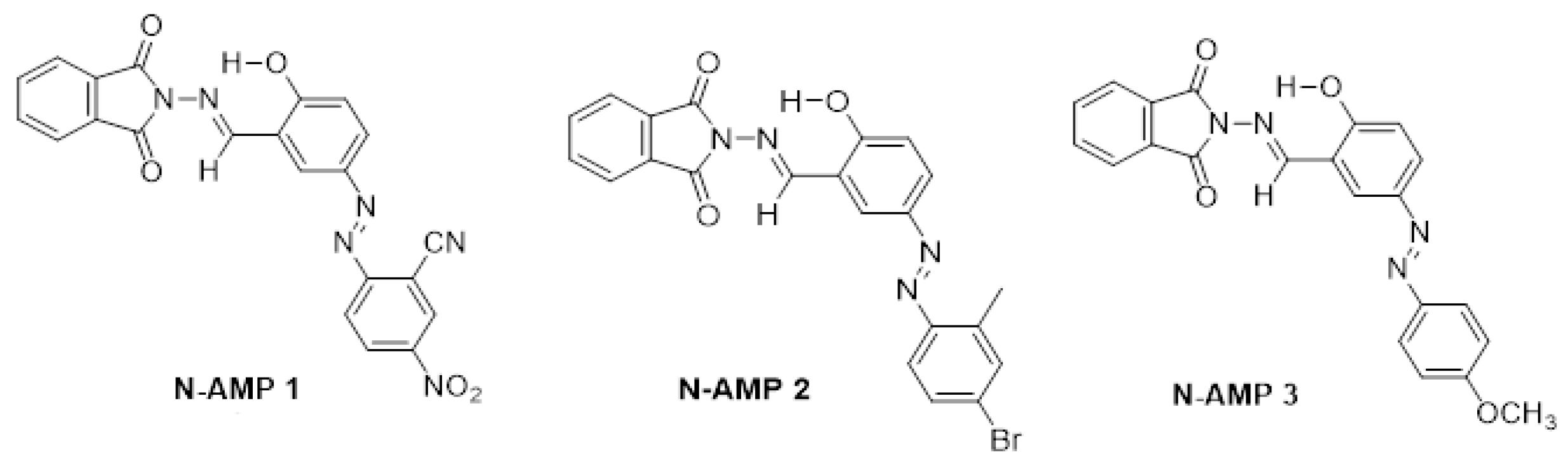
Complex Activity and Sensor Potential toward Metal Ions in Environmental Water Samples of N-Phthalimide Azo-Azomethine Dyes
Abstract
1. Introduction
2. Results and Discussion
2.1. Determination of Protonation Constants of Investigated Compounds
2.2. Testing for Sensors Activity in Acid-Base Titration
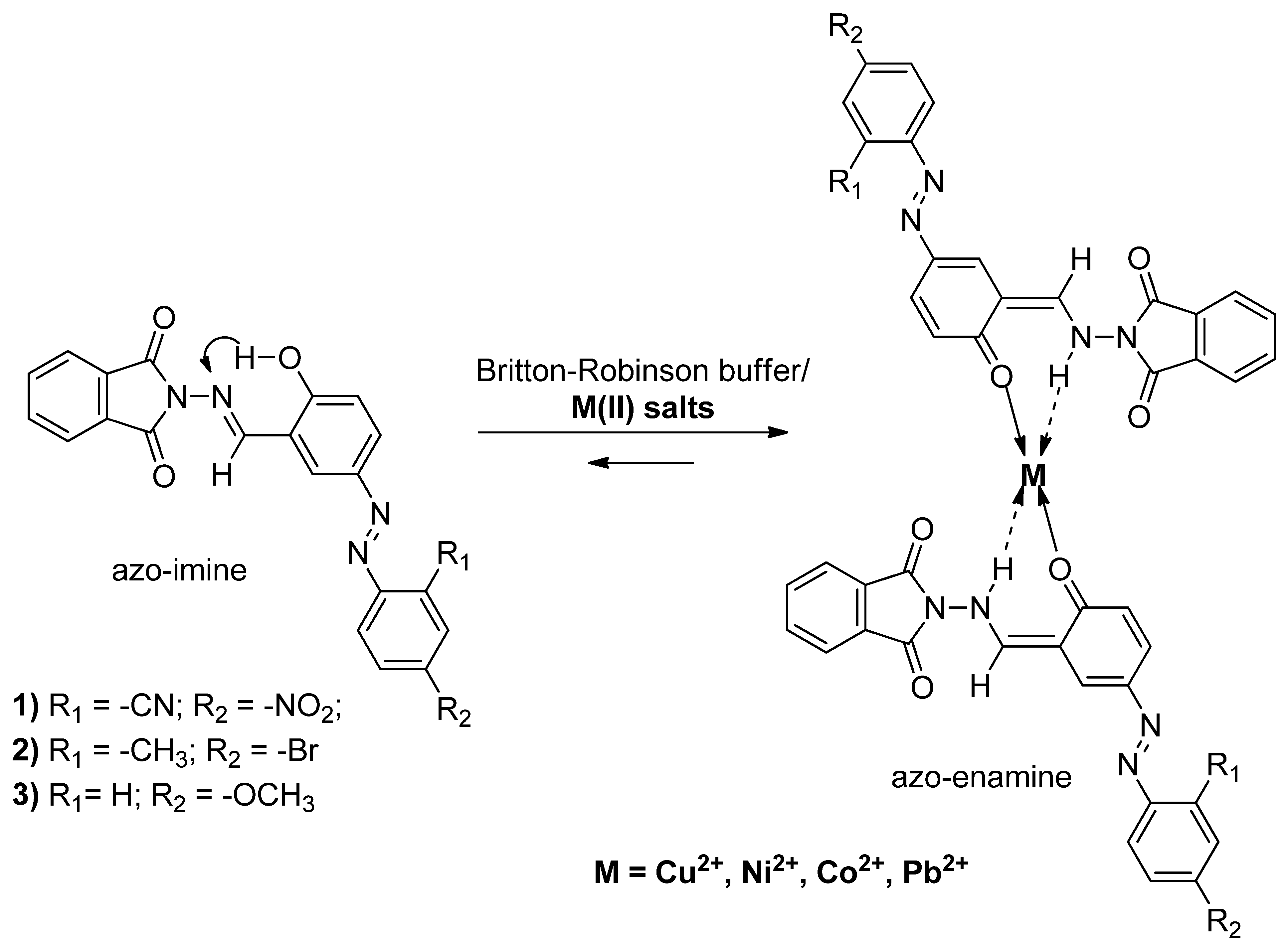
2.3. Study of Complex Formation process
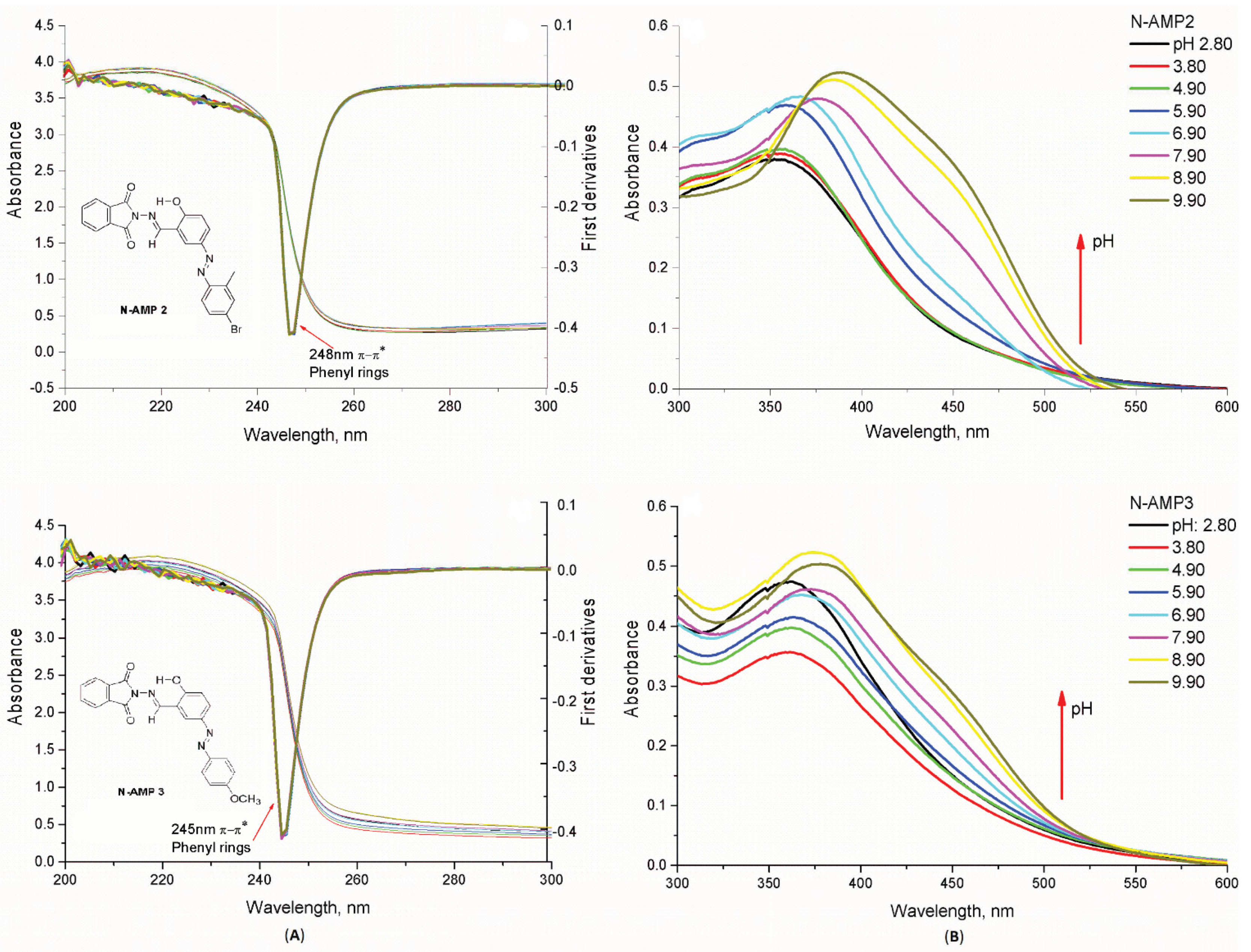
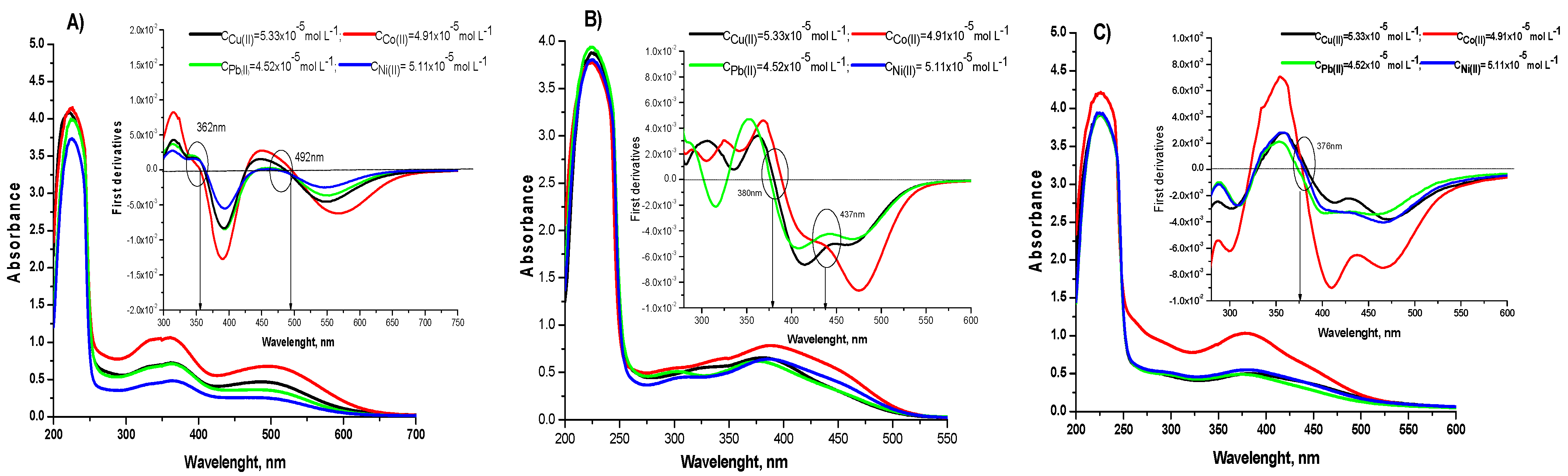
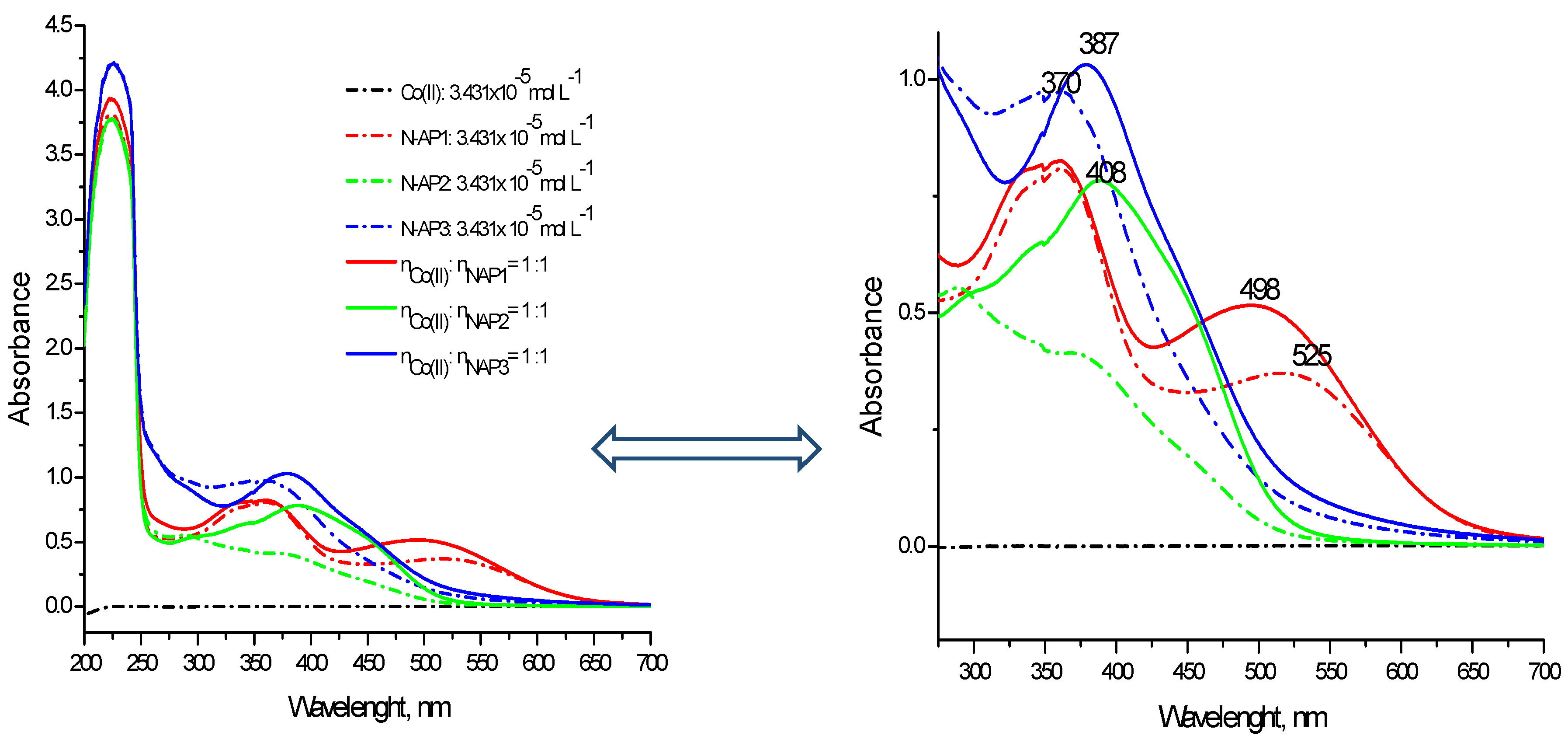
2.3.1. Spectral Characterization of the Complexes
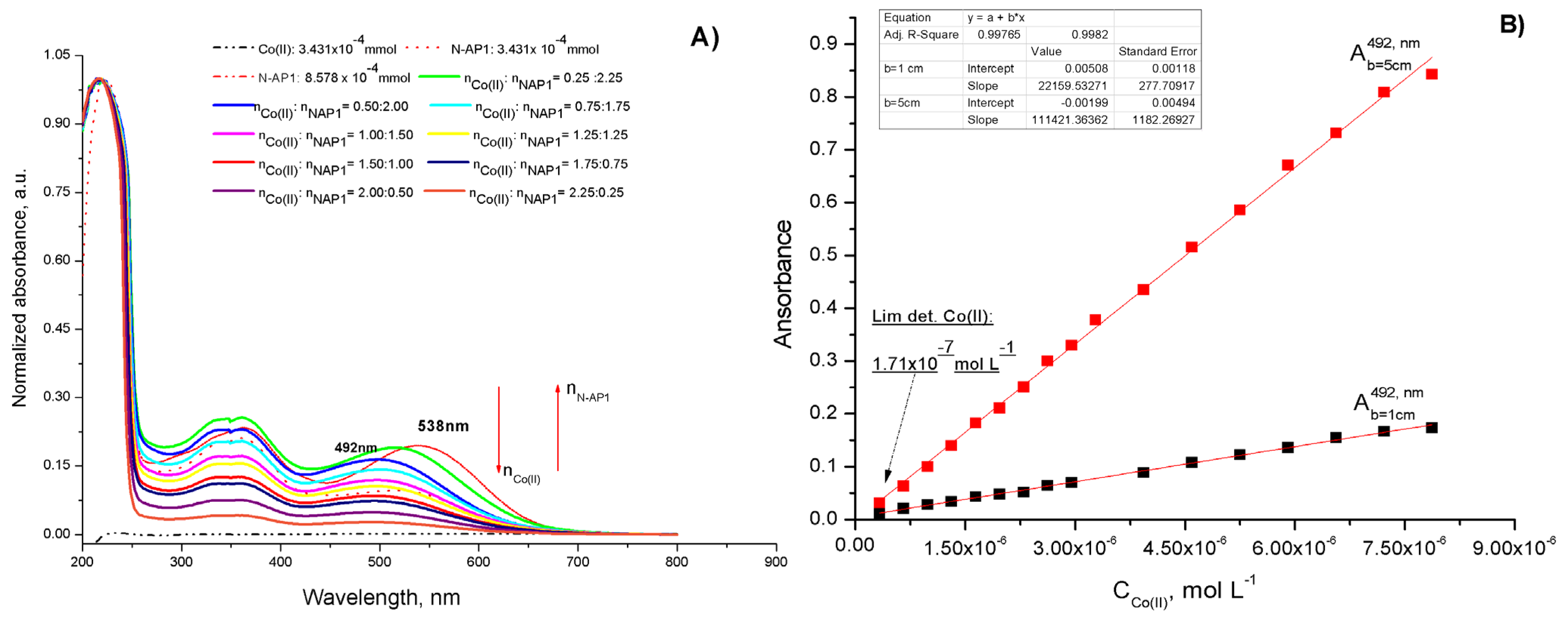
2.3.2. Determination of Complex Stoichiometry and Stability
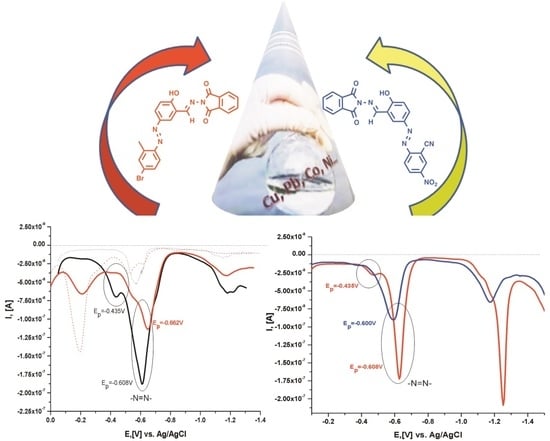
2.4. Electrochemical Characterization of the Compounds and Their Metal Complex Formation
2.5. Sensor Activity Test toward Determination of Cu(II) in Environmental Samples
2.6. Analytical Performances of the Method
2.7. Reagents and Solutions
2.8. Spectral Measurements
2.9. Electrochemistry
2.9.1. Instrumentation
2.9.2. Voltammetric Procedures
2.9.3. Potentiometric Measurements
2.10. Testing for Indicator Activity
2.11. Testing for Sensor Activity toward Copper Ions in Water Samples
2.12. Procedure for Metal Determination
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Grafe, A.; Haupt, K.; Mohr, G. Optical sensor materials for the detection of amines in organic solvents. Anal. Chim. Acta 2006, 565, 42. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, M.; Liu, Z.; Yu, M.; Li, F.; Yi, T.; Huang, C. Highly selective colorimetric sensor for cysteine and homocysteine based on azo derivatives. Tetrahedron Lett. 2006, 47, 7093. [Google Scholar] [CrossRef]
- Makedonski, P.; Brandes, M.; Grahn, W.; Kowalsky, W.; Wichern, E.; Wiese, S. Synthesis of new kinds of reactive azo dyes and their application for fibre-optical pH-measurements. Dyes Pigm. 2004, 6, 109. [Google Scholar] [CrossRef]
