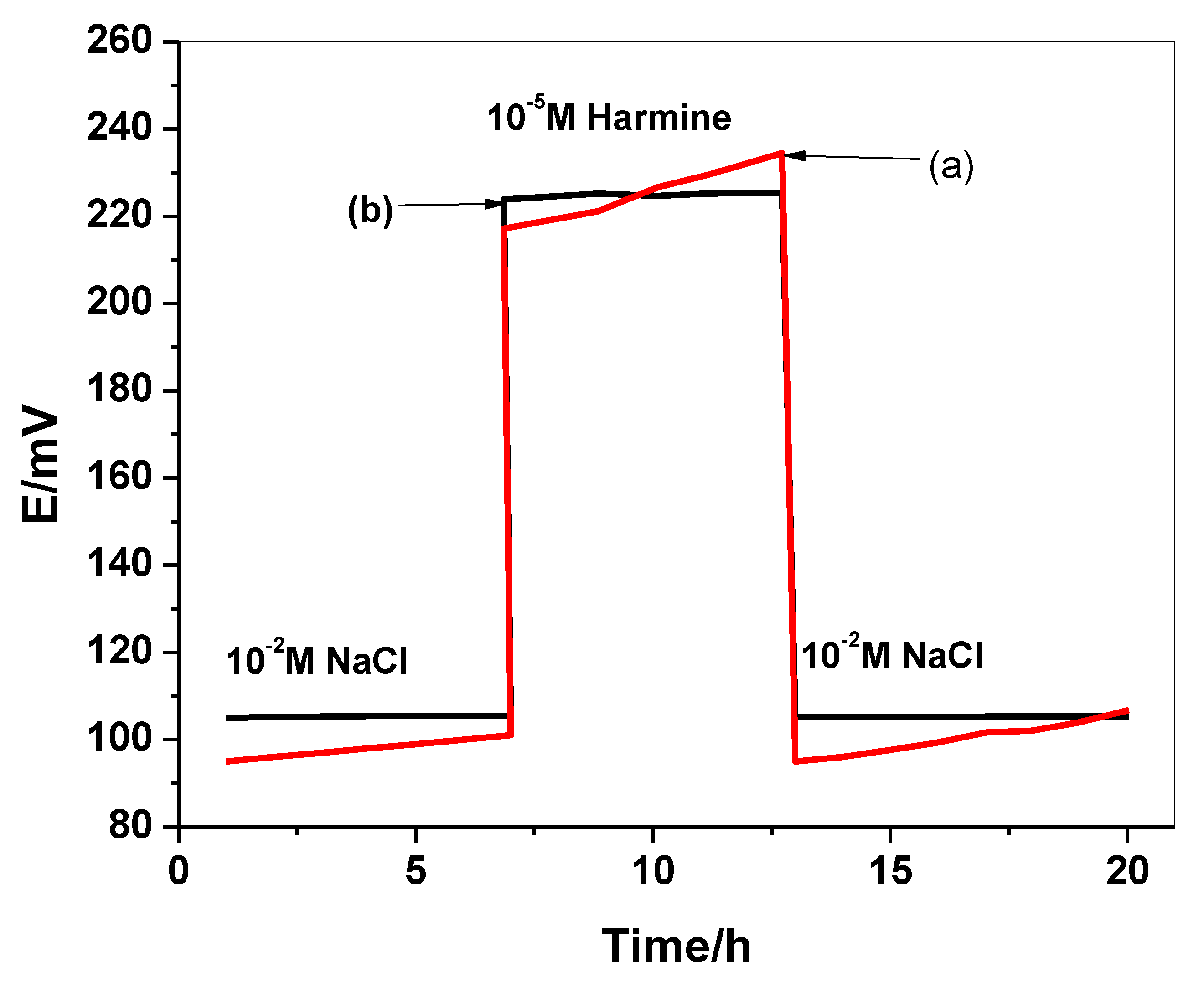
2.6. Water-Layer Test
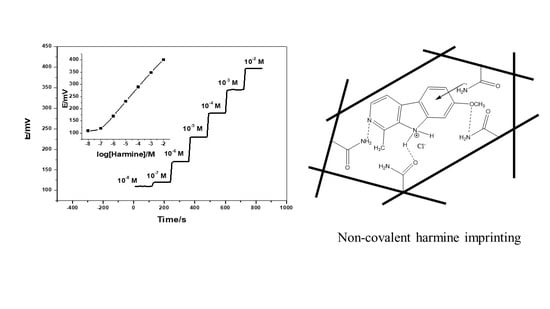
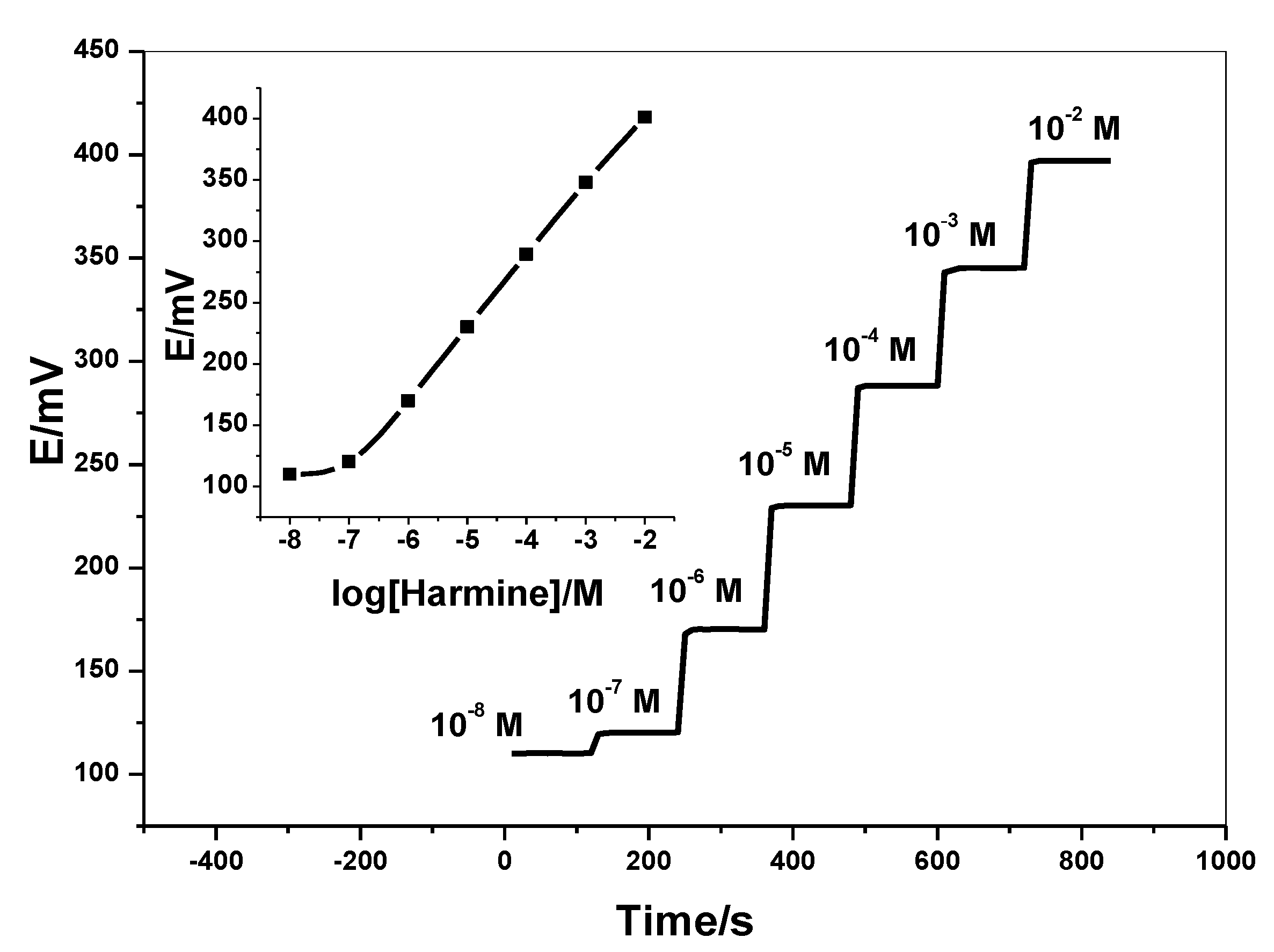
2.7. Analytical Assessment of Harmine
3. Materials and Methods
3.1. Apparatus
3.2. Chemicals and Reagents
3.3. Biomimics Synthesis
3.4. Sensors’ Fabrication
3.5. Electrochemical Measurements
3.6. Assessment of Harmine in Urinary Specimens
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Southon, I.W.; Buckingham, J. (Eds.) Dietzonary of Alkaloids; Chapman and Hall: London, UK, 1989; p. 487. [Google Scholar]
- Chen, D.; Tian, X.; Zou, X.; Xu, S.; Wang, H.; Zheng, N.; Wu, Z. Harmine, a small molecule derived from natural sources, inhibits enterovirus 71 replication by targeting NF-κB pathway. Int. Immunopharm. 2018, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, H.A.; Issa, M.Y. In vitro and in vivo activity of Peganum harmala L. alkaloids against phytopathogenic bacteria. Sci. Hortic. 2020, 264, 108940. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.; Gong, Y.; Wang, P.; Zhu, L.; Tong, L.J.; Chen, X.; Ling, Y.; Huang, C. Harmine produces antidepressant-like effects via restoration of astrocytic functions. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2017, 79, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, M.; Tan, S.; Wang, C.; Fan, S.; Huang, C. Harmine is an inflammatory inhibitor through the suppression of NF-kappaB signaling. Biochem. Biophys. Res. Commun. 2017, 489, 332–338. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, M.; Gupta, S.P.; Pandav, K.; Kumar, S. Spectroscopic and structural studies on the interaction of an anticancer beta-carboline alkaloid, harmine with GC and AT specific DNA oligonucleotides. Chem. Biol. Interact. 2016, 260, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Atefeh, J.; Farid, D.; Mohammad, M.Z. Alkaloids of Peganum harmala: Anticancer Biomarkers with Promising Outcomes. Curr. Pharma. Des. 2020. [Google Scholar] [CrossRef]
