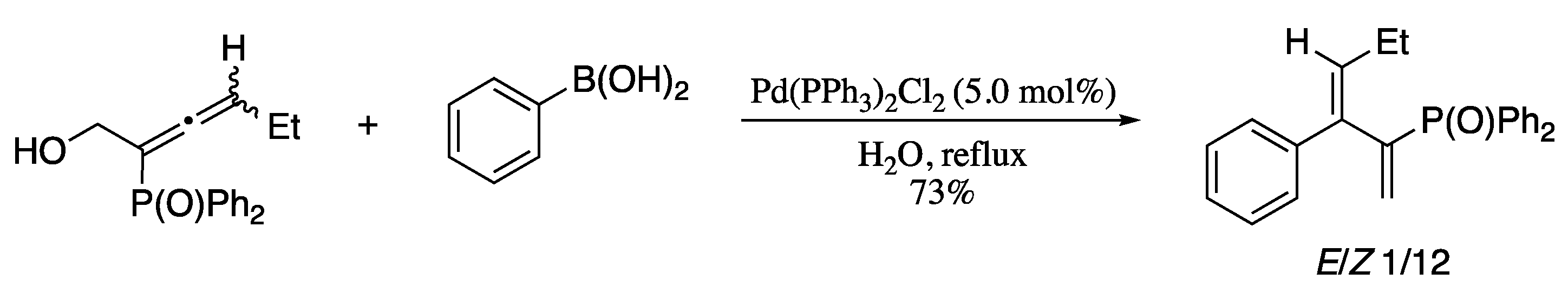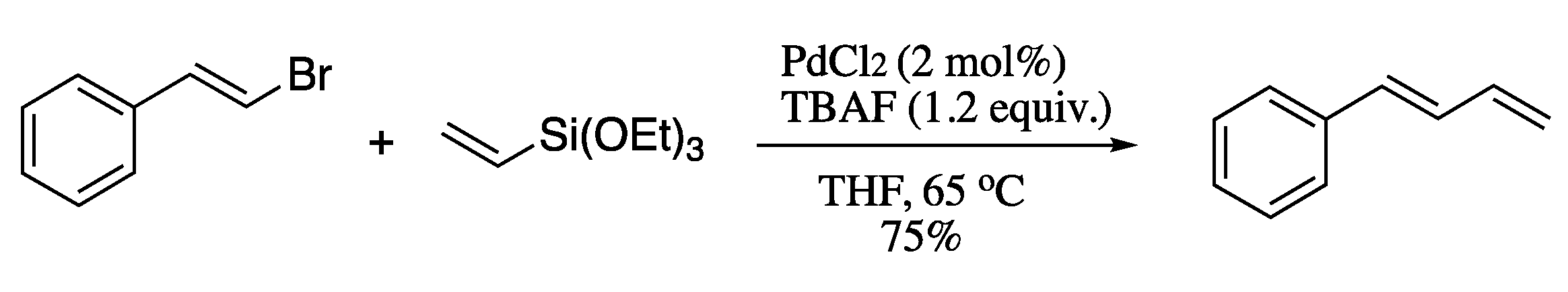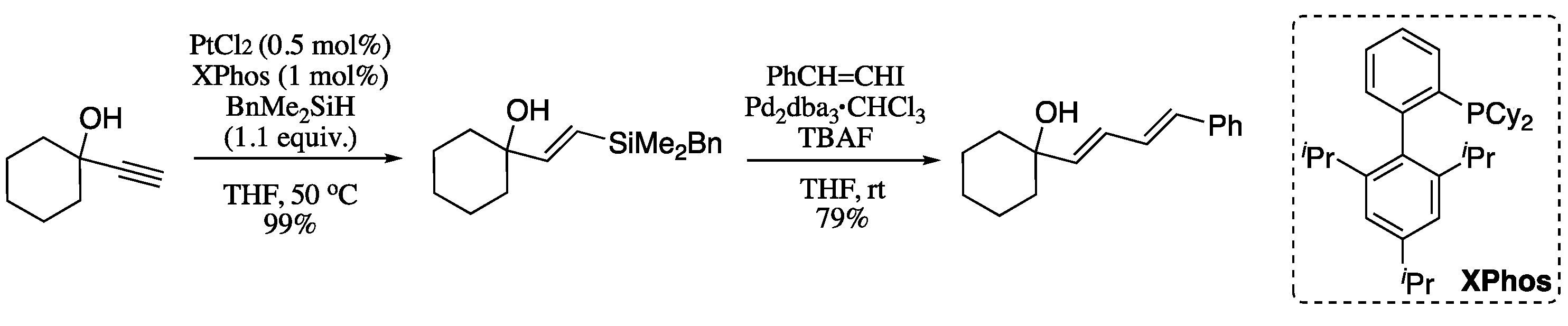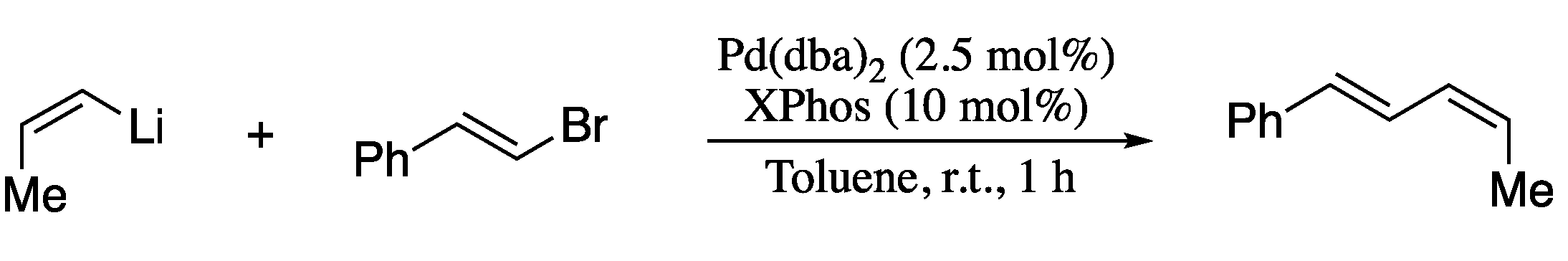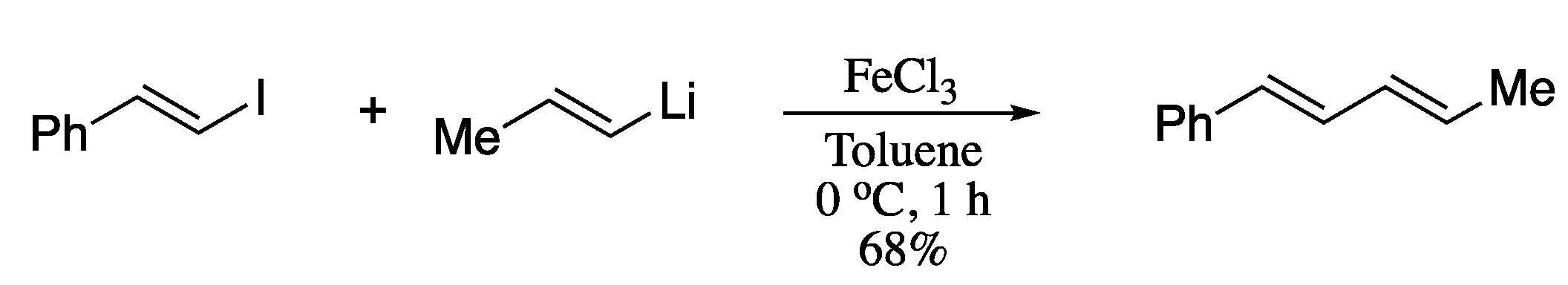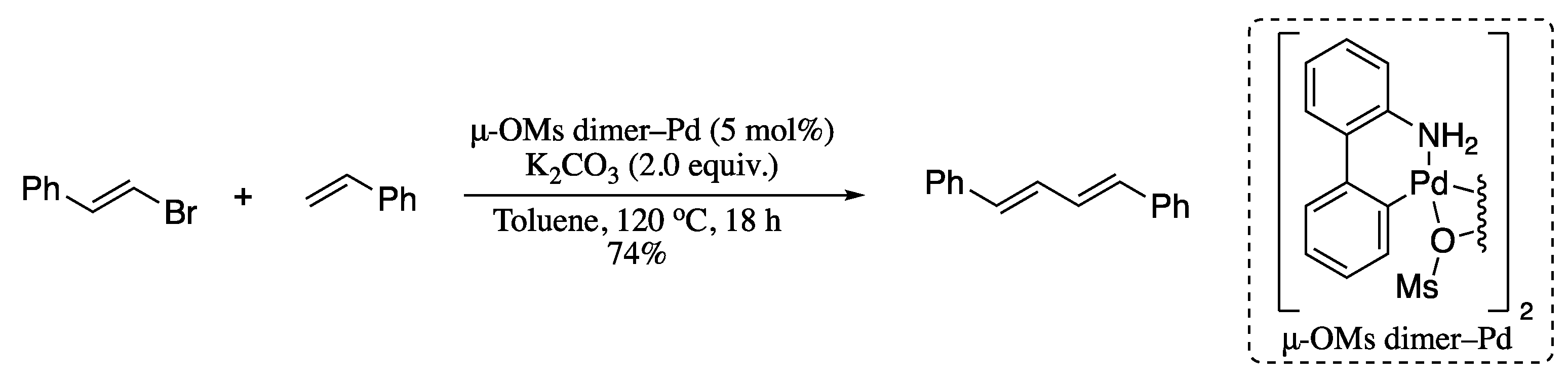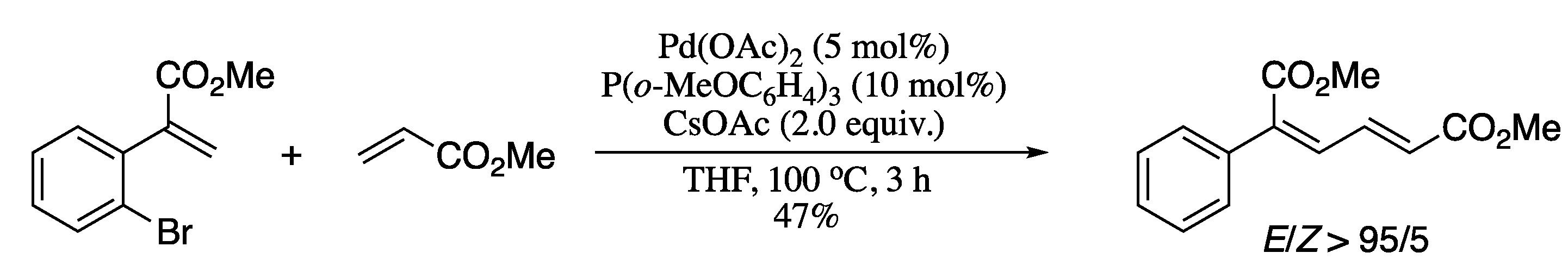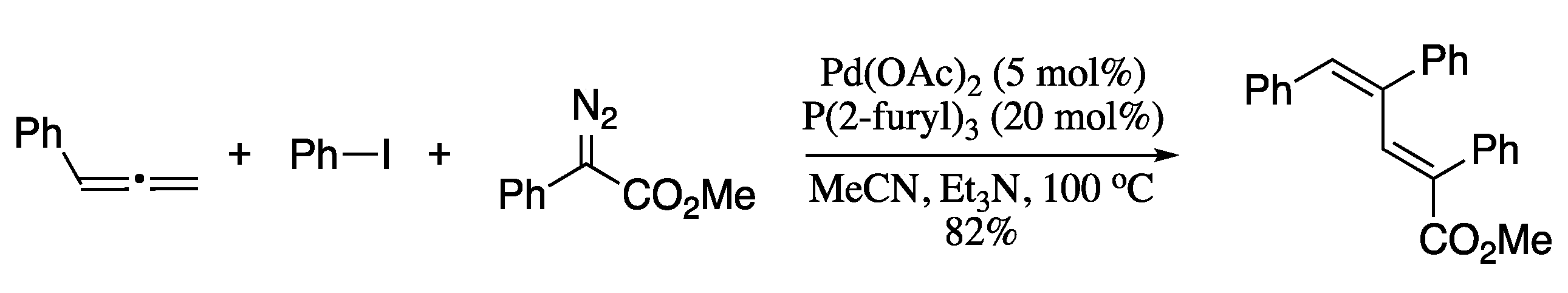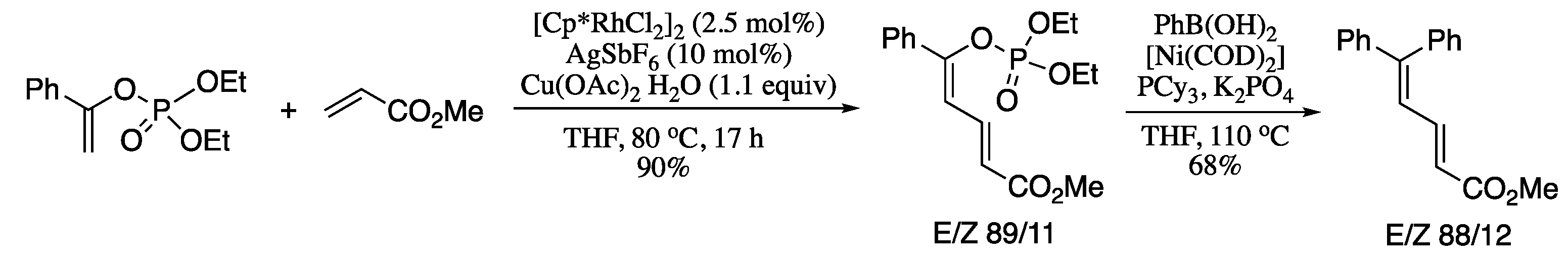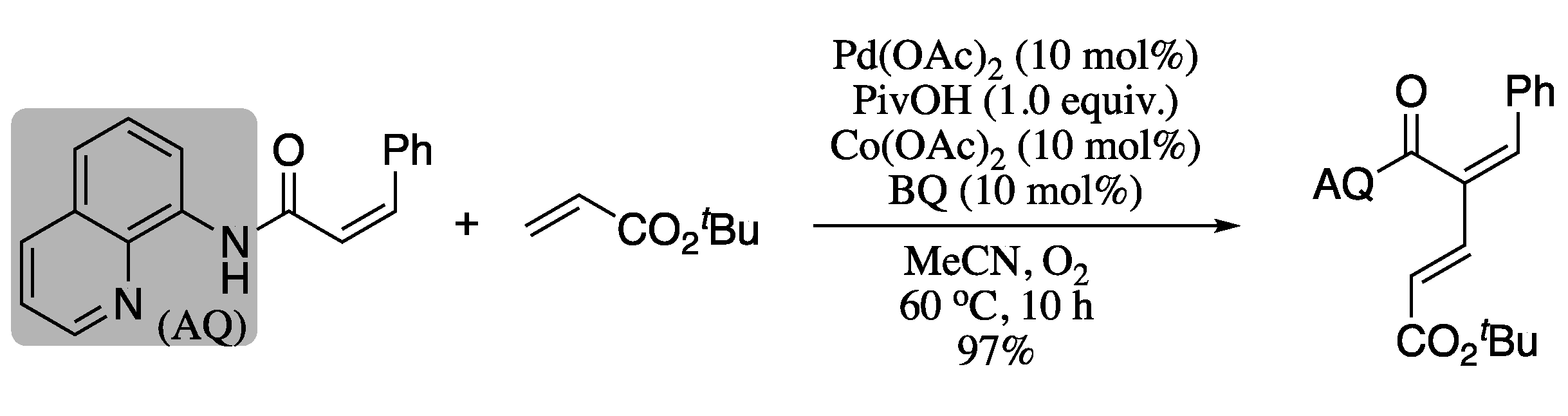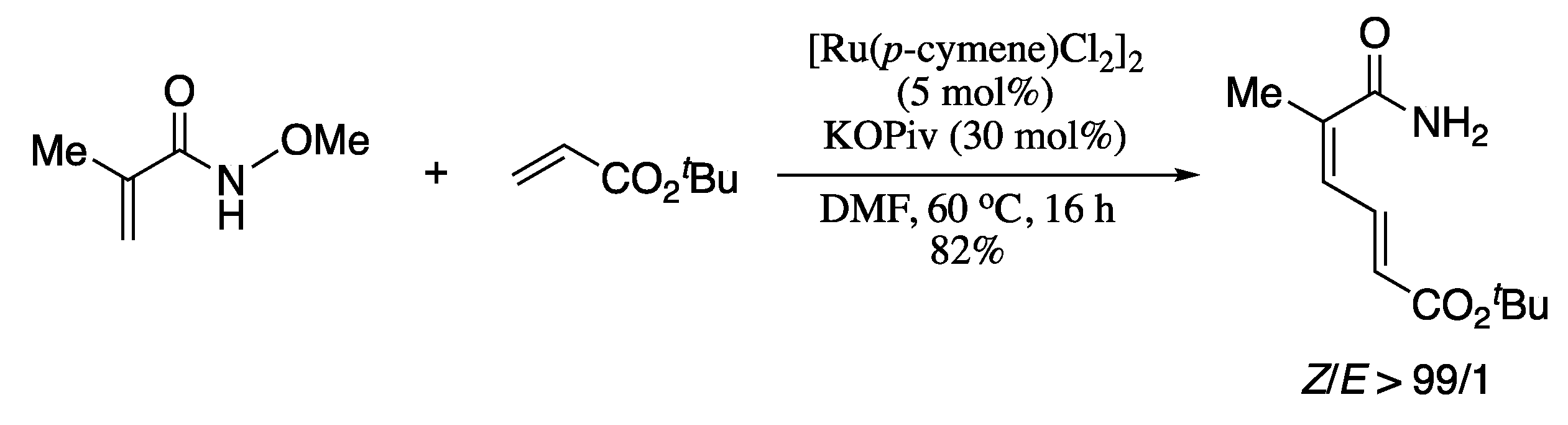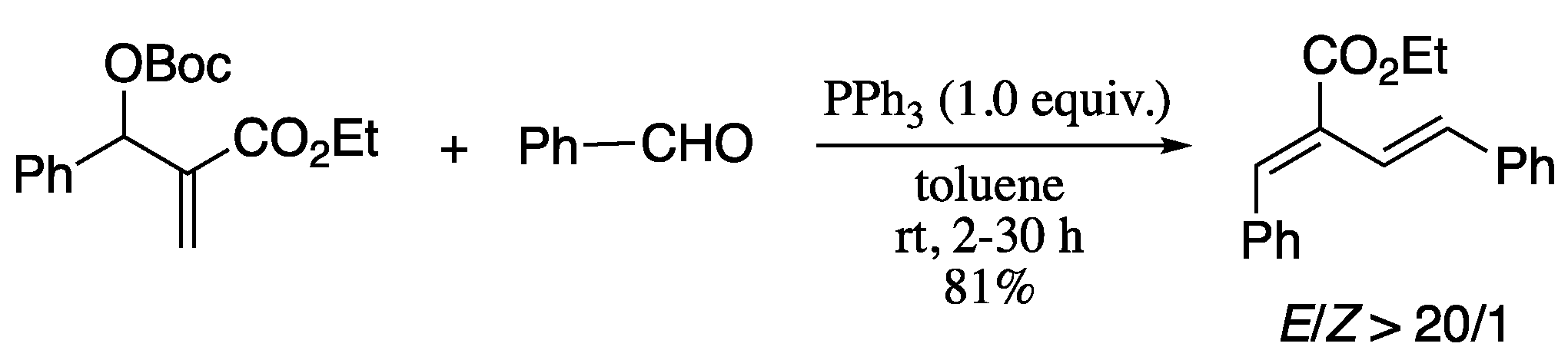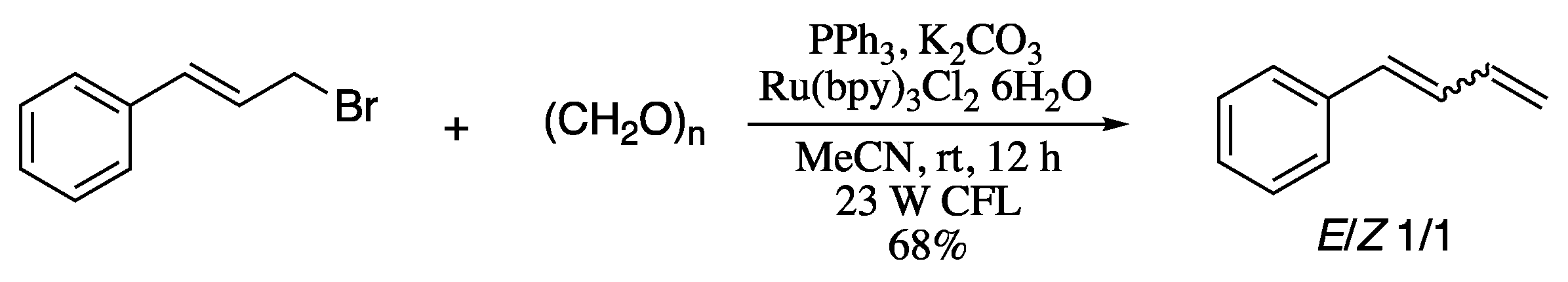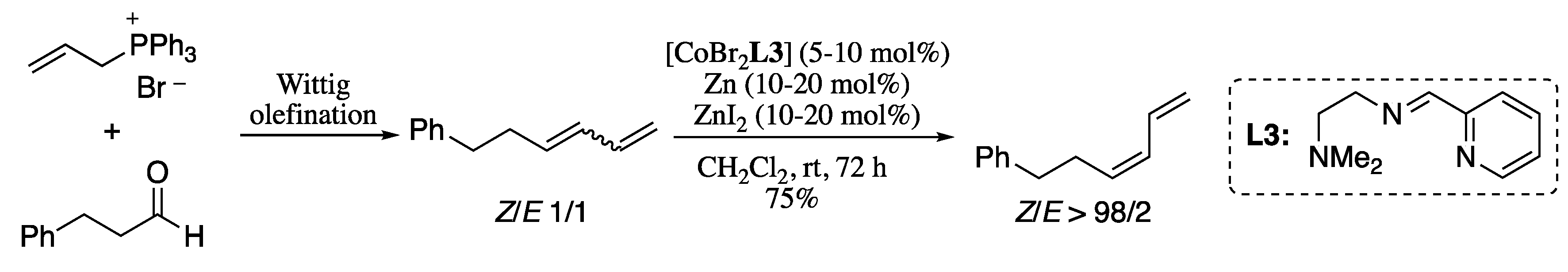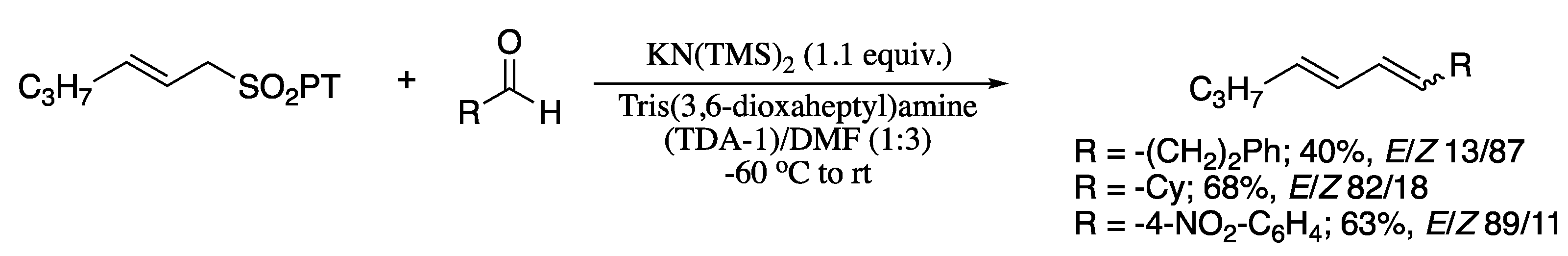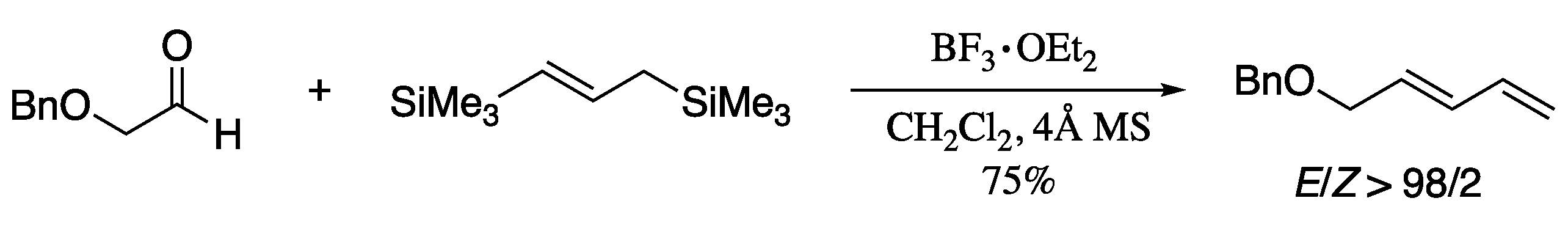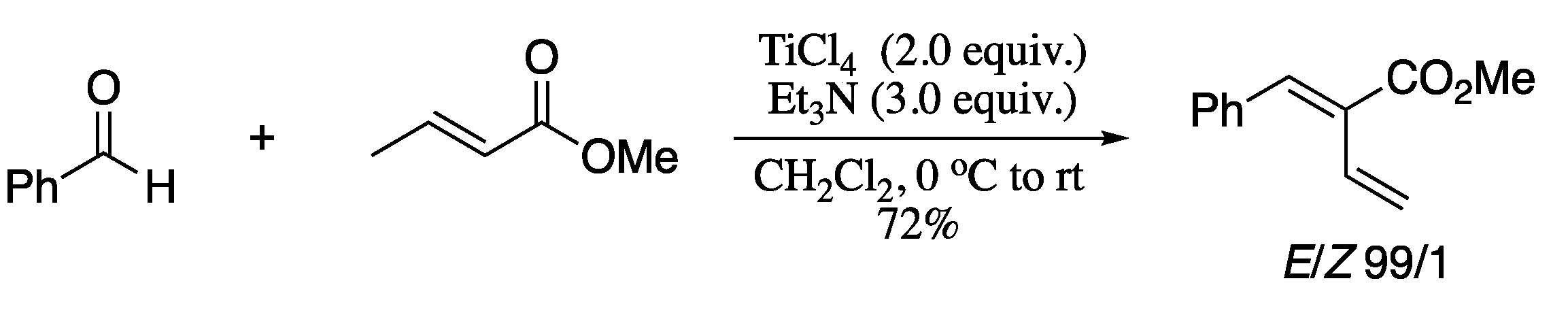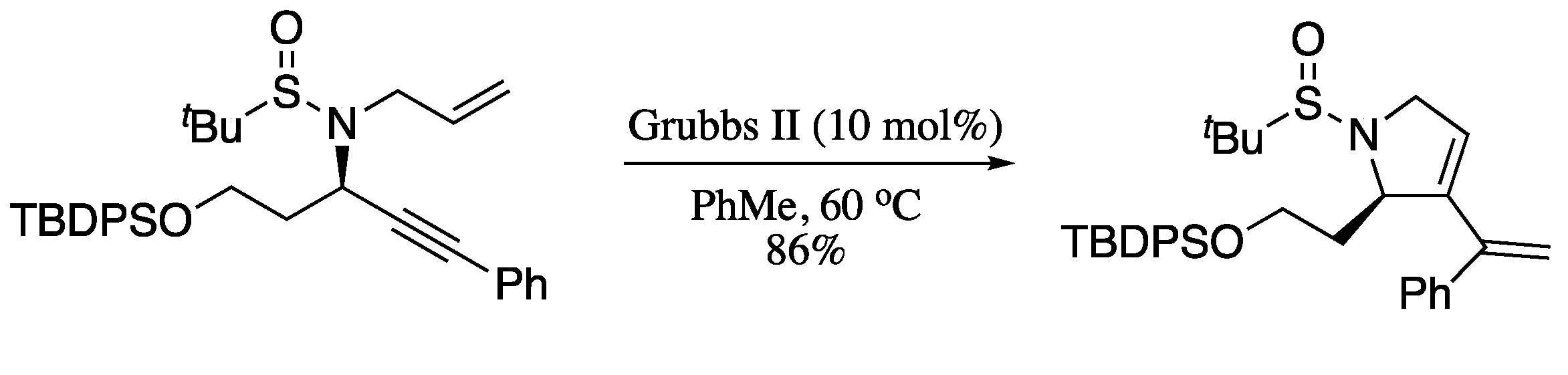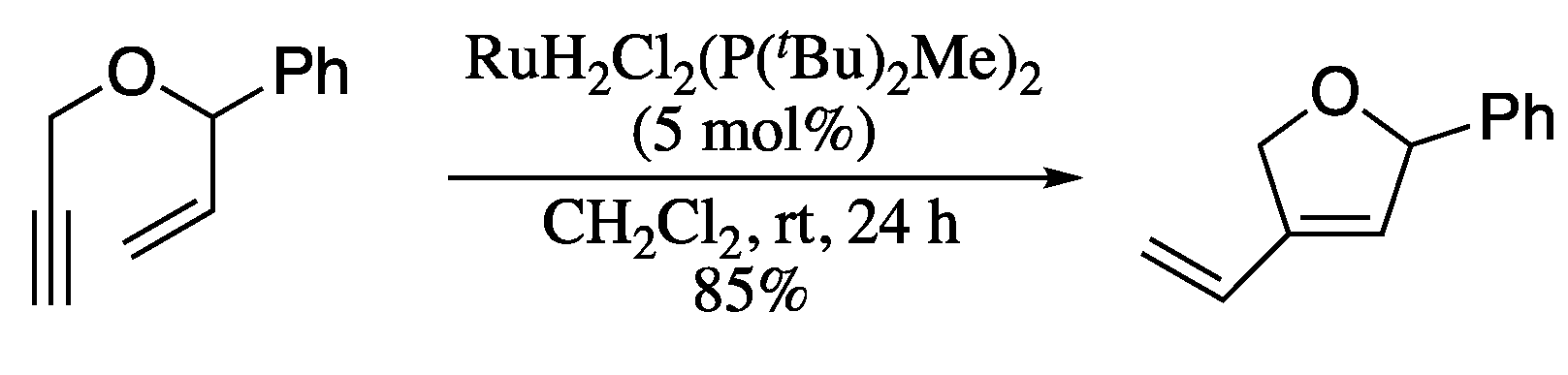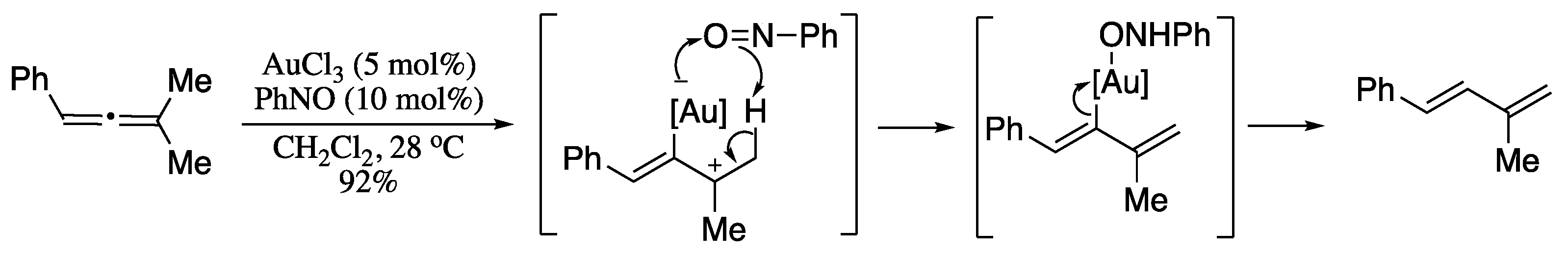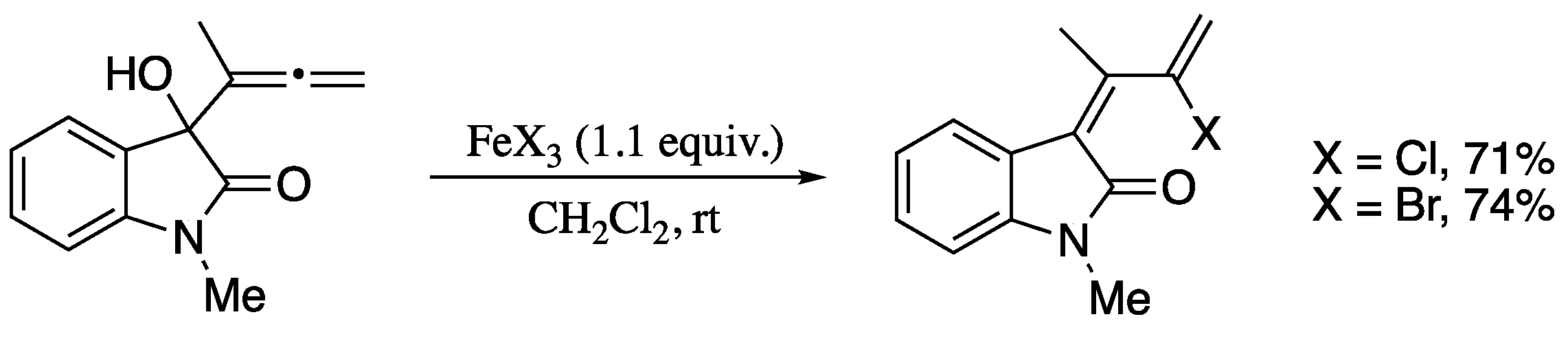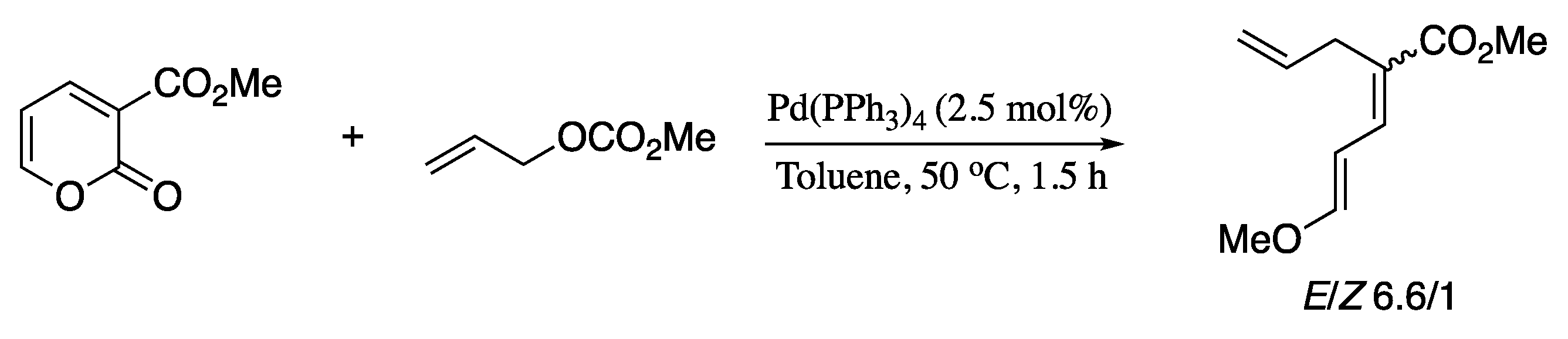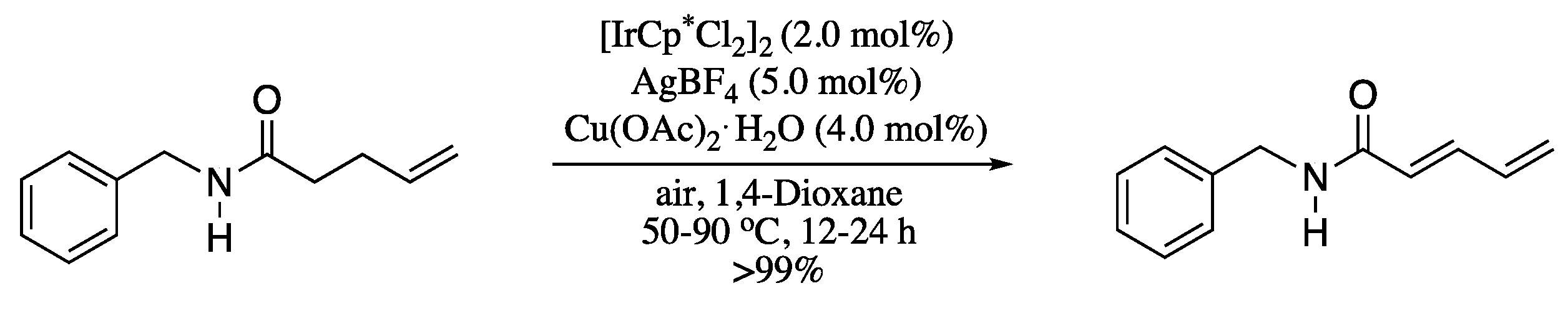Abstract
The 1,3-butadiene motif is widely found in many natural products and drug candidates with relevant biological activities. Moreover, dienes are important targets for synthetic chemists, due to their ability to give access to a wide range of functional group transformations, including a broad range of C-C bond-forming processes. Therefore, the stereoselective preparation of dienes have attracted much attention over the past decades, and the search for new synthetic protocols continues unabated. The aim of this review is to give an overview of the diverse methodologies that have emerged in the last decade, with a focus on the synthetic processes that meet the requirements of efficiency and sustainability of modern organic chemistry.