- Benkhaya, S.; El Harfi, A. Classifications, properties and applications of textile dyes: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef]
- McLaren, K.; Hilger, A. The Colour Science of Dyes and Pigments; Hilger Ltd.: Wien, Austria, 1983. [Google Scholar]
- Collier, S.W.; Storm, J.; Bronaugh, R.L. Reduction of azo dyes during in vitro percutaneous absorption. Toxicol. Appl. Pharmacol. 1993, 118, 73–79. [Google Scholar] [CrossRef]
- Benkhaya, S.; Cherkaoui, O.; Assouag, M.; Mrabet, S.; Rafik, M.; El Harfi, A. Synthesis of a New Asymmetric Composite Membrane with Bi-Component Collodion: Application in the Ultra filtration of Baths of Reagent Dyes of Fabric Rinsing/Padding. J. Mater. Environ. Sci. 2016, 7, 4556–4569. [Google Scholar]
- Al-Rubaie, L.; Mhessn, R.J. Synthesis and characterization of azo dye para red and new derivatives. J. Chem. 2012, 9, 465–470. [Google Scholar] [CrossRef]
- Georgiev, A.; Stoilova, A.; Dimov, D.; Yordanov, D.; Zhivkov, I.; Weiter, M. Synthesis and photochromic properties of some N-phthalimide azo-azomethine dyes. A DFT quantum mechanical calculation on imine-enamine tautomerism and trans-cis photoisomerization. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 210, 230–244. [Google Scholar] [CrossRef] [PubMed]
- Sahan, F.; Kose, M.; Hepokur, C.; Karakas, D.; Kurtoglu, M. New azo-azomethine-based transition metal complexes: Synthesis, spectroscopy, solid-state structure, density functional theory calculations and anticancer studies. Appl. Organomet. Chem. 2019, 33, e4954. [Google Scholar] [CrossRef]
- Chu, T.; Zhang, Y.; Liu, X.; Wang, Y.; Hu, S.; Wang, X. Synthesis and biodistribution of Tc-carbonyltechnetium-labeled fatty acids. Appl. Radiat. lsot. 2004, 60, 845–850. [Google Scholar] [CrossRef]
- Akram, D.; Elhaty, I.A.; AlNeyadi, S.S. Synthesis and Antibacterial Activity of Rhodanine-Based Azo Dyes and Their Use as Spectrophotometric Chemosensor for Fe3+ Ions. Chemosensors 2020, 8, 16. [Google Scholar] [CrossRef]