- Wang, P.; Alvarez-Perez, J.; Felsenfeld, D.P.; Liu, H.; Sivendran, S.; Bender, A.; Kumar, A.; Sanchez, R.; Scott, D.K.; Garcia-Ocaña, A.; et al. A high-throughput chemical screen reveals that harmine-mediated inhibition of DYRK1A increases human pancreatic beta cell replication. Nat. Med. 2015, 21, 383–388. [Google Scholar] [CrossRef]
- Swan, S.A. An Introduction to the Alkaloids; Blackwell: Oxford, UK, 1967; p. 487. [Google Scholar]
- Brito-da-Costa, A.M.; Dias-da-Silva, D.; Gomes, N.G.M.; Dinis-Oliveira, R.J.; Madureira-Carvalho, A. Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,N-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact. Pharmaceuticals 2020, 13, 334. [Google Scholar] [CrossRef]
- Nadkarni, K.M. (Ed.) Indian Materia Medica; Popular Prakashan: Bombay, India, 1984; Volume I, p. 928. [Google Scholar]
- Agüí, L.; Peña-Farfal, C.; Yáñez-Sedeño, P.; Pingarrón, J.M. Determination of betacarboline alkaloids in foods and beverages by high-performance liquid chromatography with electrochemical detection at a glassy carbon electrode modified with carbon nanotubes. Anal. Chim. Acta. 2007, 585, 323–330. [Google Scholar] [CrossRef]
- Herraiz, T.; Chaparro, C. Analysis of monoamine oxidase enzymatic activity by reversed-phase high performance liquid chromatography and inhibition by betacarboline alkaloids occurring in foods and plants. J. Chromatogr. A 2006, 1120, 237–243. [Google Scholar] [CrossRef]
- Herraiz, T.; Chaparro, C. Human monoamine oxidase is inhibited by tobacco smoke: Beta-carboline alkaloids act as potent and reversible inhibitors. Biochem. Biophys. Res. Commun. 2005, 326, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H. High-performance liquid chromatographic analysis of beta-carbolines in human scalp hair. J. Chromatogr. A 2004, 1031, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Stockigt, J.; Sheludko, Y.; Unger, M.; Gerasimenko, I.; Warzecha, H.; Stockigt, D. HPLC-, CE- and CE/MS analysis of selected alkaloid groups. J. Chromatogr. A 2002, 967, 85–113. [Google Scholar] [PubMed]