1. Introduction
1.1. Natural and Non-Natural 1,3-Dienes
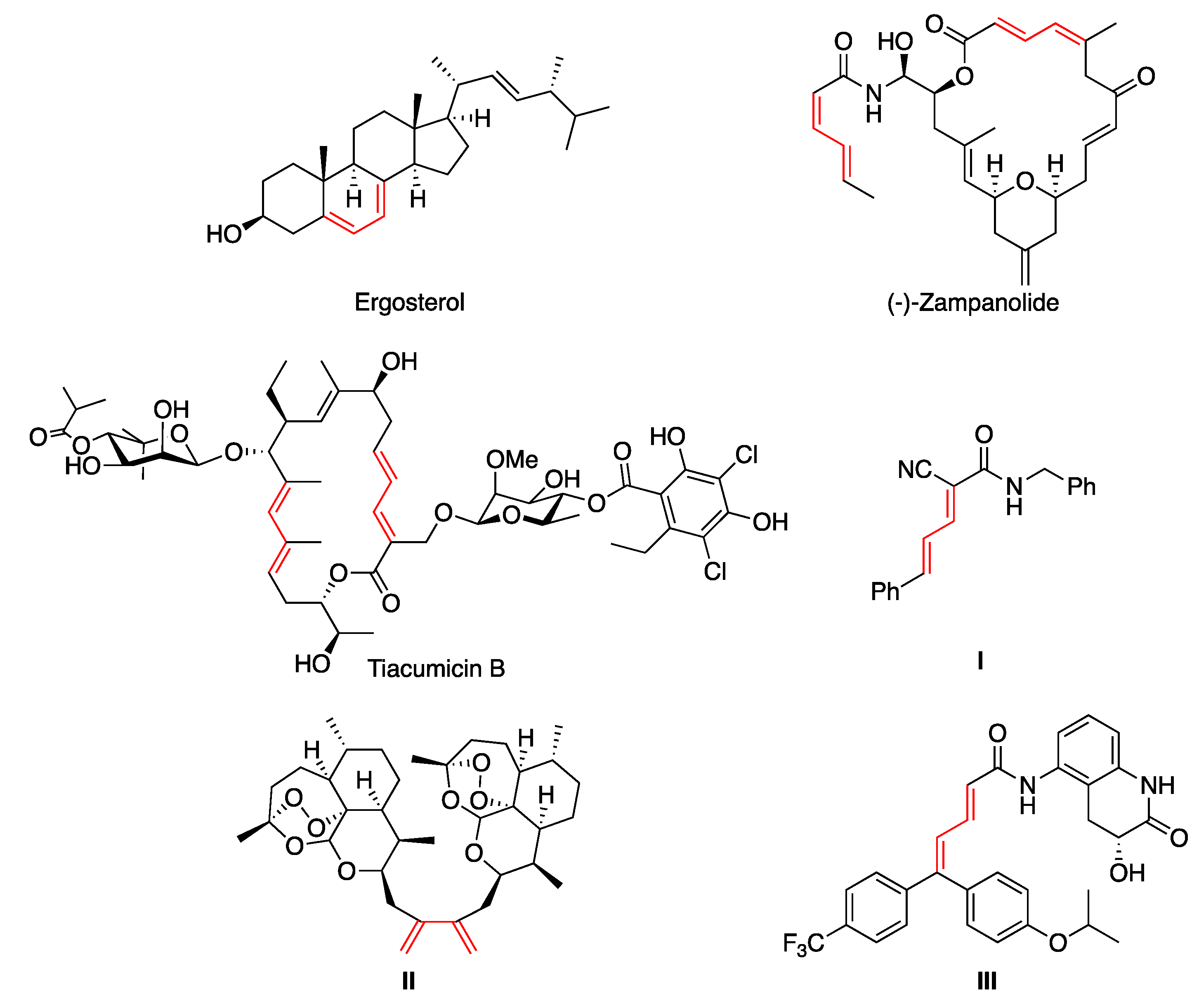
The 1,3-diene structural moiety represents the key framework of many natural [1,2,3,4] and non-natural [5] products displaying a broad spectrum of biological activities [6]. Representative examples of natural products containing the 1,3-diene motif are the vitamin D2 precursor ergosterol (ergosta-5,7,22-trien-3-β-ol) [7], the potent cytotoxic agent zampanolide [8] and the macrolide antibiotic tiacumicin B [9,10] (Figure 1). Apart from natural dienes, synthetic ones have also aroused great pharmacological interest. For instance, 3-styrylacrylonitrile I exhibited high inhibitory potency against myeloid cell leukemia sequence 1 (Mcl-1) [11], diene dimer II is a potent antimalarial [12] and 5,5-diarylpentadienamide III is a transient receptor potential vanilloid antagonist I (TRPVI), of interest for the treatment of neuropathic pain [13] (Figure 1).
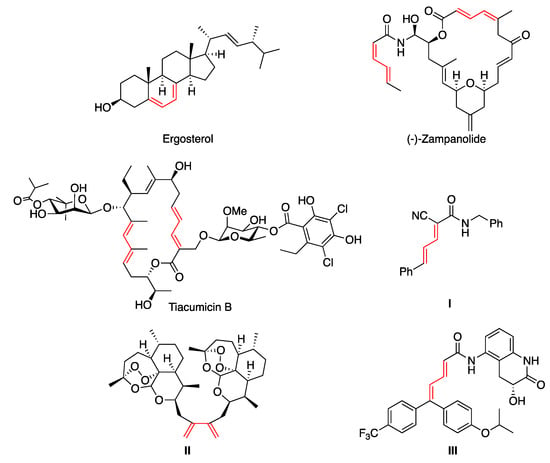
Figure 1.
Selected examples of bioactive natural and non-natural 1,3-dienes.
1.2. Applications of 1,3-Dienes
The 1,3-diene moiety have proved its usefulness and versatility in modern organic synthesis, serving as building block for the construction of important target molecules [14,15]. The reactivity of 1,3-dienes has been extensively studied, including transformations such as polymerization reactions [16,17,18], conjugate additions [19], asymmetric hydrofunctionalizations [20,21,22,23], difunctionalizations [24,25,26,27], C−H functionalizations [28,29,30,31], cycloadditions [32,33,34] and cross coupling reactions [35,36].
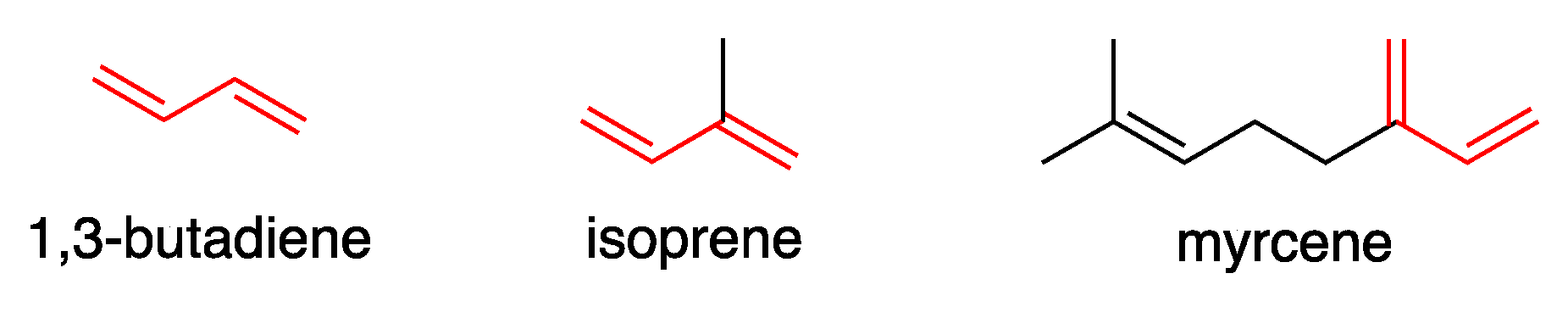
1,3-Dienes are also important industrially. Buta-1,3-diene, produced from steam crackers on a scale of more than 10 million tons per year worldwide, is a monomer in the production of synthetic rubber. [37] Other 1,3-dienes, such as isoprene and myrcene, are also produced on a multi-ton scale and used for their conversion of into more complex and useful molecules, mainly in the polymer industry [38] (Figure 2).
Figure 2.
Industrially relevant 1,3-dienes.
1.3. Stereoselective Synthesis of 1,3-Dienes
In view of the important biological roles of 1,3-dienes and their relevance as synthetic intermediates, it is not surprising that their synthesis have been at the forefront of Organic Synthesis practically from its inception [39].
The stereochemistry of dienes not only influence the outcome of further chemical transformations but also determine the physical and biological properties. Therefore, it is essential that any synthesis of substituted dienes be stereoselective. The stereoselective construction of the 1,3-dienes moiety have been typically achieved by transition-metal-catalysed cross-coupling reactions between pre-fuctionalized alkenyl cross-coupling partners of predefined stereochemistry [40]. Alternatively, well-known olefination methods for the stereoselective synthesis of alkenes have been extended to the synthesis of dienes. Additional approaches include olefin metathesis and rearrangements of enynes, alkynes or allenes, for example.
1.4. Aim of the Review
In the last ten years, the way of understanding organic chemistry has shifted dramatically. In modern organic synthesis, there is a greater focus on economic and environmental factors, such as the use of affordable and environmentally safe reagents and solvents, the recyclability of materials or the employment of unconventional more efficient energy sources. As the literature published in the last ten years attest, the preparation of 1,3-dienes is not an exception and nowadays atom economy and sustainability are aspects of central concern in most of the synthetic protocols developed. Notwithstanding this synthetic interest, no comprehensive reviews dealing with the synthesis of 1,3-dienes have appeared in the past few years and the most recent one just covers the period 2005–2010 [41]. A review on the synthesis of 1,3-dienes has been published recently, but is restricted to the synthesis of (E,Z)-1,3-dienes and its application in natural product synthesis [42].
The aim of this review is to describe the most relevant progress that has been made for the preparation of 1,3-dienes from 2010 up to the present, with a focus on the application of flourishing synthetic methodologies as, among others, C-H activation, photoredox catalysis and domino and multicomponent reactions. The synthetic routes have been grouped according to the way the 1,3-diene scaffold has been assembled. Thus, syntheses of 1,3-dienes occurring via cross-coupling reactions, both transition-metal-catalysed and metal-free, are discussed in Section 2 and Section 3, respectively. Methodologies for 1,3-diene preparation centred on aldehyde dienylation are covered in Section 4, while examples of olefin methathesis-based approaches are detailed in Section 5. Section 6 is dedicated to rearrangement/isomerization reactions and, finally, Section 7 contains several miscellaneous protocols.
The present review is not intended to be an exhaustive compendium of all the recent literature in diene chemistry, but a rational and systematic presentation the most significant developments in the stereoselective synthesis of 1,3-dienes published after 2010. The examples presented in each section correspond to the selection made by the authors in an attempt to provide representative cases of interesting recent methodologies.
2. Transition Metal-Catalysed Cross-Coupling Reactions
During the past 50 years, transition-metal-catalysed cross-couplings have revolutionized chemical science, exemplifying one of the most powerful and popular method for the formation of carbon–carbon bonds and playing a central role in the synthesis of bioactive products and synthetic building blocks. On account of the remarkable benefits for society, the 2010 Nobel Prize in Chemistry was awarded jointly to Heck, Negishi and Suzuki “for palladium-catalysed cross couplings in organic synthesis” [43].
This section is devoted to the critical analysis of the most relevant recent strategies for the construction of the 1,3-diene moiety based in transition metal-catalysed cross-coupling reactions. For this purpose, the methodologies will be broadly divided in two groups: those based on the reaction of two sp2 carbons (Cvinyl–Cvinyl cross couplings) and those resulting from the reaction of a sp carbon and a sp3 carbon (Calkynyl–Calkyl cross couplings).
2.1. Carbon(sp2)−Carbon(sp2) Cross-Coupling
Transition-metal-catalysed alkenyl–alkenyl coupling is one of the most widely used methods for the synthesis of dienes and polyenes. The classical methods usually involve either the coupling of two activated vinylic carbons (generally a vinyl halide and a organometallic halide, in Suzuki or Negishi type processes) or the coupling of just one activated vinylic carbon and an alkene in a Heck-type process. More recently, the transition-metal-catalysed cross-coupling with substitution of a C-H bond, rather than a halide (so called “C-H activation”) has emerged as a highly desirable alternative, since such processes usually generate less waste and the starting materials are often easily available and inexpensive.
2.1.1. Cross-Coupling of Two Activated Vinylic Carbons
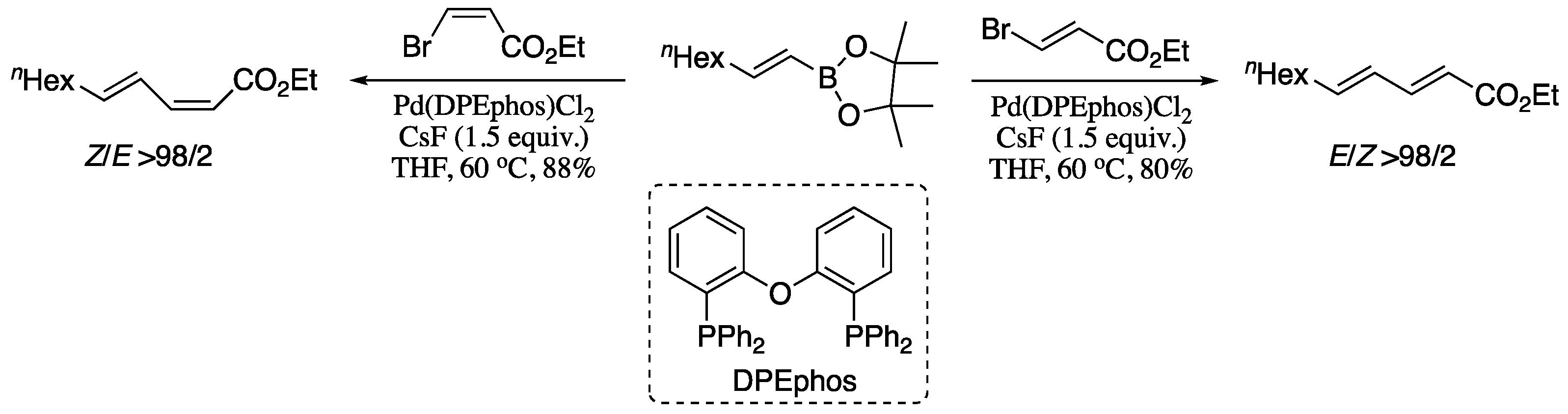
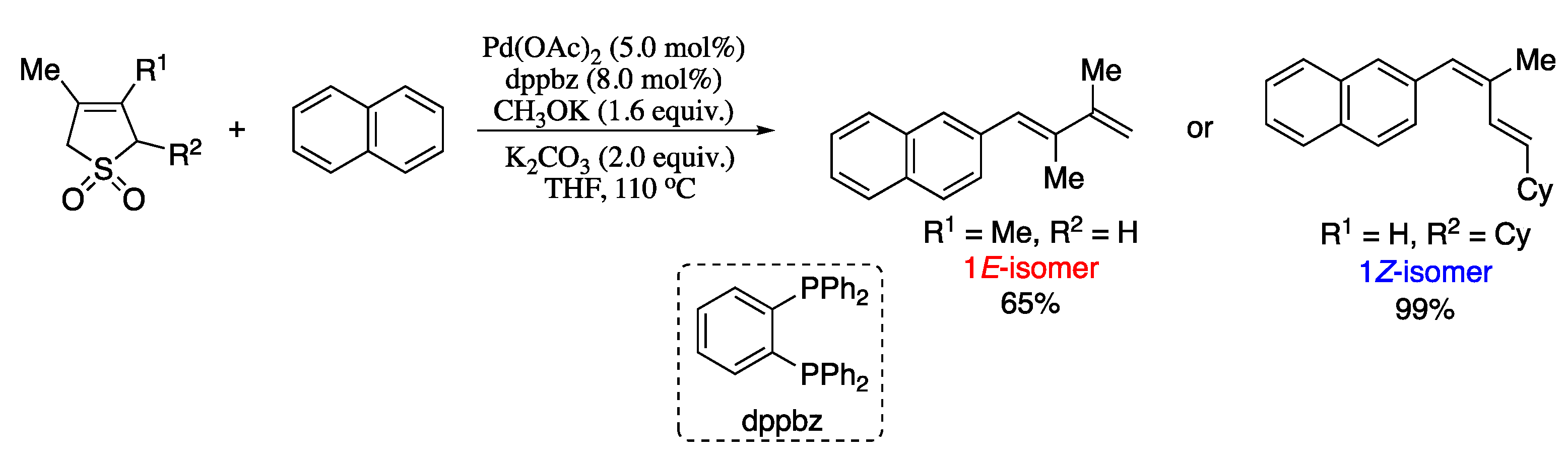
Among all the variants of the transition-metal-catalysed alkenyl–alkenyl coupling, the Pd-catalysed Suzuki-Miyaura coupling of stereodefined vinyl halides and vinylboranes reagents has long been regarded as a convenient method for the stereoselective construction of 1,3-dienes. However, stereo scrambling have been observed for several substrates. A representative example is the synthesis of dienoic esters; under Suzuki standard conditions, stereo scrambling to varying extents (≥5%) had been previously reported for the coupling of vinylboranes and 3-haloacrylates [44]. Negishi’s group found that the use of CsF as a base can suppress stereoisomerization, affording dienoic esters in good yield and excellent stereoisomeric ratio, as depicted in Scheme 1 [45].
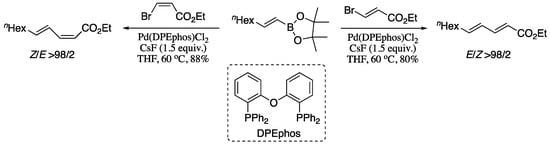
Scheme 1.
Highly selective synthesis of all four possible stereosiomers of 2,4-dienoic esters by fluoride promoted Suzuki alkenylation.
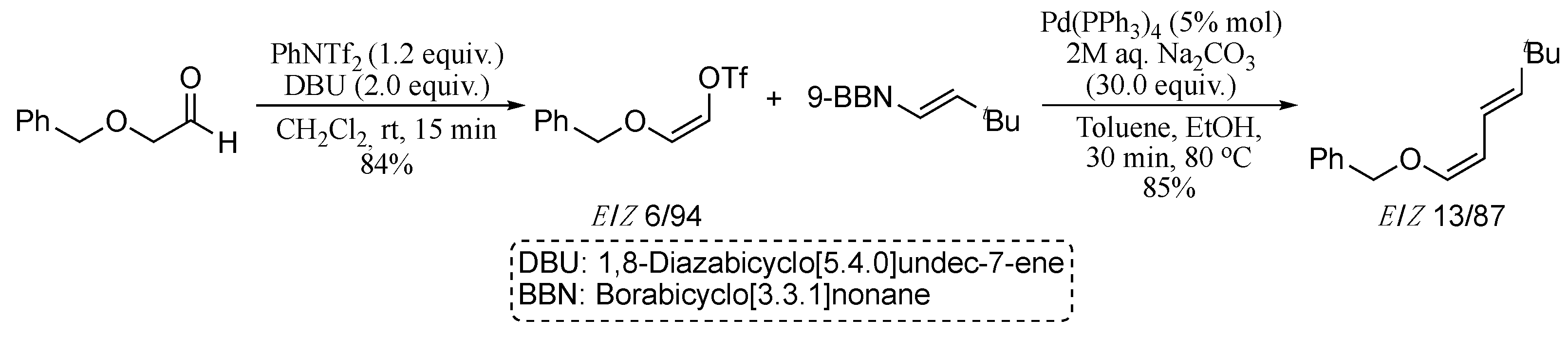
Several recent reports describe modified Suzuki coupling procedures for the highly stereoselective synthesis 1,3-dienes. In 2015, Kurosawa and co-workers described a useful synthetic sequence for the stereoselective synthesis of diverse olefin derivatives, including 1,3-dienes, based on a (Z)-selective vinyl-triflation of α-alkoxyacetoaldehydes, followed by Suzuki cross-coupling (Scheme 2) [46].
Scheme 2.
Synthesis of (Z)-dienes via Suzuki cross-coupling reaction.
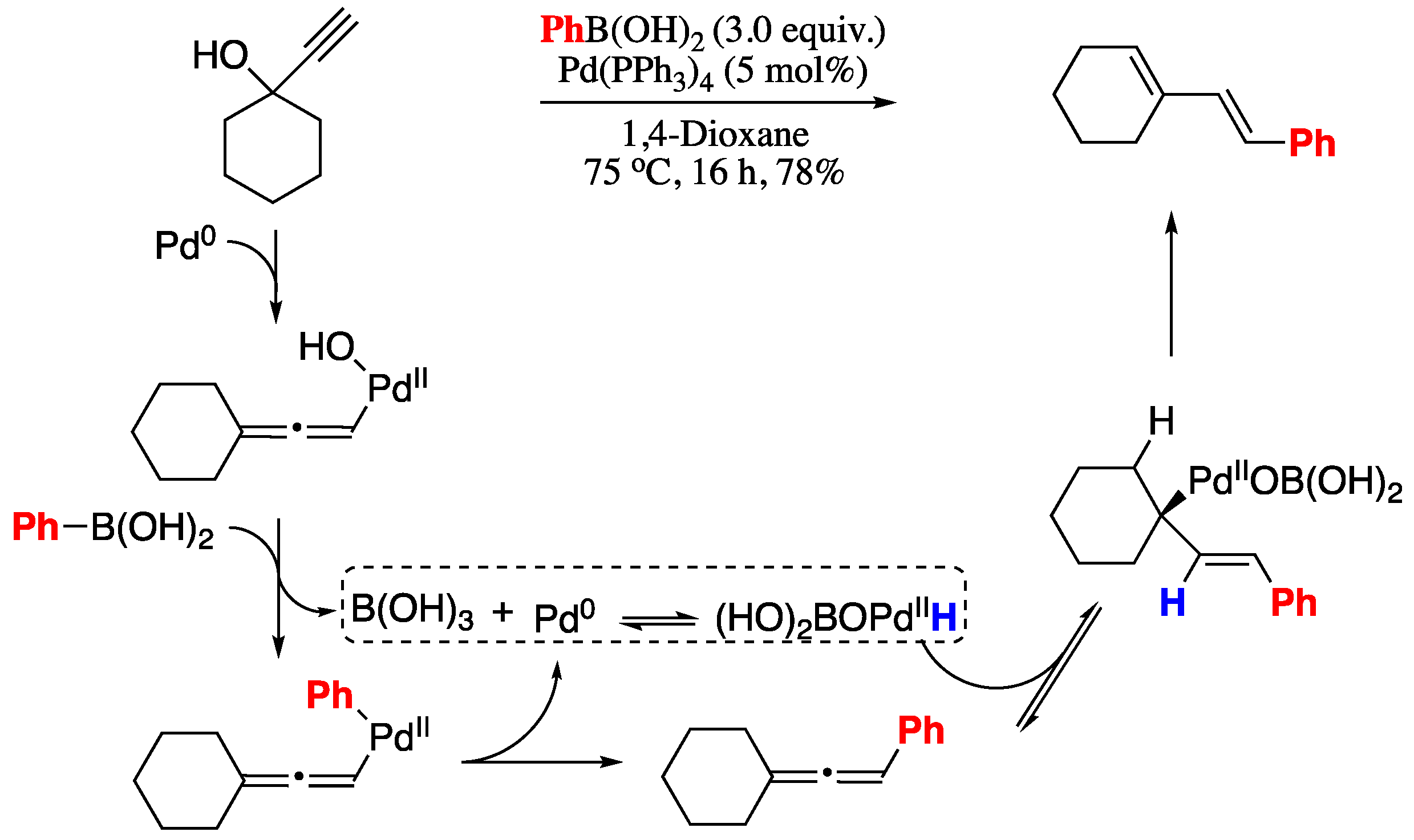
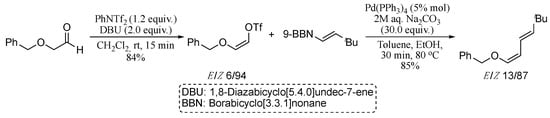
The classical Suzuki approach for the synthesis of dienes, however, requires the hydroboration of an alkyne using air and moisture sensitive boranes to generate the corresponding vinylborane, which cannot be stored for prolonged times. A recent Suzuki-based methodology which avoids the pre-formation of unstable boranes was reported in 2016 [47]. The protocol involves a palladium-mediated, base-free, Suzuki−Miyaura coupling of propargyl alcohols and boronic acids to generate the intermediate allenes, which were then converted in situ into the corresponding 1,3-dienes by means of a hydropalladation/dehydropalladation process promoted by the formation of boric acid within the base-free Suzuki−Miyaura reaction conditions (Scheme 3).
Scheme 3.
Sequential Suzuki−Miyaura coupling/palladium-mediated allene isomerization sequence.
In the past few years there have been a growing interest in the use of allenic electrophiles as alternative coupling partners in Suzuki−Miyaura cross-couplings; upon reaction with a suitable boron nucleophile, coupling occurred exclusively at the central allenic carbon generating 2-substituted 1,3-dienes [48,49]. Not only this methodology give access to functionalized dienes in good yields with excellent group tolerance [50], but also have great relevance from environmental and economic points of view. For example, Liu’s group developed new routes to phosphinoyl 1,3-butadienes based on the palladium-catalysed Suzuki-Miyaura couplings of arylboronic acids with phosphinoyl-α-allenic derivatives [51] and also demonstrated that the process could be performed “on water” without surfactants or additives to afford phosphinoyl 1,3-butadienes in good yields and stereoselectivities (Scheme 4) [52].
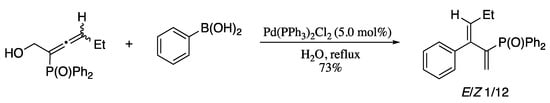
Scheme 4.
Palladium-catalysed couplings of substituted phosphinoyl-α-allenic alcohols with various arylboronic acids.
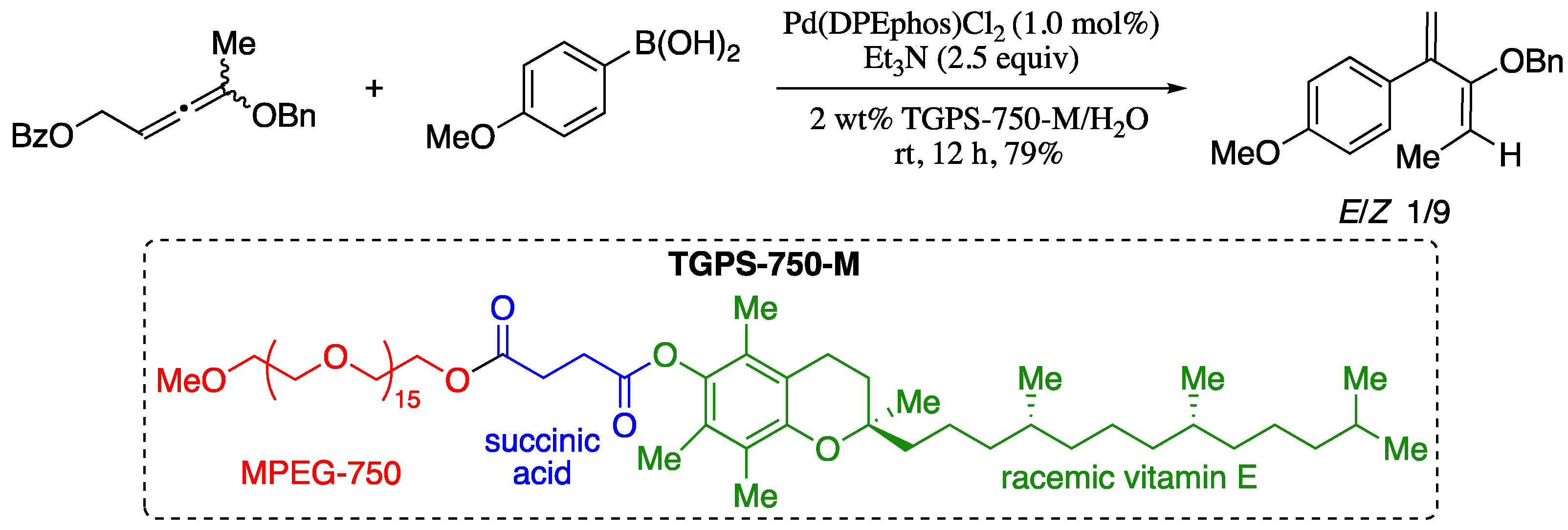
As the field of green chemistry continues to expand, a number of new technologies for improving the sustainability of transition metal-catalysed processes are becoming more and more popular. In particular, switching from an organic to an aqueous micellar medium is recognized as one of the most relevant new strategies in metal-catalysis [53,54]. Following this new trend, Lipshutz and co-workers reported an environmentally responsible methodology for the preparation of substituted 1,3-butadienes based on the palladium-catalysed cross-coupling of allenes and arylboronic acids under micellar reaction conditions (Scheme 5) [55].
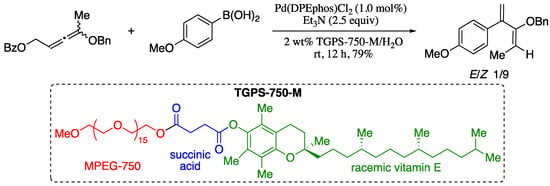
Scheme 5.
Pd-catalysed cross-couplings of substituted allenic esters in micellar medium.
Despite its potential for the construction of complex dienes and polyenes, due to the strong reducing power of boranes, Suzuki reaction is subjected to severe functional group limitations. The protection with N-methyliminodiacetic acid (MIDA) ligand is a recently developed strategy for harnessing the reactivity of boronic acids in iterative cross-coupling (ICC) reactions aimed at the preparation of complex polyenes [56]. However, this strategy has the important drawback of the additional protection-deprotection steps.
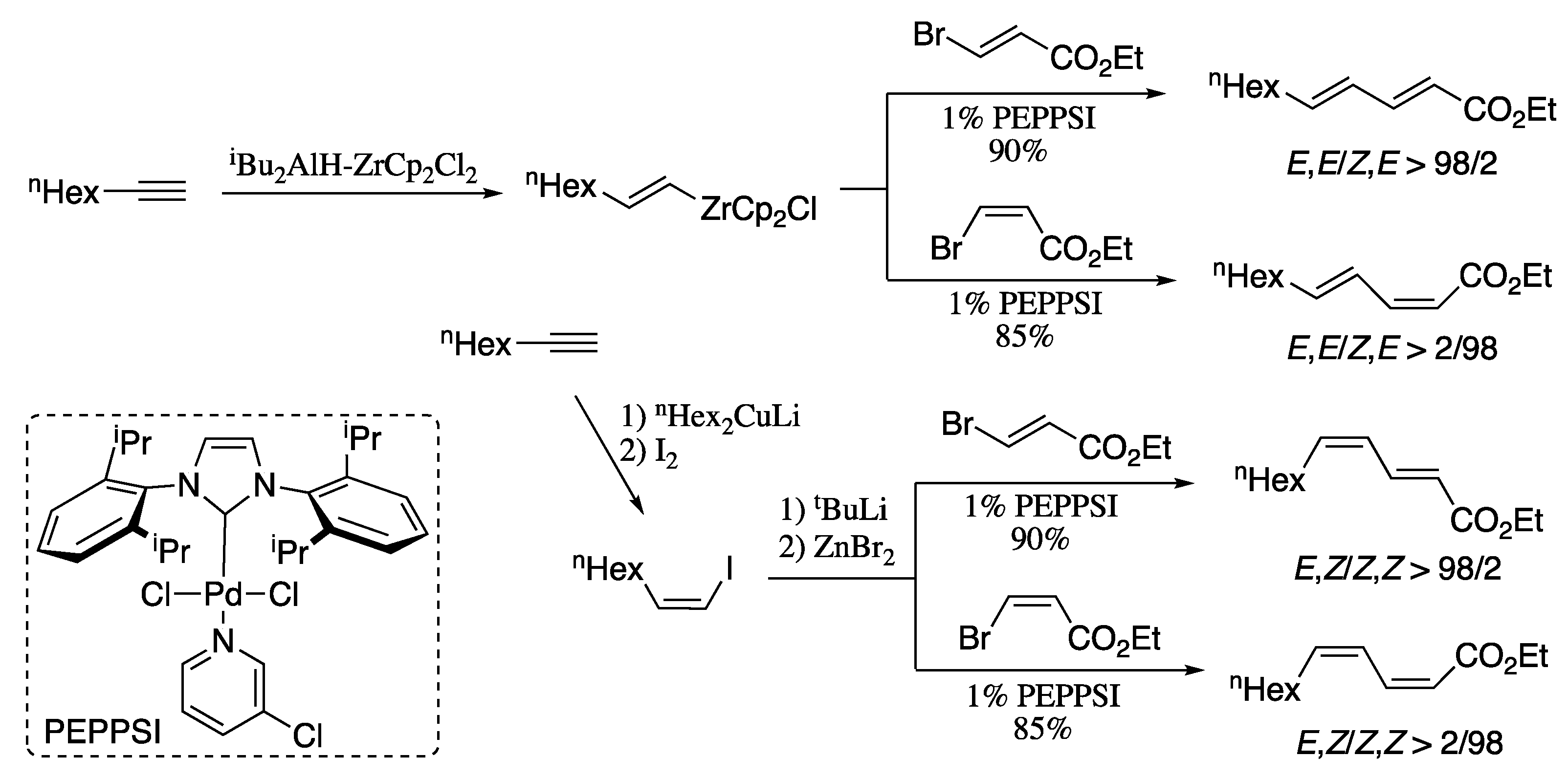
As an alternative to the Suzuki cross-coupling, Negishi developed a series of strategies for the alkyne elementometalation/Pd-catalysed alkenyl–alkenyl cross-coupling tandem processes. For example, all four stereoisomers of ethyl 2,4-undecadienoate can be prepared in high selectivity by the Negishi alkenyl–alkenyl coupling (Scheme 6) [57]. On the one hand, Negishi coupling of (E) and (Z) isomers of ethyl 3-bromoacrylate with in situ generated (E)-1-octenylzirconocene chloride afforded (2E,4E)- and (2Z,4E)-dienes in good yields and excellent diastereoisomeric ratio. On the other hand, carbocupration of 1-octyne followed by Negishi coupling of the resulting (Z)-1-octenyl iodide with both (E) and (Z) isomers of ethyl 3-bromoacrylate provided isomerically pure (2E,4Z)- and (2Z,4Z)-isomers in good yields.
