Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450
Abstract
1. Introduction
2. Results
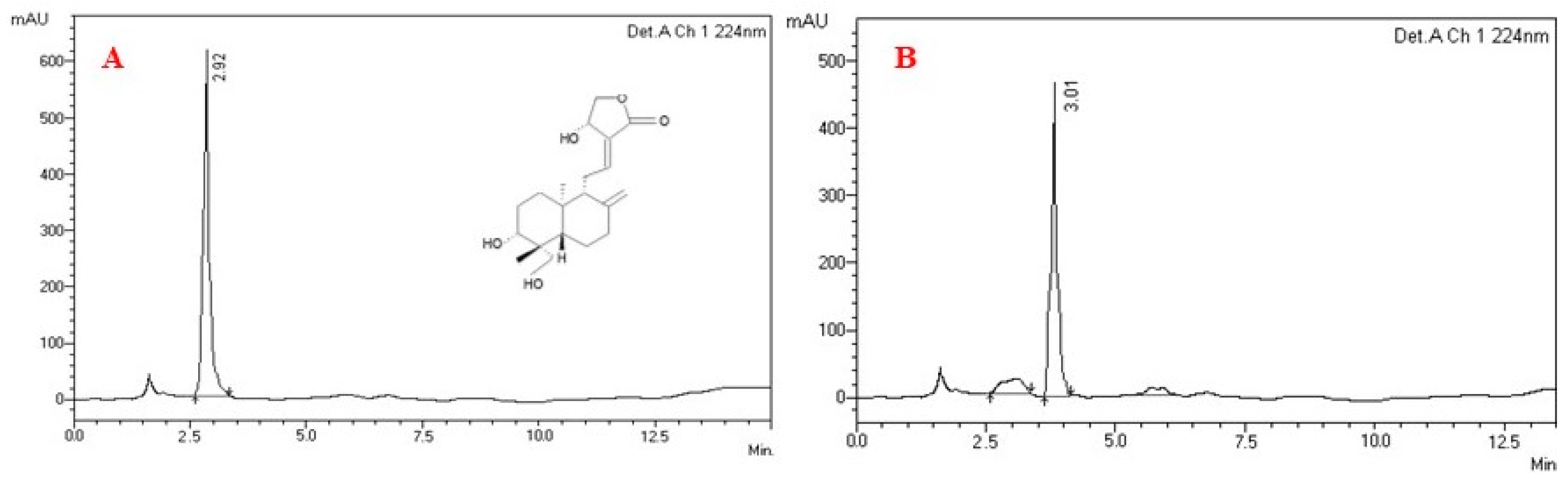
2.1. Analysis of Purified Plant Compound Andrographolide
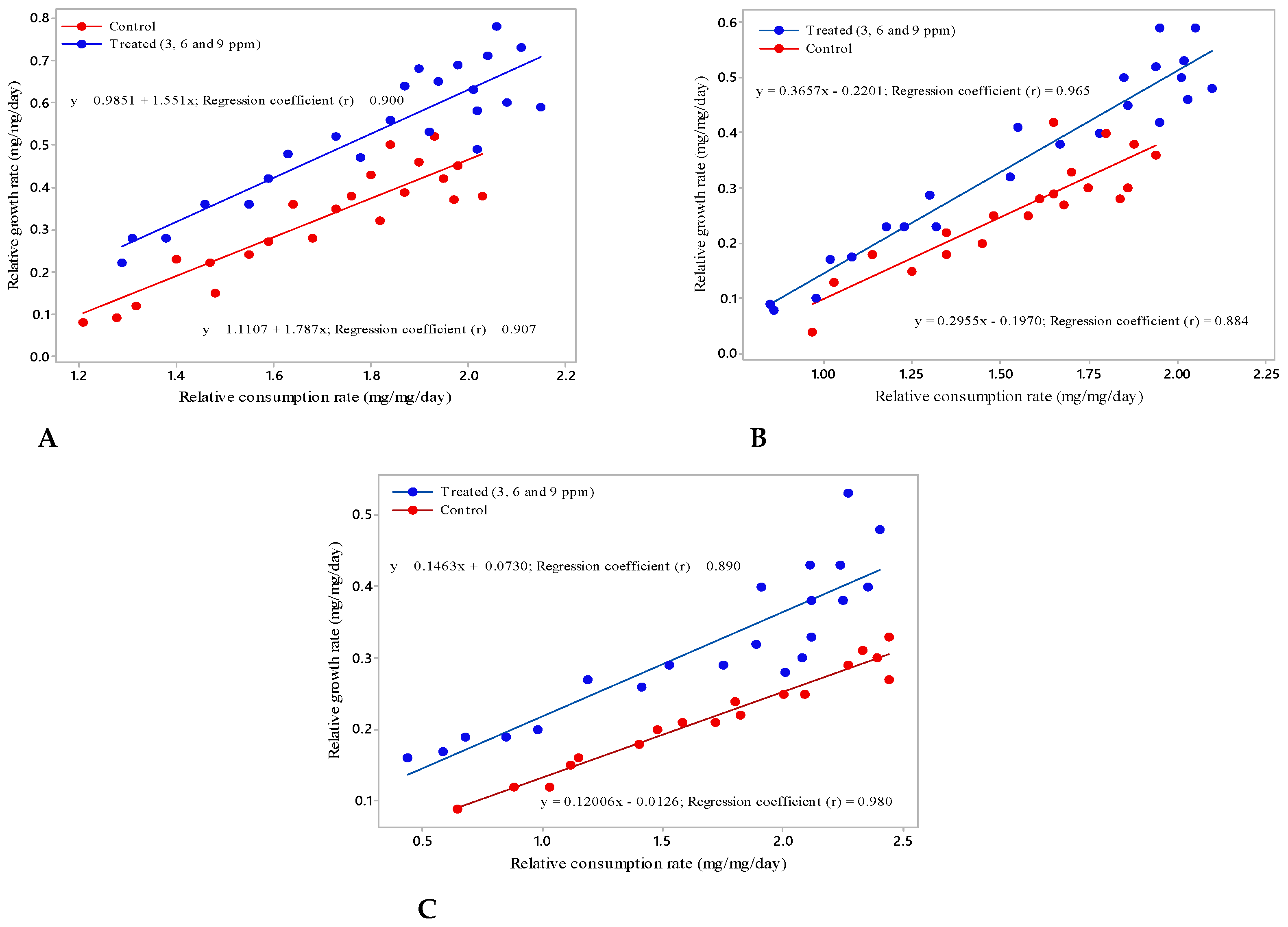
2.2. Food Utilization of S. Litura
2.3. Amylase Activity
2.4. Lipase Activity
2.5. Protease Activity
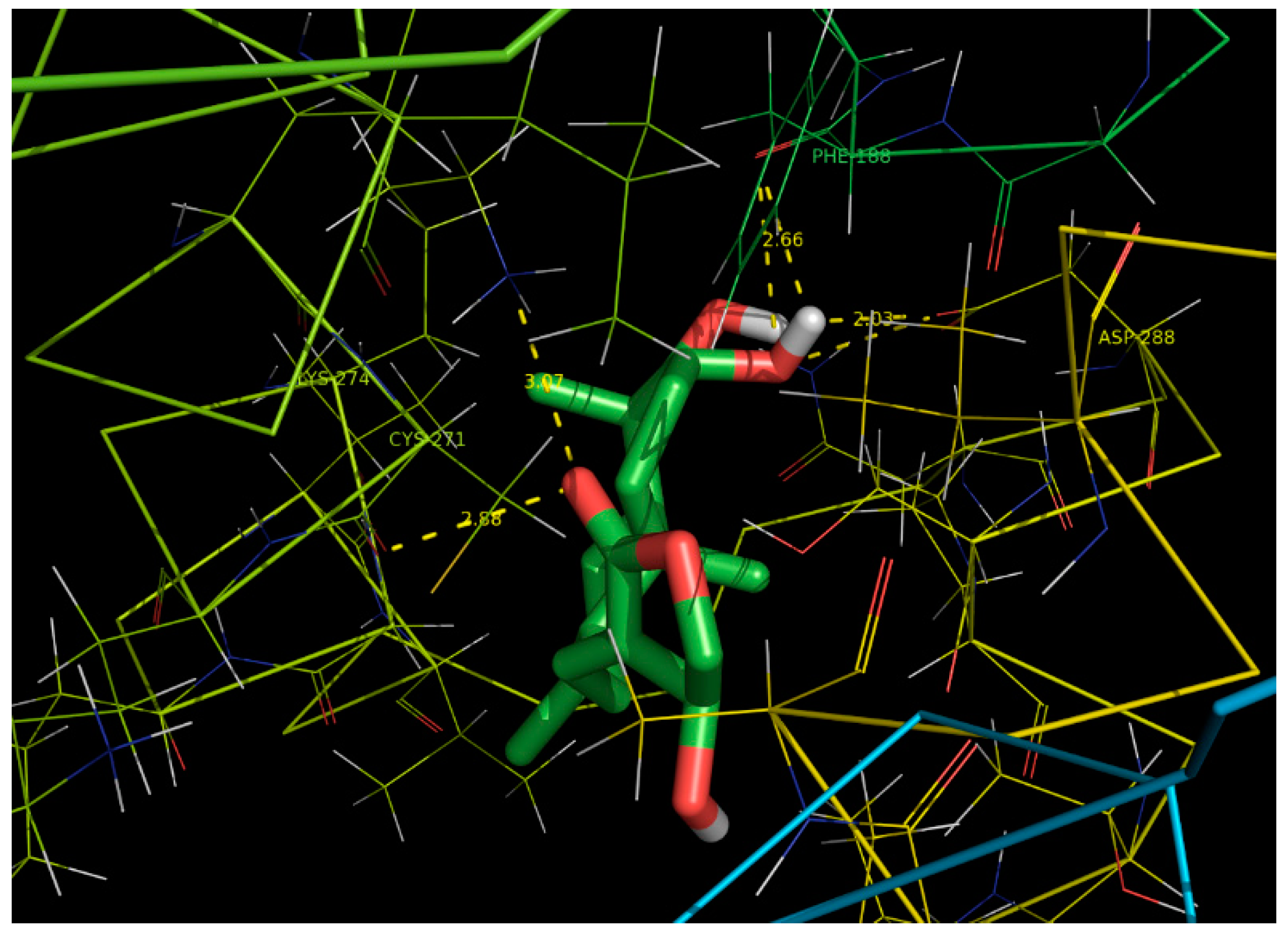
2.6. Docking Studies
3. Discussion
4. Materials and Methods
4.1. Isolation of Plant Compound Andrographolide
4.2. Insect Rearing of S. Litura
4.3. Food Utilization, Consumption and Nutritional Indices
- Relative consumption rate (RCR) = dry weight of food eaten/duration of feeding (days) × mean dry weight of the larva during the feeding period;
- Relative growth rate (RGR) = dry weight gain of the larva during the period/duration of feeding (days) × mean dry weight of the larva during the feeding period;
- Approximate digestibility (AD) = 100 × (dry weight of food eaten - dry weight of feces produced)/dry weight of food eaten;
- Efficiency of conversion of ingested food (ECI) = 100 × dry weight gain of larva/dry weight of food eaten; Efficiency of conversion of digested food (ECD) = 100 × dry weight gain of larva/dry weight of food eaten.