- Velcheva, I.; Nikolov, B. A study on the processes of distribution, accumulation and transfer of copper (Cu) in the organisms of fishes. Ecol. Balk. 2009, 1, 15–20, ISSN-1314-0213. [Google Scholar]
- Castro-Gonzales, M.I.; Mendez-Armenta, M. Heavy metals: Implications associated to fish consumption. Environ. Toxicol. Pharmacol. 2008, 26, 263–271. [Google Scholar] [CrossRef]
- Walker, C.H.; Hopkin, S.P.; Sibly, R.M.; Peakball, D.B. Principles of Ecotoxicology; CRC Press: Boca Raton, FL, USA, 2006; ISBN 0-8493-3635-X. [Google Scholar]
- American Public Health Association; American Water Works Association; Water Pollution Control Federation. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Ilieva, D.; Surleva, A.; Drochioiu, G.; Murariu, М.; Abdulah, M. Evaluation of ICP-OES method for heavy metal and metalloids determination in sterile dump material. Solid State Phenom. 2018, 273, 159. [Google Scholar] [CrossRef]
- Zevatskii, Y.; Samoilov, D.; Mchedlov-Petrosyan, N. Conthemporary methods for the experimental determination of dissociation constants of organic acids in solutions. Rus. J. General Chem. 2009, 79, 1859–1889. [Google Scholar] [CrossRef]
- Ebead, Y. Spectrophotometric investigations and computational calculations of prototropic tautomerism and acide-base properties of some new azo dyes. Dyes and Pigm. 2012, 92, 705–713. [Google Scholar] [CrossRef]
- Todorov, P.; Peneva, P.; Tchekalarova, J.; Georgieva, S. Potential anticonvulsant activity of novel VV-hemorphin-7 analogues containing unnatural amino acids: Synthesis and characterization. Amino Acids 2020, 52, 567–585. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, M.I.; Kalinkin, I.P. Practical Guide to Photometric Methods of Analysis, 5th ed.; Chemistry: Leningrad, Russia, 1986; pp. 219–221. [Google Scholar]