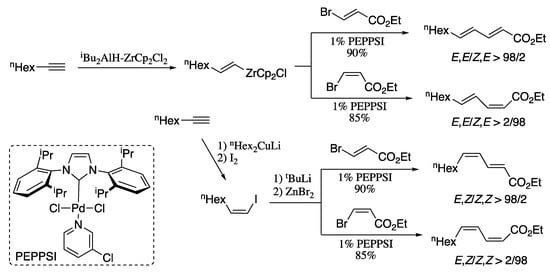
Scheme 6.
Highly selective synthesis of all four possible stereoisomers of 2,4-dienoic esters by alkyne elementometalation–Pd-catalysed Negishi coupling protocol.
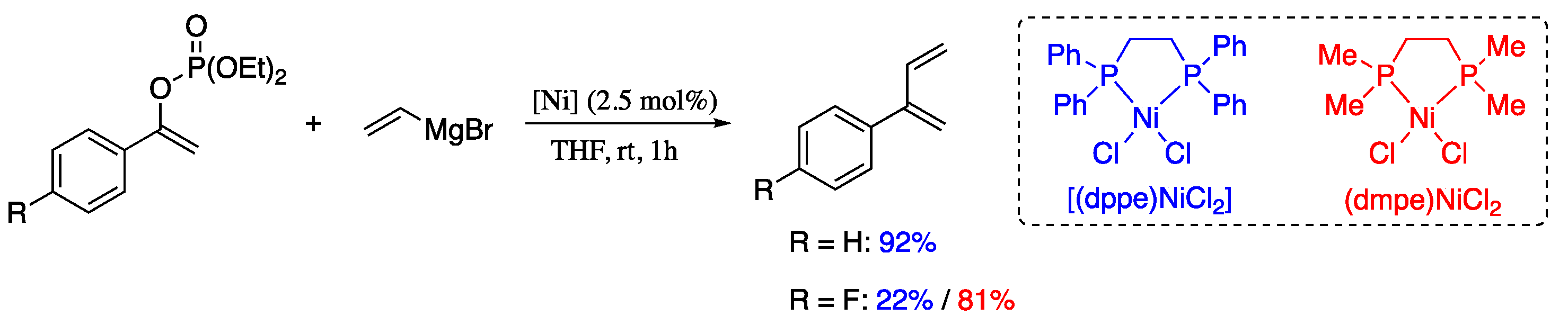
Fiorito et al. described two nickel(II) precatalysts which enable the Kumada cross-coupling between vinyl magnesium bromide and vinyl phosphates, providing 2-substituted 1,3-dienes in good yields and with high levels of stereocontrol [58]. The cross-coupling reaction between phenyl vinylphosphate and vinyl magnesium bromide in the presence of commercial precatalyst [(dppe)NiCl2] afforded 2-phenyl-1,3-diene in good yield (Scheme 7). For difficult substrates such as p-fluorophenyl vinylphosphate, complex [(dmpe)NiCl2] was used as a satisfactory alternative.
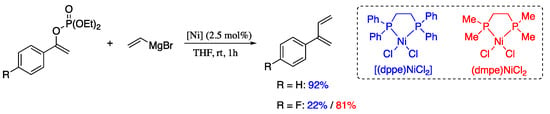
Scheme 7.
Ni-catalysed cross-coupling between vinyl phosphates and vinyl magnesium reagents.
Although effective, both the Negishi and Kumada processes suffer from similar problems to the Suzuki-based methodologies, as they require air and moisture sensitive conditions and in situ preparation of the vinyl intermediate. The Hiyama coupling is not subjected to these limitations, as vinylsilanes are non-toxic, stable and readily accessible. However, the classical version of the Hiyama coupling was severely limited by the requirement of a strong activator for the transmetallation step. Chatterjee et al. circumvented this issue utilizing metal nanoparticles as efficient catalysts in the presence of a mild activator such as tetrabutylammonium fluoride (TBAF) [59]. In a typical experimental procedure, styryl bromide was coupled with triethoxyvinylsilane in the presence of palladium(II) chloride and TBAF to produce the corresponding (E)-1,3-diene in good yields and total stereoselection (Scheme 8). The use of TBAF has a double function, as it stabilizes the Pd nanoparticles and also activates the siloxane.
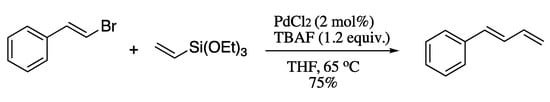
Scheme 8.
Hiyama cross-coupling catalysed by palladium nanoparticles.
Analogously to the Suzuki, Kumada and Negishi reactions, dienes can also be prepared from alkynes by the means of a hydrosilylation–Hiyama sequence. McAdam and co-workers developed an hydrosilylation methodology to form vinylsilanes from propargylic alcohols using commercially available benzyldimethylsilane; the resulting vinylsilanes are very stable and can undergo simple activation with TBAF under Trost-type conditions [60,61]. Subsequently, these authors developed a methodology for the formation of highly functionalised 1,3-dienes based on the hydrosilylation–Hiyama protocol (Scheme 9) [62]. Thus, hydrosilylation of 1-ethynylcyclohexan-1-ol afforded the corresponding vinylsilane in excellent yield and total E selectivity. Subsequent Hiyama coupling with iodostyrene afforded the corresponding (E,E)-diene in good yields and with total selectivity.

Scheme 9.
Alkyne hydrosilylation–Hiyama coupling.
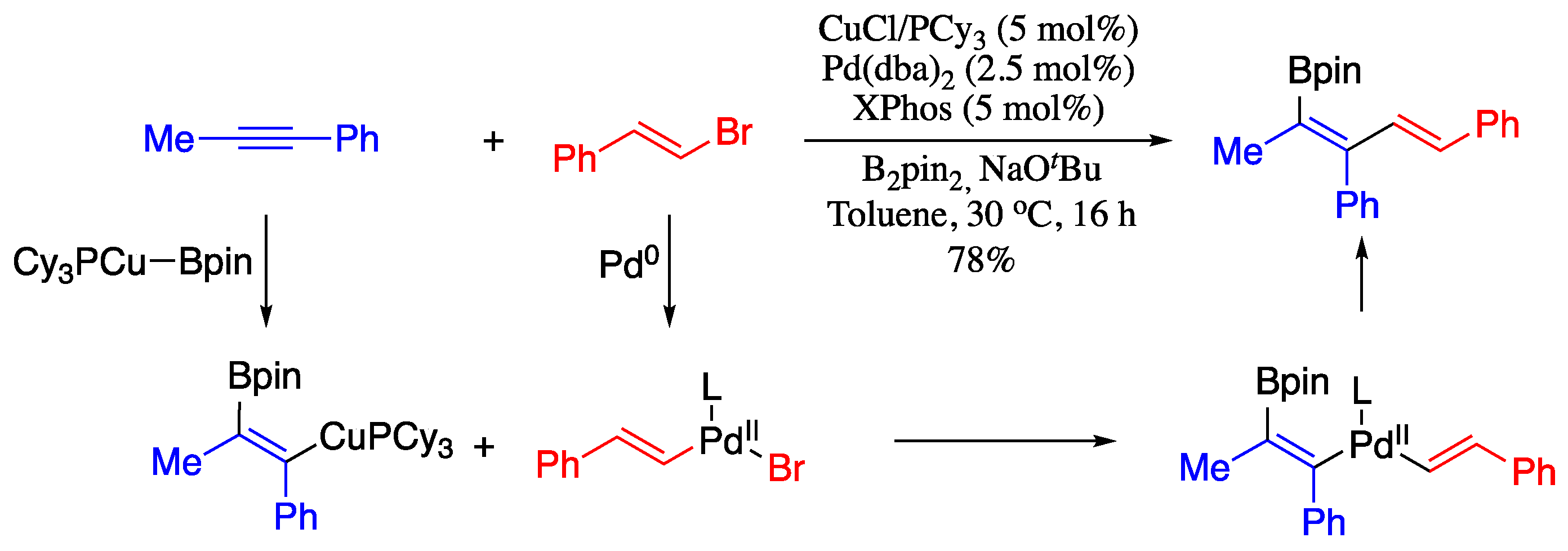
1-Boron-1,3-dienes are important building blocks in the synthesis of a broad range of bioactive natural products and organic intermediates. In 2018, Fañanás-Mastral reported notable contribution to the stereoselective synthesis of syn-borylated 1,3-dienes by Cu/Pd-catalysed alkenylboration of alkynes with alkenyl bromides and bis(pinacolato)diboron [63]. This methodology involved a Cu-catalysed carboboration of alkynes to provide β-boryl-substituted alkenylcopper intermediates, which on subsequent stereoretentive Pd-catalysed cross-coupling with alkenyl halides afforded the desired syn-borylated 1,3-dienes (Scheme 10).
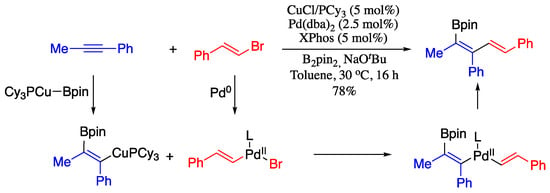
Scheme 10.
Cu/Pd-catalysed alkenylboration of alkynes.
As seen throughout this section, organozinc, organotin, and organoboron compounds that are employed in vinyl-vinyl cross-coupling reactions, are frequently prepared from the corresponding organolithium compounds by transmetalation. Notwithstanding the evident synthetic interest, the direct cross-coupling of organolithium reagents remained virtually unexplored until very recently, due to limitations such as high reactivity and low selectivity. This has changed with Feringa’s studies on the palladium-catalysed cross-coupling of organolithium nucleophiles and organic halides [64,65,66,67]. In order to take advantage of this interesting protocol for the synthesis of 1,3-dienes, the direct cross-coupling of alkenyllithium reagents with alkenyl halides was also described (Scheme 11) [68].

Scheme 11.
Palladium-catalysed cross-coupling of vinyllithium reagents and vinyl halides.
Following Feringa’s pioneering work on palladium-catalysed cross-coupling of alkenyllithium reagents, Liu and co-workers reported in 2018 a ligand-free iron-catalysed cross-coupling reaction of alkenyllithium compounds and vinyl iodides to form conjugated (E,E)-diene in good yields with full retention of stereochemistry (Scheme 12) [69].

Scheme 12.
Iron-catalysed cross-coupling of (E)-aryl vinyl iodides with (E)-propenyllithium.
Even if the use of lithium organometallics is advantageous, it is obvious that the best alternative would be to avoid completely the use vinylic organometallic reagents as starting materials. In this regard, Olivares and Weix described the synthesis of 1,3-dienes by the means pf Ni/Pd cooperative catalysis in the presence of zinc as reductant to afford tetra- and penta-substituted 1,3-dienes in good yields (Scheme 13) [70].
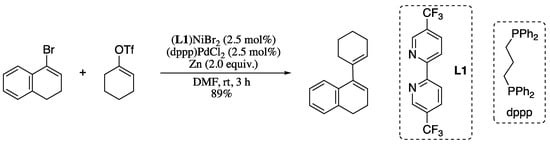
Scheme 13.
Selective cross electrophile 1,3-diene synthesis.
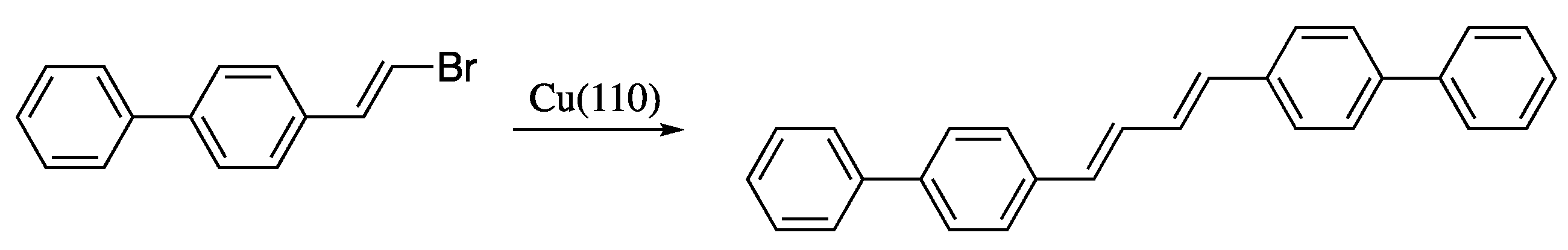
Yet another cross-coupling strategy for the synthesis of dienes is the dehydrogenative homocoupling of haloalkenes. A recent contribution to this field is the stereoselective synthesis of (E)-dienes through the dehalogenative homocoupling of alkenyl bromides on the Cu(110) surface reported by Sun et al. (Scheme 14) [71,72]. Despite the good yields and stereoselectivities and the mild conditions, this methodology is of limited interest, as it is restricted to the synthesis of symmetric dienes.
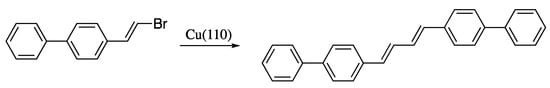
Scheme 14.
Formation of (E)-dienes by dehalogenative homocoupling of alkenyl bromides on Cu(110).
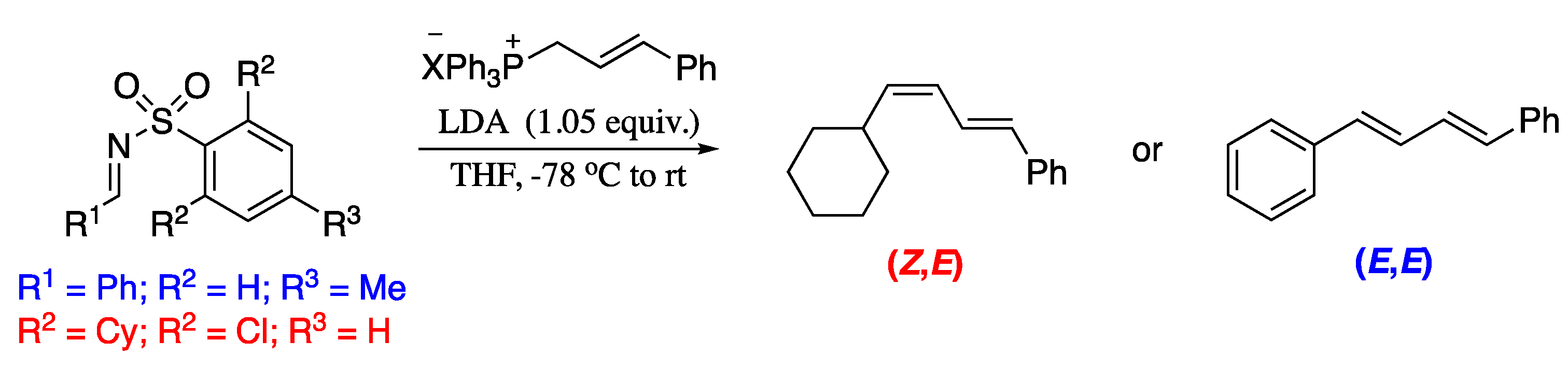
As stated before, 1,3-dienes are typically constructed by linkage of two C=C units. However, direct attachment of the 1,3-diene moiety would allow for a more convergent and efficient strategy. Notwithstanding the evident advantages, the development of this methodology has been hampered by the lack of stable and readily available dienylation reagents. Dienylboronic acids have been explored for dienylation, but they are unstable and difficult to prepare [73]. In the search for more effective dienylation agents, Nguyen and co-workers developed a simple and practical, regio- and stereoselective dienylation employing readily available sulfolenes (Scheme 15). The regio- and stereo- selectivity are determined by the substitution pattern in the starting sulfolenes. Thus, the reaction is E-selective for 3,4-dimethyl sulfolene, whereas the Z-selective dienylation is observed for 2-cyclohexyl-4-methyl sulfolene.
Scheme 15.
Dienylation of aromatic compounds with substituted sulfolenes.
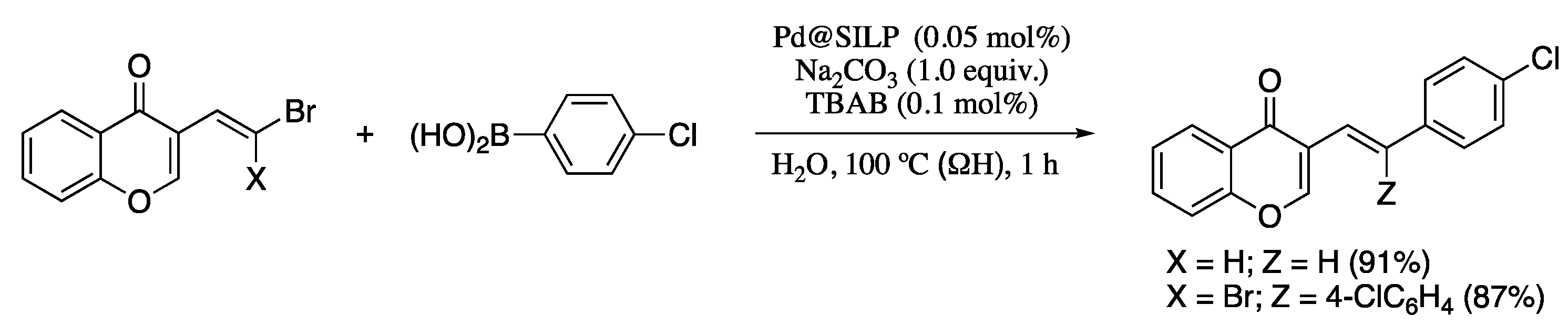
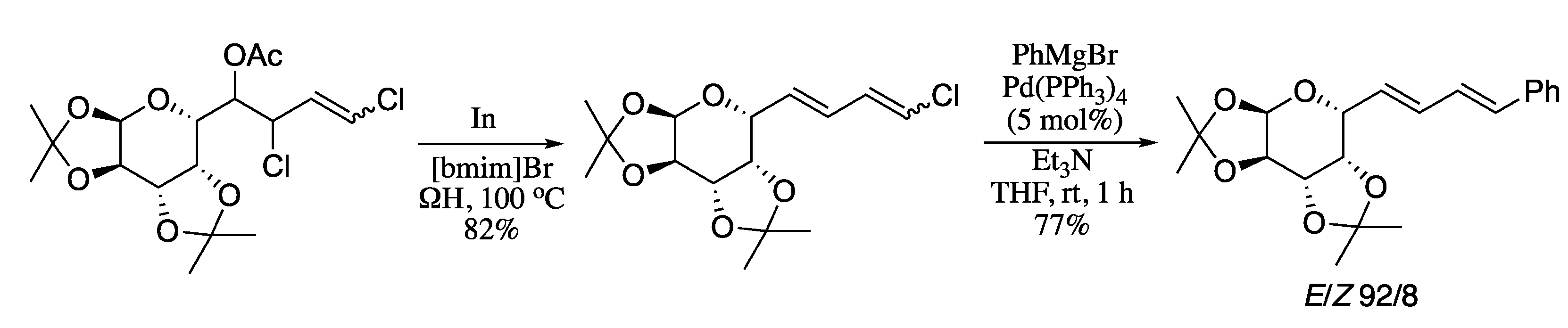
Silva and co-corkers have recently developed an environmentally benign, economically friendly, and sustainable Suzuki–Miyaura reaction of halo and dihalo dienyl derivatives and boronic acids employing Pd(II) immobilized in a silica-supported ionic liquid in water using ohmic heating (ΩH) [74]. Ohmic heating is and advanced thermal technology recently developed for organic synthesis [75,76] which leads activation on several reactions, including transition-metal catalysed cross-couplings [77]. The combination of ohmic heating with supported ionic liquid phase catalysis (SILPC) in water offers significant advantages, as the absence of undesired side reactions, the short reaction times, the mild conditions required, the wide range of functionalities tolerated, the good yields, the environment-friendly reaction conditions, and the low catalyst loading. In addition, the supported catalyst can be recovered and maintains a good activity for at least three cycles. Thus, the Pd-catalysed coupling of bromovinyl chromones and aryl boronic acids under ohmic heating afforded the corresponding dienes in high yields and total E selectivity (Scheme 16). Interestingly, the coupling of 2.0 equiv. of arylboronic acids with gem-dibromovinyl bromoflavones afforded the corresponding bisarylvinyl chromones in good yields. For the preparation of the catalyst, Pd(OAc)2 was supported on amorphous silica with the aid of the imidazolium ionic liquid 1-butyl-3-methylimidazolium hexafluorophosphate ([bmim]PF6), which was previously physisorbed in the SiO2 pores.
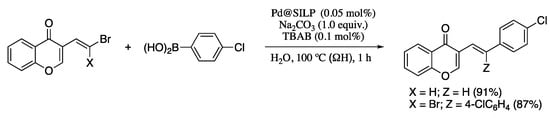
Scheme 16.
Suzuki–Miyaura reactions of chromone-based dienes with arylboronic acids.
2.1.2. Cross-Coupling of One Unactivated Vinylic Carbon
The formation of C−C bonds from vinylic C−H bonds is a powerful strategy for the synthesis of dienes. Unlike the methods reviewed above, which require activation of both coupling partners, this type of processes can deliver complex and stereodefined 1,3-dienes with prior activation of just one vinylic carbon.
Among them, the most popular is the Heck vinylation reaction [78], which enables direct formation of dienes from an activated coupling partner and a vinylic C−H bond of a terminal olefin substrate [79,80]. Depending on the activated coupling partner, there are two main types of Heck vinylation reactions: the Mirozoki-Heck reaction of alkenes and vinyl halides and the oxidative Heck reaction of alkenes and vinylboronic acids.
Despite their evident synthetic potential, the classical methods for Heck vinylation has had limited application in the preparation on biologically relevant 1,3-dienes because the scope is often limited to resonance activated olefins (e.g., α,β-unsaturated carbonyls and stryenes) and also because Pd−H isomerization usually results in moderate stereoselectivity [81,82,83]. However, the past few years have witnessed important advances in the Heck vinylation reaction, that resulted in a renewed interest in the application of this methodology to the stereoselective synthesis of 1,3-dienes [84].
One those crucial recent advances in the Heck vinylation reaction was reported in 2015. In this work, Madden and co-workers demonstrated that vinyliodides could be employed as coupling partners in the Heck–Mizoroki reaction with alkenes to access directly to dienes. Interestingly, coupling of vinyliodides with vinylboronate esters afforded the corresponding dienylboronates in good yields, which can undergo a Suzuki–Miyaura coupling with a range of aryl and alkenyl halides to furnish further functionalized dienes with total E selectivity (Scheme 17).
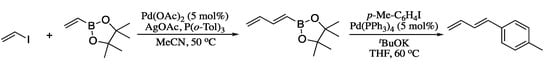
Scheme 17.
Coupling of vinyliodides with vinylboronate esters.
It is quite surprising that, until Madden’s research, vinyliodides had been almost completely overlooked in such couplings, except for one report from Heck et al. in 1975 [85].
The recent investigations aimed at overcoming the limitations of the Heck vinylation reaction for the construction of the 1,3-diene moiety were focused not only in the discovery of new coupling partners, but also in the development of novel catalyst systems. For example, Xu et al. developed a convenient methodology for the Heck reaction of alkenes and β-bromostyrenes based on the use of a μ-OMs palladium–dimer complex as promotor [86]. μ-OMs dimer is a non-phosphorus Pd-precatalyst which was developed by Buchwald for C–N/C–C coupling reactions [87]. When applied to the Heck reaction of (E)-(2-bromovinyl)benzene and styrene, the corresponding (1E,3E)-1,4-diphenylbuta-1,3-diene was isolated in good yield with total stereoselectivity (Scheme 18). It is widely assumed that the regioselectivity of the Heck vinylation reaction is substrate-controlled. Electron-deficient alkenes, such as acrylates and styrenes, afford linear dienes [88,89,90,91] whereas electron-rich alkenes, such as vinyl amides, furnish branched dienes [92,93].
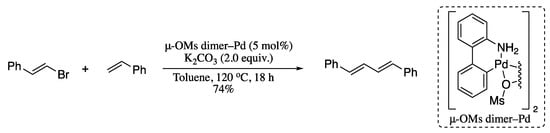
Scheme 18.
Heck reaction of alkenes and β-bromostyrenes.
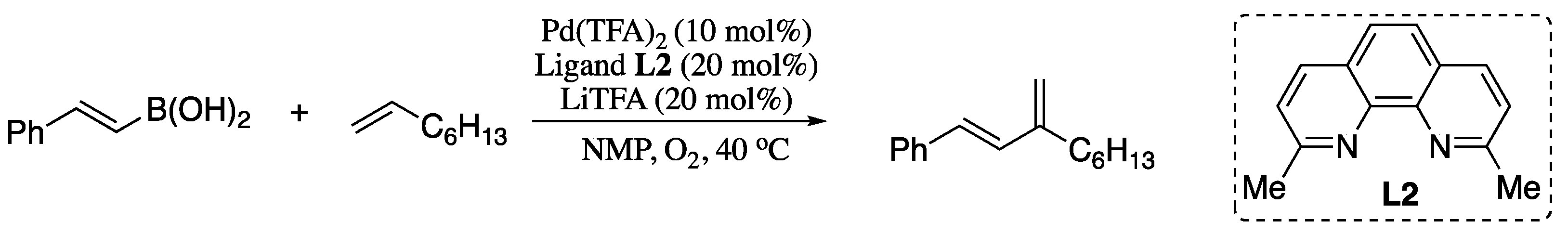
However, in 2012 Stahl and co-workers reported a oxidative coupling of alkenes and vinylboronic acids in which the regioselectivity was established via catalyst control [94]. Thus, reaction of (E)-styrenylboronic acid and 1-octene in the presence of palladium(II) trifluoroacetate [Pd(TFA)2] catalyst and neocuproine as ligand afforded the corresponding branched diene in good yield with 20/1 selectivity over the linear diene (Scheme 19).
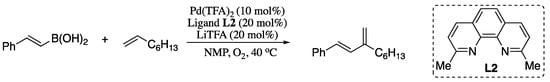
Scheme 19.
Oxidative coupling of alkenes and vinylboronic acids.
This work represented a very significant advance in the Heck vinylation, enabling access to synthetically useful branched 1,3-disubstituted conjugated dienes and expanding the scope and synthetic utility of Heck vinylation reactions.
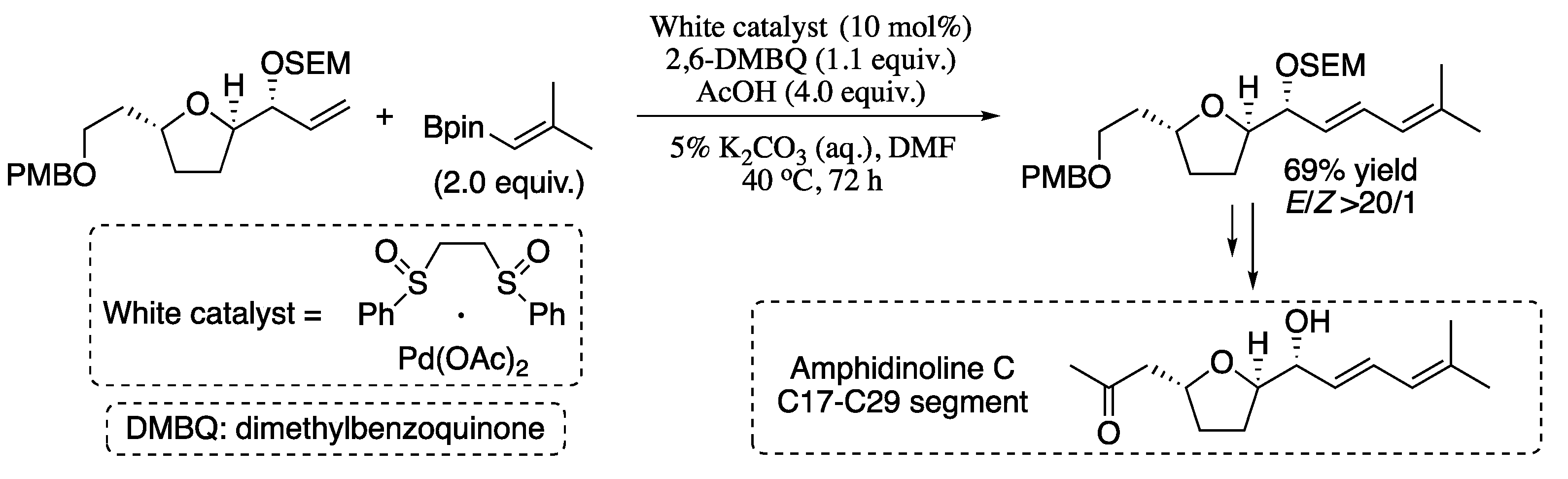
In 2013, Delcamp et al. reported an oxidative Heck vinylation for the formation of dienes and polyenes [95]. In this methodology, the reaction of limiting quantities of non-stabilized terminal olefins and slight excesses of vinyl boronic esters proceeds via oxidative Pd(II)/sulfoxide catalysis to afford 1,3-dienes in good yields and excellent stereoselectivities. The potential of this powerful cross-coupling reaction in the synthesis of medicinally relevant complex diene targets was also explored. As a representative example, the synthesis of the amphidinolide C C17−C29 segment is presented in Scheme 20.
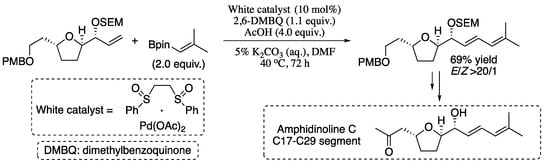
Scheme 20.
Oxidative Heck vinylation of terminal olefins and vinyl boronic esters.
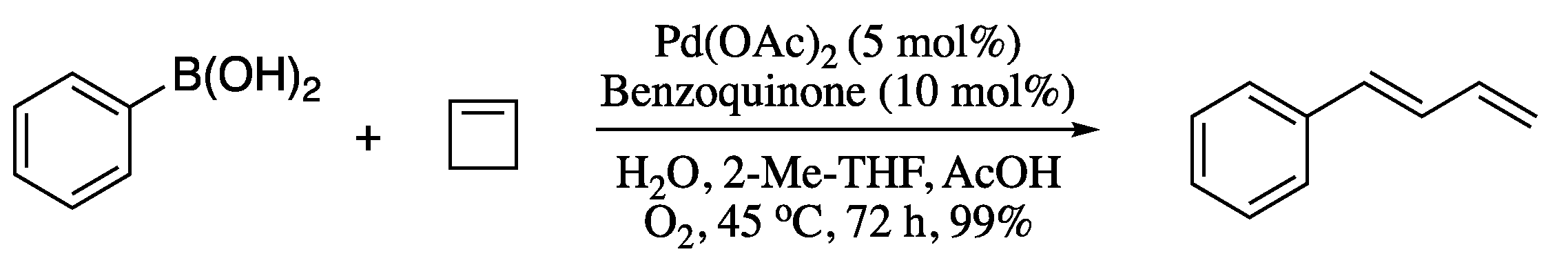
As yet another contribution to the oxidative Heck vinylation reaction, McAlpine and co-workers reported the coupling of arylboronic acids and cyclobutene to form terminal linear 1,3-dienes in near quantitative yield and total E stereoselectivity (Scheme 21) [96].

Scheme 21.
Oxidative Heck vinylation of cyclobutene with arylboronic acids.
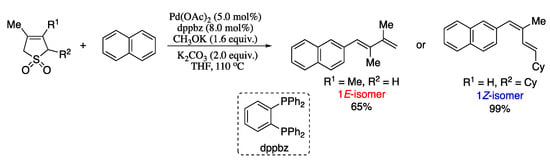
Since its discovery twenty years ago [97], 1,4-palladium migration has become an essential synthetic tool to permit the remote functionalization of C-H bonds [98,99,100,101]. Hu et al. demonstrated the usefulness of the combination of an aryl to vinyl 1,4-palladium migration with a Heck reaction for the synthesis of 1,3-dienes [102]. This method not only provides the desired dienes with high stereocontrol, but also with stereoselectivities inaccessible by previous conventional methods (Scheme 22) [103].

Scheme 22.
1,4-Palladium migration/Heck reaction of methyl 2-(2-bromoaryl)acrylates and methyl acrylate.
In 2013, Xiao et al. developed a novel methodology for the stereoselective synthesis of 1,3-dienes based on a three-component reaction of allenes, aryl iodides, and diazo compounds, furnishing the corresponding dienes in moderate to good yields as a single stereoisomer (Scheme 23) [104].
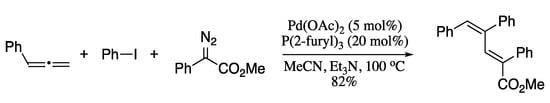
Scheme 23.
Palladium-catalysed three-component coupling of aryl iodides, allenes, and diazo compounds.