- Dry weight of feces produced were done according to Waldbauer [51]. Larval growth and food utilization were calculated after 24 h.
4.4. Preparation of Enzyme Extract
4.5. Amylase Activity
4.6. Lipase Activity (EC 3.1.1.3)
4.7. Protease Activity
4.8. Computational Docking Analysis
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rehan, A.; Freed, S. Resistance selection, mechanism and stability of Spodoptera litura (Lepidoptera: Noctuidae) to methoxyfenozide. Pestic. Biochem. Physiol. 2014, 110, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Yu, J.; Wang, L.; Hu, X.; Bao, W.; Li, G.; Chen, C.; Han, H.; Hu, S.; Yang, H. Sequence analysis of the Spodoptera litura multicapsid nucleo polyhedron virus genome. Virology 2001, 287, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Kang, T.; Zhan, S.; You, H.; Zhu, F.; Lee, K.S.; Zhao, H.; Jin, B.R.; Li, J. Peroxiredoxin 5 from common cutworm (Spodoptera litura) acts as a potent antioxidant enzyme. Comp. Biochem. Physiol. B 2014, 175, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S. A review of biopesticides and their mode of action against insect pest’s. In Environmental Sustainability-Role of Green Technologies; Springer: New York, NY, USA, 2015; pp. 49–63. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Baskar, K.; Ignacimuthu, S. Anti-feedant larvicidal and growth inhibitory effects of ononitol monohydrate isolated from Cassia tora L. against Helicoverpa armigera (Hub.) and Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). Chemosphere 2012, 88, 384–388. [Google Scholar] [CrossRef]
- Datta, R.; Kaur, A.; Saraf, I.; Singh, I.P.; Kaur, S. Effect of crude extracts and purified compounds of Alpinia galanga on nutritional physiology of a polyphagous lepidopteran pest, Spodoptera litura (Fabricius). Ecotoxicol. Environ. Saf. 2019, 168, 324–329. [Google Scholar] [CrossRef]
- Wang, J.; Yang, W.; Wang, G.; Tang, P.; Sai, Y. Determination of six components of Andrographis paniculata extract and one major metabolite of andrographolide in rat plasma by liquid chromatography tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 951, 78–88. [Google Scholar] [CrossRef]
- Edwin, E.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Thanigaivel, A.; Ponsankar, A.; Pradeepa, V.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Abel-Megeed, A.; et al. Anti-dengue efficacy of bioactive andrographolide from Andrographis paniculata (Lamiales:Acanthaceae) against the primary dengue vector Aedes aegypti (diptera: Culicidae). Acta Trop. 2016, 163, 167–178. [Google Scholar] [CrossRef]
- Akbar, S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Altern. Med. Rev. 2011, 16, 66–77. [Google Scholar]
- Widyawaruyantia, A.; Asrorya, M.; Ekasaria, W.; Setiawana, D.; Radjarama, A.; Tumewub, L.; Hafida, A.F. In vivo Antimalarial Activity of Andrographis paniculata Tablets. Procedia Chem. 2014, 13, 101–104. [Google Scholar] [CrossRef][Green Version]