- El-Attar, M.; Ismailb, I.; Ghoneima, M. Synthesis, Electrochemical, Spectrophotometric and Potentiometric Studies of Two Azo-Compounds Derived from 4-Amino-2-Methylquinoline in Ethanolic-Aqueous Buffered Solutions. J. Braz. Chem. Soc. 2012, 23, 1523–1535. [Google Scholar] [CrossRef][Green Version]
- Snigur, D.; Chebotarev, A.; Bevziuk, K. Acid–base properties of azo dyes in solution studied using spectrophotometry and colorimetry. J. Appl. Spectrosc. 2018, 85, 21–26. [Google Scholar] [CrossRef]
- Stastná, M.; Trávnícek, M.; Slais, K. New azo dyes as colored isoelectric point markers for isoelectric focusing in acidic pH region. Electrophoresis 2005, 26, 53–59. [Google Scholar] [CrossRef]
- Mabrouk, E.; Felaly, R.; El-Mossalamy, E. Distinctive Routs:Electrochemical and Spectrophotometric Studies and Dissociation Constants Determination of Some Aminopyridine Azo-Dye Derivatives in Aqueous Media. Int. J. Electrochem. Sci. 2016, 11, 4892–4908. [Google Scholar] [CrossRef][Green Version]
- Mabrouk, E.; Omary, K.; Omary, A.; El-Mossalamy, E. Electrochemical and Spectrastudies of Some Sulfa Drug Azodyes and Their Metal Complexes in Aqueous Solution. J. Adv. Chem. 2017, 14, 6021–6032. [Google Scholar] [CrossRef]
- Ahmed, S.I.; Moustafa, M.M.; Aziz, M. Mono and binuclear Ag (I), Cu (II), Zn (II) and Hg (II) complexes of a new azo-azomethine as ligand: Synthesis, potentiometric, spectral and thermal studies. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1429–1436. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Aromatic Amines, Organic Dyes, and Related Exposures. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 99, 1–658, ISSN 1017-1606. [Google Scholar]
- Dincalp, H.; Toker, F.; Durucasu, I.; Avcıbas, N.; Icli, S. New thiophene-based azo ligands containing azo methine group in the main chain for the determination of copper(II) ions. Dyes Pigm. 2007, 75, 11–24. [Google Scholar] [CrossRef]
