Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa
Abstract
1. Introduction
2. Results
2.1. Results of Evaluation of Sperm Motility, Membrane Integrity, and Chromatin Damage in Native and Frozen/Thawed Semen
2.2. Results of Chromatographic Analysis of Carbohydrate Content in Cytosol of Spermatozoa
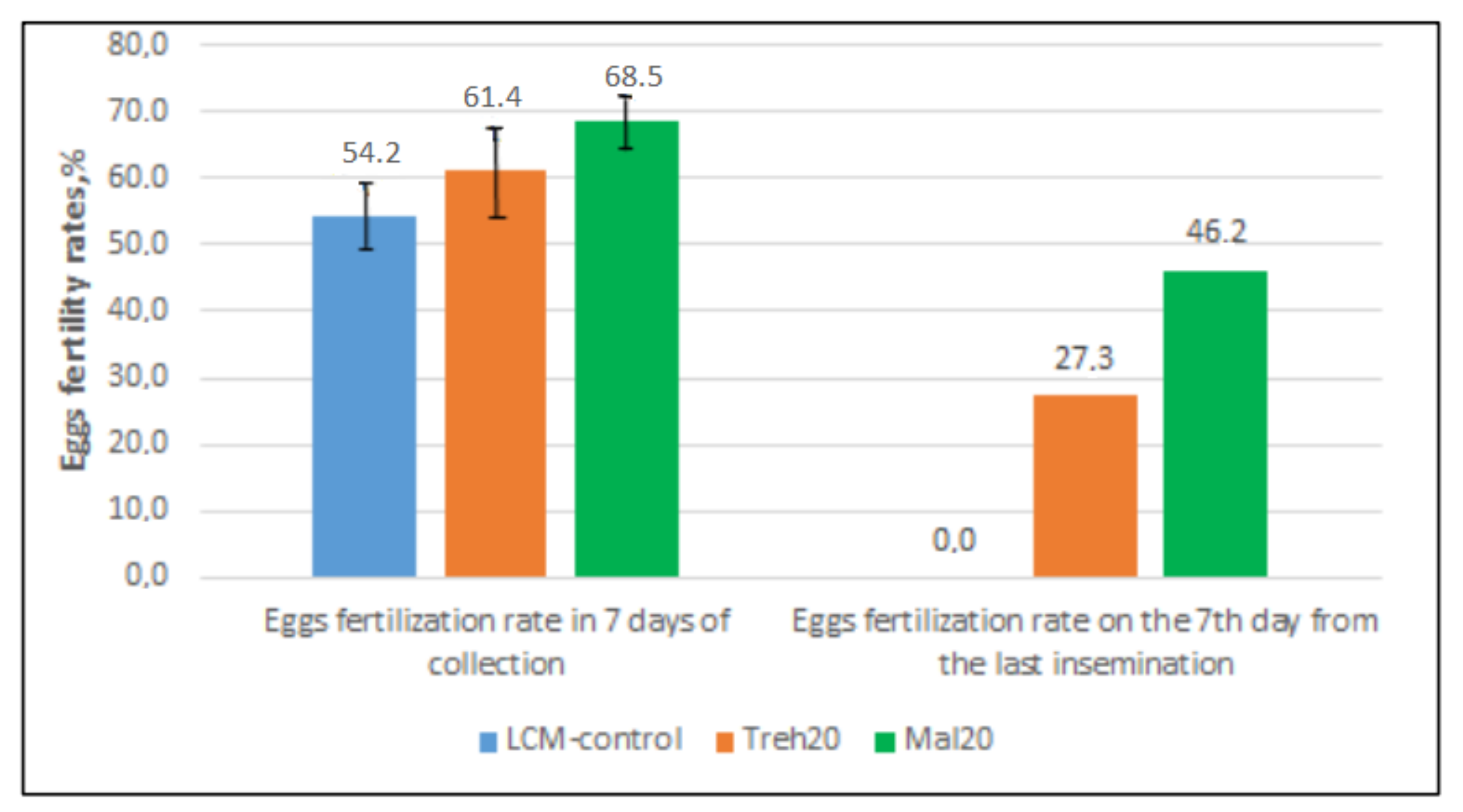
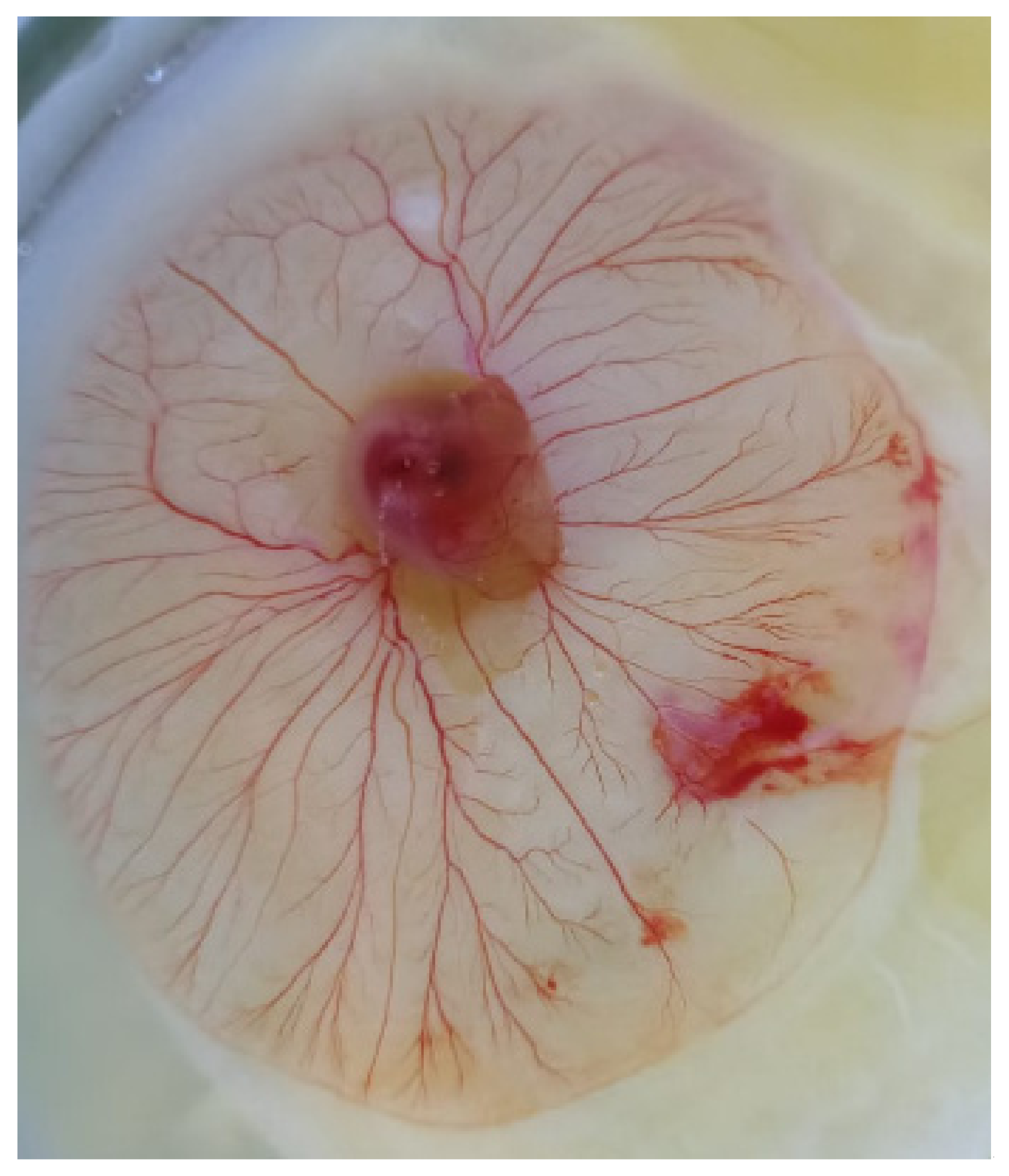
2.3. Fertility Assessment of Frozen/Thawed Semen
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Semen Collection
4.3. Spermatozoa Cryopreservation: Media and Procedure
4.4. Plasma Membrane Damage (Sperm Viability)
4.5. Sperm Chromatin Integrity
4.6. Quantitative Chromatographic Analysis of Cytosol Carbohydrates of Spermatozoa
4.7. Theoretical Calculation of the Number of Saccharide Molecules in Cryoprotective Media, Diluted Semen, and Spermatozoa Cytosol
4.8. Artificial Insemination
4.9. Ethic Statement
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seigneurin, F.; Blesbois, E. Update on Semen Cryopreservation Methods in Poultry Species. In 13th European Poultry Conference (EPC 2010); French Branch of World’s Poultry Science Association: Tours, France, 2010; p. 172. Available online: https://hal.archives-ouvertes.fr/hal-02753624/ (accessed on 13 September 2021).