- Hermawan, W.; Tsukuda, R.; Fujisaki, K.; Kobayashi, A.; Nakasuji, F. Influence of crude extracts from a tropical plant, Andrographis paniculata (Acanthaceae), on suppression of feeding by the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae) and oviposition by the azuki bean weevil, Callosobruchus chinensis (Coleoptera: Bruchidae). Appl. Entomol. Zool. 1993, 28, 251–254. [Google Scholar]
- Ramya, S.; Rajasekaran, C.; Sundararajan, G.; Alaguchamy, N.; Jayakumararaj, R. Antifeedant activity of leaf aqueous extracts of selected medicinal plants on VI instar larva of Helicoverpa armigera(Hubner). Ethnobot. Leafl. 2008, 12, 938–943. [Google Scholar]
- Ramasamy, V.; Karthi, S.; Ganesan, R.; Prakash, P.; Senthil-Nathan, S.; Umavathi, S.; Krutmuang, P.; Vasantha-Srinivasan, P. Chemical characterization of billy goat weed extracts Ageratum conyzoides (Asteraceae) and their mosquitocidal activity against three blood-sucking pests and their non-toxicity against aquatic predators. Environ. Sci Pollut. Res. 2021, 28, 28456–28469. [Google Scholar] [CrossRef]
- Chellappandian, M.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Kalaivani, K.; Hunter, W.B.; Ali, H.M.; Salem, M.Z.M.; Abdel-Megeed, A. Volatile toxin of Limonia acidissima (L.) produced larvicidal, developmental, repellent, and adulticidal toxicity effects on Aedes aegypti (L.). Toxin Rev. 2020, 1–15. [Google Scholar] [CrossRef]
- Karthi, S.; Panneerselvam, B.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Shivakumar, M.S.; Krutmuang, P. Functional identification and characterization of midgut microbial flora derived from lepidopteran larvae Spodoptera litura Fab. Biocatal. Agric. Biotechnol. 2020, 28, 101758. [Google Scholar] [CrossRef]
- Chellappandian, M.; Senthil-Nathan, S.; Vasantha-Srinivasan, P.; Karthi, S.; Thanigaivel, A.; Kalaivani, K.; Sivanesh, H.; Stanley-Raja, V.; Chanthini, K.M.-P.; Shyam-Sundar, N. Target and non-target botanical pesticides effect of Trichodesma indicum (Linn) R. Br. and their chemical derivatives against the dengue vector, Aedes aegypti L. Environ. Sci. Pollut. Res. 2019, 26, 16303–16315. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S.; Kalaivani, K.; Chung, P.G. The effects of azadirachtin and nucleopolyhedrovirus on midgut enzymatic profile of Spodoptera litura Fab. (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2005, 83, 46–57. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Vasantha-Srinivasan, P.; Edwin, E. Development of an eco-friendly mosquitocidal agent from Alangium salvifolium against the dengue vector Aedes aegypti and its biosafety on the aquatic predator. Environ. Sci. Pollut. Res. 2018, 25, 10340–10352. [Google Scholar] [CrossRef] [PubMed]
- Balaji, R.; Sabitha, K.; Thangarajan, R. Molecular modeling and docking of small molecule inhibitors against NEK2. Bioinformation 2016, 12, 62–68. [Google Scholar]
- Fjordboge, A.; Baun, A.; Vastrup, T.; Kjeldsen, P. Zero valent iron reduces toxicity and concentrations of organophosphate pesticides in contaminated groundwater. Chemosphere 2013, 90, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Abudulai, M.; Shepard, B.M.; Mitchell, P.L. Parasitism and predation on eggs of Leptoglossus phyllopus (L.) (Hemiptera: Coreidae) in cowpea: Impact of endosulfan sprays. J. Agric. Urban Entomol. 2001, 18, 105–115. [Google Scholar]