- Lee, H.; Song, X.; Park, H.; Baik, M.-H.; Lee, D. Torsionally Responsive C3-Symmetric Azo Dyes: Azo-Hydrazone Tautomerism, Conformational Switching, and Application for Chemical Sensing. J. Am. Chem. Soc. 2010, 132, 12133–12144. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, H.; Darvishpour, M. New azo ligands containing azomethine groups in the pyridazine-based chain: Synthesis and characterization. Dyes Pigm. 2009, 81, 167–173. [Google Scholar] [CrossRef]
- Georgieva, S.; Todorov, P.; Peneva, P.; Varbanov, M.; Gartsiyanova, K. VV-hemorphin-5 analogue for trace copper determination in water samples. J. Iran. Chem. Soc. 2020, 17, 2885–2894. [Google Scholar] [CrossRef]
- Jagtap, V.S. Determination of Stability Constant of Some Coordination Compound By pH Metric Technique. Pramana Res. J. 2019, 9, 22–29. [Google Scholar]
- Biata, N.; Dimpe, K.; Ramontja, J.; Mketo, N.; Nomngongo, P. Determination of thallium in water samples using inductively. coupled plasma optical emission spectrometry (ICP-OES) after ultrasonic assisted-dispersive solid phase microextraction. Microchem. J. 2017, 137, 214–222. [Google Scholar] [CrossRef]
- Chen, H.; Cho, C.; Wan, F.; Wu, T. A colorimetric sensor for Fe2+ ion. Inorg. Chem. Commun. 2014, 41, 88–91. [Google Scholar] [CrossRef]
- El-Kady, A.A.; Abdel-Wahhab, M. Occurrence of trace metals in foodstuffs and their health impact. Trends Food Sci. Technol. 2018, 75, 36–45. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Natasha, B.I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef]
- Rull-Barrull, J.; d’Halluin, M.; le Grognec, E.; Felpin, F.X. Chemically-modified cellulose paper as smart sensor device for colorimetric and optical detection of hydrogen sulfate in water. Chem. Commun. 2016, 52, 2525–2528. [Google Scholar] [CrossRef]
- Fraga, S.M.B.; Goncalves, M.S.T.; Moura, J.C.V.P.; Rani, R. Chromophoric Azo Reagents for Amino Acid and Peptide Labelling. Eur. J. Org. Chem. 2004, 8, 1750. [Google Scholar] [CrossRef]