- Casal, S.; Mendes, E.; Fernandes, J.; Oliveira, M.B.P.P.; Ferreira, M.A. Analysis of heterocyclic aromatic amines in foods by gas chromatography-mass spectrometry as their tert-butyldimethylsilyl derivatives. J. Chromatogr. A 2004, 1040, 105–113. [Google Scholar] [CrossRef]
- Ashmawy, N.H.; Almehizia, A.A.; Youssef, T.A.; El-Galil, E.A.A.; Al-Omar, M.A.; Kamel, A.H. Novel Carbon/PEDOT/PSS-Based Screen-Printed Biosensors for Acetylcholine Neurotransmitter and Acetylcholinesterase Detection in Human Serum. Molecules 2019, 24, 1539. [Google Scholar] [CrossRef]
- Guerreiro, J.R.L.; Kamel, A.H.; Sales, M.G.F. FIA potentiometric system based on periodate polymeric membrane sensors for the assessment of ascorbic acid in commercial drinks. Food Chem. 2010, 120, 934. [Google Scholar] [CrossRef][Green Version]
- Kamel, A.H.; Amr, A.E.; Galal, H.R.; Al-Omar, M.A.; Almehizia, A.A. Screen-Printed Sensor Based on Potentiometric Transduction for Free Bilirubin Detection as a Biomarker for Hyperbilirubinemia Diagnosis. Chemosensors 2020, 8, 86. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Kamel, A.H.; Amr, A.E. Article All-Solid-State Calcium Sensors Modified with Polypyrrol (PPY) and Graphene Oxide (GO) as Solid-Contact Ion-to-Electron Transducers. Chemosensors 2020, 8, 93. [Google Scholar] [CrossRef]
- Eldin, A.G.; Amr, A.E.-G.E.; Kamel, A.H.; Hassan, S.S.M. Screen-printed Microsensors Using Polyoctyl-thiophene (POT) Conducting Polymer as Solid Transducer for Ultratrace Determination of Azides. Molecules 2019, 24, 1392. [Google Scholar] [CrossRef]
- Kamel, A.H.; Hassan, A.M.E. Solid Contact Potentiometric Sensors Based on Host-Tailored Molecularly Imprinted Polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- El-Naby, E.H.; Kamel, A.H. Potential transducers based man-tailored biomimetic sensors for selective recognition of dextromethorphan as an antitussive drug. Mater. Sci. Eng. C 2015, 54, 217–224. [Google Scholar] [CrossRef] [PubMed]