- Long, J.A.; Bongalhardo, D.C.; Pelaez, J.; Saxena, S.; Settar, P.; O’Sullivan, N.P.; Fulton, J.E. Rooster semen cryopreservation: Effect of pedigree line and male age on post-thaw sperm function. Poult. Sci. 2010, 89, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Ciftci, Y.; Aygun, A. Poultry semen cryopreservation technologies. Worlds Poult. Sci. J. 2018, 74, 699–710. [Google Scholar] [CrossRef]
- Fulton, J.E. Avian genetic stock preservation: An industry perspective. Poult. Sci. 2006, 85, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Silyukova, Y.; Pleshanov, N.; Stanishevskaya, O. The influence membranes damage and activity of roosters’ sperm on the fertilization of eggs when using cured cryopreserved sperm. Reprod. Domest. Anim. 2019, 54, 101. [Google Scholar]
- Stanishevskaya, O.; Silyukova, Y.; Pleshanov, N.; Kurochkin, A.; Fedorova, E.; Fedorova, Z.; Perinek, O.; Prituzhalova, A.; Meftakh, I. Effects of Saccharides Supplementation in the Extender of Cryopreserved Rooster (Gallus domesticus) Semen on the Fertility of Frozen/Thawed Spermatozoa. Animals 2021, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Pini, T.; Leahy, T.; Graaf, S. Sublethal sperm freezing damage: Manifestations and solutions. Theriogenology 2018, 118, 172–181. [Google Scholar] [CrossRef]
- Mavrodina, T.G.; Stanishevskaya, O.I.; Cherepenov, S.V.; Silyukova, Y.L. Influence of sperm quality (cryopreserved and native) on the duration of spermatozoa storage in reproductive tracts of turkeys. Anim. Reprod. Sci. 2018, 194, 14. [Google Scholar] [CrossRef]
- Smith, A.M.J.; Bonato, M.; Dzama, K.; Malecki, I.A.; Cloete, S.W.P. Mineral profiling of Ostrich (Struthio camelus) seminal plasma and its relationship with semen traits and collection day. Anim. Reprod. Sci. 2018, 193, 98–106. [Google Scholar] [CrossRef]
- Tian, Y.; Visser, J.C.; Klever, J.S.; Woerdenbag, H.J.; Frijlink, H.W.; Hinrichs, W.L.J. Orodispersible films based on blends of trehalose and pullulan for protein delivery. Eur. J. Pharm. Biopharm. 2018, 133, 104–111. [Google Scholar] [CrossRef]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; Mocé, E.; Mercado, E. Effect of different monosaccharides and disaccharides on boar sperm quality after cryopreservation. Anim. Reprod. Sci. 2012, 133, 109–116. [Google Scholar] [CrossRef]
- Saleh, R.; Assaf, H.; Abd, W.; Maged, E.; Fawzy, M.; Elsuity, M.A. Positive effects of in-vitro Myo-inositol supplementation of cryopreserved human sperm on the outcome of cryopreservation: A randomized controlled trial. Fertil. Steril. 2017, 108, e309. [Google Scholar] [CrossRef][Green Version]
- Branca, C.; Maccarrone, S.; Magazù, S.; Maisano, G.; Bennington, S.M.; Taylor, J. Tetrahedral order in homologous disaccharide-water mixtures. J. Chem. Phys. 2005, 122, 174513. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, D.; Townrow, S.; Meunier, V.; Richardson, R.; Alam, A.; Ubbink, J. Organization and mobility of water in amorphous and crystalline trehalose. Nat. Mater. 2006, 5, 632–635. [Google Scholar] [CrossRef]
- Rabbani, G.; Choi, I. Roles of osmolytes in protein folding and aggregation in cells and their biotechnological applications. Int. J. Biol. Macromol. 2018, 109, 483–491. [Google Scholar] [CrossRef]