- Marchevsky, E.; Olsina, R.; Marone, C. 2-[2-(5-Chloropyridyl) azo]-5-dimethylaminoethanol as indicator for the complexometric determination of zinc. Talanta 1985, 32, 54–56. [Google Scholar] [CrossRef]
- Nejati, K.; Rezvani, Z.; Massoumi, B. Syntheses and investigation of thermal properties of copper complexes with azo-containing Schiff-base dyes. Dyes Pigm. 2007, 75, 653. [Google Scholar] [CrossRef]
- Peker, E.; Serin, S. Synthesis and Characterization of Some Cobalt(II), Copper(II), and Nickel(II) Complexes with New Schiff Bases from the Reaction of p-Aminoazobenzene with Salicylaldehyde. Synth. React. Inorg. Metal-Org. Chem. 2004, 34, 859–872. [Google Scholar] [CrossRef]
- Ruyffelaere, F.; Nardello, V.; Schmidt, R.; Audry, J. Photosensitizing properties and reactivity of aryl azo naphtol dyes towards singlet oxygen. J. Photochem. Photobiol. A Chem. 2006, 183, 98. [Google Scholar] [CrossRef]
- El-Mekawi, D.; Abdel-Mottaleb, S.M. The interaction and photostability of some xanthenes and selected azo sensitizing dyes with TiO2 nanoparticles. Int. J. Photoenergy 2005, 7, 95. [Google Scholar] [CrossRef]
- Khosravi, A.; Moradian, S.; Gharanjig, K.; Taromi, F. Investigation of synthesis and dyeing properties of some azonaphthalimide disperse dyestuffs for the dyeing of polyester fibres. Iran. Polym. J. 2005, 14, 667–679, ISSN: 1026-1265. [Google Scholar]
- Torfimov, A.; Schmidt, E.; Mikhaleva, A.; Vasiltsov, A.; Zaitsev, A.; Smolyanina, N.; Senotrusova, E.; Afonin, A.; Ushakov, I.; Petrushenko, K.; et al. 2-Arylazo-1-vinylpyrroles: A Novel Promising Family of Reactive Dyes. Eur. J. Org. Chem. 2006, 17, 4021. [Google Scholar] [CrossRef]
- Patel, K.; Patel, M.; Patel, R. Synthesis and studies of coloured polyesters derived from bis-azo diols. Indian J. Chem. Technol. 2000, 7, 307–311, ISSN: 0975-0991. [Google Scholar]
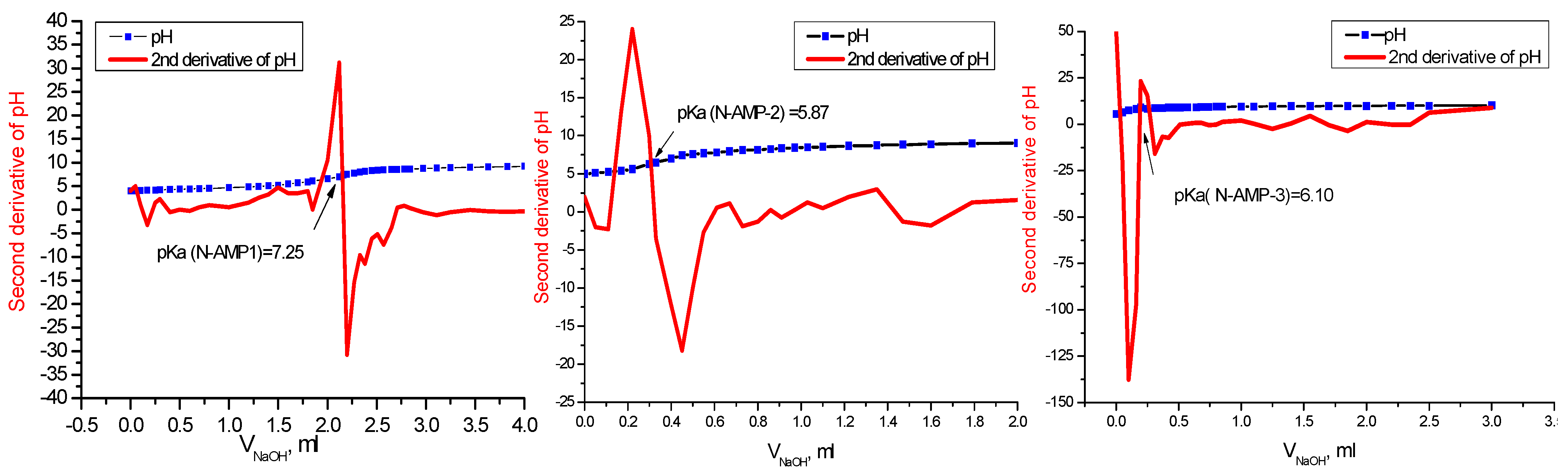


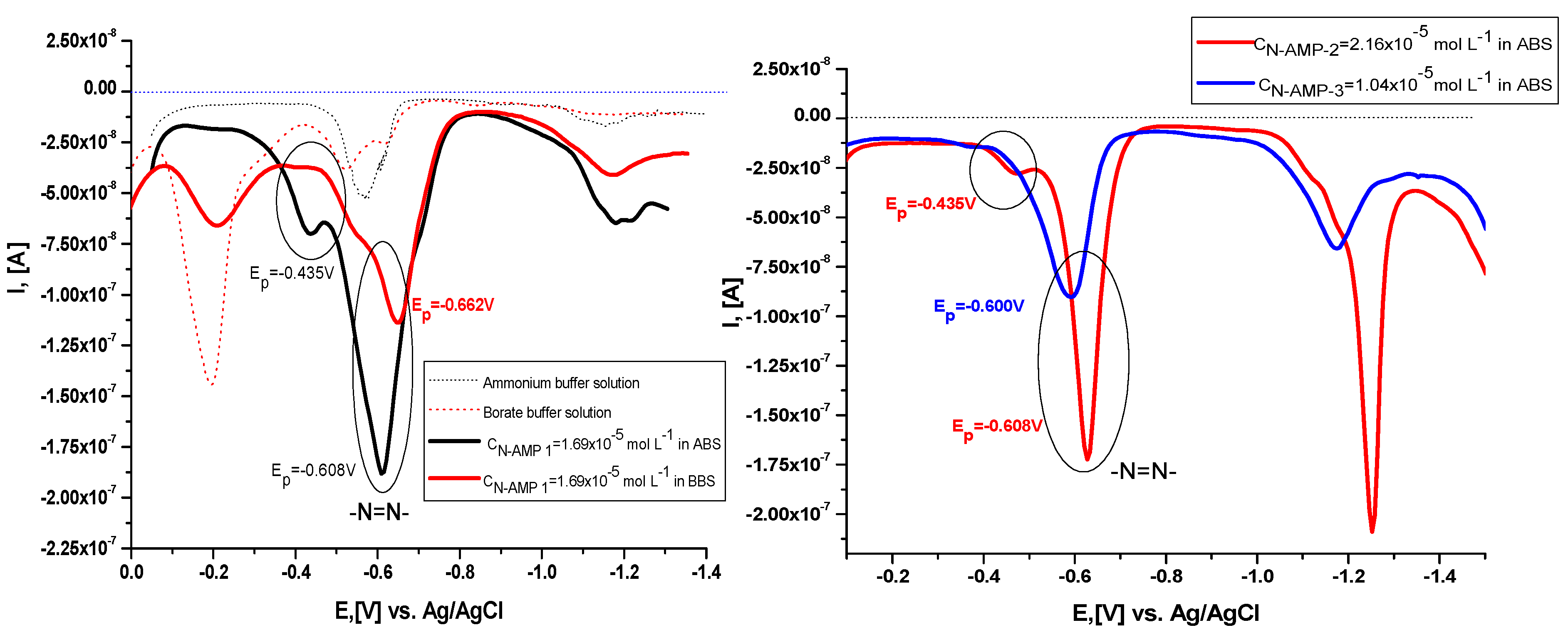