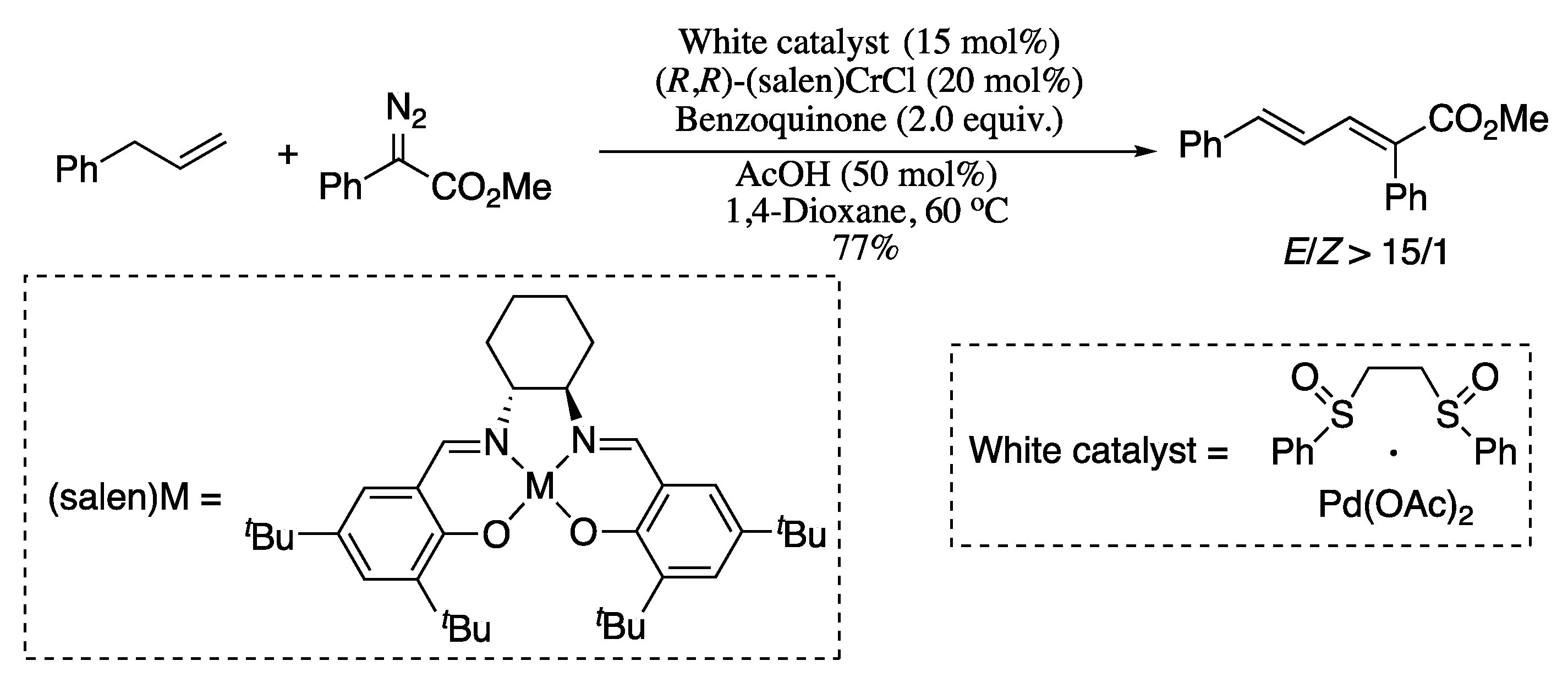
Also belonging to the subtype of transition-metal catalysed cross-coupling of one unactivated vinylic carbon is the allylic C−H olefination reported in 2014 by Wang and co-workers [105]. The olefination reaction of a wide scope of allyl compounds and α-diazo esters synergistically catalysed by a palladium(II) complex and (salen)CrCl generated diverse 1,3-diene derivatives in moderate yields and with good stereoselectivities. The (salen)CrCl was hypothesized to act as a Lewis acid to enhance the nucleophility of the α-diazo esters by coordinating to the nitrogen, thus facilitating the formation of π-allylic palladium carbenoid (Scheme 24).
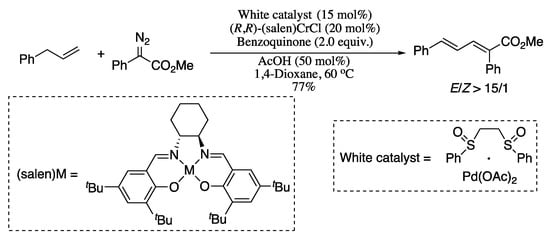
Scheme 24.
Allylic C−H olefination of α-diazo esters.
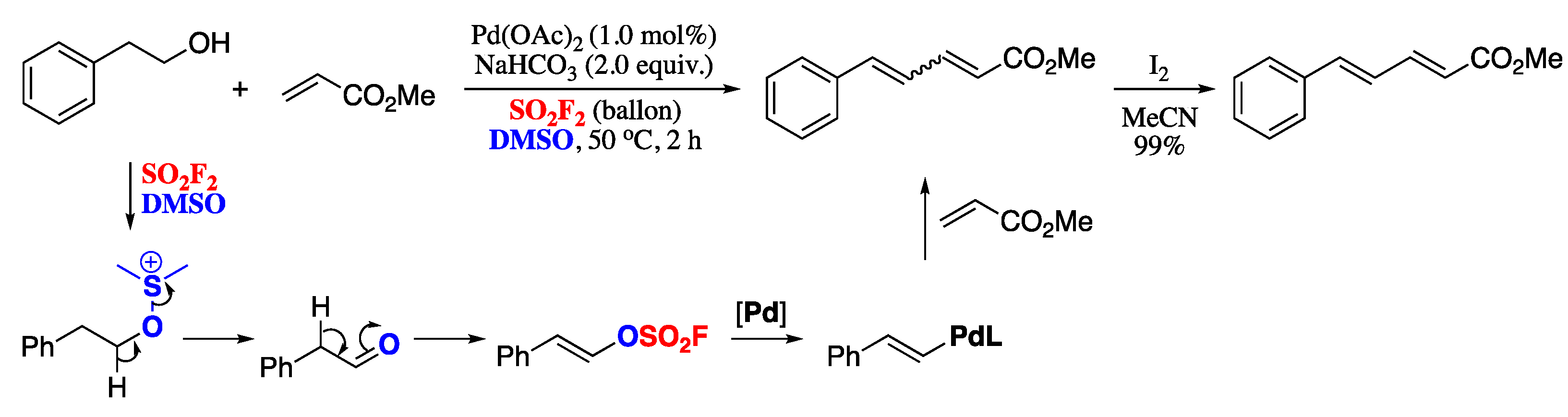
In a recent report, Revathi and co-workers developed a synthetic approach towards 1,3-dienes based on a Pd-catalysed, sulfuryl fluoride (SO2F2) mediated dehydrative cross-coupling reaction of alcohols with acrylates [106]. The process can be regarded as a Heck-type protocol, as it formally is the cross-coupling of an acrylate with an in situ formed vinyl sulfurofluoridate. Pd-catalysed reaction of an homobenzylic alcohol with methyl acrylate under a difluorosulfone atmosphere generated a mixture of E and Z dienes, which on iodine-catalysed isomerization finally furnished (E,E)-dienes in moderate yields (Scheme 25).
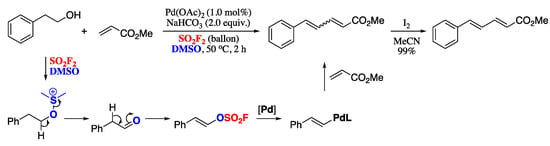
Scheme 25.
Pd-catalysed, SO2F2 mediated dehydrative cross-coupling reaction of alcohols with acrylates.
2.1.3. Cross-Coupling of Two Unactivated Vinylic Carbons
Transition-metal-catalysed direct functionalization of C–H bonds is emerging as one of the most important tools for carbon–carbon bond formation [107,108,109,110,111,112]. In particular, direct alkenylation of an inert vinylic C−H bond is particularly attractive for constructing the 1,3-diene moiety, as alkenes are naturally abundant an readily available [113]. Moreover, a one-step conversion of C-H bonds to the desired diene functionality reduces the number of synthetic steps, thereby saving reagents, solvents and time.
In 2004, Ishii reported the direct cross-coupling reaction of vinyl carboxylate acids with acrylates [114]. Although the diene motif was effectively constructed, the yields were moderate and the stereoselectivity was poor. Despite overlooked for some years, the decade started with a renewed interest in the direct olefination of double bonds, with extensive studies by Loh [115,116,117,118], Yu [119], Ge [120], Glorious [121], Georg [122], Hong [123,124,125], Gillaizeau [126] and Liu [127,128] groups. These pioneering works have been thoroughly reviewed in 2013. Therefore, in the following part of this section, the most relevant contributions from this date will be presented.
There are two basic approaches currently being adopted for the formation of vinylic C−C bonds by combining two metal-catalysed C(alkenyl)−H activations. (a) the non-directed cross-coupling of olefins via alkenyl-Pd intermediates; (b) the transition metal-catalysed olefinic C−H alkenylation based on directed syn C(alkenyl)-H activation.
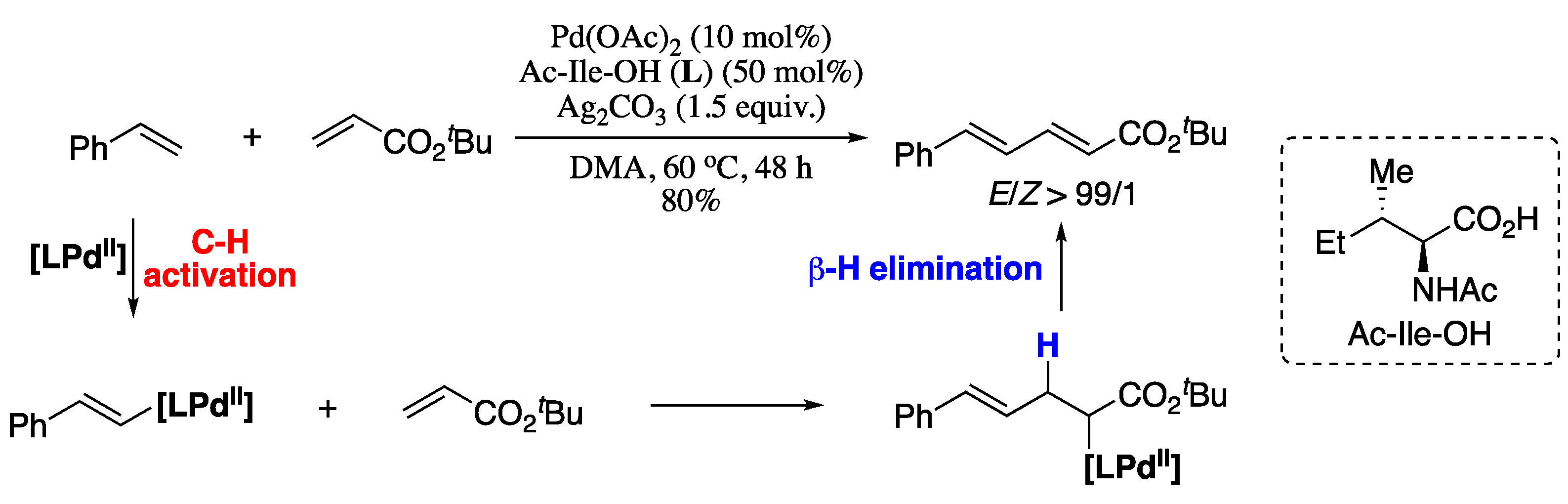
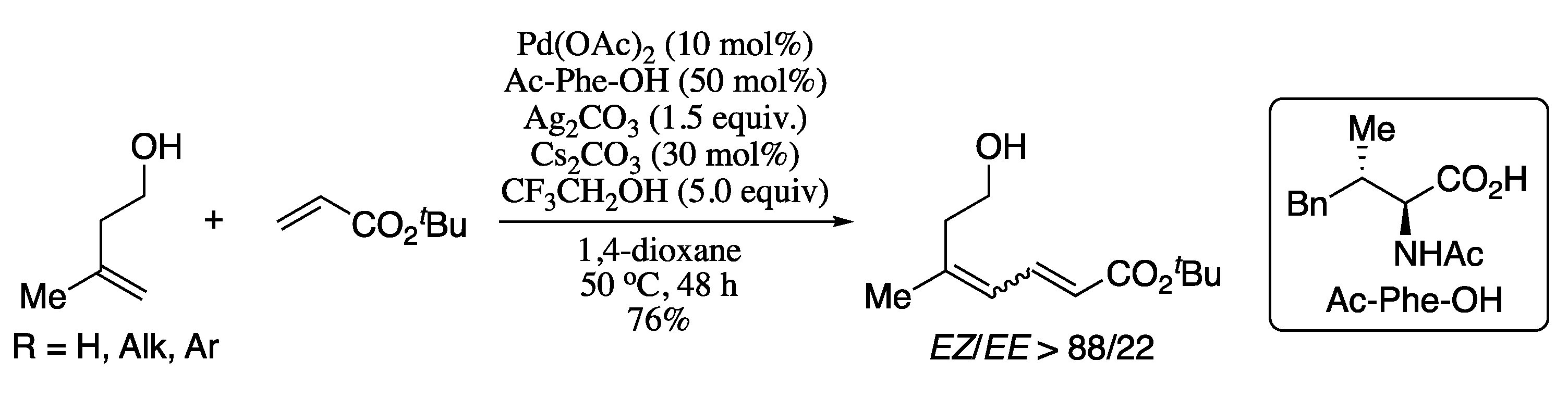
A relevant recent example of the first strategy is the palladium-catalysed cross-coupling reaction between mono-substituted common olefins and electron-deficient alkenes reported by Loh et al. in 2013 [129]. Thus, Pd-catalysed cross-coupling of alkenes with tert-butyl acrylate in the presence of an amino acid ligand (Ac-Ile-OH) furnished the corresponding (E,E)-diene products in good yield and excellent E/Z ratio (Scheme 26). Regarding the scope of the alkene coupling partners, various α,β-unsaturated amides, esters and phosphonates gave the corresponding butadiene products in moderate yields and good E/Z ratios.
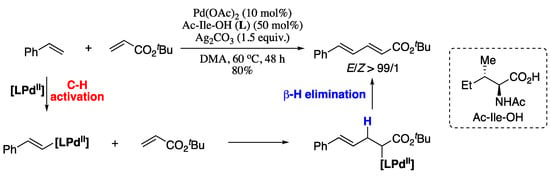
Scheme 26.
Olefination of alkenes with tert-butyl acrylate.
Notwithstanding that non-directed cross-coupling was extensively studied at the beginning of the decade, this procedure is usually restricted to the synthesis of E,E-dienes. In order to switch the selectivity to the Z-diene, Loh’s group also developed a hydroxy group chelation-assisted procedure for the oxidative cross-coupling of alkenes and acrylates, affording E,Z-dienes in moderate yields and good EZ/EE ratios (Scheme 27) [130].
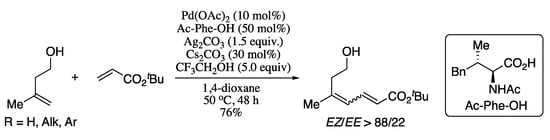
Scheme 27.
Olefination of homoallylic alcohols with tert-butyl acrylate.
Another significant contribution of Loh to the stereoselective C-H functionalization of alkenes utilizing directing groups was the Rh-catalysed regio- and stereoselective cross-couplings of enol phosphates with electron-deficient alkenes reported in 2015 [131]. The coupling products are also substrates for further cross-coupling reactions, giving rise to highly functionalized conjugated dienes. For example, reaction of enol phosphates with methyl acrylate in the presence of [{Cp*RhCl2}2], AgSbF6 and Cu(OAc) 2·H2O afforded the corresponding dienol phosphate, which on Ni-catalysed Suzuki–Miyaura cross-coupling with phenyl boronic acid generated the corresponding highly functionalized diene in moderate yield and stereoselectivity (Scheme 28).
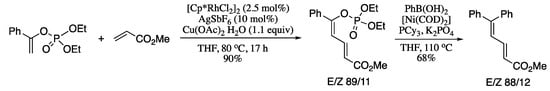
Scheme 28.
Alkenylation/Suzuki reaction of enol phosphates and acrylates.
The utilization of directing groups to improve stereoselectivity in palladium-catalysed sp2 C-H cross-couplings via chelation was further explored by Liu et al [132]. These authors reported the synthesis of 1,3-dienes from a directing-group-containing alkene (4-pentenoic acid, allyl alcohol, or 4-pentenamine derivative) and an electron-poor coupling partner using a palladium(II)-mediated directed site-selective C(alkenyl)−H activation strategy. Reoxidation of the palladium(II) catalyst was accomplished using benzoquinone (BQ), O2 and catalytic Co(OAc)2. For example, oxidative Pd-catalysed cross-coupling of a (Z)-alkenamide bearing an aminoquinoline directing group with tert-butyl acrylate gave the corresponding diene in excellent yield and stereoselectivity (Scheme 29).
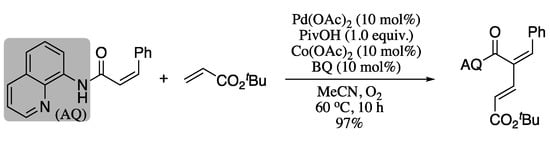
Scheme 29.
Coupling of alkene amide coupling with tert-butyl acrylate.
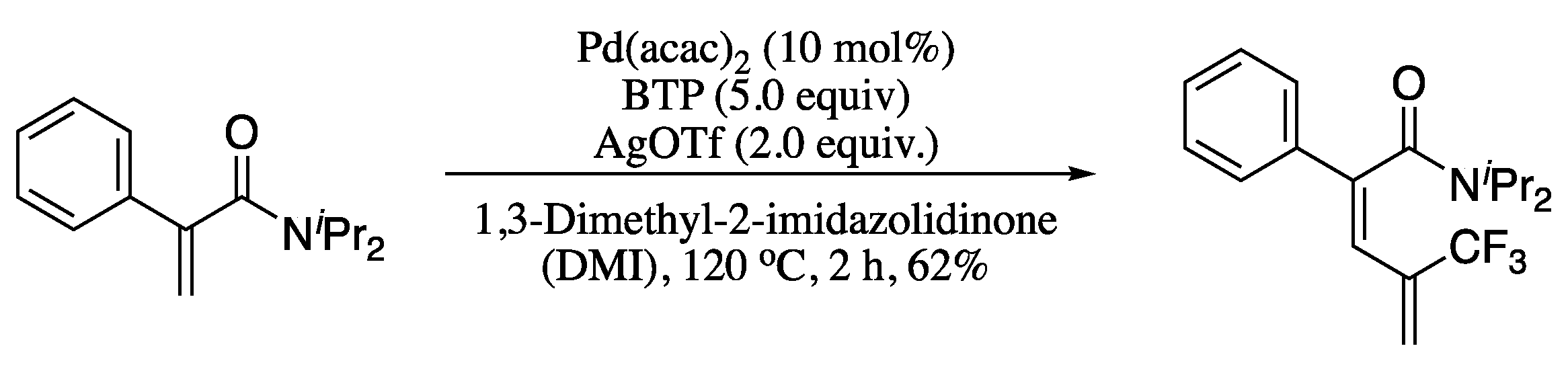
As yet another relevant example of the synthesis of 1,3-dienes by means of amide-directed C−H bond functionalization, Zhao and co-workers described the palladium-catalysed cross-coupling of α-substituted acrylamides with 2-bromo-3,3,3-trifluoropropene (BTP) to produce stereoselectively trifluoromethylated 1,3-butadienes in moderate yields (Scheme 30) [133].

Scheme 30.
Palladium-catalysed synthesis of 3-trifluoromethyl-substituted 1,3-dienes.
An emerging strategy in the domain of C–H activation chemistry is the use of an internal oxidizing directing group (DGOx) that acts as both a directing group and an internal oxidant for redox-neutral coupling reactions [134,135,136,137]. This approach avoids the use of stoichiometric amounts of metal oxidants, improving the reaction scope on account of the milder conditions and also reducing the waste formation of the oxidant. Zhang and Zhong applied this strategy to the cross-coupling of electron-deficient alkenes, which was carried out with the assistance of the oxidizing directing group CONH(OMe) and promoted by an inexpensive ruthenium catalyst to provide 1,3-butadienes with excellent selectivities (Scheme 31) [138].
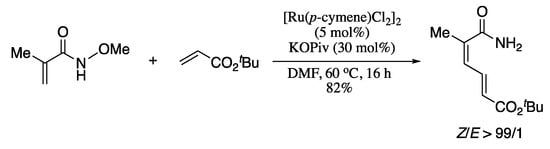
Scheme 31.
Direct cross-coupling of electron-deficient alkenes using an oxidizing directing group.
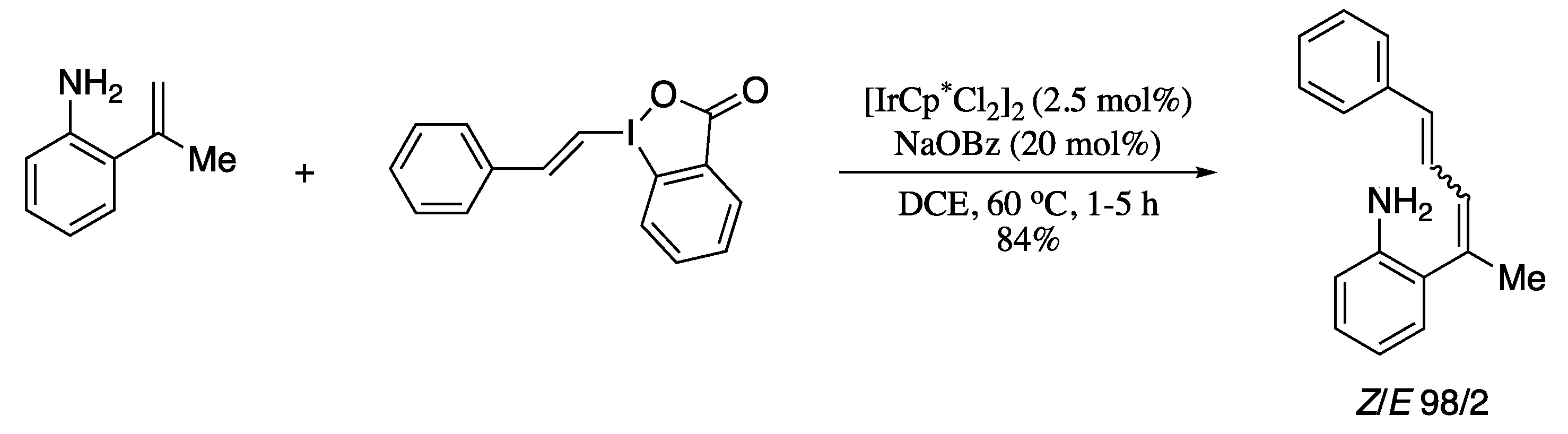
The strategy reported by Boelke and co-workers for the directing-group-mediated C−H alkenylation also avoids the use of metal oxidants, utilizing a hypervalent iodine reagent as both oxidant and coupling partner [139]. The methodology involved aromatic amines as directing groups and alkenyl-λ3-iodanes as electrophilic alkene-transfer reagents, in combination with an Ir(III) catalyst (Scheme 32).
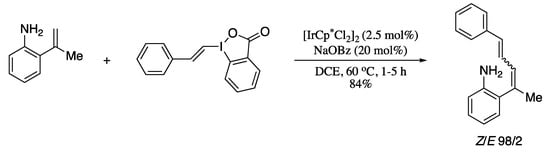
Scheme 32.
C−H Alkenylation of 2-vinylanilines with vinylbenziodoxolones.
Besides C−H alkenylation, transfer hydrogenation [140,141,142,143] is another research field of the greatest importance in modern Organic Chemistry. However, the combination of both areas remained elusive due to difficulties associated with olefin isomerization and hydrogenation of double bond. A truly game-changing report was published in 2019 and described the first example of alkene−alkene coupling integrating C−H activation and hydrogen transfer. In this work, Zhong and Zhang reported the iridium-catalysed cross-coupling between electron-deficient olefins in the presence of inexpensive chloranil as the hydrogen acceptor to provide (Z,E)-configurated dienamides [144]. Thus, reaction of acrylamides with diethyl vinylphosphonate in the presence of [IrCp*Cl2]2 (10 mol%), a silver(I) salt and chloranil as hydrogen acceptor provided stereoselectively (Z,E)-1,3-dienes in good yields (Scheme 33).

Scheme 33.
Iridium-catalysed cross-coupling reactions of alkenes.
2.2. Carbon(sp)−Carbon(sp3) Cross-Coupling
Methods based on the transition metal-catalysed alkylation of alkynes can be divided into two broad types: allylic C-H functionalization and alkyne carbometallation.
2.2.1. Allylic C-H Functionalization
Allylic C-H functionalization have attracted some recent interest for the synthesis of 1,3-dienes. Procedures based in allylic alkylation are redox-neutral and allow bypassing pre-functionalization of the substrates, which is very relevant from the perspective of atom economy, functional group tolerance and sustainability.
In 2017, a seminal article was published describing the palladium-catalysed redox-neutral allylic alkylation of pronucleophiles with unactivated skipped enynes to construct 1,3-dienes with high atom economy [145]. Indolinones, cyclic ketones, diketones, esters, nitroesters, cyanoesters, carbonates and indoline and tetrahydroquinolines are all efficiently alkylated, giving the desired 1,3-dienes in moderate to good yields and moderate E selectivity (Scheme 34).
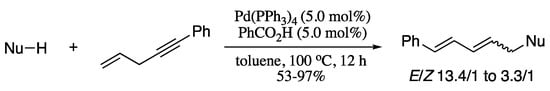
Scheme 34.
Palladium-catalysed allylic alkylation of pronucleophiles with unactivated skipped enynes.
Using a similar strategy, Lu and co-workers reported the allylic alkylation of oxindole pronucleophiles with cyclopropyl aryl acetylenes [146]. Pd-catalysed cross-coupling of aromatic-substituted cyclopropylethynyls with N-methyl-3-phenyloxindole afforded the 1,3-diene products in good yields and moderate E selectivity (Scheme 35).
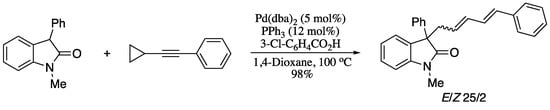
Scheme 35.
Allylic alkylation of cyclopropyl acetylene for the synthesis of 1,3-diene.
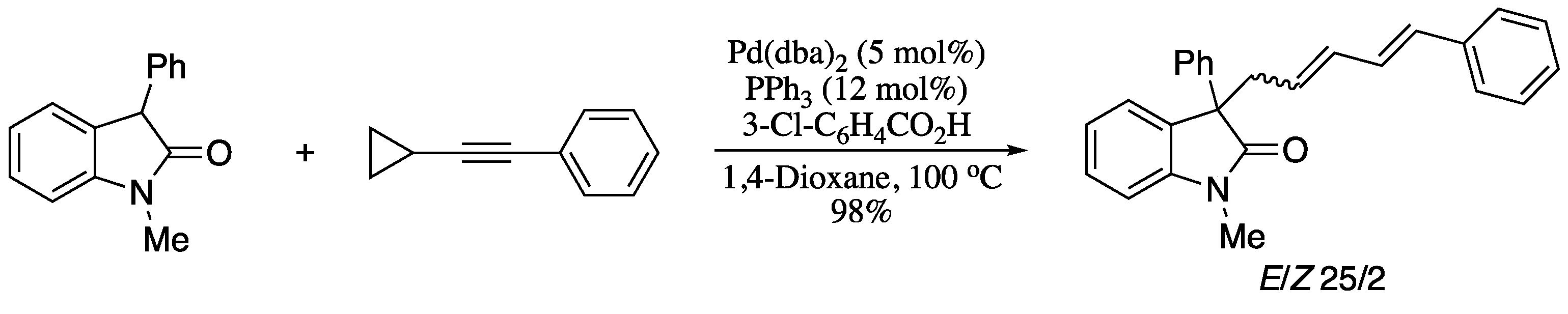
In 2018. Su et al. described the asymmetric α-allylation of aldehydes with alkynes promoted by a ternary catalyst system, consisting of an achiral palladium complex, a primary amine, and a chiral phosphoric acid [147]. The oxidative addition of Pd(0) to chiral phosphoric acid generated an hydridopalladium complex, which then reacted with acetylenes to form chiral electrophilic π-allylpalladium phosphate complexes. The enamine formed from the aldehyde and the amine catalyst then underwent asymmetric allylic substitution with the chiral π-allylpalladium complex, furnishing the corresponding dienes with good yields and enantioselectivities, albeit with moderate diastereoselectivity (Scheme 36).
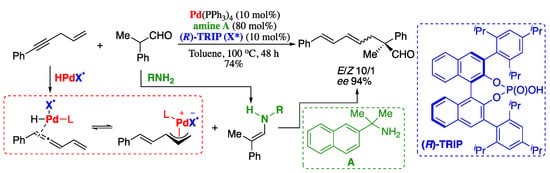
Scheme 36.
Asymmetric allylation of aldehydes with alkynes.
Despite the evident synthetic potential, one source of weakness in this strategy and a problem that should be addressed in the future is the moderate stereoselectivity.
2.2.2. Alkyne Carbometallation
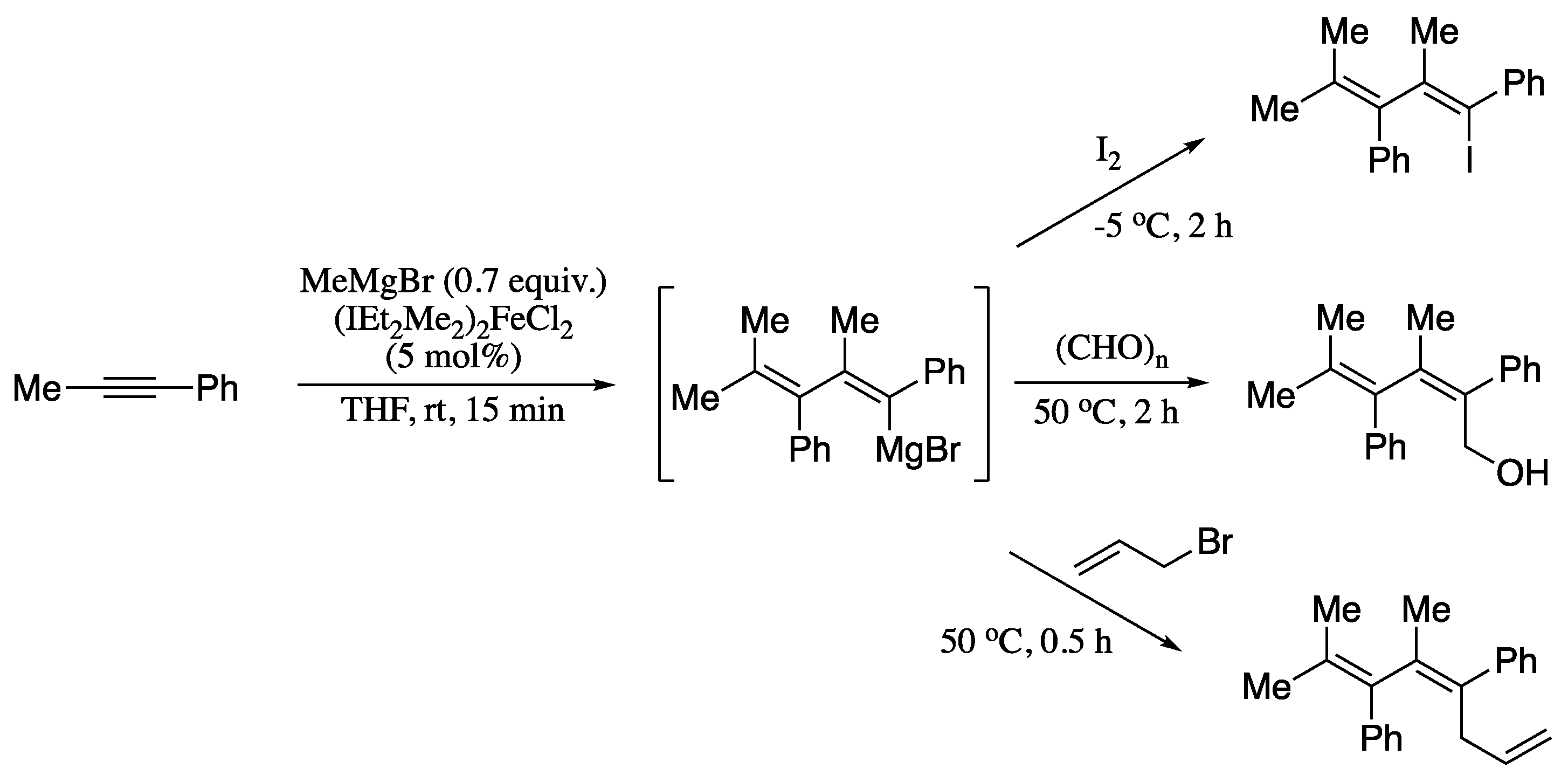
Transition-metal-catalysed carbometalation of alkynes would be an ideal methodology for generating dienyl organometallic compounds that could be further functionalized. However, the search for a catalyst enabling such transformation proved futile until a report in 2016. In this work, Liu et al. described an iron(II)-N-heterocyclic carbene (NHC) complex which can serve as a precatalyst for the double carbometalation of internal unsymmetrical alkynes with alkyl Grignard reagents, producing highly substituted 1,3-dienyl magnesium reagents with high regio- and stereoselectivity (Scheme 37) [148].
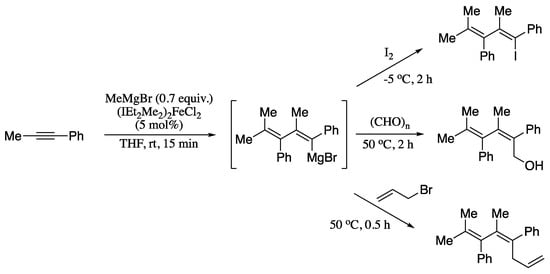
Scheme 37.
Carbometalation of phenyl(methyl)acetylene followed by transformations of the resultant 1,3-dienyl magnesium reagent.
The in situ formed 1,3-dienyl magnesium intermediate can also be trapped by electrophiles such as iodine, paraformaldehyde or allyl bromide to form the 1,3-dienyl iodide, the homoallylic alcohol or 1,3,6-triene, respectively, in good yields reflecting the synthetic utility of the 1,3-dienyl magnesium synthons.
3. Transition Metal-Free Cross-Coupling Reactions
Both the ‘classical’ transition-metal-catalysed cross-coupling methodologies based on the Heck, Negishi and Suzuki protocols and the ‘cutting-edge’ cross-coupling processes relying on the so called “C-H activation” described in the previous section have one thing in common: they are mediated by expensive late transition metals (e.g., ruthenium, rhodium, palladium and platinum). The use of these metals present two main disadvantages: on the one hand, trace metal contamination is a major problem in industry, particularly the pharmaceutical and organic electronic industries. On the other hand, the supply of these metals is at risk and it is widely assumed that their use will become unsustainable in the near future.