- El-Kosasy, A.; Kamel, A.H.; Hussin, L.; Ayad, M.F.; Fares, N. Mimicking new receptors based on molecular imprinting and their application to potentiometric assessment of 2,4-dichlorophenol as a food taint. Food Chem. 2018, 250, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Kamel, A.H.; Jiang, X.; Li, P.; Liang, R. A paper-based potentiometric sensing platform based on molecularly imprinted nanobeads for determination of bisphenol A. Anal. Methods 2018, 10, 3890–3895. [Google Scholar] [CrossRef]
- Kamel, A.H.; Soror, T.Y.; Al-Romian, F.M. Graphite Solid-Contact Mepiquat Potentiometric Sensors Based on Molecularly Imprinted Polymers and Their Application to Flow Through Analysis. Anal. Methods 2012, 4, 3007–3012. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Badr, I.H.A.; Kamel, A.H.; Mohamed, M.S. A Novel Poly (vinyl chloride) Matrix Membrane Sensor for Batch and Flow-Injection Determination of Thiocyanate, Cyanide and Some Metal Ions. Anal. Sci. 2009, 25, 911–917. [Google Scholar] [CrossRef]
- Kamel, A.H.; Sayour, H.E.M. Flow-Through Assay of Quinine Using Solid Contact Potentiometric Sensors Based on Molecularly Imprinted Polymers. Electroanalysis 2009, 21, 2701–2708. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Elnemma, E.M.; Hamada, M.A. Flow injection potentiometric determination of harmine and harmaline hallucinogens. Electroanalysis 1995, 7, 656–659. [Google Scholar] [CrossRef]
- Hassan, S.S.M.; Amr, A.E.; ElBehery, N.H.A.; Al-Omar, M.A.; Kamel, A.H. Non-Equilibrium Potential Responses towards Neutral Orcinol Using All-Solid-State Potentiometric Sensors Integrated with Molecularly Imprinted Polymers. Polymers 2019, 11, 1232. [Google Scholar] [CrossRef]
- Kamel, A.H.; Mohammad, S.G.; Awwad, N.S.; Mohammed, Y.Y. Survey on the Integration of Molecularly Imprinted Polymers as Artificial Receptors in Potentiometric Transducers for pharmaceutical Drugs. Int. J. Electrochem. Sci. 2019, 14, 2085–2124. [Google Scholar] [CrossRef]
- Kamel, A.H.; Mahmoud, W.H.; Mostafa, M.S. Biomimetic ciprofloxacin sensors made of molecularly imprinted network receptors for potential measurements. Anal. Methods 2011, 3, 957–964. [Google Scholar] [CrossRef]