- Rao, W.; Huang, H.; Wang, H.; Zhao, S.; Dumbleton, J.; Zhao, G.; He, X. Nanoparticle-Mediated Intracellular Delivery Enables Cryopreservation of Human Adipose-Derived Stem Cells Using Trehalose as the Sole Cryoprotectant. ACS Appl. Mater. Interfaces 2015, 7, 5017–5028. [Google Scholar] [CrossRef]
- Feofilova, E.P.; Mysyakina, I.S.; Usov, A.I.; Kochkina, G.A. Trehalose: Chemical structure, biological functions, and practical application. Microbiology 2014, 83, 184–194. [Google Scholar] [CrossRef]
- Rudolph, A.S.; Crowe, J.H.; Crowe, L.M. Effects of three stabilizing agents—Proline, betaine, and trehalose—On membrane phospholipids. Arch. Biochem. Biophys. 1986, 245, 134–143. [Google Scholar] [CrossRef]
- Jain, N.K.; Roy, I. Effect of trehalose on protein structure. Protein Sci. 2009, 18, 24–36. [Google Scholar] [CrossRef]
- Choi, Y.; Cho, K.W.; Jeong, K.; Jung, S. Molecular dynamics simulations of trehalose as a “dynamic reducer” for solvent water molecules in the hydration shell. Carbohydr. Res. 2006, 341, 1020–1028. [Google Scholar] [CrossRef]
- Peláez, J.; Bongalhardo, D.C.; Long, J.A. Characterizing the glycocalyx of poultry spermatozoa: III. Semen cryopreservation methods alter the carbohydrate component of rooster sperm membrane glycoconjugates. Poult. Sci. 2011, 90, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, C.; Kaya, A.; Aksoy, M.; Tekeli, T. Influence of sugar supplementation of the extender on motility, viability and acrosomal integrity of dog spermatozoa during freezing. Theriogenology 2000, 54, 579–585. [Google Scholar] [CrossRef]
- Golshahi, K.; Aramli, M.S.; Nazari, R.M.; Habibi, H. Disaccharide supplementation of extenders is an effective means of improving the cryopreservation of semen in sturgeon. Aquaculture 2018, 486, 261–265. [Google Scholar] [CrossRef]
- Xi, M.D.; Li, P.; Du, H.; Qiao, X.M.; Liu, Z.G.; Wei, Q.W. Disaccharide combinations and the expression of enolase3 and plasma membrane Ca2+ ATPase isoform in sturgeon sperm cryopreservation. Reprod. Domest. Anim. 2018, 53, 472–483. [Google Scholar] [CrossRef]
- Naing, S.W.; Wahid, H.; Mohd Azam, K.; Rosnina, Y.; Zuki, A.B.; Kazhal, S.; San, M.M. Effect of sugars on characteristics of Boer goat semen after cryopreservation. Anim. Reprod. Sci. 2010, 122, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Santos, M.R.; Martínez-Pastor, F.; García-Macías, V.; Esteso, M.C.; Soler, A.J.; de Paz, P.; Garde, J.J. Extender osmolality and sugar supplementation exert a complex effect on the cryopreservation of Iberian red deer (Cervus elaphus hispanicus) epididymal spermatozoa. Theriogenology 2007, 67, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Feiden, S.; Wolfrum, U.; Wegener, G.; Kamp, G. Expression and compartmentalisation of the glycolytic enzymes GAPDH and pyruvate kinase in boar spermatogenesis. Reprod. Fertil. Dev. 2008, 20, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Kosova, A.A.; Khodyreva, S.N.; Lavrik, O.I. Role of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in DNA repair. Biochemistry 2017, 82, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Elkina, Y.L.; Kuravsky, M.L.; El’darov, M.A.; Stogov, S.V.; Muronetz, V.I.; Schmalhausen, E.V. Recombinant human sperm-specific glyceraldehyde-3-phosphate dehydrogenase: Structural basis for enhanced stability. Biochim. Biophys. Acta 2010, 1804, 2207–2212. [Google Scholar] [CrossRef]