An important research field, therefore, involves the development of cross-coupling reactions that do not involve the use of a transition metal. There are several recent strategies for the synthesis of 1,3-dienes which are based in transition-metal free cross-coupling reactions.
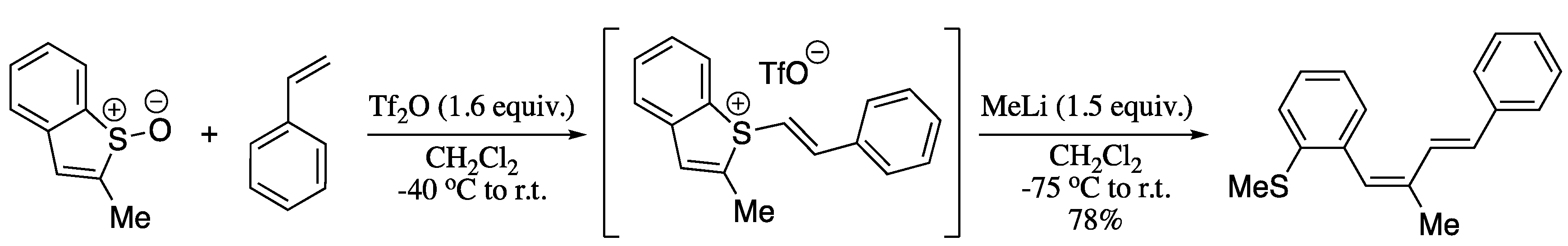
A recent strategy to achieve the transition metal free cross coupling is the use a sulphur-based directing group to set up carbon-carbon bond formation [149]. Thus, the easy formation of a carbon-sulphur bond is used to trigger the formation of a more-challenging carbon-carbon bond in a process resembling to a reductive elimination from a transition metal, with sulphur taking the place of palladium. Such methodology was applied to the selective C(sp2)-C(sp2) coupling to deliver 1,3-dienes [150]. In this process, addition of an organolithium or organomagnesium to a key sulphurane intermediate, formed in situ by means of an interrupted Pummerer reaction of sulfoxides and alkene coupling partners, provided (E,Z)-1,3-dienes in good yields with total stereocontrol (Scheme 38).
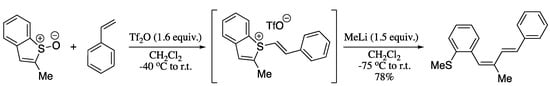
Scheme 38.
Interrupted Pummerer reaction/ligand-coupling sequence for the construction of (E,Z)-1,3-dienes.
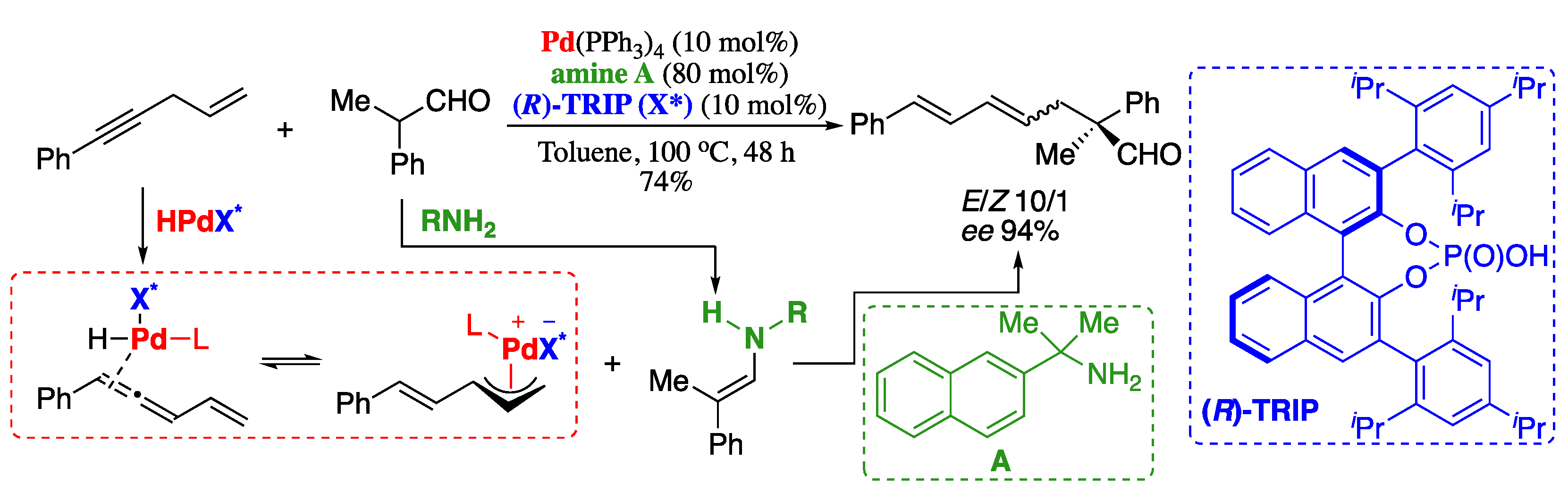
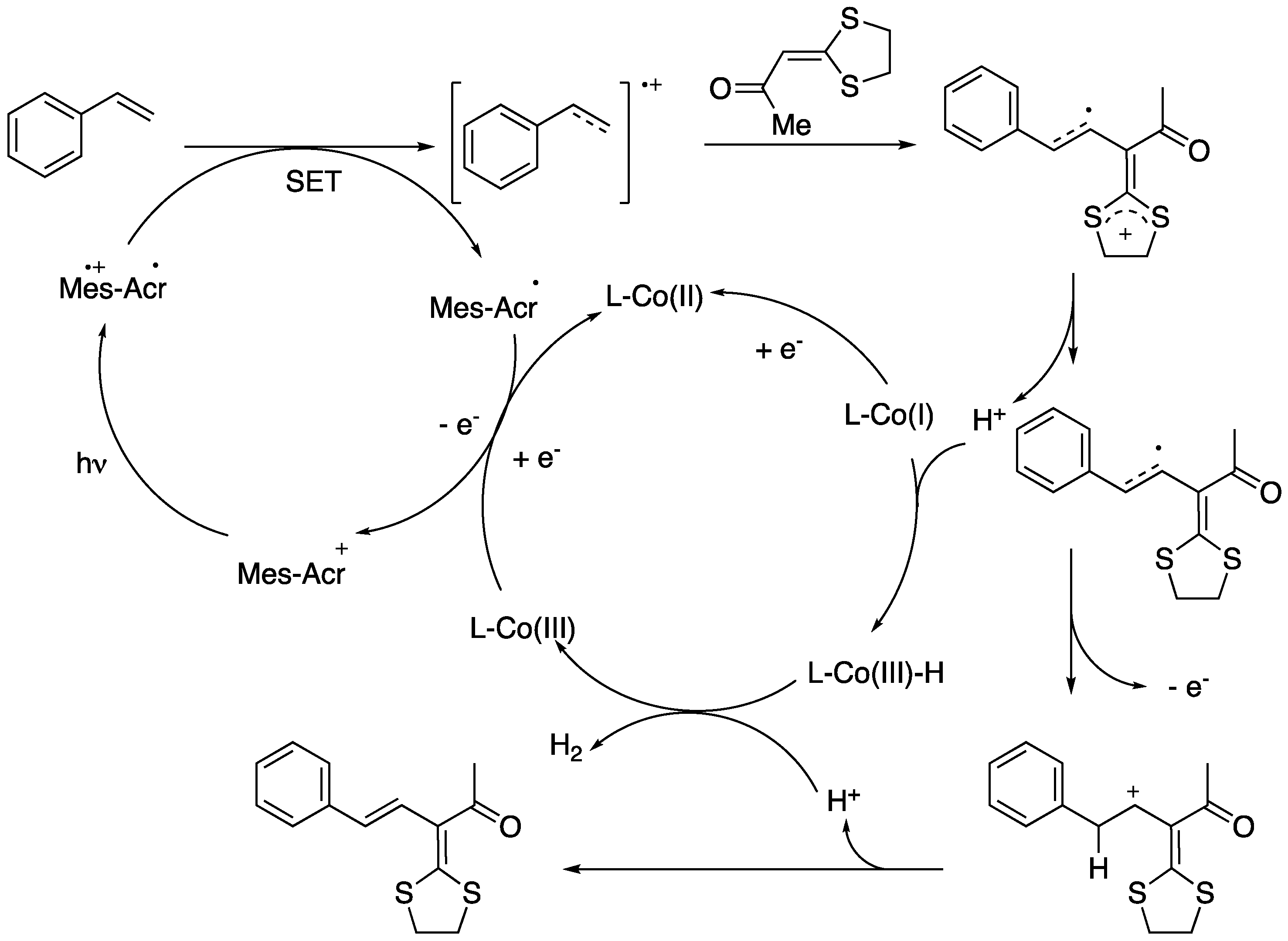
Undoubtedly, the most relevant of the emerging areas of research in synthetic chemistry is visible-light photoredox catalysis [151,152,153,154]. Photoredox catalysed cross-coupling offers an opportunity to develop greener processes and to overcome the shortcomings of using transition-metal catalysts in C−H functionalization. However, the photoredox catalysed direct C(sp2)−H/C(sp2)−H cross-coupling between two alkenes under external oxidant-free conditions was first reported in 2020. In this very recent work, Li’s group described the synthesis of substituted 1,3-dienes from vinylarenes and ketene dithioacetals under photoinduced cross-coupling reaction, providing the desired 1,3-dienes in a regio- and stereospecific manner and in good to excellent yields (Scheme 39) [155].

Scheme 39.
Photoinduced C(sp2)−H/C(sp2)−H cross-coupling of alkenes.
The proposed mechanistic cycle for the photoinduced cross-coupling reaction is depicted in Scheme 40. Initially, the styrene radical cation is formed upon electron transfer from the styrene to the excited state of the photosensitizer, generated under blue LED irradiation. Then, the nucleophilic attack of the radical cation to the α,β-unsaturated ketone would furnish a radical intermediate, which on a SET process with cobalt would form a carbocation. Finally, 1,3-diene is produced from the carbocation with the release of a proton.
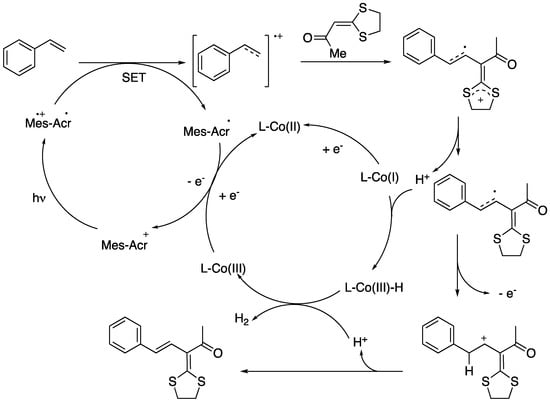
Scheme 40.
Proposed mechanism for the photoinduced synthesis of 1,3-dienes.
It is noteworthy that the reaction can be carried out using photocatalyst and cobalt dual catalyst without using noble metal and external oxidants and with hydrogen gas as the only by-product. On view of these results and also of the great recent interest in photoredox catalysis we will, no doubt, witness more advances in the near future on the photocatalytic-based synthesis of 1,3-dienes.
4. Aldehyde Dienylation
For total synthesis, the ideal scenario would be the installation of the 1,3-diene moiety in one step with high stereoselectivity. In this regard, the direct synthesis of 1,3-dienes via olefination of carbonyl groups is a very attractive alternative, which have enjoyed considerable attention. Perhaps the most common strategy for the dienylation of aldehydes is the Wittig reaction and its variants. Thus, diene formation through Wittig [156,157], Wittig-Horner [158,159], Horner–Wadsworth–Emmons (HWE) [160] olefination reactions have been thoroughly investigated. However, these methodologies often require strongly basic reaction conditions and produce dienes with moderate and substrate-dependent (E/Z)-selectivity. To avoid the basic conditions and the interference from alkali metal salt by-products inherent to the conventional Wittig reaction, much effort has been directed toward developing neutral and salt-free processes. In 2010, Zhou and co-workers reported a nearly neutral and salt-free Wittig olefination between of aldehydes in the presence of allylic carbonates and a tertiary phosphine, providing a convenient methodology for the synthesis of trisubstituted 1,3-dienes (Scheme 41) [161].
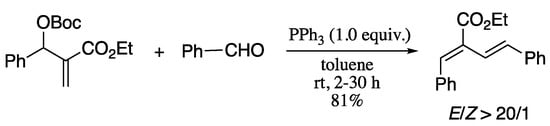
Scheme 41.
Synthesis of 1,2,4-trisubstituted 1,3-dienes from allylic carbonates and aldehydes.
In order to improve the results of the Wittig reaction, many efforts have been devoted to the modification of semi-stabilized triphenylphosphonium ylide reagents. On contrary, little attention has been paid to the electrophiles in the Wittig reaction. In this regard, Dong et al. reported a modified Wittig dienylation protocol which consist on replacing the starting aldehyde with N-sulfonyl imines, which possess distinct electronic and steric properties [162]. Thus, the reaction of in situ prepared allylidene triphenylphosphoranes with a range of p-tolyl-activated imines provided the corresponding (E,E)-1,3-dienes, whereas the use of 2,6-dichlorobenzenesulfonyl-activated imines resulted in the exclusive formation of the (Z,E)-1,3-dienes (Scheme 42).
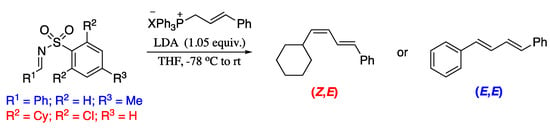
Scheme 42.
Stereoselective olefination of allylidenetriphenylphosphoranes with N-sulfonyl imines.
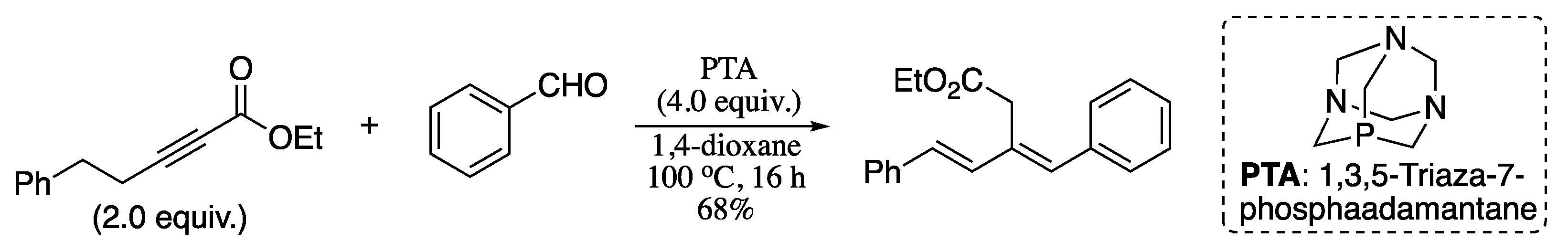
In 2011, Jacobsen and co-workers reported the Wittig olefination of phosphonium ylides generated in situ from the reaction of a 2-alkynoate and a phosphine, forming 1,3-dienes with excellent E-selectivity and good yields (Scheme 43) [163].
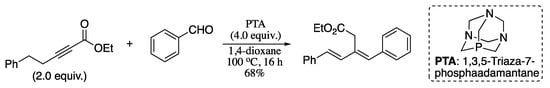
Scheme 43.
Synthesis of 1,3-dienes from aldehydes and alkynoates.
As previously stated, the past few years have seen increasingly rapid advances in the field of photoredox catalysis. Inspired by the robustness of the Wittig reaction for the formation of C=C bonds and important achievements of photoredox catalysis, Fu’s group developed a methodology for the C=C bond formation via coupling of alkyl halides with aldehydes and their derivatives using triphenylphosphine as a reductive quencher, to provided terminal 1,3-dienes in good yields albeit with poor E/Z ratio (Scheme 44) [164]. This procedure enabled access to 1,3-dienes in good yields with mild reaction conditions, operational simplicity and wide functional group tolerance. An important drawback is the moderate stereoselectivity, a factor that sure will by further considered in subsequent studies. In fact, the lack of stereoselection is a recurrent problem in the Wittig-based approaches for the synthesis of 1,3-dienes.
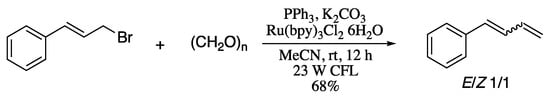
Scheme 44.
Visible-light photoredox synthesis of terminal 1,3-dienes.
In an attempt to overcome this limitation, Hilt’s group developed a procedure for the isomerization of double bonds by applying a tridentate ligand system which, in combination with a stereo-unspecific Wittig olefination, enabled the selective formation of Z-configured 1,3-dienes (Scheme 45) [165]. In this procedure, a mixture of E and Z 1,3-dienes in a 1:1 ratio, which was generated by a stereo-unspecific Wittig olefination of octanal, is converted selectively into (Z)-1,3-diene in the presence of cobalt catalyst [CoBr2(py-imine)], zinc powder, and ZnI2.
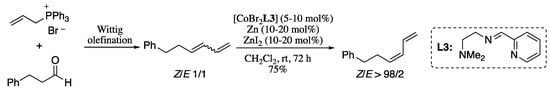
Scheme 45.
Sequence Wittig reaction/double-bond isomerization for the stereoselective generation of (Z)-dienes.
Other classical aldehyde olefination methodology that have been widely employed for the synthesis of 1,3-dienes from aldehydes is the Julia reaction. In particular, the second-generation Julia olefination developed in mid-1990s, has become a popular synthetic method for the aldehyde dienylation on account of its wide functional group tolerance and mild reaction conditions. In general terms, the Julia–Kocienski reaction yields dienes predominantly in (E)-configuration on newly formed double bond. Notwithstanding, low or even inversed selectivity was also observed in several cases. In an attempt to overcome this important drawback, Billard and co-workers developed a modification of Julia−Kocienski olefination reaction based on the use of cation-specific chelating agents which provides yields 1,3-dienes with predictable (E/Z)-selectivity [166]. Thus, reaction of the potassium salt of 1-phenyl tetrazoyl (PT) sulfone with an aldehyde afforded the corresponding dienes in moderate yields (Scheme 46). Regarding the stereoselectivity, it is substrate (aldehyde) dependent. From primary α-non-branched aldehydes the (Z)-dienes were obtained, whereas when α disubstituted or aromatic aldehydes were used, the (E)-dienes were formed as main products of the reaction.

Scheme 46.
Synthesis of 1,3-dienes via Julia−Kocienski reaction.
The studies presented so far provide a clear indication of the inherent limitations of the classic aldehyde olefination methodologies. Despite the recent advances in the field, none of the Wittig- and Julia-based aldehyde dienylation methods yet provided a universal solution in terms of yield and selectivity, which prompted the search for alternative procedures. Among them, processes based on a vinylogous Peterson elimination reaction have been widely investigated [167]; in fact, quite a few natural products displaying the 1,3-dienic moiety have been prepared by the means of the addition of a γ-silyl-substituted allylmetal reagent to an aldehyde followed by a Peterson-type olefination protocol [168]. For this purpose, silylated allylboronates [169], allyltitanates [170], allyl sulfonates [171] and allylzirconium reagents [172] have been used, affording dienes in high E-selectivity. Despite their utility, these methodologies usually lack generality and require strict reaction conditions and/or the use of highly toxic reagents. In order to overcome these limitations, a series of improved methodologies were described in the past few years.
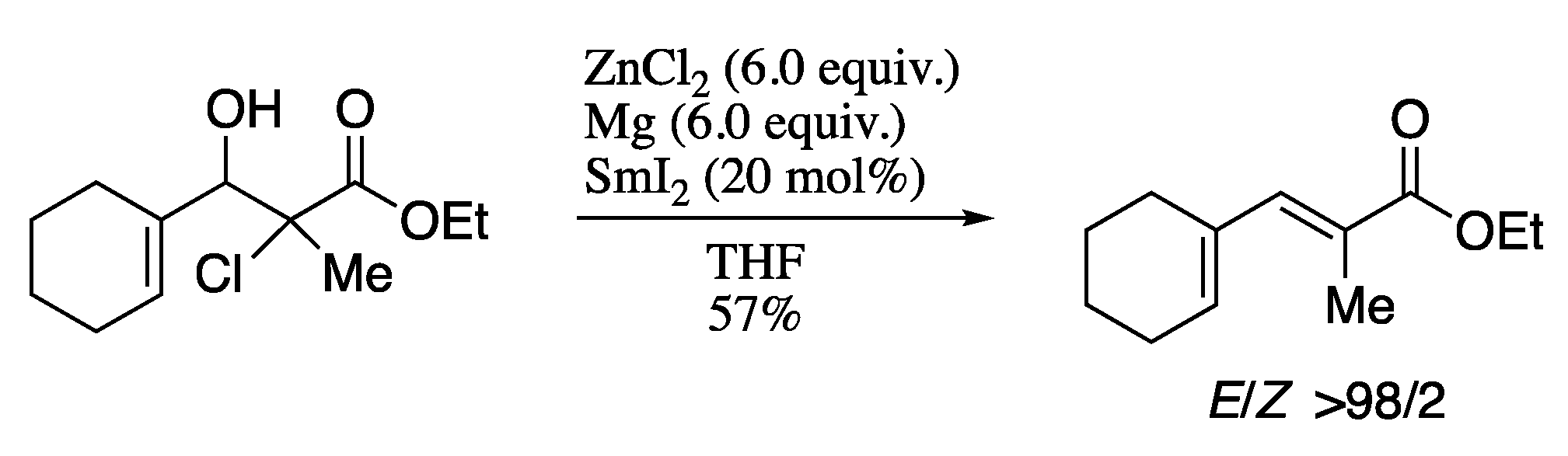
For example, in 2011 Rodríguez-Solla and co-workers described the synthesis of dienes with total E-stereoselectivity from easily available α-halo-β-hydroxy esters, with catalytic amounts of samarium diiodide in the presence of magnesium and zinc chloride (Scheme 47) [173].
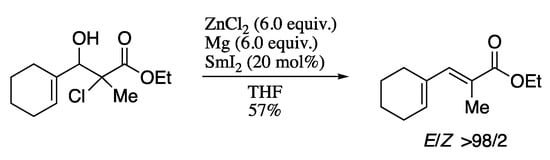
Scheme 47.
Formation of the (E)-dienes promoted by catalytic SmI2.
The same year Borg et al. reported the synthesis of (E)-1,3-dienes via Lewis acid-promoted addition of 1,3-bis(silyl)propene to aldehydes (Scheme 48) [174].
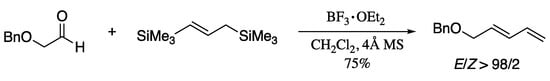
Scheme 48.
Formation of the (E)-dienes from aldehydes via allylsilanes.
The procedure is operationally straightforward but requires a 2.0 molar excess of a Lewis acid, which can be problematic from the point of view of functional group tolerance. Moreover, the starting 1,3-bis(silyl)propene is not commercially available and has to be prepared from relatively expensive allylsilane using highly flammable sec-butyllithium.
As a convenient alternative, Soengas and Rodríguez-Solla developed a methodology for stereoselective synthesis of (E)-1,3-dienes which involves the reductive β-elimination of 2-chloroallyl acetates, prepared by indium-promoted allylation of aldehydes with commercially available and unexpensive 1,3-dichloropropene [175]. Thus, reductive β-elimination of 2-chloroallyl acetates promoted by either In/CuI or Mg/ZnCl2 afforded (E)-1-substituted-1,3-dienes in good yields with high control of the stereoselectivity (Scheme 49).
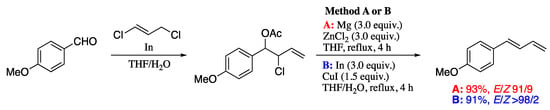
Scheme 49.
Synthesis of highly functionalized 1-substituted (E)-1,3-dienes by reductive β-elimination of 2-chloroallyl acetates.
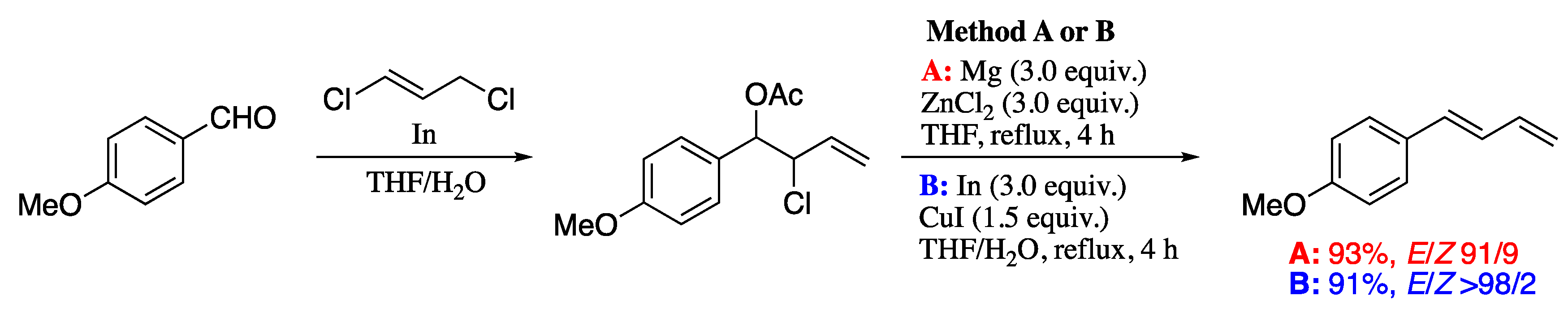
In a further improvement of this methodology, Soengas and Rodríguez-Solla demonstrated the beneficial effects of the combination of a novel and increasingly popular reaction media such as an ionic liquid with a cutting-edge heating technology like ohmic heating (ΩH) in the dienylation reaction [176]. In this process, synthetically useful (1E)-4-chloro-1-substituted-1,3-dienes were prepared from 2,4-dichlorohomoallyl acetates, derived from the indium-promoted allylation of aldehydes with 3-bromo-1,3-dichloro-propene and subsequent acetylation. The elimination process was carried out by reductive β-elimination promoted by indium and, in contrast to previously reported procedures, by using ionic liquid media and ohmic heating desired 4-chloro-1,3-dienes were obtained in good yields and excellent E-selectivities without need of any additive. The presence of the 4-chloro-1,3-diene moiety makes these compounds ideal precursors for palladium-catalysed coupling reactions, which can be exploited for the introduction of new substituents and the elongation of the carbon chain. For example, indium-promoted allylation of a galactose-derived aldehyde, followed by acetylation, afforded the chloroacetate intermediate. Indium-promoted elimination under ohmic heating furnished the corresponding 1-chlorodiene, which on Kumada cross-coupling gave the (E,E)-diene in good yield (Scheme 50).
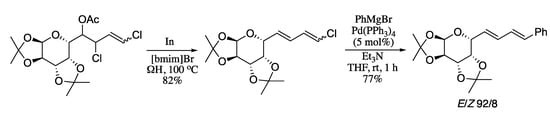
Scheme 50.
Synergic combination of ohmic heating and ionic liquids for the indium-based synthesis of 1,3-dienes.
More recently, Soengas and Rodríguez-Solla reported the stereoselective synthesis of highly functionalized (E)-1,3-dienes from carbonyl compounds and 1,3-dichloropropene through a cascade allylation/β-elimination reaction promoted by a cooperative zinc/catalytic indium system [177]. Thus, aldehydes or ketones were effectively dienylated in the presence of zinc and catalytic indium trichloride, affording the corresponding (E)-dienes in good yields and selectivities (Scheme 51).
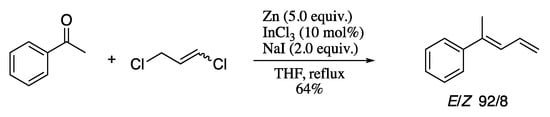
Scheme 51.
Cooperative zinc/catalytic indium system for the synthesis of highly functionalized (E)-1,3-dienes from carbonyl compounds.
Another recent example of aldehyde dienylation is the TiCl4/Et3N-mediated condensation of aldehydes with α,β-unsaturated carboxylates to generate 1,3-dienes in good yields and stereoselectivities reported in 2016 (Scheme 52) [178]. Despite the satisfactory results, the procedure is restricted to the synthesis of butadiene-2-carboxylates.
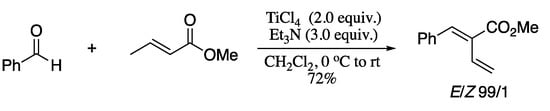
Scheme 52.
Titanium(IV) chloride-mediated stereoselective α-alkylidenation of aldehydes.
5. Olefin Methathesis
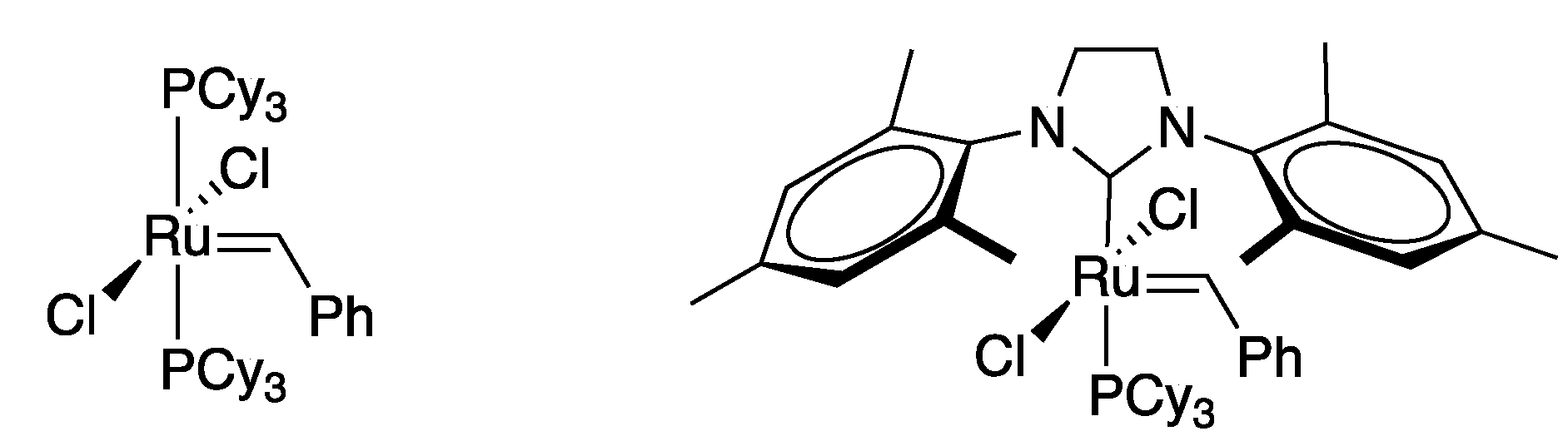
Olefin metathesis is one of the most powerful existing methods for the formation of C-C double bonds. It basically provides the same end goal of the Wittig reaction but through a catalytic pathway. In this regard, is an extraordinarily atom-economical reaction, compatible with many functional groups. Although olefin metathesis has been known since mid-1950s, it was Grubbs’ work on ruthenium based catalysts what put this reaction at the forefront of organic synthesis [179,180] (Figure 3).
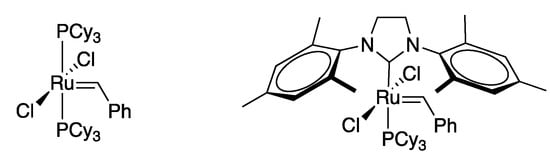
Figure 3.
Grubbs’ catalysts.
There are two basic types of olefin metathesis for the formation of C-C bonds: the cross-metathesis and the ring closing metathesis (RCM).
A recent example of the application of olefin cross-metathesis to the synthesis of 1,3-dienes is the cross-metathesis/elimination sequence reported by Bilel and co-workers in 2014 [181]. Thus, olefin cross-metathesis of allylic chlorides and alkenes afforded chloroallylic substrates, which on Ru-catalysed elimination generated the corresponding dienes in good yields and moderate E-selectivity (Scheme 53).
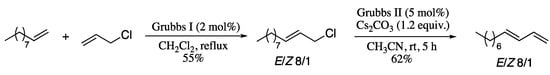
Scheme 53.
Ru-catalysed cross-metathesis/elimination sequence.
When the ring closing metathesis is performed on an enyne system, the so-called ring closing enyne metathesis (RCEYM), it provides cyclic 1,3-dienes with one exocyclic double bond, which are of special interest for the synthesis of bioactive compounds. Both heterocyclic and carbocyclic 1,3-dienes can be prepared by means of a RCEYM reaction. For example, Tan and co-workers reported the Ru-catalysed RCEYM of chiral propargyl amines bearing an allyl group bonded to the nitrogen atom, to provide vinylpyrrolines in high yields (Scheme 54) [182].

Scheme 54.
Ru-catalysed RCEYM of chiral propargyl amines.
More recently, Dolan et al. described the use of ruthenium (IV) dihydride complexes as new catalysts for the metathesis of oxygenated and nitrogenated enynes (Scheme 55) [183].
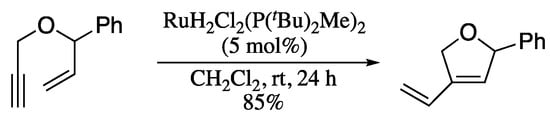
Scheme 55.
Ru-catalysed metathesis of hetero-substituted enynes.
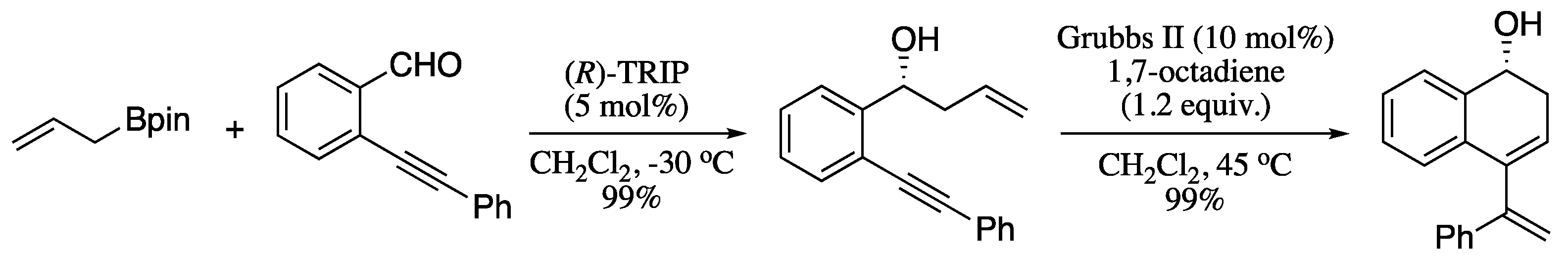
Some recent contributions on the synthesis of 1,3-diene carbocyclic rings includes the work of Fustero’s group on the synthesis of enantiopure cyclic dienes through the ring-closing metathesis of homoallylic benzylic or amines with an alkenyl group at ortho-position of the aromatic ring [184,185]. For example, chiral ω-alkynyl homoallylic(homopropargylic)alcohols, easily available from asymmetric allyl(propargyl)-boration of ortho-alkynyl benzaldehydes, were transformed to the corresponding cyclic 1,3-dienes via ring-closing enyne metathesis (RCEYM) (Scheme 56).