- Guan, G.; Liu, B.; Wang, Z.; Zhang, Z. Imprinting of Molecular Recognition Sites on Nanostructures and Its Applications in Chemosensors. Sensors 2008, 8, 8291–8320. [Google Scholar] [CrossRef] [PubMed]
- Mahony, J.O.; Nolan, K.; Smyth, M.R.; Mizaikoff, B. Molecularly Imprinted Polymers—Potential and Challenges in Analytical Chemistry. Anal. Chim. Acta 2005, 36, 31–39. [Google Scholar] [CrossRef]
- Moreira, F.T.C.; Guerreiro, J.R.L.; Vera, L.O.; Azevedo, L.O.V.; Kamel, A.H.; Sales, M.G.F. New potentiometric sensors for the determination of tetracycline in biological samples: Batch and flow mode operations. Anal. Methods 2010, 2, 2039. [Google Scholar] [CrossRef][Green Version]
- Abdalla, N.S.; Youssef, M.A.; Algarni, H.; Awwad, N.S.; Kamel, A.H. All Solid-State Poly (Vinyl Chloride) Membrane Potentiometric Sensor Integrated with Nano-Beads Imprinted Polymers for Sensitive and Rapid Detection of Bispyribac Herbicide as Organic Pollutant. Molecules 2019, 24, 712. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, N.S.; Amr, A.E.; El-Tantawy, A.S.M.; Al-Omar, M.A.; Kamel, A.H.; Khalifa, N.M. Tailor-Made Specific Recognition of Cyromazine Pesticide Integrated in a Potentiometric Strip Cell for Environmental and Food Analysis. Polymers 2019, 11, 1526. [Google Scholar] [CrossRef]
- Kartal, M.; Altun, M.L.; Kurucu, S. HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J. Pharm. Biomed. Anal. 2003, 31, 263–269. [Google Scholar] [CrossRef]
- Kang, S.; Green, J.P. Steric and Electronic Relationships among Some Hallucinogenic Compounds. Proc. Natl. Acad. Sci. USA 1970, 67, 62–67. [Google Scholar] [CrossRef]
- Rose, A.; Zhu, Z.; Madigan, C.F.; Swager, T.M.; Bulović, V. Sensitivity gains in chemosensing by lasing action inorganic polymers. Nature 2005, 434, 876–879. [Google Scholar] [CrossRef]
- Renkecz, T.; Mistlberger, G.; Pawlak, M.; Horváth, V.; Bakker, E. Molecularly Imprinted Polymer Microspheres Containing Photoswitchable Spiropyran-Based Binding Sites. ACS Appl. Mater. Interfaces 2013, 5, 8537–8545. [Google Scholar] [CrossRef]