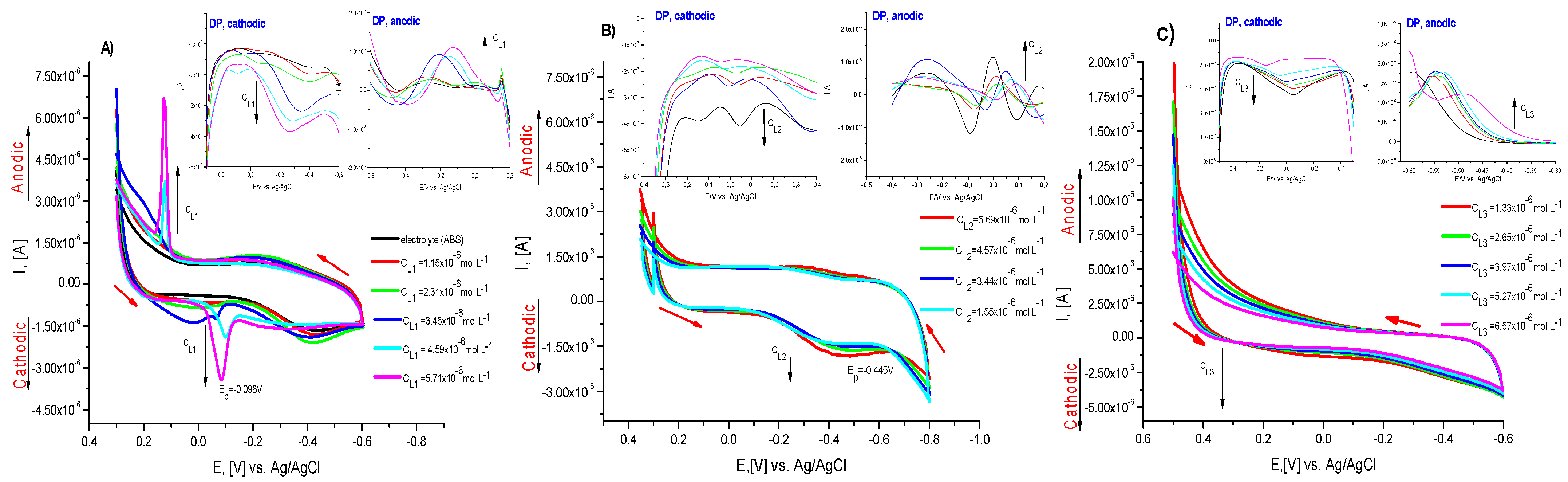

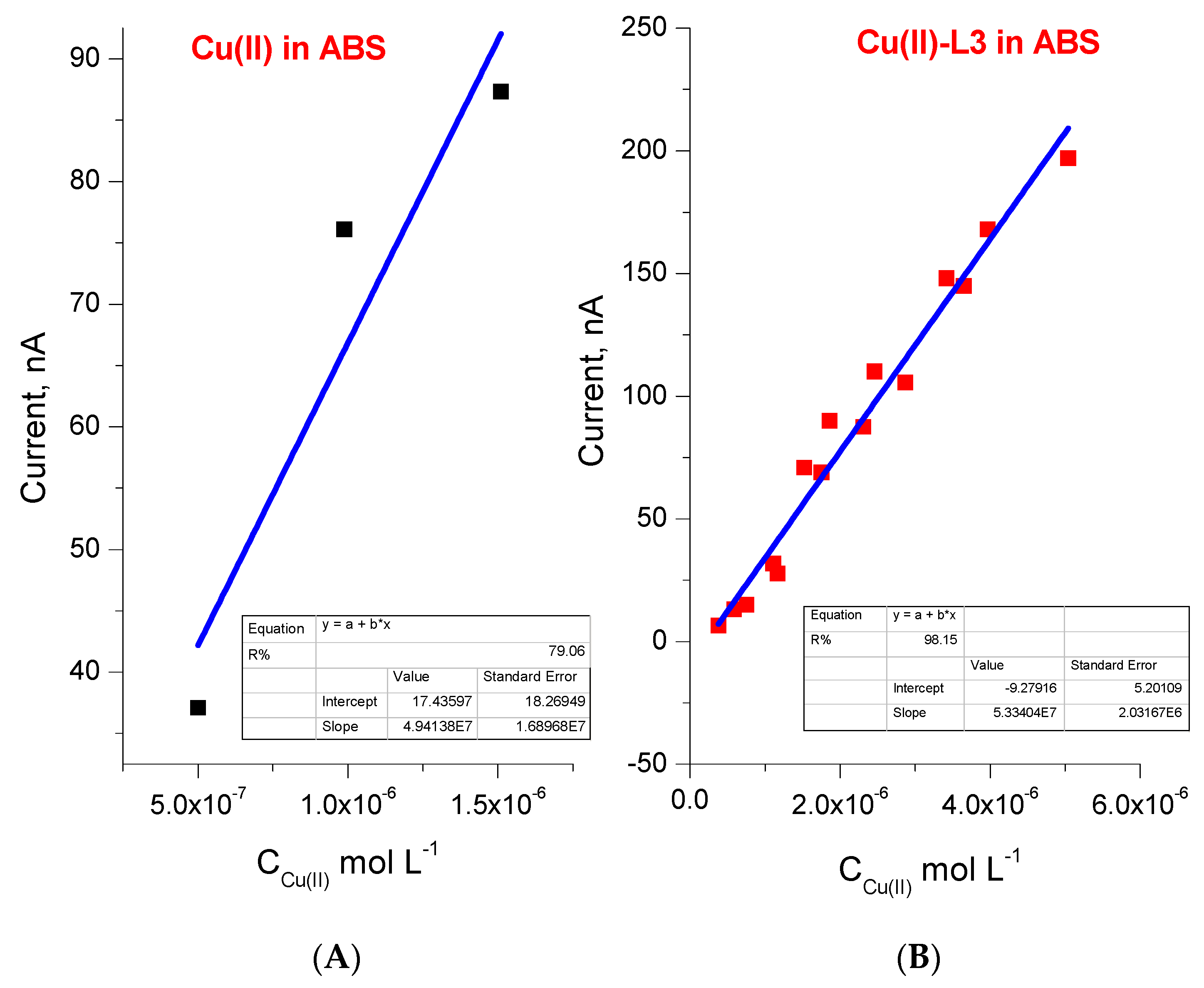
| Compound | pK Determination by Potentiometry; pH = f(VNaOH) | pK Determination by Potentiometry: | pK Determine by UV–Vis [35] | Mean pKa Value |
|---|---|---|---|---|
| NAP-1 | 7.25 ± 0.15 * | 6.89 | 7.09 ± 0.08 | 7.08 |
| NAP-2 | 5.87 ± 0.08 | 5.57 | 5.75 ± 0.04 | 5.73 |
| NAP-3 | 6.10 ± 0.08 | 6.32 | 6.21 ± 0.06 | 6.21 |
| Compounds | λabs (nm) | λem (nm) | Stokes Shift (cm−1) |
|---|---|---|---|
| N-AMP1 | 362 | 428 | 4260 |
| N-AMP1 + Cu(II) | 363 | 431 | 4340 |
| N-AMP1 + Ni(II) | 364 | 412 | 3200 |
| N-AMP1 + Co(II) | 362 | 410 | 3230 |
| N-AMP1 + Pb(II) | 363 | 411 | 3217 |
| N-AMP2 | 398 | 397 | 3620 |
| N-AMP2 + Cu(II) | 383 | 397 | 920 |
| N-AMP2 + Ni(II) | 389 | 436 | 2770 |
| N-AMP2 + Co(II) | 387 | 436 | 2900 |
| N-AMP2 + Pb(II) | 377 | 423 | 2884 |
| N-AMP3 | 385 | 496 | 5812 |
| N-AMP3 + Cu(II) | 383 | 496 | 5950 |
| N-AMP3 + Ni(II) | 381 | 492 | 5920 |
| N-AMP3 + Co(II) | 378 | 493 | 6180 |
| N-AMP3 + Pb(II) | 377 | 490 | 6111 |
| Sample/Volume for Analysis, mL | Concentration of Cu(II) (Real Sample + Amound Added), mol L−1 | Concentration Obtained, mol L−1 | Concentration Recovered, mol L−1 | Recovery (R, %) *, % | Relative Error, % |
|---|---|---|---|---|---|
| S1/0.050 mL | 1.299 × 10−5 | 1.312 × 10−5 | 1.034 × 10−5 | 101.3 | 1.3 |
| S1/0.100 mL | 1.854 × 10−5 | 1.834 × 10−5 | 1.001 × 10−5 | 98.05 | −1.9 |
| S2/0.050 mL | 1.198 × 10−5 | 1.208 × 10−5 | 1.031 × 10−5 | 100.9 | 0.4 |
| S2/0.100 mL | 1.376 × 10−5 | 1.388 × 10−5 | 1.033 × 10−5 | 101.2 | 1.2 |
| Sampling Stations | Location |
|---|---|
| S1: Topolnitsa River, before Pirdopska River | GPS: N42 39 27 E24 08 15 |
| S2: Medets’ka River at HPP: (hydroelectric power station) | GPS: N42 39 05 E24 09 |
| Metal Component | Al | Ba | Be | Bi | Cd | Ca | Cr | Co | Cu | |
| Concentration, mg L−1 | S1 | 35.69(0.04) * | <0.010 | <0.005 | <0.010 | <0.002 | 122.8(0.7) | 0.012(0.000) | 0.423(0.002) | 24.70(0.03) |