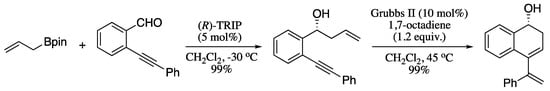
Scheme 56.
Sequential asymmetric allyl(propargyl)boration/RCEYM for the synthesis of chiral enantiopure cyclic dienes.
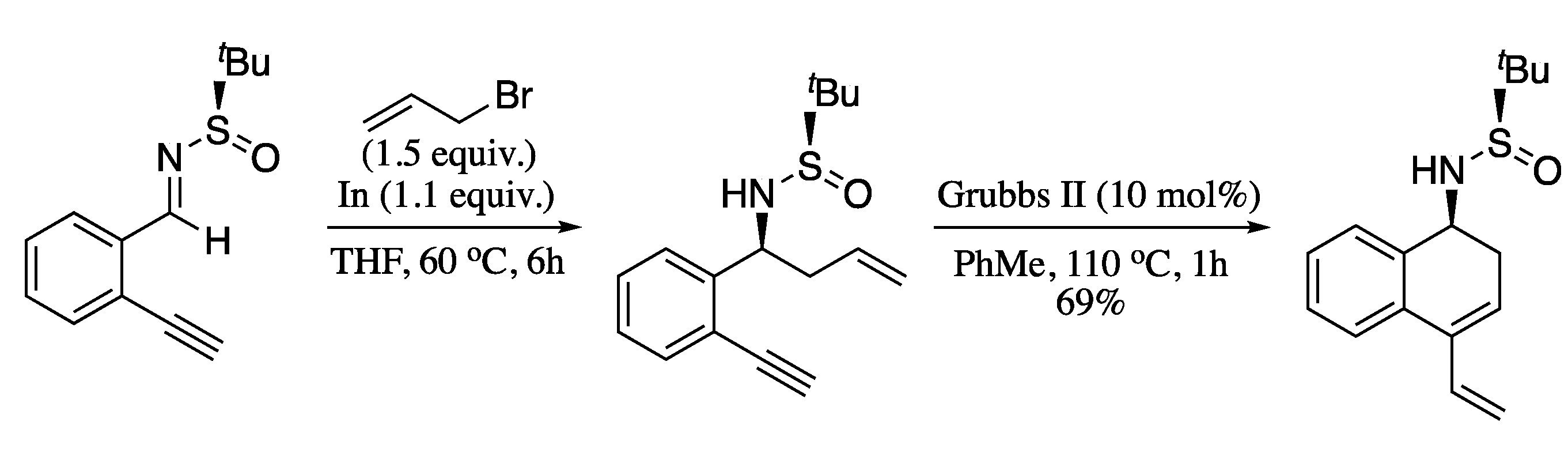
More recently, Yus and Foubelo performed the ring-closing metathesis of chiral N-tert-butanesulfinyl amino derivatives, prepared by means of an indium-promoted enantioselective propargylation protocol developed in their group, [186,187] producing the enantiopure cyclic dienes in high yields (Scheme 57) [188].
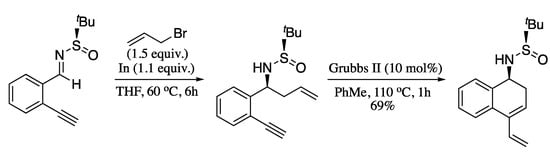
Scheme 57.
Ring-closing metathesis of enantioenriched enynes.
6. Rearrangement/Isomerization
Synthetic methodologies based in isomerizations or rearrangements of other chemical motifs have been extensively investigated as atom-economic alternatives for the synthesis of 1,3-dienes. Taking into account that other unsaturated functions as allenes and alkynes have the same oxidation state as 1,3-dienes, their transformation into dienes just requires some internal hydrogen reorganization. This is considerably more atom economical than external sequential reduction-oxidation operations, hence the considerable amount of literature that has been published on this matter. Rearrangements of other diene derivatives as well as other functional groups, as enols or cyclobutenes, has also been considered for the synthesis of dienes. The next section presents some major studies on this field that were conducted in the past decade.
6.1. From Allenes
Translation of allene derivatives is a particularly effective method for the synthesis of functionalized dienes, thereby being a research area of much recent interest [189,190,191,192,193,194,195]. Isomerization of an allenic system to a conjugated diene was first demonstrated by Trost and co-workers [196]. Inspired by Trost’s seminal study, Tin et al. developed a gold-catalysed isomerization of unactivated allenes into 1,3-dienes [197]. Reaction of and tetrasubstituted allenes with catalytic gold(III) in the presence of nitrosobenzene as additive afforded highly functionalized dienes in excellent yields in the E-form only. The most likely mechanism would involve a gold allylic cation intermediate, which on an intramolecular proton transfer mediated by nitrosobenzene, generates the final 1,3-diene (Scheme 58).
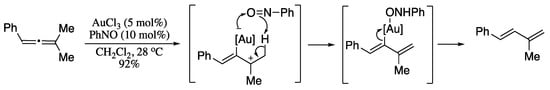
Scheme 58.
Gold-catalysed isomerization of unactivated allenes.
In 2014, Hampton and Harmata reported the isomerization of allenic sulfones to arylsulfonyl 1,3-dienes [198]. Under conditions of palladium catalysis in the presence of a weak acid, allenic sulfones were converted to 1-arylsulfonyl 1,3-dienes in good yields and total stereo- and regio-control (Scheme 59).
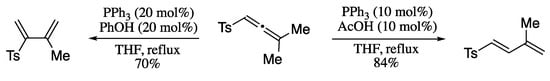
Scheme 59.
Regiodivergent synthesis of 1- and 2-arylsulfonyl 1,3-dienes.
On the other hand, nucleophilic catalysis using triphenylphosphine in the presence of a proton shuttle yields 2-arylsulfonyl 1,3-dienes.
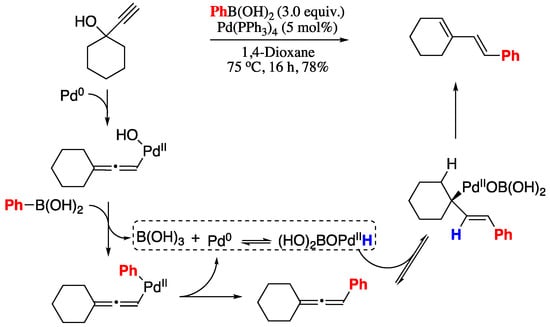
More recently, Kimber’s group developed the rearrangement of an allene to a 1,3-diene by means of a palladium hydride complex generated in situ from a Pd(0) source and boric acid. [199] Under these conditions, allenes were transformed in 1,3-dienes in good yields and total E-stereoselectivity (Scheme 60). The reaction was hypothesized to occur via formation of a π-allylpalladium complex and subsequent syn-β-hydride elimination to give the 1,3-diene products.
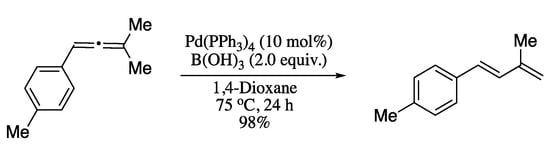
Scheme 60.
Rearrangement of unactivated allenes to 1,3-dienes.
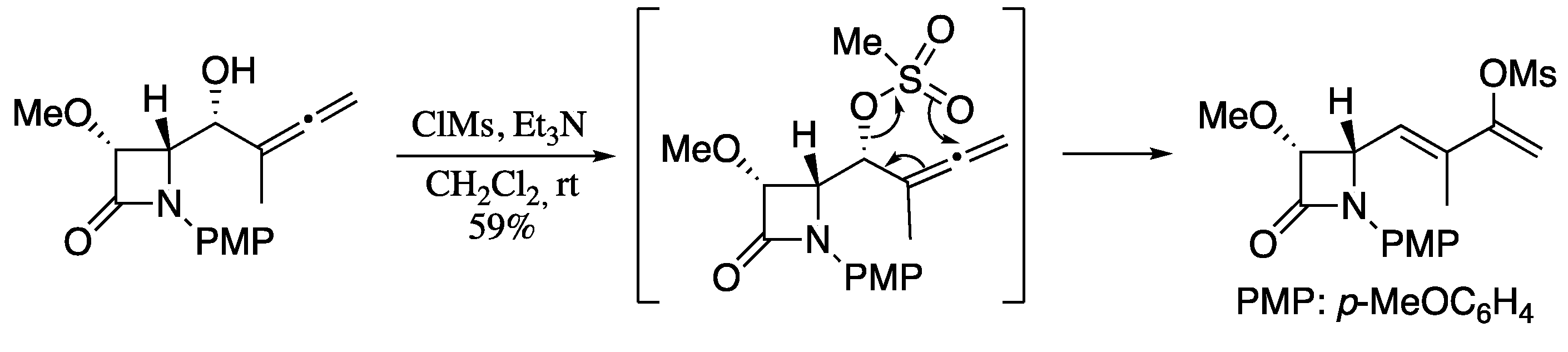
1,3-Transposition of C-O bonds across a π-system is an important reaction in organic synthesis and has been widely studied for allylic and propargylic alcohols. On contrary, the rearrangement of allenic alcohols and their derivatives remained underdeveloped until several recent examples highlighted the interest in this transformation for the stereoselective synthesis of 1,3-dienes. In one of this pioneering works, Alcaide, observed the formation of mesylated 1,3-dienes upon treating allenic alcohols with methanesulfonyl chloride and triethylamine (Scheme 61) [200].
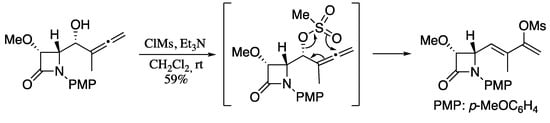
Scheme 61.
Rearrangement of allenols to 1,3-dienes.
Later in 2011, Kraft’s group developed a gold-catalysed Claisen-type rearrangement of allenyl vinyl ethers to give 1,3-dienes with different substitution patterns (Scheme 62) [201].
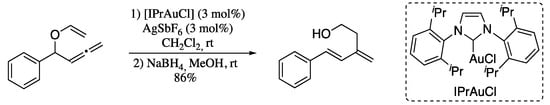
Scheme 62.
Gold (I)-catalysed Claisen rearrangement of allenyl vinyl ethers.
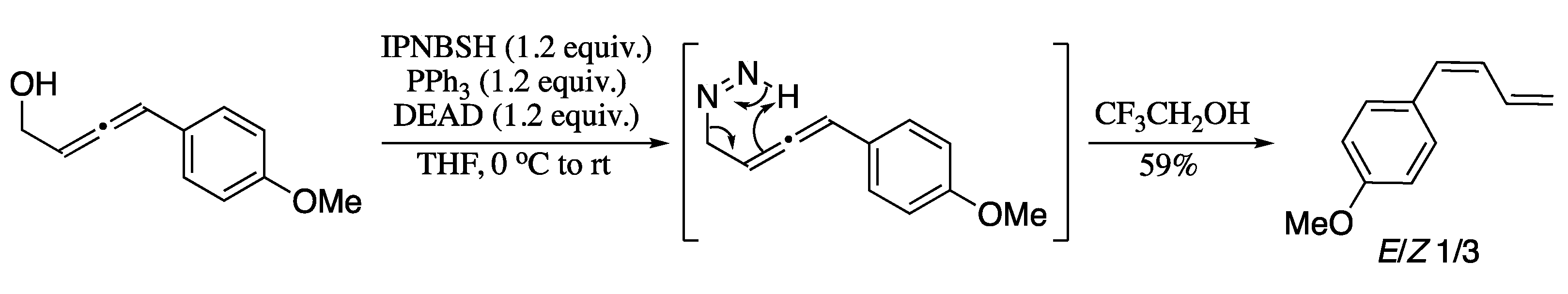
More recently, Rinaolo et al. developed a methodology for the synthesis of 1,3-dienes via reductive transposition of allenols (Scheme 63) [202]. Reaction of 1,2-allenols with N-isopropylidene-N′-2-nitrobenzenesulfonyl hydrazine (IPNBSH) under Mitsunobu conditions [PPh3/diethyl azodicarboxylate(DEAD)] generated in situ the corresponding monoalkyl diazene intermediate, which upon treatment with trifluoroethanol provided through the loss of dinitrogen, 1,3-dienes in good yields albeit in moderate Z-selectivity.
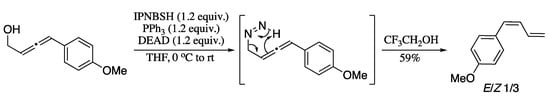
Scheme 63.
Reductive transposition of allenols.
As yet another contribution of Alcaide’s group, the synthesis of 2-halo-1,3-dienes was achieved on rearrangement of terminal allenic alcohols [203] in the presence of a stoichiometric amount of an iron(III) halide (Scheme 64) [204].
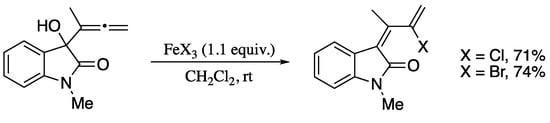
Scheme 64.
Halogenation/rearrangement of indolinone allenols.
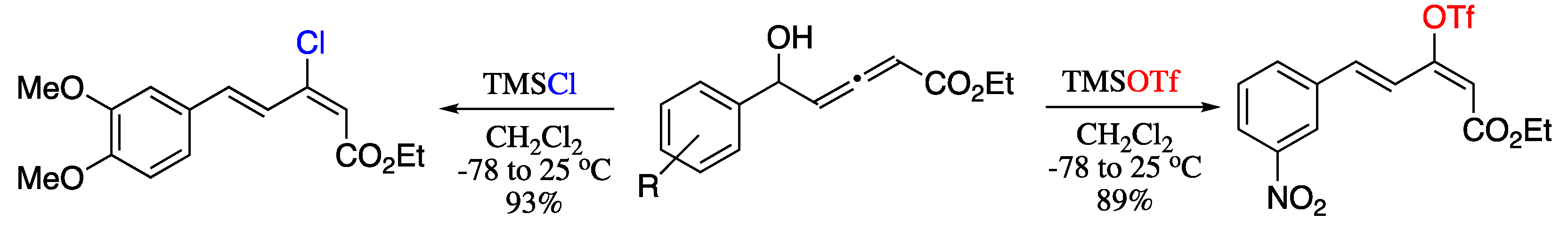
In 2013, Sabbasani et al. reported a metal-free rearrangement of allenic alcohols to (E,E)-1,3-dien-2-yl triflates or chlorides by using trimethylsilyl triflate (TMSOTf) or trimethylsilyl chloride (TMSCl) respectively [205]. It was observed that the outcome of the reaction was strongly dependent on the electronic effects of the substituents. Therefore, vinyl triflates were obtained from electron-deficient substrates and TMSOTf whereas vinyl chlorides were generated on reaction of electron-rich substrates and TMSCl (Scheme 65).
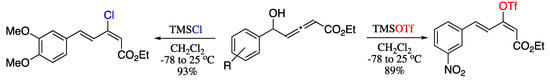
Scheme 65.
Allylic 1,3-transposition of allenols with TMSOTf or TMSCl.
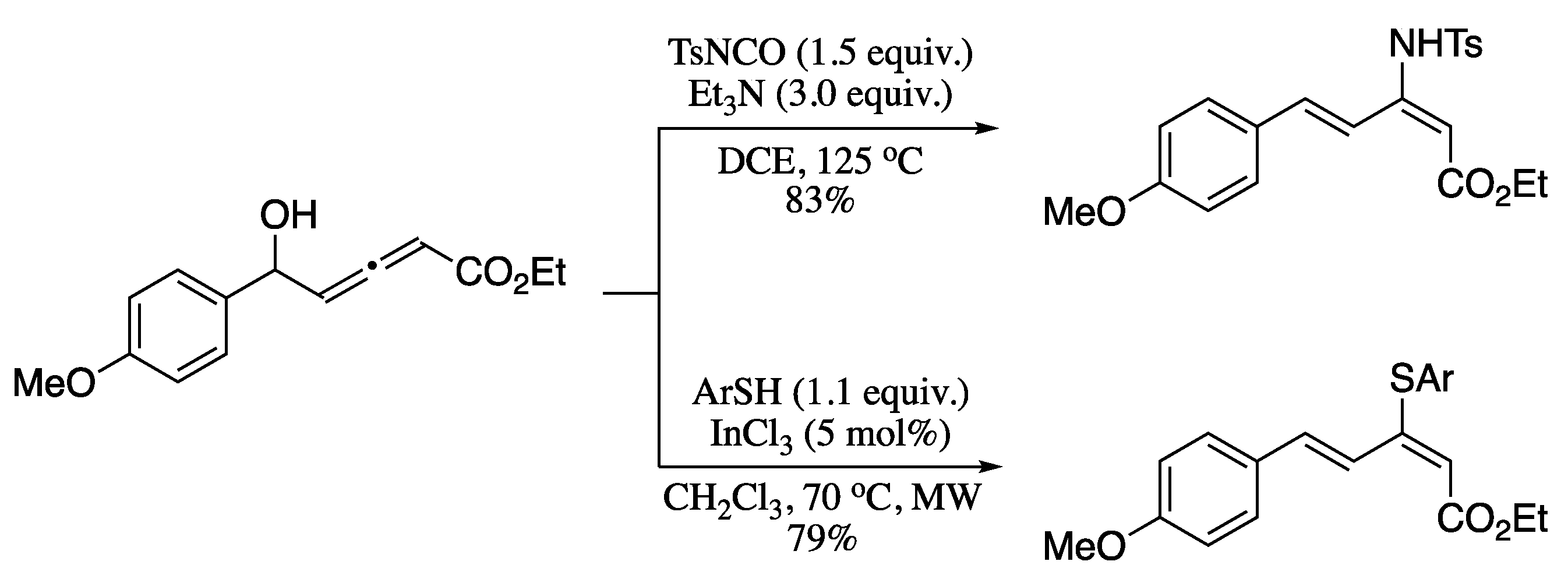
Nucleophilic additions to allenols offer a unique opportunity for the synthesis of trisubstituted dienes. To date, several studies have investigated different strategies to attain this goal. For example, Wu and co-workers prepared 2-amino-1,3-dienes via metal-free decarboxylative amination of allenols by TsNCO [206], while Lee’s group described the indium(III)-catalysed addition of thiols to allenols to provide 2-thio-1,3-dienes (Scheme 66) [207].
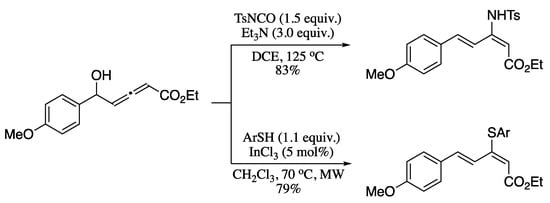
Scheme 66.
Nucleophilic additions to allenols.
6.2. From Alkynes
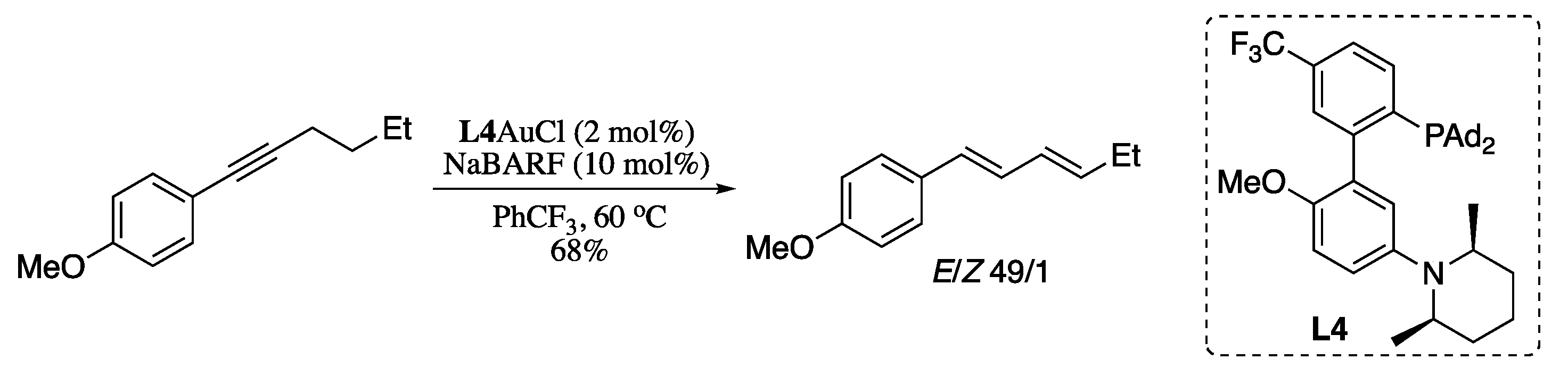
Metal-catalysed alkyne isomerization has long been regarded as an efficient, atom-economical approach for the synthesis of 1,3-dienes. Several transition-metal complexes (e.g., Ru, Ir, and Pd) are known to catalyse the isomerization of acylalkynes and related substrates with strongly electron-withdrawing groups [208]. On the other hand, until recently little progress had been described for the use of unactivated alkynes as substrates [209,210,211]. During the last decade, the research on the field of alkyne isomerization was mainly focused on achieving a larger scope by developing improved strategies for the isomerization of unactivated alkynes to the corresponding 1,3-dienes. Zhang envisioned that a gold(I) complex possessing orthogonally positioned “push” and “pull” forces would enable soft propargylic deprotonation, which could led to efficient isomerization of alkynes into 1,3-dienes [212]. Thus, Zhang’s group designed a gold(I) cationic complex combining a LAu+ soft Lewis acid as the “pull” force with an optimally positioned basic site “pushing” force, both linked by a rigid ligand framework. Treating alkynes with the gold complex L4AuCl in the presence of chloride scavenger NaBARF (sodium tetrakis [3,5-bis(trifluoromethyl)phenyl]borate) resulted in the formation of the (1E,3E)-dienes in good yields and stereoselectivities (Scheme 67).
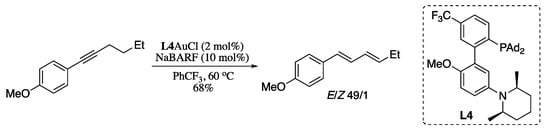
Scheme 67.
Gold-catalysed isomerization of alkynes to dienes.
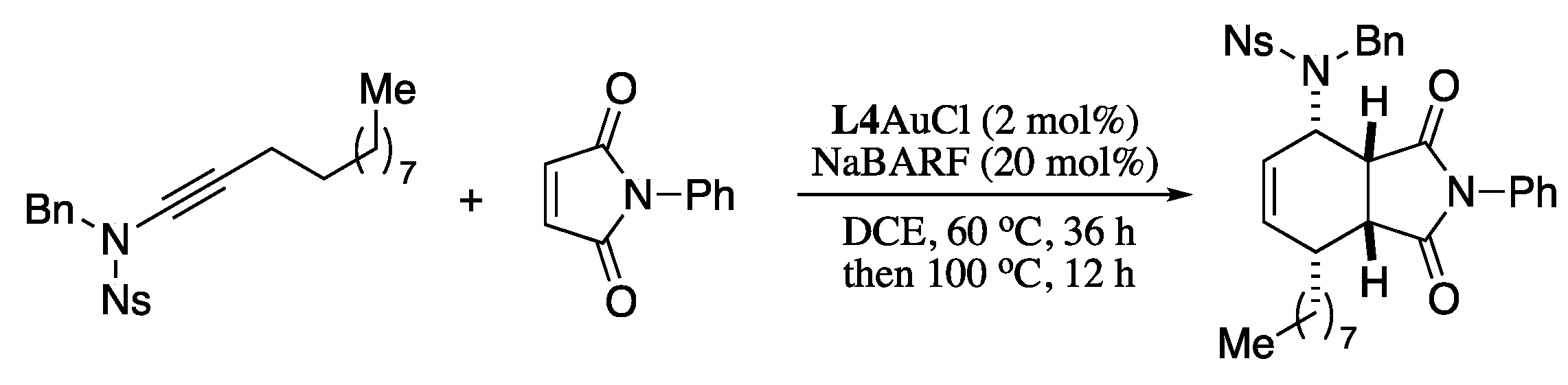
In a further application of this methodology, N-alkynyl-o-nosylamides were converted to (1E,3E)-1-amido-1,3-dienes with excellent regio and stereoselectivities under gold catalysis, in a process that can be combined with a one-pot Diels−Alder reaction leading to valuable bicyclic compounds, as depicted in Scheme 68 [213].
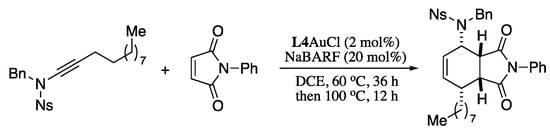
Scheme 68.
Ynamide isomerization−cycloaddition domino reaction.
In these Au-catalysed isomerizations, as well as in the classical Pd-, Ru- and Rh-catalysed reactions, the electron density of the π-system extends in a single direction. In contrast, Cera et al. disclosed in a recent report a bidirectional dual isomerization of alkynes to dienes via Pd(0)/carboxylic acid catalysis. [214] In this protocol, a palladium hydride catalyst formed in situ on combination of palladium(0) with cheap benzoic acid, enables a bi-directional p-walk involving the two α-C(sp3) of a 2-butynyl fragment to form the 1,3-diene moiety in moderate yields with total E-stereoselectivity (Scheme 69).
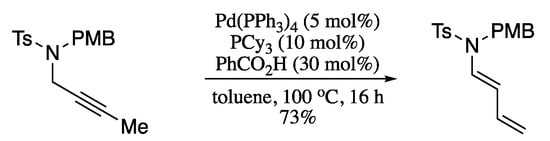
Scheme 69.
Bi-directional alkyne tandem isomerization.
The past decade has also witnessed some relevant advances in the synthesis of 1,3-dienes by means of rearrangements of propargyl alcohols. For example, Vidhani and co-workers developed a Rh(I)-catalysed approach to functionalized (E,Z)-1,3-dienals based on a tandem Claisen rearrangement/stereoselective hydrogen transfer (Scheme 70) [215]. Z-Stereochemistry of the first double bond suggests the involvement of a six-membered cyclic intermediate whereas the E-stereochemistry of the second double bond stems from the subsequent protodemetalation step giving an (E,Z)-dienal. Overall, this procedure enables access to stereodefined dienals with great atom economy and minimal waste generation.
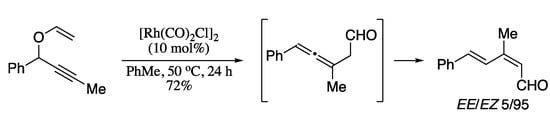
Scheme 70.
Rh(I)-catalysed tandem Claisen rearrangement/stereoselective hydrogen transfer.
In 2012, Gevorgyan and co-workers reported the synthesis of 1,3-dienes by means of a double migratory cascade reaction of α-halogen-substituted propargylic phosphates [216]. The main feature of this transformation is a 1,3-phosphatyloxy group migration followed by a 1,3-shift of an halogen, a relevant example of an extraordinarily unusual double migration of two different functionalizable groups. Moreover, this transformation is stereodivergent: copper-catalysed reactions produced (Z)-1,3-dienes, whereas using a gold catalyst resulted in the formation of the corresponding E-diene derivatives (Scheme 71).
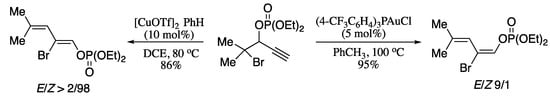
Scheme 71.
Cu-catalysed synthesis of (Z)-dienes and Au-catalysed synthesis of (E)-dienes.
6.3. From Dienes
1,3-Dienes can be also obtained from isomerization other dienyl derivatives. For example, it has been described that 1,4-dienols can undergo facile isomerization to the thermodynamically favored 1,3-dienols via an acid catalysed 2-oxonia-Cope rearrangement [217]. Capel et al. took advantage of this reaction to develop the synthesis of 1,3-dienols from aldehydes by means of a synthetic sequence involving the addition of pentadienylindium followed by the indium-catalysed 2-oxonia Cope rearrangement of the resulting 1,4-dienol product to the corresponding 1,3-dienol (Scheme 72) [218].
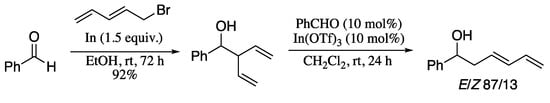
Scheme 72.
2-Oxonia Cope rearrangement of 1,4-dienols to 1,3-dienols.
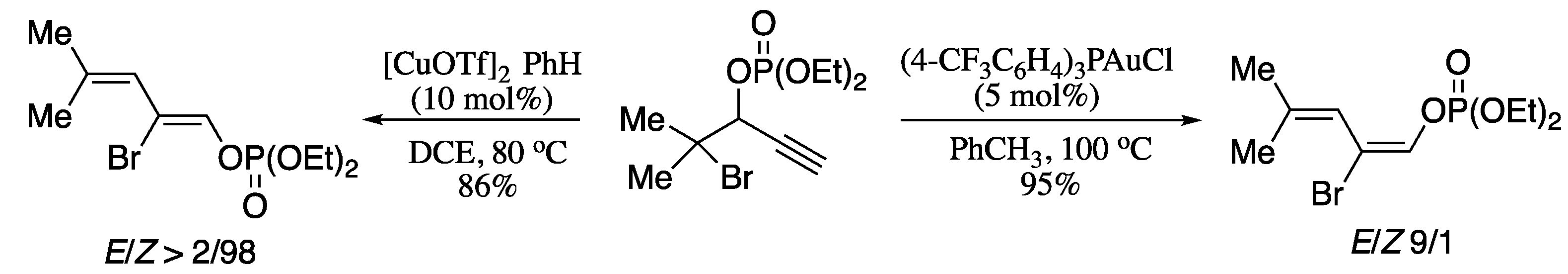
In a recent report, Tang et al. described an iridium-catalysed regio-divergent allylic amination of unactivated dienyl allylic alcohols [219]. The protocol proceeds under mild conditions and tolerates a wide scope of substrates, but probably the most relevant feature is the possibility of producing either branched or linear amino-1,3-dienes just by slightly tuning the reaction conditions. Thus, treatment of secondary dienyl allylic alcohols with secondary amines in the presence of an iridium catalyst and scandium triflate as additive in dichloromethane at room temperature afforded C5 amination products in good yields (Scheme 73).
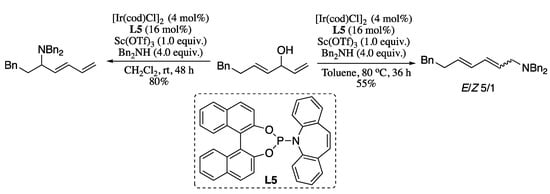
Scheme 73.
Regio-divergent allylic amination of dienyl allylic alcohols.
On contrary, when the reaction was performed in toluene at 80 °C, the corresponding C1 amination products were obtained in moderate yields and stereoselectivities.
6.4. From Other Functions
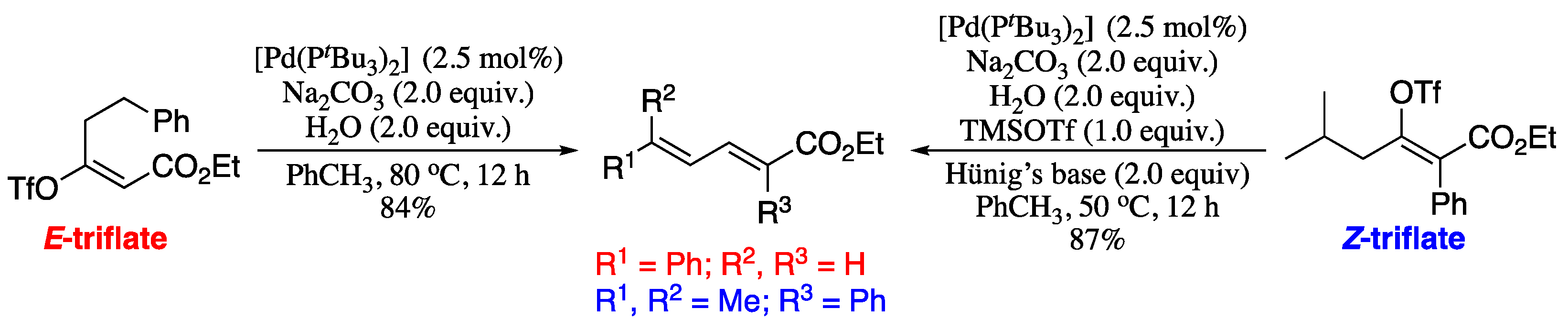
In 2011 Crouch and co-workers reported a conceptually new route to substituted 1,3-dienes via palladium-catalysed elimination/isomerization of enol triflates [220]. Both E- and Z-enol triflates are capable of providing the corresponding functionalized, highly substituted 1,3-dienes. However, Z-enol triflates are considerably less reactive and require a strong Lewis acid, such as TMSOTf, to generate the corresponding dienes in synthetically useful yields. Thus, two separate sets of reaction conditions were used based on the starting olefin geometry of the enol triflate. E-enol triflates were treated with sodium carbonate and water at 50 °C in toluene in the presence of a palladium catalyst. Under these conditions, the corresponding (E,E)-dienes were obtained in good yields, as depicted in Scheme 74. For the Z-enol triflates, the above reagents and conditions were used, plus addition of trimethylsilyl triflate (TMSOTf) and Hünig’s base. Again, the (E,E)-dienes were obtained in good yields.
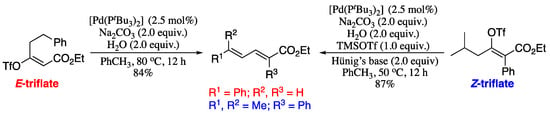
Scheme 74.
Palladium-catalysed elimination/isomerization of enol triflates.
Ukaji group developed the sequence 1,4-elimination/[1,2]-Wittig rearrangement for the selective synthesis of (Z)-dienyl alcohols. Initially, δ-benzyloxy-substituted allylic sulfones were used as starting materials, obtaining the corresponding (Z)-2,4- pentadien-1-ols in good yields and stereoselectivities. [221] The Z-selectivity was attributed to the “syn-effect”, namely, a stereoelectronic effect owing to σC−H→π*C=C interaction in the transition step of the 1,4-elimination. Notwithstanding the good results, the protocol was restricted to α,α-disubstituted allylic sulfones. When subjected to the same reaction conditions, the more acidic α-proton of α-monosubstituted allylic sulfones was deprotonated first, causing the elimination the benzyloxy group instead the sulfone moiety. In order to overcome this limitation and improve the reaction scope, Ukaji and co-workers applied the sequential 1,4-elimination and [1,2]-Wittig rearrangement δ-benzyloxy-substituted allylic benzoates, providing (2Z,4E)-2,4-pentadien-1-ols in moderate yields and stereoselectivities (Scheme 75) [222].