- Miki, K. Energy metabolism and sperm function. Soc. Reprod. Fertil. Suppl. 2007, 65, 309–325. [Google Scholar]
- Salehi, M.; Mahdavi, A.H.; Sharafi, M.; Shahverdi, A. Cryopreservation of rooster semen: Evidence for the epigenetic modifications of thawed sperm. Theriogenology 2020, 142, 15–25. [Google Scholar] [CrossRef]
- Chen, T.; Acker, J.P.; Eroglu, A.; Cheley, S.; Bayley, H.; Fowler, A.; Toner, M. Beneficial Effect of Intracellular Trehalose on the Membrane Integrity of Dried Mammalian Cells. Cryobiology 2001, 43, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Silyukova, Y.I.; Stanishevskaya, O.I.; Pleshanov, N.V.; Kurochkin, A.A. Efficiency of using a combination of mono- and disac-charides in a diluent for freezing rooster semen. Sel’skokhozyaistvennaya Biol. 2020, 55, 1148–1158. [Google Scholar] [CrossRef]
- Pintado, B.; de la Fuente, J.; Roldan, E.R.S. Permeability of boar and bull spermatozoa to the nucleic acid stains propidium iodide or Hoechst 33258, or to eosin: Accuracy in the assessment of cell viability. Journal of reproduction and fertility. J. Reprod. Fertil. 2000, 118, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Banaszewska, D.; Andraszek, K.; Zdrowowicz, E.; Czubaszek, M.; Walczak-Jędrzejowska, R. The role of staining techniques in seminological analysis of mammalian semen. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2015, 320, 5–20. [Google Scholar]
- Kazerooni, T.; Asadi, N.; Jadid, L.; Kazerooni, M.; Ghanadi, A.; Ghaffarpasand, F.; Kazerooni, Y.; Zolghadr, J. Evaluation of sperm’s chromatin quality with acridine orange test, chromomycin A3 and aniline blue staining in couples with unexplained recurrent abortion. J. Assist. Reprod. Genet. 2009, 26, 591–596. [Google Scholar] [CrossRef]
- Somogui, M. Determination of blood sugar. J. Biol. Chem. 1945, 160, 69. [Google Scholar] [CrossRef]
- Brobst, K.M.; Scobell, H.D. Modern Chromatographic Methods for the Analysis of Carbohydrate Mixtures. Starch-Stärke 1982, 34, 117–121. [Google Scholar] [CrossRef]

| Quality Indicators | Fresh Semen | Frozen/Thawed Sperm | ||
|---|---|---|---|---|
| LCM Control * | Mal20 * | Treh20 * | ||
| Total motility (TM), % | 86.3 ± 0.04 | 52.7 ± 0.7 | 55.4 ± 1.5 | 52.9 ± 0.9 |
| Progressive motility (PM), % | 65.7 ± 0.14 | 34.3 ± 2.6 a | 40.2 ± 3.8 b | 33.4 ± 2.6 a |
| Membrane integrity, % | 90.4 ± 0.01 | 48.0 ± 7.7 a | 67.2 ± 2.3 b | 69.3 ± 2.8 b |
| Chromatin integrity, % | 95.6 ± 0.01 | 79.5 ± 5.2 | 74.5 ± 4.1 a | 92.4 ± 5.9 b |
| LCM Control | Mal20 | Treh20 | |||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 2 | 1 | 2 | ||
| % of dry biomass | Glycerol | 0.010 | 0.010 | 0.008 | 0.008 | 0.009 | 0.008 |
| Fructose | 0.005 | 0.008 | 0.008 | 0.006 | 0.003 | 0.002 | |
| Glucose | 0.002 | 0.003 | 0.000 | 0.000 | 0.000 | 0.001 | |
| Inositol | 0.022 | 0.021 | 0.020 | 0.020 | 0.035 | 0.035 | |
| Maltose | 0.000 | 0.000 | 0.001 | 0.002 | 0.000 | 0.000 | |