- Wood, H.A.; Granados, R.R. Genetically engineered baculoviruses as agents for pest control. Annu. Rev. Microbiol. 1991, 45, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Hermawan, W.; Kajiyama, S.; Tsukuda, R.; Fujisaki, K.; Kobayashi, A.; Nakasuji, F. Antifeedant and antioviposition activities of the fractions of extract from a tropical plant, Andrographis paniculata (Acanthaceae), against the diamondback moth, Plutella xylostella (Lepidoptera: Yponomeutidae). Appl. Entomol. Zool. 1994, 29, 533–538. [Google Scholar] [CrossRef]
- Elango, G.; Zahir, A.A.; Bagavan, A.; Kamaraj, C.; Rajakumar, G.; Santhoshkumar, T.; Marimuthu, S.; Rahuman, A.A. Efficacy of indigenous plant extracts on the malaria vector Anopheles subpictus Grassi (Diptera: Culicidae). Indian. J. Med. Res. 2011, 134, 375–383. [Google Scholar] [PubMed]
- Hasheminia, S.M.; Sendi, J.J.; Jahromi, K.T.; Moharramipour, S. The effects of Artemisia annua L. and Achillea millefolium L. crude leaf extracts on the toxicity, development, feeding efficiency and chemical activities of small cabbage Pieris rapae L. (Lepidoptera: Pieridae). Pestic. Biochem. Physiol. 2011, 99, 244–249. [Google Scholar] [CrossRef]
- Haouas, D.; Flamini, G.; Halima-Kamel, M.B.; Hamouda, M.H.B. Feeding perturbation and toxic activity of five Chrysanthemum species crude extracts against Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). Crop Prot. 2010, 29, 992–997. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.H.; Seo, H.Y. Food consumption, utilization and detoxification enzyme activity of the rice leaffolder larvae after treatment with Dysoxylum limonoids. Pestic. Biochem. Physiol. 2007, 88, 260–267. [Google Scholar] [CrossRef]
- Montenegro, I.J.; Corral, S.D.; Napal, G.N.D.; Carpinella, M.C.; Mellado, M.; Madrid, A.M.; Villena, J.; Palacios, S.M.; Cuellar, M.A. Antifeedant effect of polygodial and drimenol derivatives against Spodoptera frugiperda and Epilachna paenulata and quantitative structure-activity analysis. Pest. Manag. Sci. 2018, 74, 1623–1629. [Google Scholar] [CrossRef]
- Koul, O.; Singh, G.; Singh, R.; Multani, J.S. Bioefficacy and mode-of-action of aglaroxin A from Aglaia elaeagnoidea (syn. A. roxburghiana) against Helicoverpa armigera and Spodoptera litura. Entomol. Exp. Appl. 2005, 114, 197–204. [Google Scholar] [CrossRef]
- Khosravi, R.; Sendi, J.J. Effect of neem pesticide (achook) on midgut enzymatic activities and selected biochemical compounds in the hemolymph of lesser mulberry pyralid, Glyphodes pyloalis walker (Lepidoptera: Pyralidae). J. Plant Prot. Res. 2013, 53, 238–247. [Google Scholar] [CrossRef]
- Senthil-Nathan, S.; Kalaivani, K. Efficacy of nucleopolyhedrovirus and azadirachtin on Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Biol. Control. 2005, 34, 93–98. [Google Scholar] [CrossRef]
- Koul, O. A global perspective. In Today and in the New Millennium; Neem Koul, O., Wahab, S., Eds.; Kluwar Academic Publisher: Dordrecht, The Netherlands, 2004; Volume 53, pp. 1–19. [Google Scholar]