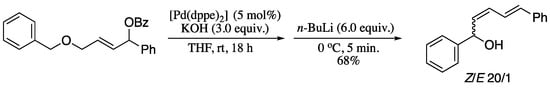
Scheme 75.
Z-Selective synthesis of dienyl alcohols.
Campbell and Sammis developed in 2014 a protocol for the diastereoselective synthesis of E dienes based on the radical cyclization of bromoallyl hydrazones [223]. This process involves a radical 6-endo-cyclization, followed by an elimination and a cycloreversion assisted by the release of nitrogen. Interestingly, this methodology was further extended to the direct synthesis of 1,3-dienes from aldehydes by means of a one-pot condensation/radical cyclization/cycloreversion cascade (Scheme 76).
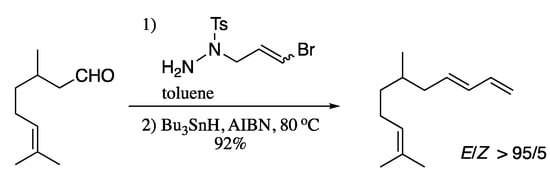
Scheme 76.
One-pot hydrazone formation followed by one-electron/pericyclic cascade for the synthesis of dienes.
A classic example pericyclic rearrangement is the thermal conrotatory 4π-electrocyclic ring opening of cyclobutenes to afford 1,4-butadienes [224]. As the cyclobutene configuration is transferred into the diene geometry, this process generates the double bonds with high stereocontrol, hence its relevance in the context of natural products synthesis [225]. Maulide et al. decided to take advantage of the 4π-electrocyclic ring opening of cyclobutene derivatives to prepare diene carboxylates. Starting from simple bicyclic lactones, the synthesis of functionalized dienoic carboxylates was achieved by a domino allylic alkylation/4π- electrocyclic ring opening using oxygen- or nitrogen-based nucleophiles [226]. This approach has shown interesting applications in the total synthesis of diverse polyene natural products [227]. For example, a double cyclobutene electrocyclic ring opening was employed for the preparation of the southeastern fragment of macrolactin A (Scheme 77).
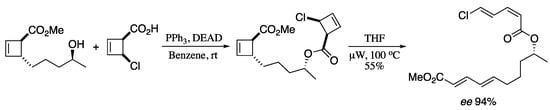
Scheme 77.
Synthesis of the southeastern fragment of Macrolactin A.
Kim and Oh extensively investigated the eliminative reaction pathways of (E)-β-chlorovinyl ketones and discovered that, in the presence catalytic amount of Lewis base and triphenylphosphine, 1,3-dienones were formed in high yields (Scheme 78) [228].
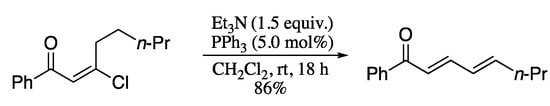
Scheme 78.
α-Vinyl enolization of (E)-chloroenones.
7. Miscellaneous
Maji and Tunge described an elegant palladium-catalysed decarboxylative allylation protocol for the synthesis of conjugated acyclic dienes utilizing pyrones as C4 synthons [229]. The process occurs via addition of a nucleophile generated by decarboxylation of an allylic carbonate to highly electrophilic 2-carboxypyrones (Scheme 79).
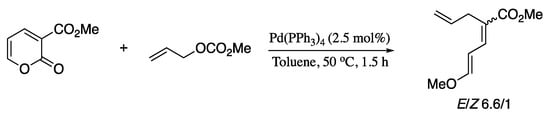
Scheme 79.
Palladium(0)-catalysed allylation of pyrones.
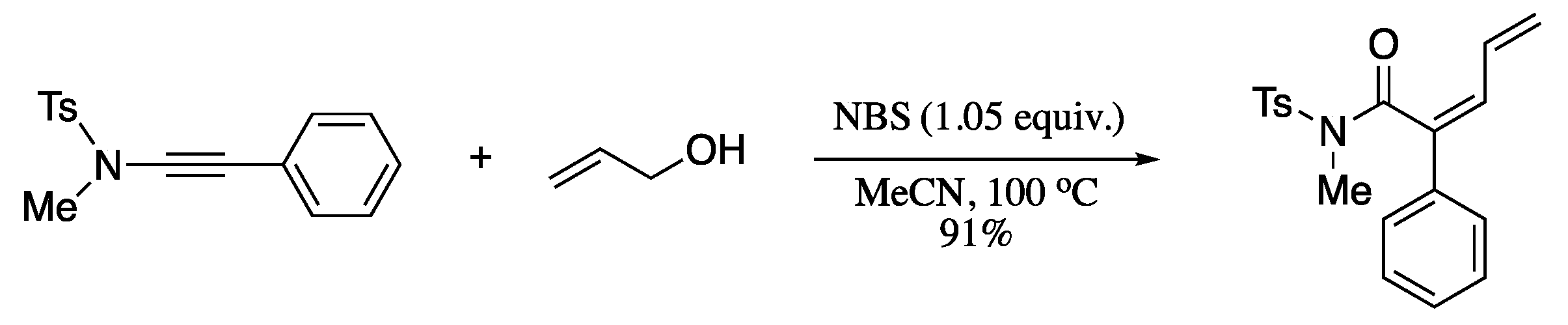
Ding et al. reported a NBS-promoted allyloxyl addition−Claisen rearrangement−dehydrobromination cascade reaction of alkynylsulfonamides and allyl alcohols to provide (Z)-2,4-dienamides in moderate to high yields (Scheme 80) [230].
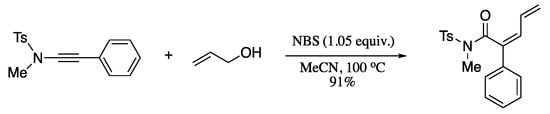
Scheme 80.
Allyloxyl addition−Claisen rearrangement−dehydrobromination cascade reaction.
In 2018, Wang and co-workers described the preparation of conjugated dienamides and dienoic acids by the means of a direct aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids using a simple iridium/copper relay catalysis system [231]. The reaction proceeds via allyl−Ir intermediate species, which on direct hydride elimination or ketone tautomerization, followed by 1,5-hydrogen shift, afforded the desired dehydrogenation products preserving the olefin geometry of the starting material (Scheme 81).

Scheme 81.
α,β-Dehydrogenation of γ,δ-unsaturated amides.
8. Conclusions
The 1,3-diene moiety has played a central role in chemical synthesis since the inception of organic chemistry as a field. This vast interest in 1,3-dienes is mainly motivated by the ubiquity of the butadiene moiety in natural and synthetic compounds of pharmacological relevance, by the emergence of diene feedstock for applications in industry and by their long-known usefulness as synthetic intermediates in a broad range of chemical processes. In this regard, the stereochemistry of dienes is crucial, since it determines the physical and biological properties but also control the outcome of further chemical transformations. Nevertheless, to date it remains challenging to target specific 1,3-diene geometries and to position substituents and functional groups along the C4 framework at prescribed locations. As it has been stated before, the synthetical interest on the stereoselective construction of the 1,3-butadienyl moiety has shown a great interest by the synthetical community since is present in many natural and non-natural products displaying a broad spectrum of biological activities. To this end, these synthetic challenges continue to fuel the interest of organic chemists in the development of novel methodologies for the stereoselective synthesis of dienes and we expect further exciting developments in coming years.
Funding
This work has received financial support from the Principado de Asturias (FICYT IDI/2018/000181), the European Union (European Regional Development Fund-ERDF) and MINECO (PID2019-109253RB-I00). Partial financial support by Arcelor Mittal (R&D-Principado de Asturias; FUO-20-296) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mevers, E.; Saurí, J.; Liu, Y.; Moser, A.; Ramadhar, T.R.; Varlan, M.; Clardy, J. Homodimericin A: A complex hexacyclic fungal metabolite. J. Am. Chem. Soc. 2016, 138, 12324–12327. [Google Scholar] [CrossRef] [PubMed]
- Harned, A.-M.; Volp, K.-A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011, 28, 1790–1810. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Xanthane sesquiterpenoids: Structure, synthesis and biological activity. Nat. Prod. Rep. 2011, 28, 824–842. [Google Scholar] [CrossRef] [PubMed]
- Thirsk, C.; Whiting, A. Polyene natural products. J. Chem. Soc. Perkin Trans. 2002, 1, 999–1023. [Google Scholar] [CrossRef]
- Schöffmann, A.; Wimmer, L.; Goldmann, D.; Khom, S.; Hintersteiner, J.; Baburin, I.; Schwarz, T.; Hintersteininger, M.; Pakfeifer, P.; Oufir, M.; et al. Efficient modulation of γ-amino-butyric acid type A receptors by piperine derivatives. J. Med. Chem. 2014, 57, 5602–5619. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-L.; Li, Y.-F.; Yu, B.; Shan, L.-H.; Liu, H.-M. Recent progress on the synthesis and bioactivity of marine naturally occurring dienamides and related derivatives. Synth. Commun. 2015, 45, 2159–2180. [Google Scholar] [CrossRef]
- Rajakumar, K.; Greenspan, S.L.; Thomas S., B.; Holick, M.F. Solar ultraviolet radiation and vitamin D: A historical perspective. Am. J. Public Health 2007, 97, 1746–1754. [Google Scholar] [CrossRef]
- Tanaka, J.-I.; Higa, T. Zampanolide, a new cytotoxic marcrolide from a marine sponge. Tetrahedron Lett. 1996, 37, 5535–5538. [Google Scholar] [CrossRef]
- Norsikian, S.; Tresse, C.; François-Eude, M.; Jeanne-Julien, L.; Masson, G.; Servajean, V.; Genta-Jouve, G.; Beau, J.-M.; Roulland, E. Total synthesis of Tiacumicin B: Implementing hydrogen bond directed acceptor delivery for highly selective β-glycosylations. Angew. Chem. Int. Ed. 2020, 59, 6612–6616. [Google Scholar] [CrossRef]
- Erb, W.; Zhu, J. From natural product to marketed drug: The Tiacumicin odyssey. Nat. Prod. Rep. 2013, 30, 161–174. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, T.; Li, X.; Wu, Z.; Feng, Y.; Xie, F.; Liu, C.; Qin, J.; Chen, H. Novel soluble myeloid cell leukemia sequence 1 (Mcl-1) inhibitor (E,E)-2-(benzylaminocarbonyl)-3-styrylacrylonitrile (4g) developed using a fragment-based approach. Eur. J. Med. Chem. 2013, 59, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Paik, I.H.; Xie, S.; Shapiro, T.A.; Labonte, T.; Narducci Sarjeant, A.A.; Baege, A.C.; Posner, G.H. Second generation, orally active, antimalarial, artemisinin-derived trioxane dimers with high stability, efficacy, and anticancer activity. J. Med. Chem. 2006, 49, 2731–2734. [Google Scholar] [CrossRef] [PubMed]
- Saku, O.; Ishida, H.; Atsumi, E.; Sugimoto, Y.; Kodaira, H.; Kato, Y.; Shirakura, S.; Nakasato, Y. Discovery of novel 5,5-diarylpentadienamides as orally available transient receptor potential vanilloid 1 (TRPV1) antagonists. J. Med. Chem. 2012, 55, 3436–3451. [Google Scholar] [CrossRef] [PubMed]
- Eschenbrenner-Lux, V.; Kumar, K.; Waldmann, H. The asymmetric hetero-Diels-Alder reaction in the syntheses of biologically relevant compounds. Angew. Chem. Int. Ed. 2014, 53, 11146–11157. [Google Scholar] [CrossRef] [PubMed]
- Sherburn, M.; Mackay, E. The Diels–Alder reaction in steroid synthesis. Synthesis 2014, 47, 1–21. [Google Scholar] [CrossRef]
- Friebe, L.; Nuyken, O.; Obrecht, W. Neodymium-based Ziegler/Natta catalysts and their application in diene polymerization. Adv. Polym. Sci. 2006, 204, 1–154. [Google Scholar] [CrossRef]
- Fischbach, A.A.; Anwander, R. Rare-earth metals and aluminum getting close in Ziegler-Type organometallics. Adv. Polym. Sci. 2006, 204, 155–281. [Google Scholar] [CrossRef]
- Arndt, S.; Beckerle, K.; Zeimentz, P.M.; Spaniol, T.P.; Okuda, J. Cationic yttrium methyl complexes as functional models for polymerization catalysts of 1,3-dienes. Angew. Chem. Int. Ed. 2005, 44, 7473–7477. [Google Scholar] [CrossRef]
- Bäckvall, J.-E.; Chinchilla, R.; Najera, C.; Yus, M. The use of sulfonyl 1,3-dienes in organic synthesis. Chem. Rev. 1998, 98, 2291–2312. [Google Scholar] [CrossRef]
- Yang, X.H.; Dong, V.M. Rhodium-catalyzed hydrofunctionalization: Enantioselective coupling of indolines and 1,3-dienes. J. Am. Chem. Soc. 2017, 139, 1774–1777. [Google Scholar] [CrossRef]
- Adamson, N.J.; Hull, E.; Malcolmson, S.J. Enantioselective intermolecular addition of aliphatic amines to acyclic dienes with a Pd-PHOX catalyst. J. Am. Chem. Soc. 2017, 139, 7180–7183. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Wolf, L.M.; Zielinski, A.; Thiel, W.; Alcarazo, M. α-Dicationic chelating phosphines: Synthesis and application to the hydroarylation of dienes. J. Am. Chem. Soc. 2017, 139, 4948–4953. [Google Scholar] [CrossRef] [PubMed]
- Adamson, N.J.; Wilbur, K.C.E.; Malcolmson, S.J. Enantioselective intermolecular Pd-catalyzed hydroalkylation of acyclic 1,3-dienes with activated pronucleophiles. J. Am. Chem. Soc. 2018, 140, 2761–2764. [Google Scholar] [CrossRef] [PubMed]
- Sardini, S.R.; Brown, M.K. Catalyst controlled regiodivergent arylboration of dienes. J. Am. Chem. Soc. 2017, 139, 9823–9826. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Smith, K.B.; Brown, M.K. Copper-catalyzed borylacylation of activated alkenes with acid chlorides. Angew. Chem. Int. Ed. 2017, 56, 13314–13318. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gong, L.-Z. Palladium(0)-catalyzed difunctionalization of 1,3-dienes: From racemic to enantioselective. Synthesis 2019, 51, 122–134. [Google Scholar] [CrossRef]
- Perry, G.J.P.; Jia, T.; Procter, D.J. Copper-catalyzed functionalization of 1,3-dienes: Hydrofunctionalization, borofunctionalization, and difunctionalization. ACS Catal. 2020, 10, 1485–1499. [Google Scholar] [CrossRef]
- Li, H.; Caire da Silva, L.; Schulz, M.D.; Rojas, G.; Wagener, K.B. A review of how to do an acyclic diene metathesis reaction. Polym. Int. 2016, 66, 7–12. [Google Scholar] [CrossRef]
- Liao, L.; Guo, R.; Zhao, X. Organoselenium-catalyzed regioselective C-H pyridination of 1,3-dienes and alkenes. Angew. Chem. Int. Ed. 2017, 56, 3201–3205. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Wang, Y.; Ge, Y.; Liu, J.; Luan, X. Diastereoselective synthesis of dibenzo[b,d]azepines by Pd(II)-catalyzed [5 + 2] annulation of o-arylanilines with dienes. Org. Lett. 2017, 19, 1734–1737. [Google Scholar] [CrossRef]
- Chen, S.S.; Wu, M.S.; Han, Z.Y. Palladium-catalyzed cascade sp2 C-H functionalization/ intramolecular asymmetric allylation: From aryl ureas and 1,3-dienes to chiral indolines. Angew. Chem. Int. Ed. 2017, 56, 6641–6645. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.; Kim, J.; Son, J.Y.; Baek, Y.; Um, K.; Lee, P.H. One-pot synthesis of indolizines via sequential rhodium-catalyzed [2 + 1]-cyclopropanation, palladium-catalyzed ring expansion and oxidation reactions from pyridotriazoles and 1,3-dienes. Org. Lett. 2017, 19, 5677–5680. [Google Scholar] [CrossRef] [PubMed]
- Lang, B.; Zhu, H.; Wang, C.; Lu, P.; Wang, Y. Rhodium-catalyzed cycloadditions between 3-diazoindolin-2-imines and 1,3-dienes. Org. Lett. 2017, 19, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, H.; Um, K.; Lee, P.H. Synthesis of azepinoindoles via rhodium-catalyzed formal aza-[4 + 3] cycloaddition reaction of 3-diazoindolin-2-imines with 1,3-dienes in one-pot. J. Org. Chem. 2017, 82, 9808–9815. [Google Scholar] [CrossRef] [PubMed]
- Sargent, B.T.; Alexanian, E.J. Cobalt-catalyzed carbonylative cross-coupling of alkyl tosylates and dienes: Stereospecific synthesis of dienones at low pressure. J. Am. Chem. Soc. 2017, 139, 12438–12440. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.-C.; Wang, P.-S.; Tao, Z.-L.; Han, Z.-Y.; Gong, L.-Z. An enantioselective multicomponent carbonyl allylation of aldehydes with dienes and alkynyl bromides enabled by chiral palladium phosphate. Adv. Synth. Catal. 2017, 359, 2383–2389. [Google Scholar] [CrossRef]
- White, W.C. Butadiene production process overview. Chem. Biol. Interact. 2007, 166, 10–14. [Google Scholar] [CrossRef]
- Matos, C.T.; Gouveia, L.; Morais, A.R.C.; Reis, A.; Bogel-Łukasik, R. Green metrics evaluation of isoprene production by microalgae and bacteria. Green Chem. 2013, 15, 2854–2864. [Google Scholar] [CrossRef]
- Mehta, G.; Prakash Rao, H.S. Synthesis of conjugated dienes and polyenes. In Patai’s Chemistry of Functional Groups; Rappoport, Z., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 1997. [Google Scholar]
- Woerly, E.; Roy, J.; Burke, M.D. Synthesis of most polyene natural product motifs using just 12 building blocks and one coupling reaction. Nat. Chem. 2014, 6, 484–491. [Google Scholar] [CrossRef]
- De Paolis, M.; Chataigner, I.; Maddaluno, J. Recent advances in stereoselective synthesis of 1,3-dienes. Top. Curr. Chem. 2012, 327, 87–146. [Google Scholar] [CrossRef]
- Hubert, P.; Seibel, E.; Beemelmanns, C.; Campagne, J.-M.; de Figueiredo, R.M. Stereoselective construction of (E,Z)-1,3-dienes and its application in natural product synthesis. Adv. Synth. Catal. 2020, 362, 5532–5575. [Google Scholar] [CrossRef]
- Seechurn, C.C.C.J.M.; Kitching, O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 Nobel Prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, T.; Ohe, T.; Miyaura, N.; Suzuki, A. Stereoselective synthesis of conjugated 2,4-alkadienoates via the palladium-catalyzed cross-coupling of 1-alkenylboronates with 3-bromo-2-alkenoates. Bull. Chem. Soc. Jpn. 1989, 62, 3892–3895. [Google Scholar] [CrossRef]
- Negishi, E.; Tobrman, T.; Rao, H.; Xu, S.; Lee, C.-T. Highly (≥98%) selective trisubstituted alkene synthesis of wide applicability via fluoride-promoted Pd-catalyzed cross-coupling of alkenylboranes. Isr. J. Chem. 2011, 50, 696–701. [Google Scholar] [CrossRef]
- Kurosawa, F.; Nakano, T.; Soeta, T.; Endo, K.; Ukaji, Y. (Z)-Selective enol triflation of α-alkoxyacetoaldehydes: Application to synthesis of (Z)-allylic alcohols via cross-coupling reaction and [1,2]-Wittig rearrangement. J. Org. Chem. 2015, 80, 5696–5703. [Google Scholar] [CrossRef]
- Al-Jawaheri, Y.; Kimber, M.C. Synthesis of 1,3-dienes via a sequential Suzuki−Miyaura coupling/palladium-mediated allene isomerization sequence. Org. Lett. 2016, 18, 3502–3505. [Google Scholar] [CrossRef]
- Moriya, T.; Furuuchi, T.; Miyaura, N.; Suzuki, A. A new facile synthesis of 2-substituted 1,3-butadiene derivatives via palladium-catalyzed cross-coupling reaction of 2,3-alkadienyl carbonates with organoboron compounds. Tetrahedron 1994, 50, 7961–7968. [Google Scholar] [CrossRef]
- Yoshida, M.; Gotou, T.; Ihara, M. Palladium-catalysed coupling reaction of allenic alcohols with aryl- and alkenylboronic acids. Chem. Commun. 2004, 1124–1125. [Google Scholar] [CrossRef]
- Brown, R.W.; Zamani, F.; Gardiner, M.G.; Pyne, S.G.; Hyland, C.J.T. Divergent Pd-catalyzed cross-coupling of allenyloxazolidinones to give chiral 1,3-dienes and vinyloxazolidinones. Chem. Sci. 2019, 10, 9051–9056. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Zhang, L.; Lu, A.-M.; Yang, F.; Wu, L. α-Allenyl ethers as starting materials for palladium catalyzed Suzuki—Miyaura couplings of allenylphosphine oxides with arylboronic acids. J. Org. Chem. 2015, 80, 673–680. [Google Scholar] [CrossRef]
- Liu, T.; Dong, J.; Cao, S.-J.; Guo, L.-C.; Wu, L. Suzuki-Miyaura coupling of phosphinoyl-α-allenic alcohols with arylboronic acids catalyzed by palladium complex “on water”: An efficient method to generate phosphinoyl 1,3-butadienes and derivatives. RSC Adv. 2014, 4, 61722–61726. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Abela, A.R.; Moser, R.; Nishikata, T.; Duplais, C.; Krasovskiy, A.; Gaston, R.D.; Gadwood, R.C. TPGS-750-M: A second-generation amphiphile for metal-catalyzed cross-couplings in water at room temperature. J. Org. Chem. 2011, 76, 4379–4391. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B. Synthetic chemistry in a water world. New rules ripe for discovery. Curr. Opin. Green Sustain. Chem. 2018, 11, 1–8. [Google Scholar] [CrossRef]
- Lippincott, D.J.; Linstadt, R.T.H.; Maser, M.R.; Gallou, F.; Lipshutz, B.H. Synthesis of functionalized 1,3-butadienes via Pd-catalyzed cross-couplings of substituted allenic esters in water at room temperature. Org. Lett. 2018, 20, 4719–4722. [Google Scholar] [CrossRef]
- Li, J.; Grillo, A.S.; Burke, M.D. From synthesis to function via iterative assembly of N‑methyliminodiacetic acid boronate building blocks. Acc. Chem. Res. 2015, 48, 2297–2307. [Google Scholar] [CrossRef]
- Wang, G.; Mohan, S.; Negishi, E. Highly selective synthesis of conjugated dienoic and trienoic esters via alkyne elementometalation-Pd-catalyzed cross-coupling. Proc. Natl. Acad. Sci. USA 2011, 108, 11344–11349. [Google Scholar] [CrossRef]
- Fiorito, D.; Folliet, S.; Liu, Y.; Mazet, C. A general nickel-catalyzed Kumada vinylation for the preparation of 2-substituted 1,3-dienes. ACS Catal. 2018, 8, 1392–1398. [Google Scholar] [CrossRef]
- Chatterjee, T.; Dey, R.; Ranu, B.C. An easy access to styrenes: Trans aryl 1,3-, 1,4- and 1,5-dienes, and 1,3,5-trienes by Hiyama cross-coupling catalyzed by palladium nanoparticles. New J. Chem. 2011, 35, 1103–1110. [Google Scholar] [CrossRef]
- McLaughlin, M.G.; Cook, M.J. Highly diastereoselective hydrosilylations of allylic alcohols. Chem. Commun. 2014, 50, 3501–3504. [Google Scholar] [CrossRef]
- Reid, J.P.; McAdam, C.A.; Johnston, A.J.S.; Grayson, M.N.; Goodman, J.M.; Cook, M.J. Base-mediated cascade rearrangements of aryl-substituted diallyl ethers. J. Org. Chem. 2015, 80, 1472–1498. [Google Scholar] [CrossRef]
- McAdam, C.A.; McLaughlin, M.G.; Cook, M.J. An alkyne hydrosilylation–Hiyama coupling approach to highly functionalised 1,3-dienes. Org. Chem. Front. 2015, 2, 510–514. [Google Scholar] [CrossRef]
- Vázquez-Galiñanes, N.; Fañanás-Mastral, M. Stereoselective synthesis of borylated 1,3-dienes by synergistic Cu/Pd catalysis. ChemCatChem 2018, 10, 4817–4820. [Google Scholar] [CrossRef]
- Giannerini, M.; Fañanás-Mastral, M.; Feringa, B.L. Direct catalytic cross-coupling of organolithium compounds. Nat. Chem. 2013, 5, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Vila, C.; Giannerini, M.; Hornillos, V.; Fañanás-Mastral, M.; Feringa, B.L. Palladium-catalysed direct cross-coupling of secondary alkyllithium reagents. Chem. Sci. 2014, 5, 1361–1367. [Google Scholar] [CrossRef]
- Vila, C.; Hornillos, V.; Giannerini, M.; Fañanaś-Mastral, M.; Feringa, B.L. Palladium-catalysed direct cross-coupling of organo- lithium reagents with aryl and vinyl triflates. Chem.-Eur. J. 2014, 20, 13078–13083. [Google Scholar] [CrossRef]
- Heijnen, D.; Hornillos, V.; Corbet, B.P.; Giannerini, M.; Feringa, B.L. Palladium-catalyzed C(sp3)-C(sp2) cross-coupling of (trimethylsilyl)methyllithium with (hetero)aryl halides. Org. Lett. 2015, 17, 2262–2265. [Google Scholar] [CrossRef]
- Hornillos, V.; Giannerini, M.; Vila, C.; Fañanaś-Mastral, M.; Feringa, B.L. Direct catalytic cross-coupling of alkenyllithium compounds. Chem. Sci. 2015, 6, 1394–1398. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, Z.-Y.; Peng, X.-S.; Wong, H.N.C. Ligand-free iron-catalyzed carbon(sp2)−carbon(sp2) cross-coupling of alkenyllithium with vinyl halides. J. Org. Chem. 2018, 83, 6325–6333. [Google Scholar] [CrossRef]
- Olivares, A.M.; Weix, D.J. Multimetallic Ni- and Pd-catalyzed cross-electrophile coupling to form highly substituted 1,3-dienes. J. Am. Chem. Soc. 2018, 140, 2446–2449. [Google Scholar] [CrossRef]
- Sun, Q.; Cai, L.; Ma, H.; Yuan, C.; Xu, W. The stereoselective synthesis of dienes through dehalogenative homocoupling of terminal alkenyl bromides on Cu(110). Chem. Commun. 2016, 52, 6009–6012. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Cai, L.; Ding, Y.; Xie, L.; Zhang, C.; Tan, Q.; Xu, W. Dehydrogenative homocoupling of terminal alkenes on copper surfaces: A route to dienes. Angew. Chem. Int. Ed. 2015, 54, 4549–4552. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.T.; Dang, H.T.; Pham, H.H.; Nguyen, V.D.; Flores-Hansen, C.; Arman, H.D.; Larionov, O.V. Highly regio- and stereoselective catalytic synthesis of conjugated dienes and polyenes. J. Am. Chem. Soc. 2018, 140, 8434–8438. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.L.; Soengas, R.G.; Silva, A.S.M. Ionic liquids and ohmic heating in combination for Pd-catalyzed cross-coupling reactions: Sustainable synthesis of flavonoids. Molecules 2020, 25, 1564. [Google Scholar] [CrossRef] [PubMed]
- Pinto, J.; Silva, V.L.M.; Silva, A.M.G.; Silva, A.M.S.; Costa, J.C.S.; Santos, L.M.N.B.F.; Enes, R.; Cavaleiro, J.A.S.; Vicente, A.A.M.O.S.; Teixeira, J.A.C. Ohmic heating as a new efficient process for organic synthesis in water. Green Chem. 2013, 15, 970–975. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Santos, L.M.N.B.F.; Silva, A.M.S. Ohmic heating: An emerging concept in organic synthesis. Chem. Eur. J. 2017, 23, 7853–7865. [Google Scholar] [CrossRef]
- Silva, V.L.M.; Silva, A.M.S.; Santos, L.M.N.B.F.; Silva, A.M.G.; Pinto, J.; Enes, R.; Cavaleiro, J.A.S.; Vicente, A.A.M.O.S.; Teixeira, J.A.C.; Morais, A. Reator Para Síntese Química Com Aquecimento Óhmico, Método e Suas Aplicações. PT105,908. 27 September 2011. [Google Scholar]
- Littke, A.F.; Fu, G.C. A versatile catalyst for Heck reactions of aryl chlorides and aryl bromides under mild conditions. J. Am. Chem. Soc. 2001, 123, 6989–7000. [Google Scholar] [CrossRef] [PubMed]
- McConville, M.; Saidi, O.; Blacker, J.; Xiao, J. Regioselective Heck vinylation of electron-rich olefins with vinyl halides: Is the neutral pathway in operation? J. Org Chem. 2009, 74, 2692–2698. [Google Scholar] [CrossRef]
- Battace, A.; Zair, T.; Doucet, H.; Santelli, M. Heck vinylations using vinyl sulfide, vinyl sulfoxide, vinyl sulfone, or vinyl sulfonate derivatives and aryl bromides catalyzed by a Palladium complex derived from a tetraphosphine. Synthesis 2006, 3495–3505. [Google Scholar] [CrossRef]
- Knowles, J.P.; O’Conner, V.E.; Whiting, A. Studies towards the synthesis of the northern polyene of viridenomycin and synthesis of Z-double bond analogues. Org. Biomol. Chem. 2011, 9, 1876–1886. [Google Scholar] [CrossRef]
- Grushin, V.V. Hydrido complexes of palladium. Chem. Rev. 1996, 96, 2011–2034. [Google Scholar] [CrossRef]
- Hills, I.D.; Fu, G.C. Elucidating reactivity differences in palladium-catalyzed coupling processes: the chemistry of palladium hydrides. J. Am. Chem. Soc. 2004, 126, 13178–13179. [Google Scholar] [CrossRef] [PubMed]
- Fayol, A.; Fang, Y.-Q.; Lautens, M. Synthesis of 2-vinylic indoles and derivatives via a Pd-catalyzed tandem coupling reaction. Org. Lett. 2006, 8, 4203–4206. [Google Scholar] [CrossRef] [PubMed]
- Heck, R.F.; Dieck, H.A. Palladium-catalyzed conjugated diene synthesis from vinylic halides and olefinic compounds. J. Org. Chem. 1975, 40, 1083–1090. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Zhu, Q.; Chen, H.; Wu, N.; Wen, X.; Xu, Z. Synthesis of (1E,3E)-1,4-diarylbuta-1,3-dienes promoted by μ-OMs palladium–dimer complex. BMC Chem. 2019, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Bruno, N.C.; Tudge, M.T.; Buchwald, S.L. Design and preparation of new palladium precatalysts for C–C and C–N cross-coupling reactions. Chem. Sci. 2013, 4, 916–920. [Google Scholar] [CrossRef]
- Hansen, A.L.; Ebran, J.P.; Ahlquist, M.; Norrby, P.O.; Skrydstrup, T. Heck coupling with nonactivated alkenyl tosylates and phosphates: Examples of effective 1,2-migrations of the alkenyl palladium(II) intermediates. Angew. Chem. Int. Ed. 2006, 45, 3349–3353. [Google Scholar] [CrossRef]
- Yoo, K.S.; Yoon, C.H.; Jung, K.W. Oxidative palladium(II) catalysis: a highly efficient and chemoselective cross-coupling method for carbon−carbon bond formation under base-free and nitrogenous-ligand conditions. J. Am. Chem. Soc. 2006, 128, 16384–16393. [Google Scholar] [CrossRef]
- Ebran, J.-P.; Hansen, A.L.; Gøgsig, T.M.; Skrydstrup, T. Studies on the Heck reaction with alkenyl phosphates: can the 1,2-migration be controlled? Scope and limitations. J. Am. Chem. Soc. 2007, 129, 6931–6942. [Google Scholar] [CrossRef]
- Lemhadri, M.; Battace, A.; Berthiol, F.; Zair, T.; Doucet, H.; Santelli, M. Palladium-tetraphosphine complex catalysed Heck reaction of vinyl bromides with alkenes: A powerful access to conjugated dienes. Synthesis 2008, 1142–1152. [Google Scholar] [CrossRef]
- Andappan, M.M.S.; Nilsson, P.; von Schenck, H.; Larhed, M. Dioxygen-promoted regioselective oxidative Heck arylations of electron-rich olefins with arylboronic acids. J. Org. Chem. 2004, 69, 5212–5218. [Google Scholar] [CrossRef]
- Ruan, J.; Xiao, J. From α-arylation of olefins to acylation with aldehydes: A journey in regiocontrol of the Heck reaction. Acc. Chem. Res. 2011, 44, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, D.; Stahl, S.S. Catalyst-controlled regioselectivity in the synthesis of branched conjugated dienes via aerobic oxidative Heck reactions. J. Am. Chem. Soc. 2012, 134, 16496–16499. [Google Scholar] [CrossRef] [PubMed]
- Delcamp, J.H.; Gormisky, P.E.; White, M.C. Oxidative Heck vinylation for the synthesis of complex dienes and polyenes. J. Am. Chem. Soc. 2013, 135, 8460–8463. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, N.J.; Wang, L.; Carrow, B.P. A diverted aerobic Heck reaction enables selective 1,3-diene and 1,3,5-triene synthesis through C−C bond scission. J. Am. Chem. Soc. 2018, 140, 13634–13639. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Larock, R.C. Synthesis of 9-alkylidene-9H-fluorenes by a novel palladium-catalyzed rearrangement. Org. Lett. 2000, 2, 3329–3332. [Google Scholar] [CrossRef]
- Shi, F.; Larock, R.C. Remote C-H activation via through-space palladium and rhodium migrations. Top. Curr. Chem. 2010, 292, 123–164. [Google Scholar] [CrossRef]
- Zhou, J.; He, J.; Wang, B.; Yang, W.; Ren, H. 1,7-Palladium migration via C−H activation, followed by intramolecular amination: Regioselective synthesis of benzotriazoles. J. Am. Chem. Soc. 2011, 133, 6868–6870. [Google Scholar] [CrossRef]
- Piou, T.; Bunescu, A.; Wang, Q.; Neuville, L.; Zhu, J.P. Pd-catalyzed through-space double C(sp3)-H and C(sp2)-H bond activation via 1,4-Pd migration of transient C(sp3)-Pd(II) intermediate: Efficient synthesis of [3,4]-fused oxindoles. Angew. Chem. Int. Ed. 2013, 52, 12385–12389. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Zhuang, Y.-X.; Xu, Y.-H.; Loh, T.-P. Pd-catalyzed intramolecular C–N bond cleavage, 1,4-migration, sp3 C–H activation, and Heck reaction: Four controllable diverse pathways depending on the judicious choice of the base and ligand. J. Am. Chem. Soc. 2015, 137, 1341–1347. [Google Scholar] [CrossRef]
- Hu, T.-J.; Li, M.-Y.; Zhao, Q.; Feng, C.-G.; Lin, G.-Q. Highly stereoselective synthesis of 1,3-dienes through an aryl to vinyl 1,4-palladium migration/Heck sequence. Angew. Chem. Int. Ed. 2018, 57, 5871–5875. [Google Scholar] [CrossRef]