| Trehalose | 0.000 | 0.000 | 0.000 | 0.000 | 0.008 | 0.007 | |
| Σ % of dry biomass | 0.040 | 0.042 | 0.036 | 0.036 | 0.054 | 0.053 | |
| % of Σ | Glycerol | 24.3 | 23.3 | 21.1 | 22.3 | 16.3 | 16.0 |
| Fructose | 13.8 | 19.7 | 21.1 | 16.3 | 5.3 | 3.4 | |
| Glucose | 5.6 | 6.3 | 0.0 | 0.0 | 0.9 | 1.4 | |
| Inositol | 56.3 | 50.7 | 55.0 | 55.2 | 63.5 | 65.7 | |
| Maltose | 0.0 | 0.0 | 2.7 | 6.1 | 0.0 | 0.0 | |
| Trehalose | 0.0 | 0.0 | 0.0 | 0.0 | 14.0 | 13.5 | |
| Saccharide | LCM Control * | Mal20 * | Treh20 * | |||
|---|---|---|---|---|---|---|
| The Number of Molecules | ||||||
| In the Media (per 1 mL) | Introduced into the Native Semen (per 1 mL) | In the Media (per 1 mL) | Introduced into the Native Semen (per 1 mL) | In the Media (per 1 mL) | Introduced into the Native Semen (per 1 mL) | |
| Fructose | 0.265 × 1020 | 1.463 × 1014 | 0.216 × 1020 | 0.517 × 1014 | 0.216 × 1020 | 0.675 × 1014 |
| Maltose | − | − | 0.057 × 1020 | 0.233 × 1014 | − | − |
| Trehalose | − | − | − | − | 0.057 × 1020 | 0.875 × 1014 |
| Saccharide | The Number of Molecules in the Cytosol of the Spermatozoa after Equilibration, pcs | ||
|---|---|---|---|
| LCM Control * | Mal20 * | Treh20 * | |
| Fructose | 6.65 × 104 | 2.35 × 104 | 3.07 × 104 |
| Maltose | − | 1.06 × 104 | − |
| Trehalose | − | − | 3.98 × 104 |
| Medium Composition | Medium | ||
|---|---|---|---|
| LCM Control * | Treh20 * | Mal20 * | |
| Monosodium glutamate | 1.92 g (114 mM) | 1.92 g (114 mM) | 1.92 g (114 mM) |
| Fructose | 0.8 g (44 mM) | 0.64 g (36 mM) | 0.64 g (36 mM) |
| Potassium acetate | 0.5 g (51 mM) | 0.5 g (5 mM) | 0.5 g (5 mM) |
| Polyvinylpyrrolidone | 0.3 g (8.3 mM) | 0.3 g (8.3 mM) | 0.3 g (8.3 mM) |
| Protamine sulfate | 0.032 g (3.27 mM) | 0.032 g (3.27 mM) | 0.032 g (3.27 mM) |
| Trehalose | − | 0.326 g (9.5 mM) | − |
| Maltose | − | − | 0.326 g (9.5 mM) |
| Distilled water | 100 mL | ||
| Osmolarity | 339 mOsm | 344 mOsm | 334 mOsm |
| Day of Experiment | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | 8th | 9th | 10th | 11th | 12th | 13th | 14th | 15th | |
| Insemination | + | + | + | + | |||||||||||
| Collecting eggs for 1st incubation | + | + | + | + | + | + | + | ||||||||
| Collecting eggs for 2nd incubation | + | ||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanishevskaya, O.; Silyukova, Y.; Pleshanov, N.; Kurochkin, A. Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa. Molecules 2021, 26, 5920. https://doi.org/10.3390/molecules26195920
Stanishevskaya O, Silyukova Y, Pleshanov N, Kurochkin A. Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa. Molecules. 2021; 26(19):5920. https://doi.org/10.3390/molecules26195920
Chicago/Turabian StyleStanishevskaya, Olga, Yulia Silyukova, Nikolai Pleshanov, and Anton Kurochkin. 2021. "Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa" Molecules 26, no. 19: 5920. https://doi.org/10.3390/molecules26195920
APA StyleStanishevskaya, O., Silyukova, Y., Pleshanov, N., & Kurochkin, A. (2021). Role of Mono- and Disaccharide Combination in Cryoprotective Medium for Rooster Semen to Ensure Cryoresistance of Spermatozoa. Molecules, 26(19), 5920. https://doi.org/10.3390/molecules26195920