- Bahrami, B.M.; Mikani, A.; Moharramipour, S. Effect of Achillea millefolium and Teucrium polium extracts on nutritional indices and α-amylase and protease activities of Egyptian cotton leafworm, Spodoptera littoralis (Lepidoptera: Noctuidae). J. Crop Prot. 2018, 7, 183–190. [Google Scholar]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.H.; Seo, H.Y.; Kalaivani, K.; Kim, J.D. Toxicity and behavioural effect of 3b, 25, 26-trihydroxycycloaratane and beddomei lactone on the rice leaffolder Cnaphalocrocis medinalis (Guenee) (Lepidoptera: Pyralidae). Ecotoxicol. Environ. Saf. 2009, 72, 1156–1162. [Google Scholar] [CrossRef]
- Handrasekaran, R.; Revathi, K.; Nisha, S.; Kirubakaran, S.A.; Sathish-Narayanan, S.; Senthil-Nathan, S. Physiological effect of chitinase purified from Bacillus subtilis against the tobacco cutworm Spodoptera litura Fab. Pestic. Biochem. Physiol. 2012, 104, 65–71. [Google Scholar] [CrossRef]
- Rath, S.; Prasad, B.; Sinha, B. Food utilization efficiency in fifth instar larvae of Antheraea mylitta (Lepidoptera: Saturniidae) infected with Nosema sp. and its effect on reproductive potential and silk production. J. Invertebr. Pathol. 2003, 83, 1–9. [Google Scholar] [CrossRef]
- Broadway, R.M.; Duffey, S.S. The effect of plant protein quality on insect digestive physiology and the toxicity of plant proteinase inhibitors. J. Insect Physiol. 1988, 34, 1111–1117. [Google Scholar] [CrossRef]
- Shekari, M.; Sendi, J.J.; Etebari, K.; Zibaee, A.; Shadparvar, A. Effects of Artemisia annua L. (Asteracea) on nutritional physiology and enzyme activities of elm leaf beetle,Xanthogaleruca Luteola Mull. (Coleoptera: Chrysomellidae). Pestic. Biochem. Physiol. 2008, 91, 66–74. [Google Scholar] [CrossRef]
- Senthil-Nathan, S. Effects of Melia azedarach on nutritional physiology and enzyme activities of the rice leaffolder Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Pestic. Biochem. Physiol. 2006, 84, 98–108. [Google Scholar] [CrossRef]
- Araujo, C.L.; Bezerra, I.W.; Oliveira, A.S.; Moura, F.T.; Macedo, L.L.; Gomes, C.E.; Barbosa, A.E.; Macedo, F.P.; Souza, T.M.; Franco, O.L.; et al. In vivo bio-insecticidal activity toward Ceratitis capitata (fruit fly) and Callosobruchus maculatus (cowpea weevil) and in vitro bioinsecticidal activity toward different orders of insect pests of a trypsin inhibitor purified from tamarind tree (Tamarindus indica) seeds. J. Agric. Food Chem. 2005, 53, 4381–4387. [Google Scholar] [PubMed]
- De-Leo, F.; Bonade-Botino, M.; Ceci, R.L.; Galerani, R.; Jouanin, L. Effects of mustard trypsin inhibitors expressed in different plants on three different lepidopteran pests. Insect Biochem. Molec. Biol. 2001, 31, 593–602. [Google Scholar] [CrossRef]
- Macedo, M.L.R.; Sa, C.M.; Freire, M.G.M.; Parra, J.R.P. A Kunitz-type inhibitor from Adenanthera pavonia L. seeds active against coleopteran pest proteases and its effect on the development of Callosobruchus maculatus (Coleoptera: Bruchidae). J. Agric. Food Chem. 2004, 52, 2533–2540. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Zhang, X.T.; Gao, X.W. Molecular cloning and recombinant expression of cytochrome P450 CYP6B6 from Helicoverpa armigera in Escherichia coli. Mol. Biol. Rep. 2013, 40, 1211–1217. [Google Scholar] [CrossRef]