| S2 | 20.86(0.06) * | <0.010 | <0.005 | <0.010 | <0.002 | 118.9(0.8) | 0.015(0.000) | 0.261(0.002) | 15.79(0.04) | |
| Metal Component | Fe | Pb | Mg | Mn | Ni | K | Se | Na | Sr | |
| Concentration, mg L−1 | S1 | 0.583(0.003) | <0.010 | 111.7(0.3) | 5.678(0.004) | 0.126(0.002) | 4.839(0.007) | <0.010 | 20.60 (0.06) | 0.787(0.001) |
| S2 | 0.512(0.003) | <0.010 | 80.21(0.04) | 3.674(0.004) | 0.088(0.002) | 7.559(0.011) | <0.010 | 27.60 (0.06) | 0.637(0.001) | |
| Metal Component | Sb | As | Mo | P | Si | Tl | S | Sn | Zn | |
| Concentration, mg L−1 | S1 | <0.010 | <0.010 | <0.010 | <0.010 | 7.898(0.005) | <0.010 | 324.1(1.4) | 0.075(0.003) | 1.445(0.004) |
| S2 | <0.010 | <0.010 | <0.010 | <0.010 | 5.866(0.005) | <0.010 | 232.5(1.5) | 0.114(0.004) | 0.934(0.003) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Georgieva, S.; Bezfamilnyi, A.; Georgiev, A.; Varbanov, M. Complex Activity and Sensor Potential toward Metal Ions in Environmental Water Samples of N-Phthalimide Azo-Azomethine Dyes. Molecules 2021, 26, 5885. https://doi.org/10.3390/molecules26195885
Georgieva S, Bezfamilnyi A, Georgiev A, Varbanov M. Complex Activity and Sensor Potential toward Metal Ions in Environmental Water Samples of N-Phthalimide Azo-Azomethine Dyes. Molecules. 2021; 26(19):5885. https://doi.org/10.3390/molecules26195885
Chicago/Turabian StyleGeorgieva, Stela, Artem Bezfamilnyi, Anton Georgiev, and Marian Varbanov. 2021. "Complex Activity and Sensor Potential toward Metal Ions in Environmental Water Samples of N-Phthalimide Azo-Azomethine Dyes" Molecules 26, no. 19: 5885. https://doi.org/10.3390/molecules26195885
APA StyleGeorgieva, S., Bezfamilnyi, A., Georgiev, A., & Varbanov, M. (2021). Complex Activity and Sensor Potential toward Metal Ions in Environmental Water Samples of N-Phthalimide Azo-Azomethine Dyes. Molecules, 26(19), 5885. https://doi.org/10.3390/molecules26195885