- Wulff, C. The role of binding-site interactions in the molecular imprinting of polymers. Trends Biotechnol. 1993, 11, 85–87. [Google Scholar] [CrossRef]
- IUPAC. Analytical chemistry division, commission on analytical nomenclature. Pure Appl. Chem. 1995, 67, 507. [Google Scholar]
- Bakker, E. Determination of Improved Selectivity Coefficients of Polymer Membrane Ion-Selective Electrodes by Conditioning with a Discriminated Ion. J. Electrochem. Soc. 1996, 143, L83–L85. [Google Scholar] [CrossRef]
- Bobacka, J. Potential Stability of All-Solid-State Ion-Selective Electrodes Using Conducting Polymers as Ion-to-Electron Transducers. Anal. Chem. 1999, 71, 4932–4937. [Google Scholar] [CrossRef] [PubMed]
| Parameter | C/Harmine-ISE | C/PEDOT:PSS/Harmine-ISE (o-NPOE) | ||
|---|---|---|---|---|
| o-NPOE | DOP | TBP | ||
| Slope (mV/decade) | 58.0 ± 0.7 | 50.1 ± 0.3 | 45.2 ± 0.6 | 59.2 ± 0.8 |
| Correlation coefficient (r2) | 0.9997 | 0.9994 | 0.9995 | 0.9996 |
| Detection limit (µg/mL) | 0.03 | 0.23 | 0.68 | 0.02 |
| Linear range (M) | 6.0 × 10−7–1.0 × 10−2 | 4.0 × 10−6–1.0 × 10−2 | 7.0 × 10−6–1.0 × 10−2 | 2.0 × 10−7–1.0 × 10−2 |
| Working pH range (pH) | 3–7.5 | 3–7.5 | 3–7.5 | 3–7.5 |
| Response time (s) | <10 | <10 | <10 | <10 |
| Repeatability (% mV) | 0.9 | 1.2 | 1.1 | 0.6 |
| Reproducibility (% mV) | 1.3 | 1.1 | 0.9 | 0.8 |
| Accuracy (%) | 99.1 | 98.8 | 98.4 | 99.6 |
| Interfering Ion | Log KpotHarmine,J + SD * | ||
|---|---|---|---|
| o-NPOE | DOP | TBP | |
| K+ | −5.4 ± 0.2 | −5.4 ± 0.3 | −5.4 ± 0.1 |
| Na+ | −5.7 ± 0.3 | −5.7 ± 0.2 | −5.7 ± 0.1 |
| Harmaline | −1.3 ± 0.2 | −1.1 ± 0.1 | −0.9 ± 0.2 |
| Strychnine | −3.3 ± 0.1 | −3.2 ± 0.1 | −3.0 ± 0.2 |
| Caffeine | −3.7 ± 0.2 | −3.5 ± 0.3 | −3.2 ± 0.3 |
| Atropine | −3.4 ± 0.4 | −3.1 ± 0.4 | −2.9 ± 0.6 |
| Quinine | −3.8 ± 0.3 | −3.7 ± 0.2 | −3.6 ± 0.1 |
| Ephedrine | −3.6 ± 0.2 | −3.5 ± 0.1 | −3.5 ± 0.2 |
| Adrenaline | −3.2 ± 0.3 | −3.1 ± 0.4 | −3.0 ± 0.3 |
| Glycine | −3.9 ± 0.2 | −3.8 ± 0.3 | −3.7 ± 0.6 |
| Interfering Ion | Log KPotHarmine,J ± SD * | ||
|---|---|---|---|