- Zeng, R.S.; Wen, Z.; Niu, G.; Schuler, M.A.; Berenbaum, M.R. Allelochemical induction of cytochrome P450 monooxygenases and amelioration of xenobiotic toxicity in Helicoverpa zea. J. Chem. Ecol. 2007, 33, 449–461. [Google Scholar] [CrossRef]
- Feyereisen, R. Insect cytochrome P450. In Comprehensive Molecular Insect Science; Gilbert, L.I., Iatrou, K., Gill, S.S., Eds.; Elsevier: Oxford, UK, 2005; Volume 4, pp. 1–77. [Google Scholar]
- Bullangpoti, V.; Wajnberg, E.; Audant, P.; Feyereisen, R. Antifeedant activity of Jatrophaossypifolia and Meliaazedarach senescent leaf extracts on Spodoptera frugiperda (Lepidoptera: Noctuidae) and their potential uses synergists. Pest. Manag. Sci. 2012, 268, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Entomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Wang, R.L.; Xia, Q.Q.; Baerson, S.R.; Ren, Y.; Wang, J.; Su, Y.J.; Zheng, S.C.; Zeng, R.S. A novel cytochrome P450 CYP6AB14 gene in Spodoptera litura (Lepidoptera: Noctuidae) and its potential role in plant allelochemical detoxification. J. Insect Physiol. 2015, 75, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Senthil-Nathan, S.; Choi, M.Y.; Paik, C.H.; Kalaivani, K. The toxicity and physiological effect of goniothalamin, a styryl-pyrone, on the generalist herbivore, Spodoptera exigua Hubner. Chemosphere 2008, 72, 1393–1400. [Google Scholar] [CrossRef] [PubMed]
- Waldbauer, G.P. The consumption, digestion and utilization of solanaceous and non-solanaceous plants by larvae of the tobacco hornworm, Protoparce sexta (Johan.) (Lepidoptera: Sphingidae). Entomol. Exp. Appl. 1964, 7, 253–269. [Google Scholar] [CrossRef]
- Applebaum, S.W.; Jankovic, M.; Birk, Y. Studies on the midgut amylase activity of Tenebrio molitor L. larvae. J. Insect. Physiol. 1961, 7, 100–108. [Google Scholar] [CrossRef]
- Applebaum, S.W. The action pattern and physiological role of Tenebrio larval amylase. J. Insect. Physiol. 1964, 10, 897–906. [Google Scholar] [CrossRef]
- Ishaaya, I.; Swirski, E. Invertase and amylase activity in the armoured scales Chrysomphalus aonidum and Aonidiella aurantii. J. Insect Physiol. 1970, 16, 1599–1606. [Google Scholar] [CrossRef]
- Snell, F.D.; Snell, C.T. Calorimetric Methods of Analysis, 3rd ed.; Van Nostrand Company: New York, NY, USA, 1949; p. 145. [Google Scholar]
- Sanner, M.F. Python: A programming language for software integration and development. Mol. J. Graph. Model. 1999, 17, 57–61. [Google Scholar]
- Stierand, K.; Rarey, M. Drawing the PDB: Protein-Ligand Complexes in Two Dimensions. Med. Chem. Lett. 2010, 1, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A method for computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989. [Google Scholar]
- SAS Institute. The SAS System for Windows Release 8.1; SAS Publisher: Cary, NC, USA, 2001. [Google Scholar]
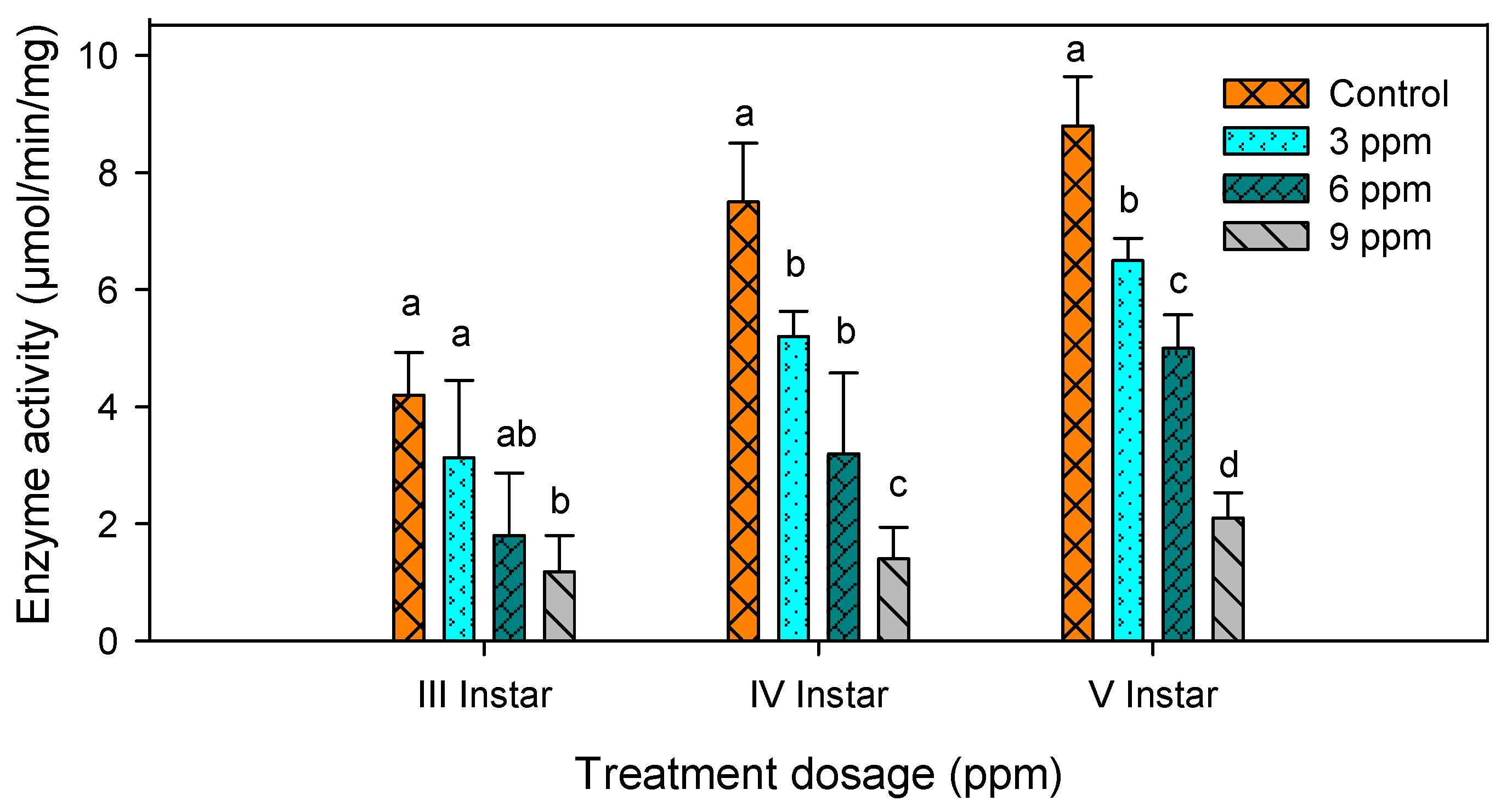