- Hu, X.-H.; Zhang, J.; Yang, X.-F.; Xu, Y.-H.; Loh, T.-P. Stereo- and chemoselective cross-coupling between two electron-deficient acrylates: An efficient route to (Z,E)-muconate derivatives. J. Am. Chem. Soc. 2015, 137, 3169–3172. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, B.; Tian, L.; Yang, Y.; Ma, J.; Zhang, Y.; Chen, S.; Wang, J. Palladium-catalyzed three-component reaction of allenes, aryl iodides, and diazo compounds: Approach to 1,3-dienes. Angew. Chem. Int. Ed. 2013, 52, 9305–9308. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.-S.; Lin, H.-C.; Zhou, X.-L.; Gong, L.-Z. Palladium(II)/Lewis acid synergistically catalyzed allylic C−H olefination. Org. Lett. 2014, 16, 3332–3335. [Google Scholar] [CrossRef] [PubMed]
- Revathi, L.; Ravindar, L.; Balakrishna, M.; Qin, H.-L. SO2F2 mediated dehydrative cross-coupling of alcohols with electron-deficient olefins in DMSO using a Pd-catalyst: One-pot transformation of alcohols into 1,3-dienes. Org. Chem. Front. 2019, 6, 796–800. [Google Scholar] [CrossRef]
- Giri, R.; Shi, B.-F.; Engle, K.M.; Maugel, N.; Yu, J.Q. Transition metal-catalyzed C–H activation reactions: Diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 2009, 38, 3242–3272. [Google Scholar] [CrossRef]
- Lyons, T.W.; Sanford, M.S. Palladium-catalyzed ligand-directed C−H functionalization reactions. Chem. Rev. 2010, 110, 1147–1169. [Google Scholar] [CrossRef]
- Gutekunst, W.R.; Baran, P.S. C–H functionalization logic in total synthesis. Chem. Soc. Rev. 2011, 40, 1976–1991. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, B.; Zhang, J.; Yu, W.; Liu, Z.; Zhang, Y. Transition metal-catalyzed C–H bond functionalizations by the use of diverse directing groups. Org. Chem. Front. 2015, 2, 1107–1295. [Google Scholar] [CrossRef]
- Shang, R.; Ilies, L.; Nakamura, E. Iron-catalyzed C–H bond activation. Chem. Rev. 2017, 117, 9086–9139. [Google Scholar] [CrossRef]
- Basu, D.; Kumar, S.; Sudhir, S.; Bandichhor, R. Transition metal catalyzed C-H activation for the synthesis of medicinally relevant molecules: A review. J. Chem. Sci. 2018, 130, 71. [Google Scholar] [CrossRef]
- Kang, M.; Nielsen, J. Biobased production of alkanes and alkenes through metabolic engineering of microorganisms. J. Ind. Microbiol. Biotech. 2017, 44, 3253–3260. [Google Scholar] [CrossRef] [PubMed]
- Hatamoto, Y.; Sakaguchi, S.; Ishii, Y. Oxidative cross-coupling of acrylates with vinyl carboxylates catalyzed by a Pd(OAc)2/HPMoV/O2 system. Org. Lett. 2004, 6, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Lu, J.; Loh, T.P. Direct cross-coupling reaction of simple alkenes with acrylates catalyzed by palladium catalyst. J. Am. Soc. Chem. 2009, 131, 1372–1373. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.H.; Chok, Y.K.; Loh, T.P. Synthesis and characterization of a cyclic vinylpalladium(II) complex: Vinylpalladium species as the possible intermediate in the catalytic direct olefination reaction of enamide. Chem. Sci. 2011, 2, 1822–1825. [Google Scholar] [CrossRef]
- Wen, Z.K.; Xu, Y.H.; Loh, T.P. Palladium-catalyzed cross-coupling of unactivated alkenes with acrylates: Application to the synthesis of the C13–C21 fragment of Palmerolide A. Chem. Eur. J. 2012, 18, 13284–13287. [Google Scholar] [CrossRef]
- Zhang, J.; Loh, T.P. Ruthenium- and rhodium-catalyzed cross-coupling reaction of acrylamides with alkenes: Efficient access to (Z,E)-dienamides. Chem. Commun. 2012, 48, 11232–11234. [Google Scholar] [CrossRef]
- Yu, H.; Jin, W.; Sun, C.; Chen, J.; Du, W.; He, S.; Yu, Z. Palladium-catalyzed cross-coupling of internal alkenes with terminal alkenes to functionalized 1,3-butadienes using C-H bond activation: Efficient synthesis of bicyclic pyridones. Angew. Chem., Int. Ed. 2010, 49, 5792–5797. [Google Scholar] [CrossRef]
- Li, M.Z.; Li, L.X.; Ge, H.B. Direct C-3-Alkenylation of quinolones via palladium-catalyzed C-H functionalization. Adv. Synth. Catal. 2010, 352, 2445–2449. [Google Scholar] [CrossRef]
- Besset, T.; Kuhl, N.; Patureau, F.W.; Glorius, F. Rh(III)-catalyzed oxidative olefination of vinylic C-H bonds: Efficient and selective access to di-unsaturated α-amino acid derivatives and other linear 1,3-butadienes. Chem. Eur. J. 2011, 17, 7167–7171. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Niphakis, M.J.; Georg, G.I. Palladium(II)-catalyzed dehydrogenative alkenylation of cyclic enaminones via the Fujiwara–Moritani reaction. Org. Lett. 2011, 13, 5932–5935. [Google Scholar] [CrossRef]
- Moon, Y.; Kwon, D.; Hong, S. Palladium-catalyzed dehydrogenation/oxidative cross-coupling sequence of β-heteroatom-substituted ketones. Angew. Chem. Int. Ed. 2012, 51, 11333–11336. [Google Scholar] [CrossRef]
- Min, M.; Kim, Y.; Hong, S. Regioselective palladium-catalyzed olefination of coumarins via aerobic oxidative Heck reactions. Chem. Commun. 2013, 49, 196–198. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hong, S. Palladium(II)-catalyzed direct intermolecular alkenylation of chromones. Org. Lett. 2011, 13, 4466–4469. [Google Scholar] [CrossRef] [PubMed]
- Gigant, N.; Gillaizeau, I. Palladium(II)-catalyzed direct alkenylation of nonaromatic enamides. Org. Lett. 2012, 14, 3304–3307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.X.; Cui, Z.J.; Li, Z.J.; Liu, Z.Q. Pd(II)-catalyzed dehydrogenative olefination of vinylic C–H bonds with allylic esters: General and selective access to linear 1,3-butadienes. Org. Lett. 2012, 14, 1838–1841. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Liu, Z.-Q. Transition metal-catalyzed Cvinyl-Cvinyl bond formation via double Cvinyl-H bond activation. Chem. Soc. Rev. 2013, 42, 3253–3260. [Google Scholar] [CrossRef]
- Wen, Z.-K.; Xu, Y.-H.; Loh, T.-P. Palladium(II)-catalyzed cross-coupling of simple alkenes with acrylates: A direct approach to 1,3-dienes through C–H activation. Chem. Sci. 2013, 4, 4520–4524. [Google Scholar] [CrossRef]
- Liang, Q.-J.; Yang, C.; Meng, F.-F.; Jiang, B.; Xu, Y.-H.; Loh, T.-P. Chelation versus non-chelation control in the stereoselective alkenyl sp2 C-H bond functionalization reaction. Angew. Chem. Int. Ed. 2017, 56, 5091–5095. [Google Scholar] [CrossRef]
- Hu, X.-H.; Yang, X.-F.; Loh, T.-P. Selective alkenylation and hydroalkenylation of enol phosphates through direct C-H functionalization. Angew. Chem. Int. Ed. 2015, 54, 15535–15539. [Google Scholar] [CrossRef]
- Liu, M.; Yang, P.; Karunananda, M.K.; Wang, Y.; Liu, P.; Engle, K.M. C(alkenyl)−H activation via six-membered palladacycles: Catalytic 1,3-diene synthesis. J. Am. Chem. Soc. 2018, 140, 5805–5813. [Google Scholar] [CrossRef]
- Zhao, Q.; Tognetti, V.; Joubert, L.; Besset, T.; Pannecoucke, X.; Bouillon, J.-P.; Poisson, T. Palladium-catalyzed synthesis of 3-trifluoromethyl-substituted 1,3-butadienes by means of directed C−H bond functionalization. Org. Lett. 2017, 19, 2106–2109. [Google Scholar] [CrossRef] [PubMed]
- Patureau, F.W.; Glorius, F. Oxidizing directing groups enable efficient and innovative C-H activation reactions. Angew. Chem. Int. Ed. 2011, 50, 1977–1979. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, D.; Duan, P.; Ben, R.; Dai, L.; Shao, X.; Hong, M.; Zhao, J.; Huang, Y. A multitasking functional group leads to structural diversity using designer C–H activation reaction cascades. Nat. Commun. 2014, 5, 4610. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, K.; Wang, B.; Yi, H.; Hu, F.; Li, C.; Zhang, Y.; Wang, J. Rhodium(III)-catalyzed transannulation of cyclopropenes with N-phenoxyacetamides through C-H activation. Angew. Chem. Int. Ed. 2014, 53, 13234–13238. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, S.; Lan, Y.; Wan, B.; Li, X. Rhodium-catalyzed C–H activation of phenacyl ammonium salts assisted by an oxidizing C–N bond: A combination of experimental and theoretical studies. J. Am. Chem. Soc. 2015, 137, 1623–1631. [Google Scholar] [CrossRef]
- Yu, C.; Li, F.; Zhang, J.; Zhong, G. A direct cross-coupling reaction of electron-deficient alkenes using an oxidizing directing group. Chem. Commun. 2017, 53, 533–536. [Google Scholar] [CrossRef]
- Boelke, A.; Caspers, L.D.; Nachtsheim, B.J. NH2-directed C−H alkenylation of 2-vinylanilines with vinylbenziodoxolones. Org. Lett. 2017, 19, 5344–5347. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The golden age of transfer hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Ebe, Y.; Nishimura, T. Iridium-catalyzed branch-selective hydroarylation of vinyl ethers via C−H bond activation. J. Am. Chem. Soc. 2015, 137, 5899–5902. [Google Scholar] [CrossRef]
- Iglesias, M.; Oro, L.A. A leap forward in iridium−NHC catalysis: New horizons and mechanistic insights. Chem. Soc. Rev. 2018, 47, 2772–2808. [Google Scholar] [CrossRef]
- Zhou, X.; Xia, J.; Zheng, G.; Kong, L.; Li, X. Divergent coupling of anilines and enones by integration of C−H activation and transfer hydrogenation. Angew. Chem. Int. Ed. 2018, 57, 6681–6685. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Sun, Y.; Zhang, J.; Zhang, K.; Ji, X.; Ding, L.; Zhong, G. Iridium-catalyzed cross-coupling reactions of alkenes by hydrogen transfer. Org. Lett. 2019, 21, 8219–8224. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, H.; Yang, C.; Fu, Z.; Yao, H.; Lin, A. Accessing 1,3-dienes via palladium-catalyzed allylic alkylation of pronucleophiles with skipped enynes. Org. Lett. 2017, 19, 4710–4713. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-J.; Yu, X.; Wang, H.; Chen, Y.-T.; Song, Q.-B.; Yang, Z.-P.; Wang, H. Palladium-catalyzed allylation of cyclopropyl acetylenes with oxindoles to construct 1,3-dienes. Eur. J. Org. Chem. 2020, 680–688. [Google Scholar] [CrossRef]
- Su, Y.-L.; Li, L.-L.; Zhou, X.-L.; Dai, Z.-Y.; Wang, P.-S.; Gong, L.-Z. Asymmetric α-allylation of aldehydes with alkynes by integrating chiral hydridopalladium and enamine catalysis. Org. Lett. 2018, 20, 2403–2406. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Deng, L. Selective double carbomagnesiation of internal alkynes catalyzed by iron-N-heterocyclic carbene complexes: A convenient method to highly substituted 1,3-dienyl magnesium reagents. J. Am. Chem. Soc. 2016, 138, 112–115. [Google Scholar] [CrossRef]
- Dean, W.M.; Šiauciulis, M.; Storr, T.E.; Lewis, W.; Stockman, R.A. Versatile C(sp2)-C(sp3) ligand couplings of sulfoxides for the enantioselective synthesis of diarylalkanes. Angew. Chem. Int. Ed. 2016, 55, 10013–10016. [Google Scholar] [CrossRef]
- Šiauciulis, M.; Ahlsten, N.; Pulis, A.P.; Procter, D.J. Transition-metal-free cross-coupling of benzothiophenes and styrenes in a stereoselective synthesis of substituted (E,Z)-1,3-dienes. Angew. Chem. Int. Ed. 2019, 58, 8779–8783. [Google Scholar] [CrossRef]
- Crisenza, G.E.M.; Melchiorre, P. Chemistry glows green with photoredox catalysis. Nat. Commun. 2020, 11, 803. [Google Scholar] [CrossRef]
- Hu, X.-Q.; Liu, Z.-K.; Xiao, W.-J. Radical carbonylative synthesis of heterocycles by visible light photoredox catalysis. Catalysts 2020, 10, 1054. [Google Scholar] [CrossRef]
- Shaw, M.H.; Twilton, J.; MacMillan, D.W.C. Photoredox catalysis in organic chemistry. J. Org. Chem. 2016, 81, 6898–6926. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zheng, B.; Zhou, X.; Pan, L.; Liu, Q.; Li, Y. Photoinduced C(sp2)−H/C(sp2)−H cross-coupling of alkenes: Direct synthesis of 1,3-dienes. Org. Lett. 2020, 22, 1692–1697. [Google Scholar] [CrossRef]
- Maryanoff, B.E.; Reitz, A.B. The Wittig olefination reaction and modifications involving phosphoryl-stabilized carbanions. Stereochemistry, mechanism, and selected synthetic aspects. Chem. Rev. 1989, 89, 863–927. [Google Scholar] [CrossRef]
- Tamura, R.; Saegusa, K.; Kakihana, M.; Oda, D. Stereoselective E and Z olefin formation by Wittig olefination of aldehydes with allylic phosphorus ylides. Stereochemistry. J. Org. Chem. 1988, 53, 2723–2728. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ukai, J.; Ikeda, N.; Yamamoto, H. Stereoselective synthesis of (Z)- and (E)-1,3-alkadienes from aldehydes using organotitanium and lithium reagents. Tetrahedron 1987, 43, 723–730. [Google Scholar] [CrossRef]
- Cramer, C.J.; Harmata, M.; Rashatasakhon, P. Intramolecular 4 + 3 cycloadditions. Theoretical and experimental evaluation of endo/exo preferences of a cyclopentenyl cation. J. Org. Chem. 2001, 66, 5641–5644. [Google Scholar] [CrossRef]
- Wang, Y.F.; West, G. A convenient method for the synthesis of terminal (E)-1,3-dienes. Synthesis 2002, 99–103. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, C.; Song, H.; He, Z. Wittig olefination between phosphine, aldehyde, and allylic carbonate: A general method for stereoselective synthesis of trisubstituted 1,3-dienes with highly variable substituents. Org. Lett. 2010, 12, 976–979. [Google Scholar] [CrossRef]
- Dong, D.-J.; Li, H.-H.; Tian, S.-K. A highly tunable stereoselective olefination of semistabilized triphenylphosphonium ylides with N-sulfonyl imines. J. Am. Chem. Soc. 2010, 132, 5018–5020. [Google Scholar] [CrossRef]
- Jacobsen, M.J.; Funder, E.D.; Cramer, J.R.; Gothelf, K.V. β-Olefination of 2-alkynoates leading to trisubstituted 1,3-dienes. Org. Lett. 2011, 13, 3418–3421. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yang, H.; Lefebvre, Q.; Su, J.; Fu, H. Olefination of alkyl halides with aldehydes by merging visible-light photoredox catalysis and organophosphorus chemistry. iScience 2018, 6, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Pünner, F.; Schmidt, A.; Hilt, G. Up the hill: Selective double-bond isomerization of terminal 1,3-dienes towards Z-1,3-dienes or 2Z,4E-dienes. Angew. Chem. Int. Ed. 2012, 51, 1270–1273. [Google Scholar] [CrossRef] [PubMed]
- Billard, F.; Robiette, R.; Pospíšil, P. Julia–Kocienski reaction-based 1,3-diene synthesis: Aldehyde-dependent (E,E/E,Z)-selectivity. J. Org. Chem. 2012, 77, 6358–6364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fiorito, D.; Mazet, C. Exploring site selectivity of iridium hydride insertion into allylic alcohols: Serendipitous discovery and comparative study of organic and organometallic catalysts for the vinylogous Peterson elimination. ACS Catal. 2017, 7, 1554–1562. [Google Scholar] [CrossRef]
- Van Staden, L.F.; Gravestock, D.; Ager, D.J. New developments in the Peterson olefination reaction. Chem. Soc. Rev. 2002, 31, 195–200. [Google Scholar] [CrossRef]
- Tsai, D.J.S.; Matteson, D.S. A stereocontrolled synthesis of Z and E terminal dienes from pinacol E-1-trimethylsilyl-1-propene-3-boronate. Tetrahedron Lett. 1981, 22, 2751–2752. [Google Scholar] [CrossRef]
- Yamamoto, H.; Ikeda, Y. A practical synthesis of 1,3-diene using allyltriphenylsilane and titanium tetraisopropoxide. Bull. Chem. Soc. Jpn. 1986, 59, 657–658. [Google Scholar] [CrossRef]
- Meagher, T.P.; Yet, L.; Hsiao, C.-N.; Shechter, H. (E)- and (Z)-1-(Phenylsulfonyl)-4-(trimethylsilyl)-2-butenes: synthetic equivalents for the 1-(1,3-butadienyl) anion and the 1,1-(1,3-butadienyl) dianion. J. Org. Chem. 1998, 63, 4181–4192. [Google Scholar] [CrossRef]
- Maeta, H.; Hasegawa, T.; Suzuki, K. Hydrozirconation of allenylstannane for generating bimetallic three-carbon species: Synthesis of (E)-1,3-dienes from carbonyl compounds. Synlett 1993, 341–343. [Google Scholar] [CrossRef]
- Concellón, J.M.; Rodríguez-Solla, H.; Concellón, C.; Díaz-Pardo, A.; Llavona, R. A convenient synthesis of (E)-α,β-unsaturated esters with total stereoselectivity promoted by catalytic samarium diiodide. Synlett 2011, 262–264. [Google Scholar] [CrossRef]
- Borg, T.; Tuzina, P.; Somfai, P. Lewis acid-promoted addition of 1,3-bis(silyl)propenes to aldehydes: A route to 1,3-dienes. J. Org. Chem. 2011, 76, 8070–8075. [Google Scholar] [CrossRef] [PubMed]
- Soengas, R.G.; Rodríguez-Solla, H.; Díaz-Pardo, A.; Acúrcio, R.; Concellón, C.; del Amo, V.; Silva, A.M.S. General preparation of 1-substituted (E)-1,3-dienes under mild conditions. Eur. J. Org. Chem. 2015, 2524–2530. [Google Scholar] [CrossRef]
- Soengas, R.G.; Silva, V.L.; Pinto, J.; Rodríguez-Solla, H.; Silva, A.M.S. Ohmic heating and ionic liquids in combination for the indium-promoted synthesis of 1-halo alkenyl compounds: Applications to Pd-catalysed cross-coupling reactions. Eur. J. Org. Chem. 2016, 99–107. [Google Scholar] [CrossRef]
- González-Rodríguez, J.; Soengas, R.G.; Rodríguez-Solla, H. Cooperative zinc/catalytic indium system for the stereoselective sequential synthesis of (E)-1,3-dienes from carbonyl compounds. Org. Chem. Front. 2021, in press. [Google Scholar] [CrossRef]
- Sun, R.; Song, W.; Ma, C.; Zhang, H.; Yu, X. Titanium(IV) chloride-mediated stereoselective α-alkylidenation to efficiently assemble multisubstituted 1,3-dienes. Adv. Synth. Catal. 2016, 358, 3977–3982. [Google Scholar] [CrossRef]
- Schwab, P.; Grubbs, R.H.; Ziller, J.W. Synthesis and applications of RuCl2(=CHR’)(PR3)2: The influence of the alkylidene moiety on metathesis activity. J. Am. Chem. Soc. 1996, 118, 100–110. [Google Scholar] [CrossRef]
- Sanford, M.S.; Love, J.A.; Grubbs, R.H. Mechanism and activity of ruthenium olefin metathesis catalysts. J. Am. Chem. Soc. 2001, 123, 6543–6554. [Google Scholar] [CrossRef]
- Bilel, H.; Hamdi, N.; Zagrouba, F.; Fischmeister, C.; Bruneau, C. Terminal conjugated dienes via a ruthenium- catalyzed cross-metathesis/elimination sequence: Application to renewable resources. Catal. Sci. Technol. 2014, 4, 2064–2071. [Google Scholar] [CrossRef]
- Bauer, R.A.; Diblasi, C.M.; Tan, D.S. The tert-butylsulfinamide lynchpin in transition-metal-mediated multiscaffold library synthesis. Org. Lett. 2010, 12, 2084–2087. [Google Scholar] [CrossRef]
- Dolan, M.A.; Dixon, A.D.C.; Chisholm, J.D.; Clark, D.A. Ruthenium dihydride complexes as enyne metathesis catalysts. Tetrahedron Lett. 2018, 59, 4471–4474. [Google Scholar] [CrossRef]
- Rodríguez, E.; Grayson, M.N.; Asensio, A.; Barrio, P.; Houk, K.N.; Fustero, S. Chiral Brønsted acid-catalyzed asymmetric allyl(propargyl)boration reaction of ortho-alkynyl benzaldehydes: Synthetic applications and factors governing the enantioselectivity. ACS Catal. 2016, 6, 2506–2514. [Google Scholar] [CrossRef]
- Lázaro, R.; Barrio, P.; Finamore, C.; Román, R.; Fustero, S. Homoallylic o-halobenzylamines: Asymmetric diversity-oriented synthesis of benzo-fused cyclic amines. Struct. Chem. 2017, 28, 445–452. [Google Scholar] [CrossRef]
- Sirvent, A.; Foubelo, F.; Yus, M. Diastereoselective indium-mediated allylation of N-tert-butanesulfinyl ketimines: Easy access to asymmetric quaternary stereocenters bearing nitrogen atoms. Chem. Commun. 2012, 48, 2543–2545. [Google Scholar] [CrossRef] [PubMed]
- García-Muñoz, M.J.; Zacconi, F.; Foubelo, F.; Yus, M. Indium-promoted diastereo- and regioselective propargylation of chiral sulfinylimines. Eur. J. Org. Chem. 2013, 1287–1295. [Google Scholar] [CrossRef]
- García-Muñoz, M.J.; Sirvent, A.; Foubelo, F.; Yus, M. Synthesis of chiral 1,3-dienes through ring-closing metathesis of enantioenriched enynes: Potential precursors of morphane analogs. An. Acad. Bras. Ciênc. 2018, 90, 1059–1072. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhou, L.; Zeng, S.; Ma, R.; Wang, Z.; He, Z. Phosphine-mediated olefination between aldehydes and allenes: An efficient synthesis of trisubstituted 1,3-dienes with high E-selectivity. Org. Lett. 2009, 11, 3498–3501. [Google Scholar] [CrossRef]
- Yu, F.; Lian, X.; Ma, S. Pd-catalyzed regio- and stereoselective cyclization−Heck reaction of monoesters of 1,2-allenyl phosphonic acids with alkenes. Org. Lett. 2007, 9, 1703–1706. [Google Scholar] [CrossRef]
- Pacheco, M.C.; Gouverneur, V. Electrophilic fluorodesilylation of allenylmethylsilanes: a novel entry to 2-fluoro-1,3-dienes. Org. Lett. 2005, 7, 1267–1270. [Google Scholar] [CrossRef]
- Ma, S. Some typical advances in the synthetic applications of allenes. Chem. Rev. 2005, 105, 2829–2872. [Google Scholar] [CrossRef]
- Deng, Y.; Jin, X.; Fu, C.; Ma, S. Efficient highly selective synthesis of methyl 2-(ethynyl)alk-2(E)-enoates and 2-(1′-chlorovinyl)alk-2(Z)-enoates from 2-(methoxycarbonyl)-2,3-allenols. Org. Lett. 2009, 11, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Buzas, A.K.; Istrate, F.M.; Gagosz, F. Gold(I)-catalyzed isomerization of allenyl carbinol esters: an efficient access to functionalized 1,3-butadien-2-ol esters. Org. Lett. 2007, 9, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gu, Z. Scavenging byproducts in the sulfoxide glycosylation reaction: application to the synthesis of Ciclamycin. J. Am. Chem. Soc. 2005, 127, 6176–6182. [Google Scholar] [CrossRef]
- Trost, B.M.; Kazmaier, U. Internal redox catalyzed by triphenylphosphine. J. Am. Chem. Soc. 1992, 114, 7933–7935. [Google Scholar] [CrossRef]
- Ting, C.-M.; Hsu, Y.-L.; Liu, R.-S. Gold-catalyzed isomerization of unactivated allenes into 1,3-dienes under ambient conditions. Chem. Commun. 2012, 48, 6577–6579. [Google Scholar] [CrossRef]
- Hampton, C.S.; Harmata, M. Regiodivergent synthesis of 1- and 2-arylsulfonyl 1,3-dienes. Org. Lett. 2014, 16, 1256–1259. [Google Scholar] [CrossRef]
- Al-Jawaheri, Y.; Turner, M.; Kimber, M.C. Enabling the rearrangement of unactivated allenes to 1,3-dienes by use of a palladium (0)/boric acid system. Synthesis 2018, 50, 2329–2336. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Aragoncillo, C.; Redondo, M.C. Stereoselective synthesis of 1,2,3-trisubstituted 1,3-dienes through novel [3,3]-sigmatropic rearrangements in α-allenic methanesulfonates: Application to the preparation of fused tricyclic systems by tandem rearrangement/Diels−Alder reaction. Eur. J. Org. Chem. 2005, 98–106. [Google Scholar] [CrossRef]
- Krafft, M.E.; Hallal, K.M.; Vidhani, D.V.; Cran, J.W. Gold (I)-catalyzed Claisen rearrangement of allenyl vinyl ethers; synthesis of substituted 1,3-dienes. Org. Biomol. Chem. 2011, 9, 7535–7538. [Google Scholar] [CrossRef]
- Rinaolo, V.J.; Robinson, E.E.; Diagne, A.B.; Schaus, S.E.; Thomson, R.J. Diene synthesis by the reductive transposition of 1,2-allenols. Synlett 2019, 30, 2073–2076. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Rodríguez-Acebes, R. Metal-mediated entry to functionalized 3-substituted 3-hydroxyindolin-2-ones via regiocontrolled carbonylallylation, bromoallylation, 1,3-butadien-2-ylation, propargylation, or allenylation reactions of isatins in aqueous media. J. Org. Chem. 2005, 70, 3198–3204. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Almendros, P.; Luna, A.; Prieto, N. Direct FeX3-based stereocontrolled access to (Z)-3-alkenyl-oxindoles from allenols. J. Org. Chem. 2012, 77, 11388–11392. [Google Scholar] [CrossRef] [PubMed]
- Sabbasani, V.R.; Mamidipalli, P.; Lu, H.; Xia, Y.; Lee, D. Subtle electronic effects in metal-free rearrangement of allenic alcohols. Org. Lett. 2013, 15, 1552–1555. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, H.; Liang, Y.; Cheng, P. Regio- and stereoselective synthesis of 2-amino-dienes via decarboxylative amination of 4-(ethoxycarbonyl)-2,3-allenols by TsNCO. J. Org. Chem. 2014, 79, 11264–11269. [Google Scholar] [CrossRef]
- Webster, S.; Young, P.C.; Barker, G.; Rosair, G.M.; Lee, A.L. Dehydrative thiolation of allenols: Indium vs gold catalysis. J. Org. Chem. 2015, 80, 1703–1718. [Google Scholar] [CrossRef]
- Trost, B.M.; Schmidt, T. A simple synthesis of dienones via isomerization of alkynones effected by palladium catalysts. J. Am. Chem. Soc. 1988, 110, 2301–2303. [Google Scholar] [CrossRef]
- Shiotsuki, M.; Ura, Y.; Ito, T.; Wada, K.; Kondo, T.; Mitsudo, T. Ruthenium-catalyzed formal [4 + 2] cycloaddition of alkynes with alkenes: Formation of cyclohexenedicarboxylates via isomerization of alkynes and successive Diels–Alder reaction. J. Organomet. Chem. 2004, 689, 3168–3172. [Google Scholar] [CrossRef]
- Shintani, R.; Diang, W.-L.; Park, S.; Hayashi, T. Rhodium-catalyzed isomerization of unactivated alkynes to 1,3-dienes. Chem. Commun. 2006, 3646–3647. [Google Scholar] [CrossRef]
- Yasui, H.; Yorimitsu, H.; Oshima, K. Isomerization of alkynes to 1,3-dienes under rhodium or palladium catalysis. Synlett 2006, 1783–1785. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, L. Soft propargylic deprotonation: Designed ligand enables Au-catalyzed isomerization of alkynes to 1,3-dienes. J. Am. Chem. Soc. 2014, 136, 8887–8890. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Ma, X.; Liu, P.; Zhang, L. Designed bifunctional phosphine ligand-enabled gold-catalyzed isomerizations of ynamides and allenamides: Stereoselective and regioselective formation of 1-amido-1,3-dienes. Org. Lett. 2017, 19, 5744–5747. [Google Scholar] [CrossRef] [PubMed]
- Cera, G.; Lanzi, M.; Bigi, F.; Maggi, R.; Maestri, G.; Malacria, M. Bi-directional alkyne tandem isomerization via Pd(0)/carboxylic acid joint catalysis: Expedient access to 1,3-dienes. Chem. Commun. 2018, 54, 14021–14024. [Google Scholar] [CrossRef] [PubMed]
- Vidhani, D.V.; Krafft, M.E.; Alabugin, I.V. Stereocontrolled synthesis of (E,Z)-dienals via tandem Rh(I)-catalyzed rearrangement of propargyl vinyl ethers. Org. Lett. 2013, 15, 4463–4465. [Google Scholar] [CrossRef]
- Shiroodi, R.K.; Dudnik, A.S.; Gevorgyan, V. Stereocontrolled 1,3-phosphatyloxy and 1,3-halogen migration relay toward highly functionalized 1,3-dienes. J. Am. Chem. Soc. 2012, 134, 6928–6931. [Google Scholar] [CrossRef][Green Version]
- Nokami, J.; Nomiyama, K.; Matsuda, S.; Imai, N.; Kataoka, K. Highly enantioselective alk-2-enylation of aldehydes through an allyl-transfer reaction. Angew. Chem. Int. Ed. 2003, 42, 1273–1276. [Google Scholar] [CrossRef] [PubMed]
- Capel, N.J.; Lindley, M.R.; Pritchard, G.J.; Kimber, M.C. Indium-mediated 2-oxonia Cope rearrangement of 1,4-dienols to 1,3-dienols. ACS Omega 2019, 4, 785–792. [Google Scholar] [CrossRef]
- Tang, S.; Li, Z.; Shao, Y.; Sun, J. Ir-catalyzed regiocontrolled allylic amination of di-/trienyl allylic alcohols with secondary amines. Org. Lett. 2019, 21, 7228–7232. [Google Scholar] [CrossRef]
- Crouch, I.T.; Dreier, T.; Frantz, D.E. Palladium-catalyzed elimination/isomerization of enol triflates into 1,3-dienes. Angew. Chem. Int. Ed. 2011, 50, 6128–6132. [Google Scholar] [CrossRef]
- Horii, S.; Ishimaru, I.; Ukaji, Y.; Inomata, K. Stereoselective one-pot 1,4-elimination and the [1,2]-Wittig rearrangement of (E)-δ-(arylmethoxy or 3-silyl-2-propynyloxy)-substituted allylic sulfones. Chem. Lett. 2011, 40, 521–523. [Google Scholar] [CrossRef]
- Nakano, T.; Soeta, T.; Endo, K.; Inomata, K.; Ukaji, Y. Stereoselective synthesis of (2Z,4E)-2,4-pentadien-1-ols via sequential 1,4-elimination reaction and [1,2]-Wittig rearrangement starting from (E)-4-alkoxy-2-butenyl benzoates. J. Org. Chem. 2013, 78, 12654–12661. [Google Scholar] [CrossRef]
- Campbell, N.E.; Sammis, G.M. Single-electron/pericyclic cascade for the synthesis of dienes. Angew. Chem. Int. Ed. 2014, 53, 6228–6231. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B.; Hoffmann, R. The conservation of orbital symmetry. Angew. Chem. Int. Ed. 1969, 8, 781–853. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Vega, J.A.; Vassilikogiannakis, G. cis-3,4-Dichlorocyclobutene as a versatile synthon in organic synthesis. Rapid entry into complex polycyclic systems with remarkably stereospecific reactions. Angew. Chem. Int. Ed. 2001, 40, 4441–4445. [Google Scholar] [CrossRef]
- Maulide, N.; Souris, C.; Frébault, F.; Luparia, M.; Audisio, D. Direct synthesis of stereodefined and functionalized dienes as valuable building blocks. Chimia 2014, 68, 248–251. [Google Scholar] [CrossRef]
- Souris, C.; Misale, A.; Chen, Y.; Luparia, M.; Maulide, N. From stereodefined cyclobutenes to dienes: Total syntheses of Ieodomycin D and the southern fragment of Macrolactin A. Org. Lett. 2015, 17, 4486–4489. [Google Scholar] [CrossRef]
- Kim, H.Y.; Oh, K. 1,3-Dienones and 2H-pyran-2-ones from soft α-vinyl enolization of β-chlorovinyl ketones: Defined roles of Brönsted and Lewis base. Org. Lett. 2015, 17, 6254–6257. [Google Scholar] [CrossRef]
- Maji, T.; Tunge, J.A. Palladium-catalyzed double-decarboxylative addition to pyrones: Synthesis of conjugated dienoic esters. Org. Lett. 2015, 17, 4766–4769. [Google Scholar] [CrossRef]
- Ding, R.; Li, Y.; Tao, C.; Cheng, B.; Zhai, H. Stereoselective synthesis of (2Z)-2,4-dienamides via NBS-mediated allyloxyl addition—Claisen rearrangement—dehydrobromination cascade reaction of ynsulfonamides. Org. Lett. 2015, 17, 3994–3997. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Zhang, L.; Huang, Y. Iridium-catalyzed aerobic α,β-dehydrogenation of γ,δ-unsaturated amides and acids: Activation of both α- and β-C—H bonds through an allyl–iridium intermediate. J. Am. Chem. Soc. 2018, 140, 735–740. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).