| a C/PEDOT:PSS/harmine-ISE (o-NPOE Plasticizer) | b Harmine/Tetratphenyl Borate-ISE (TBP Plasticizer) [30] | b Harmine/Reineckate-ISE (DOP Plasticizer) [30] | |
| K+ | −5.5 ± 0.1 | −3.6 | −3.1 |
| Na+ | −5.6 ± 0.2 | −2.8 | −3.2 |
| Harmaline | −1.4 ± 0.1 | −0.1 | −0.1 |
| Strychnine | −3.4 ± 0.2 | −2.0 | −1.2 |
| Caffeine | −3.8 ± 0.1 | −1.8 | −2.0 |
| Atropine | −3.6 ± 0.3 | −1.6 | −1.8 |
| Quinine | −3.9 ± 0.2 | −1.3 | −1.1 |
| Ephedrine | −3.5 ± 0.3 | −1.9 | −1.9 |
| Adrenaline | −3.1 ± 0.5 | −1.6 | −1.3 |
| Glycine | −4.0 ± 0.1 | −3.5 | −2.9 |
| Sample No. | Spiked Amount, µg/mL | Found, µg/mL | t-Student Test | F-Test | |||
|---|---|---|---|---|---|---|---|
| Potentiometry | Recovery a (%) ± SD | HPLC | Recovery b (%) ± SD | ||||
| 1 | 2 | 1.9 ± 0.3 | 95 ± 0.5 | 2.1 ± 0.2 | 105 ± 1.1 | 2.7 | 6.1 |
| 2 | 5 | 5.1 ± 0.2 | 102 ± 0.4 | 4.9 ± 0.3 | 98 ± 0.3 | 2.1 | 4.6 |
| 3 | 10 | 9.4 ± 0.3 | 94 ± 0.3 | 10.1 ± 0.2 | 101 ± 1.2 | 3.4 | 4.5 |
| 4 | 20 | 20.3 ± 0.2 | 101.5 ± 0.2 | 19.6 ± 0.3 | 98 ± 0.4 | 1.2 | 3.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amr, A.E.-G.E.; Kamel, A.H.; Almehizia, A.A.; Sayed, A.Y.A.; Abd-Rabboh, H.S.M. Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens. Molecules 2021, 26, 324. https://doi.org/10.3390/molecules26020324
Amr AE-GE, Kamel AH, Almehizia AA, Sayed AYA, Abd-Rabboh HSM. Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens. Molecules. 2021; 26(2):324. https://doi.org/10.3390/molecules26020324
Chicago/Turabian StyleAmr, Abde El-Galil E., Ayman H. Kamel, Abdulrahman A. Almehizia, Ahmed Y. A. Sayed, and Hisham S. M. Abd-Rabboh. 2021. "Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens" Molecules 26, no. 2: 324. https://doi.org/10.3390/molecules26020324
APA StyleAmr, A. E.-G. E., Kamel, A. H., Almehizia, A. A., Sayed, A. Y. A., & Abd-Rabboh, H. S. M. (2021). Solid-Contact Potentiometric Sensors Based on Main-Tailored Bio-Mimics for Trace Detection of Harmine Hallucinogen in Urine Specimens. Molecules, 26(2), 324. https://doi.org/10.3390/molecules26020324