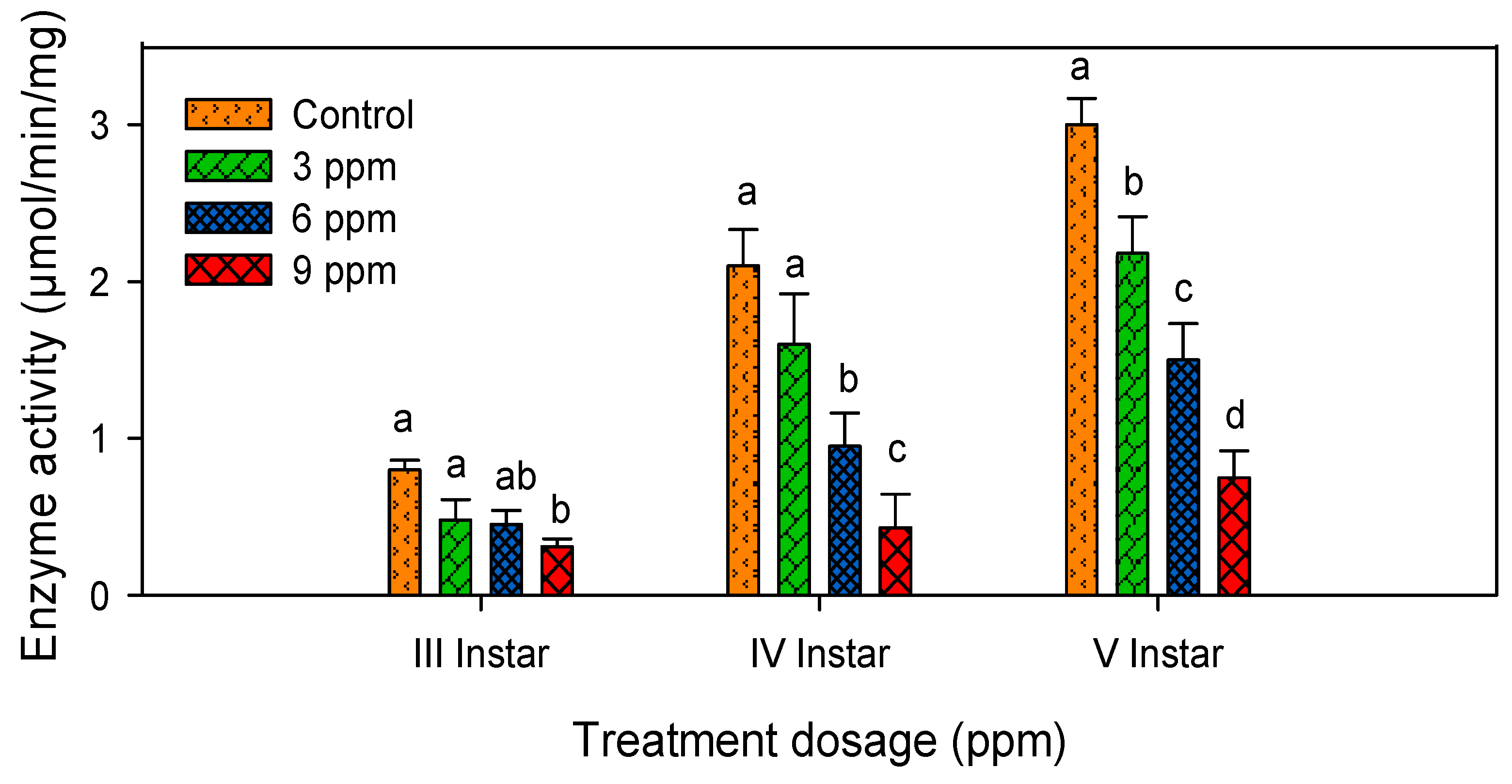

| S. No | Treatments | Third Instar (µmol/min/mg) | Fourth Instar (µmol/min/mg) | Fifth Instar (µmol/min/mg) |
|---|---|---|---|---|
| 1 | Control | 4.20 ± 0.73 a | 7.50 ± 1.03 a | 8.80 ± 0.83 a |
| 2 | 3 ppm | 3.00 ± 1.32 ab | 5.20 ± 0.29 b | 6.50 ± 0.38 b |
| 3 | 6 ppm | 1.80 ± 1.07 bc | 3.20 ± 1.38 c | 5.00 ± 0.57 c |
| 4 | 9 ppm | 1.18 ± 0.62 c | 1.40 ± 0.80 d | 2.10 ± 0.94 d |
| S. No | Treatments | Third Instar (µmol/min/mg) | Fourth Instar (µmol/min/mg) | Fifth Instar (µmol/min/mg) |
|---|---|---|---|---|
| 1 | Control | 0.80 ± 0.06 a | 2.10 ± 0.94 a | 3.00 ± 1.32 a |
| 2 | 3 ppm | 0.48 ± 0.13 b | 1.60 ± 0.72 ab | 2.18 ± 0.83 ab |
| 3 | 6 ppm | 0.45 ± 0.09 bc | 0.95 ± 0.20 bc | 1.50 ± 0.87 ab |
| 4 | 9 ppm | 0.31 ± 0.05 c | 0.43 ± 0.09 c | 0.75 ± 0.17 b |
| S. No | Treatments | Third Instar (µmol/min/mg) | Fourth Instar (µmol/min/mg) | Fifth Instar (µmol/min/mg) |
|---|---|---|---|---|
| 1 | Control | 11.50 ± 3.15 a | 17.50 ± 2.24 a | 21.20 ± 2.77 a |
| 2 | 3 ppm | 8.20 ± 1.09 ab | 13.80 ± 0.58 b | 15.80 ± 1.03 b |
| 3 | 6 ppm | 6.30 ± 1.44 b | 10.30 ± 1.03 c | 11.90 ± 1.67 c |
| 4 | 9 ppm | 2.40 ± 1.22 c | 5.10 ± 0.56 d | 7.00 ± 1.07 d |
| Ligand | Protein PDB ID | Binding Amino Acid Residues | Binding Energy (kcal/mol) | Inhibition Constant (uM) | RMSD (Ǻ) | Ligand Efficiency |
|---|---|---|---|---|---|---|
| Andrographolide | CYP6B | PHE`188/O with 42 atoms,
CYS`271/O with 10 atoms, LYS`274/NZ with 22 atoms, ASP`288/OD2 with 54 atoms | −6.37 | 21.42 | 12.16 | 0.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edwin, E.-S.; Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Chellappandian, M.; Karthi, S.; Narayanaswamy, R.; Stanley-Raja, V.; Sivanesh, H.; Ramasubramanian, R.; Al-Huqail, A.A.; et al. Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450. Molecules 2021, 26, 5982. https://doi.org/10.3390/molecules26195982
Edwin E-S, Vasantha-Srinivasan P, Senthil-Nathan S, Chellappandian M, Karthi S, Narayanaswamy R, Stanley-Raja V, Sivanesh H, Ramasubramanian R, Al-Huqail AA, et al. Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450. Molecules. 2021; 26(19):5982. https://doi.org/10.3390/molecules26195982
Chicago/Turabian StyleEdwin, Edward-Sam, Prabhakaran Vasantha-Srinivasan, Sengottayan Senthil-Nathan, Muthiah Chellappandian, Sengodan Karthi, Radhakrishnan Narayanaswamy, Vethamonickam Stanley-Raja, Haridoss Sivanesh, Ramakrishnan Ramasubramanian, Asma A. Al-Huqail, and et al. 2021. "Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450" Molecules 26, no. 19: 5982. https://doi.org/10.3390/molecules26195982
APA StyleEdwin, E.-S., Vasantha-Srinivasan, P., Senthil-Nathan, S., Chellappandian, M., Karthi, S., Narayanaswamy, R., Stanley-Raja, V., Sivanesh, H., Ramasubramanian, R., Al-Huqail, A. A., Khan, F., Krutmuang, P., Abdel-Megeed, A., Ghaith, A., & Paik, C.-H. (2021). Toxicity of Bioactive Molecule Andrographolide against Spodoptera litura Fab and Its Binding Potential with Detoxifying Enzyme Cytochrome P450. Molecules, 26(19), 5982. https://doi.org/10.3390/molecules26195982











