Exploring the Dual Characteristics of CH3OH Adsorption to Metal Atomic Structures on Si (111)-7 × 7 Surface
Abstract
:1. Introduction
2. Materials and Methods
- (1)
- Three Si (111) substrates were degassed in the preparation chamber at about 450 °C. Then, the substrate was repeatedly flashed at 1250 °C until a clean well-ordered 7 × 7 reconstructed surface was obtained. Then, the Si (111)-7 × 7 sample was cooled down to room temperature in the preparation chamber.
- (2)
- Three Si (111)-7 × 7 samples were successively sent into the observation chamber, which was filled with CH3OH, C2H5OH and C3H7OH gas, respectively. During the adsorption time, the mass spectrometer was operated to monitor the gaseous ion composition in the observation chamber. When the gas was converted into ions, the peak intensity of each ion spectrum could be measured and analyzed online.
- (3)
- STM is used to scan the Si atoms on the top layer of the substrate surface before and after the alcohol gases’ introduction. Since each Si atom corresponds to a gas adsorption site, the number of Si atoms adsorbed by alcohol gas can be observed, that is, the coverage rate of Si adatoms. Besides, by analyzing the mass spectrometer data, an atomic model for the CH3OH adsorption process should be established and discussed.
- (4)
- After exploring and summarizing the alcohol adsorption law, Si (111)-7 × 7-CH3OH samples were moved back to the preparation chamber. In some experiments, Si(111)-7 × 7-CH3OH samples were heated in a 0.2 A current with an external power source. Additionally, through controlling the steaming current and time, metal atoms can be evaporated by heating a W filament with Au, Fe and Sn wire (purity > 99.995%). In the preparation chamber, different metal atoms were steamed on the surface of the sample with or without preheating.
- (5)
- Based on the Si (111)-7 × 7 model, we established a CH3OH adsorption model. By scanning different Si (111)-7 × 7-CH3OH-metal surfaces by STM, the influence of CH3OH on the deposition process was analyzed, including the change of deposition sites and the atomic structure of metal clusters. Then, a theoretical model was established to adjust and optimize the growth process of metal clusters.
- (6)
- In the observation chamber, XPS equipment was used to detect chemical bonds among metal and Si atoms. Since the sample was not taken out of the vacuum chamber, external interference, such as an oxidation reaction, could be avoided. With the aim of improving the signal-to-noise ratio of the data, the area of the XPS measurement was kept to 10 × 10 µm2 for all tests.
3. Results
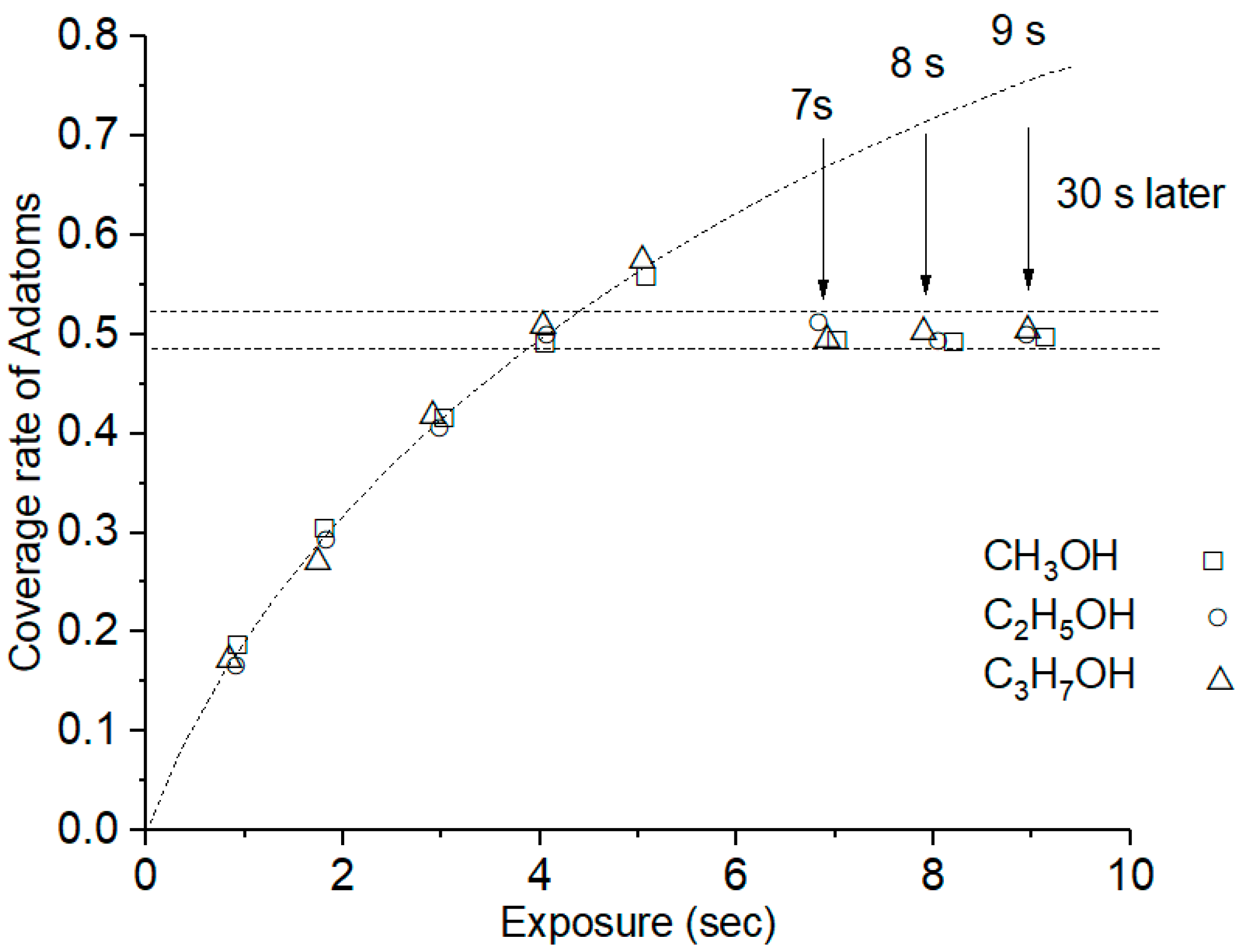
3.1. Adsorption and Dissociation Process of the Alcohol Gases
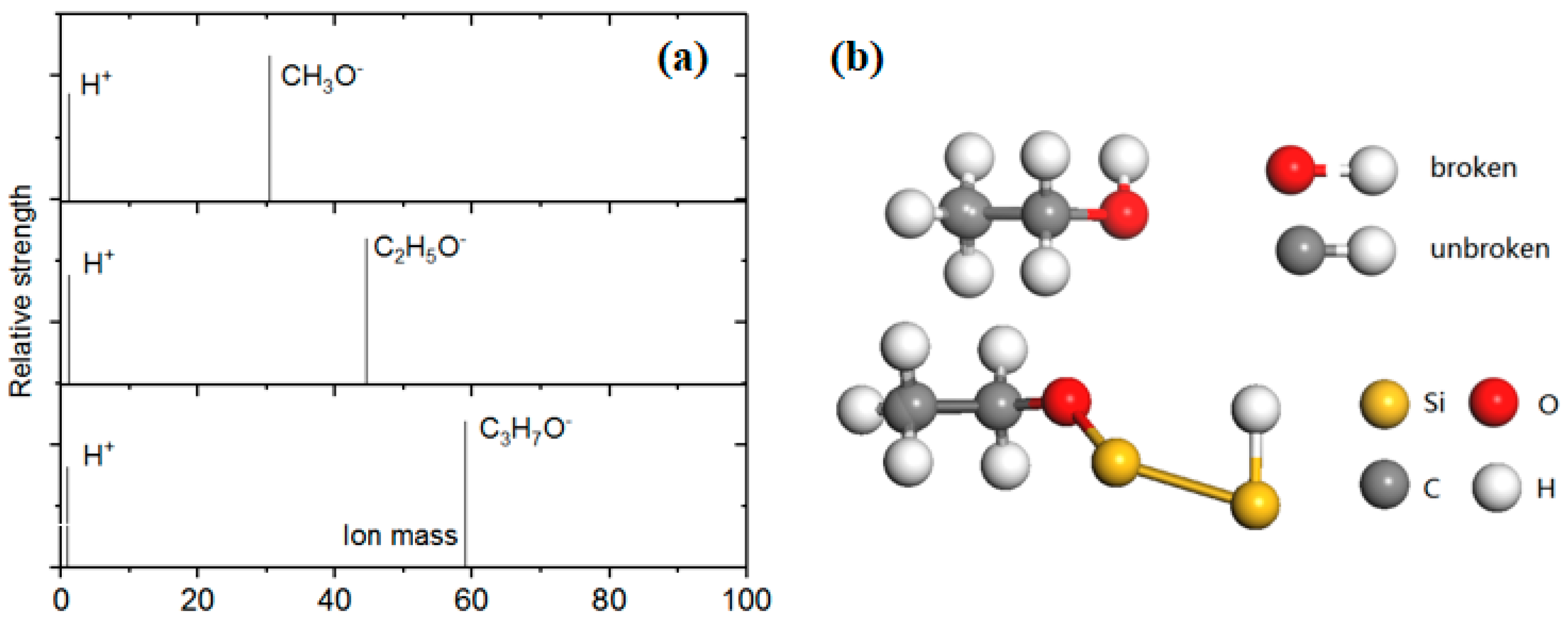
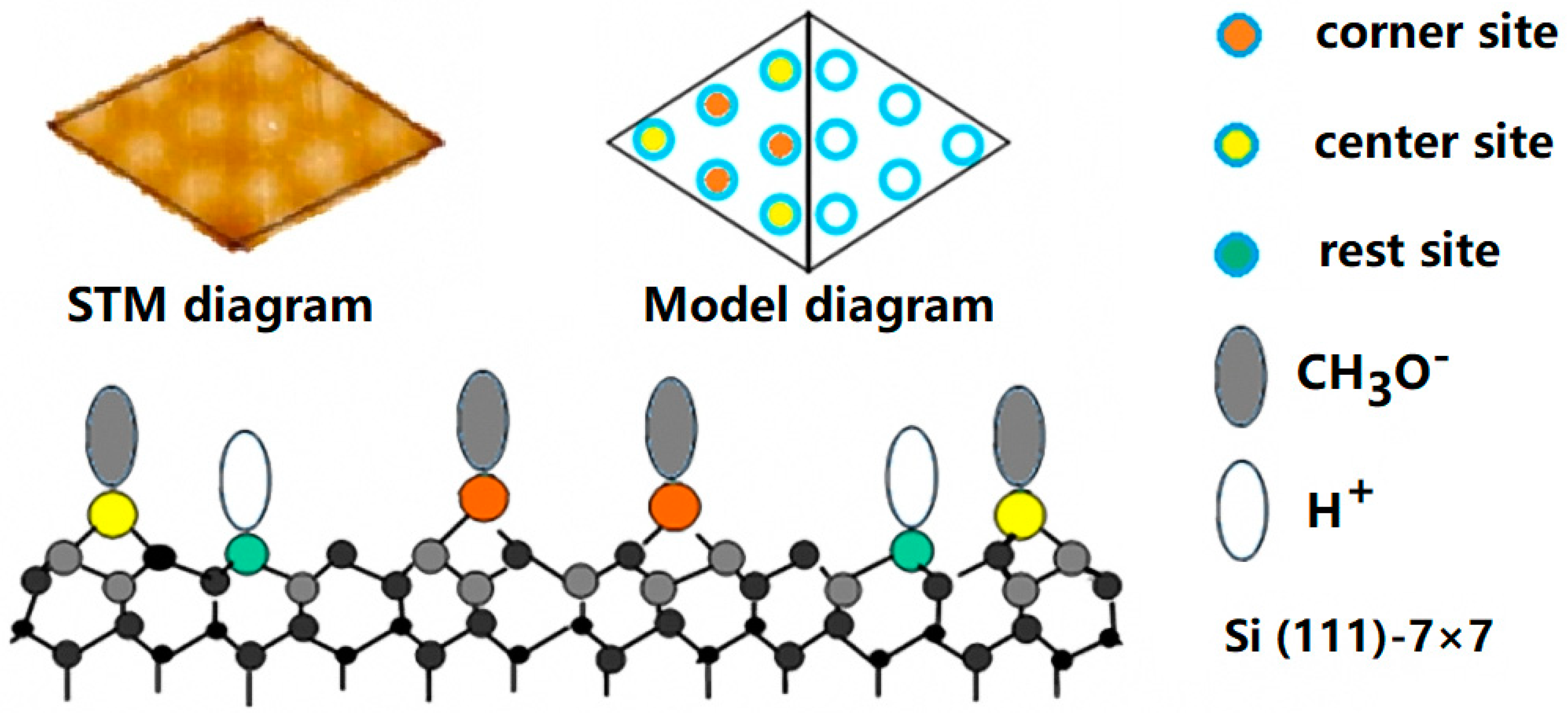
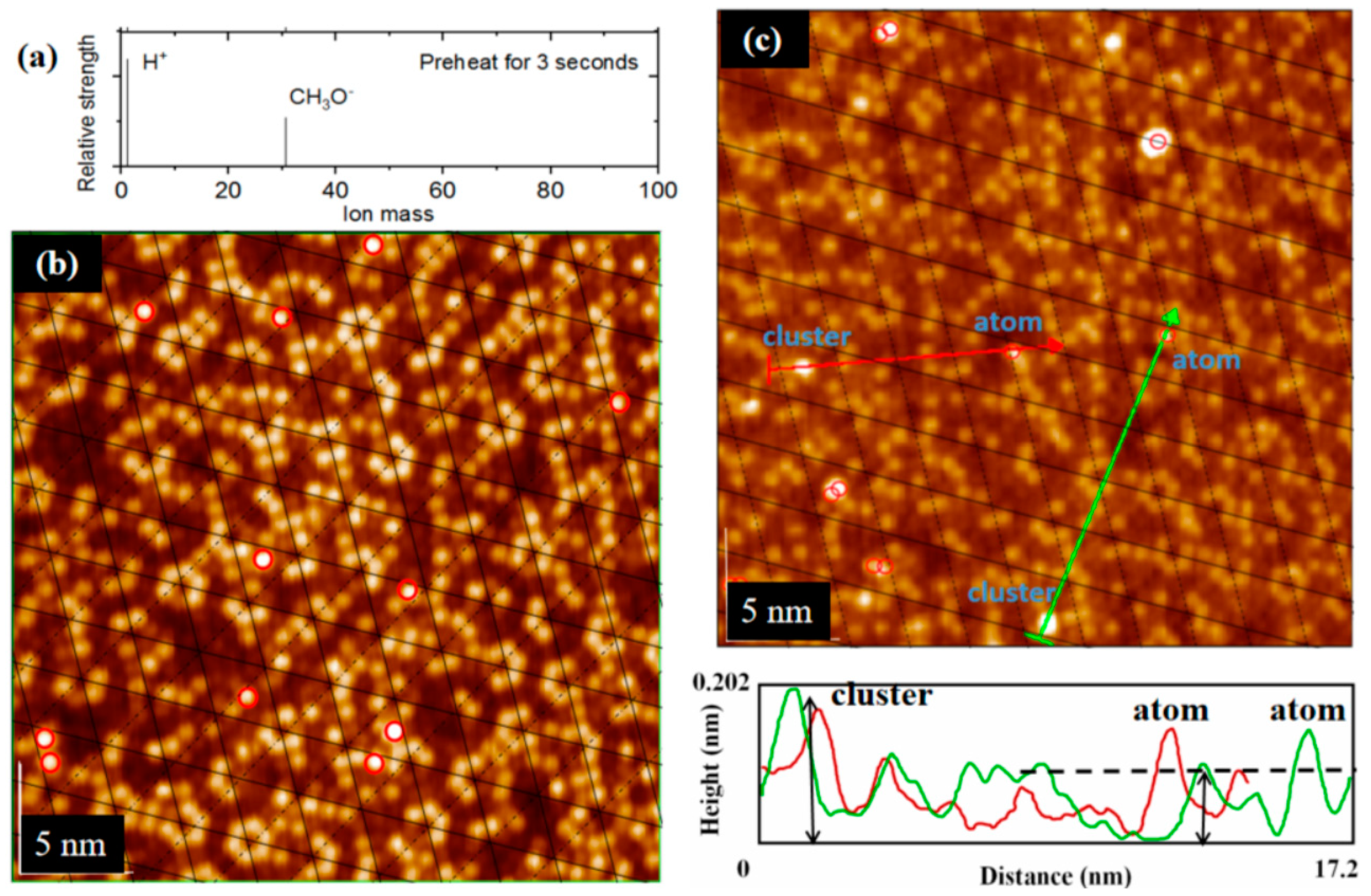
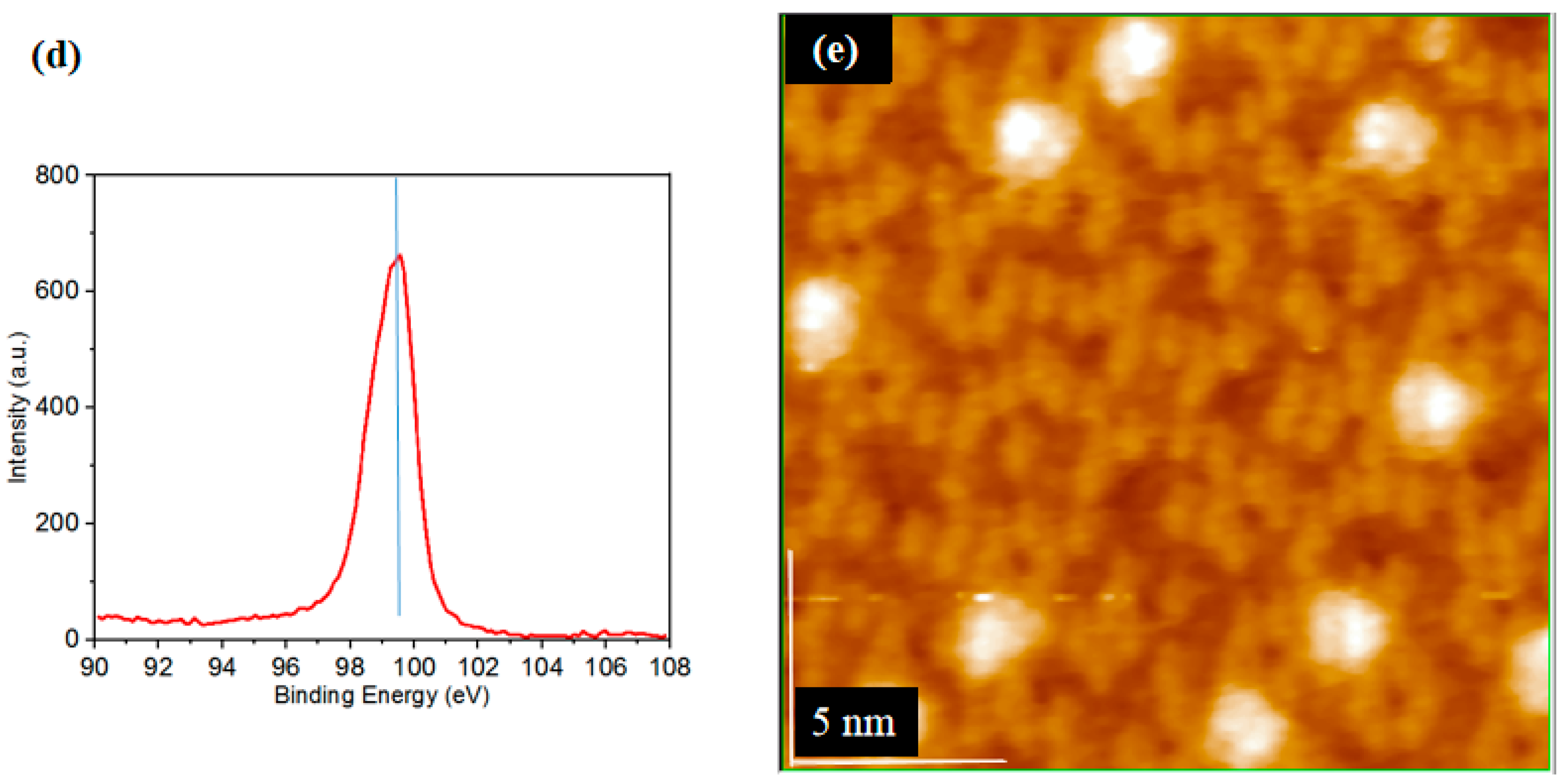
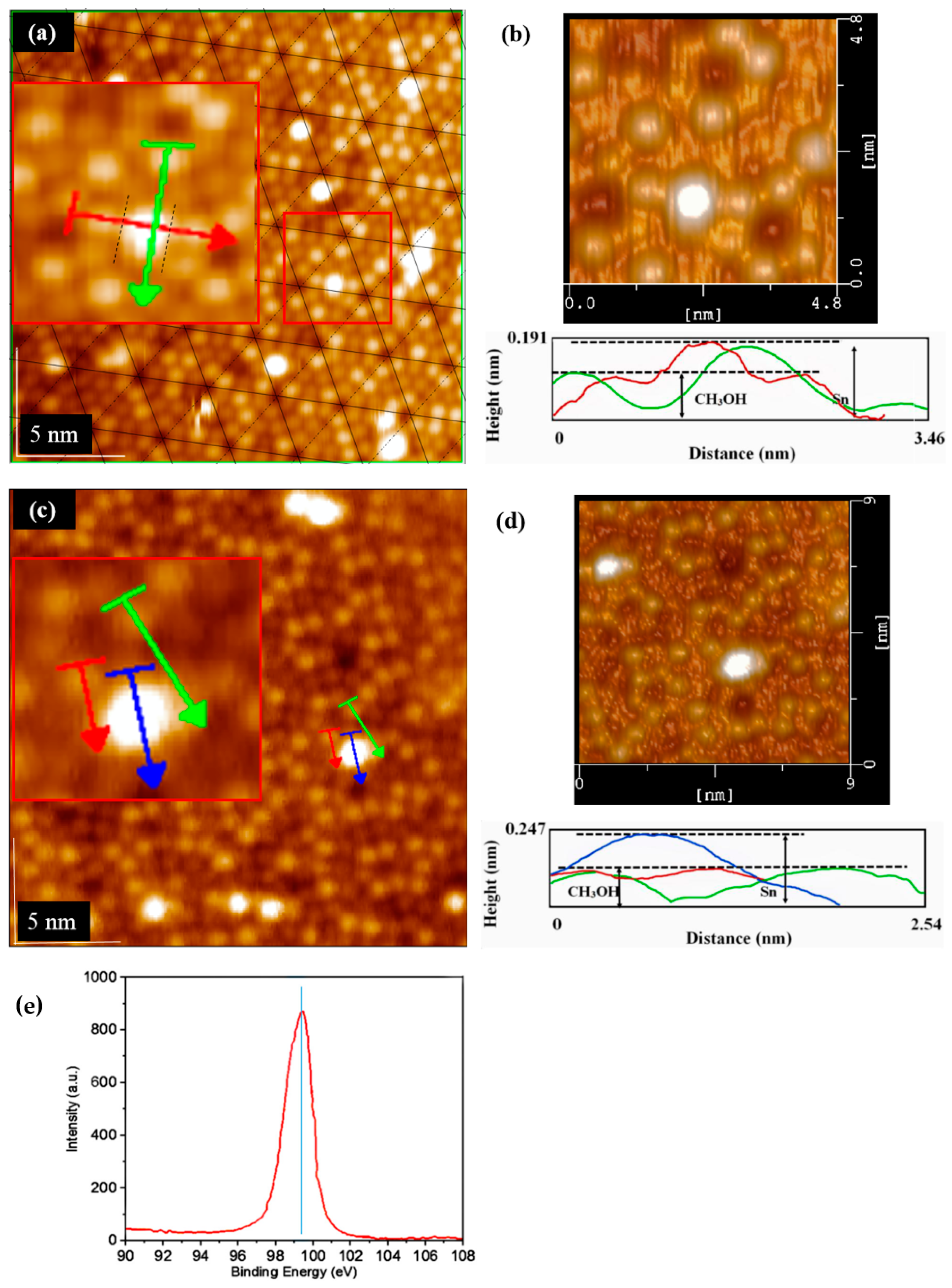
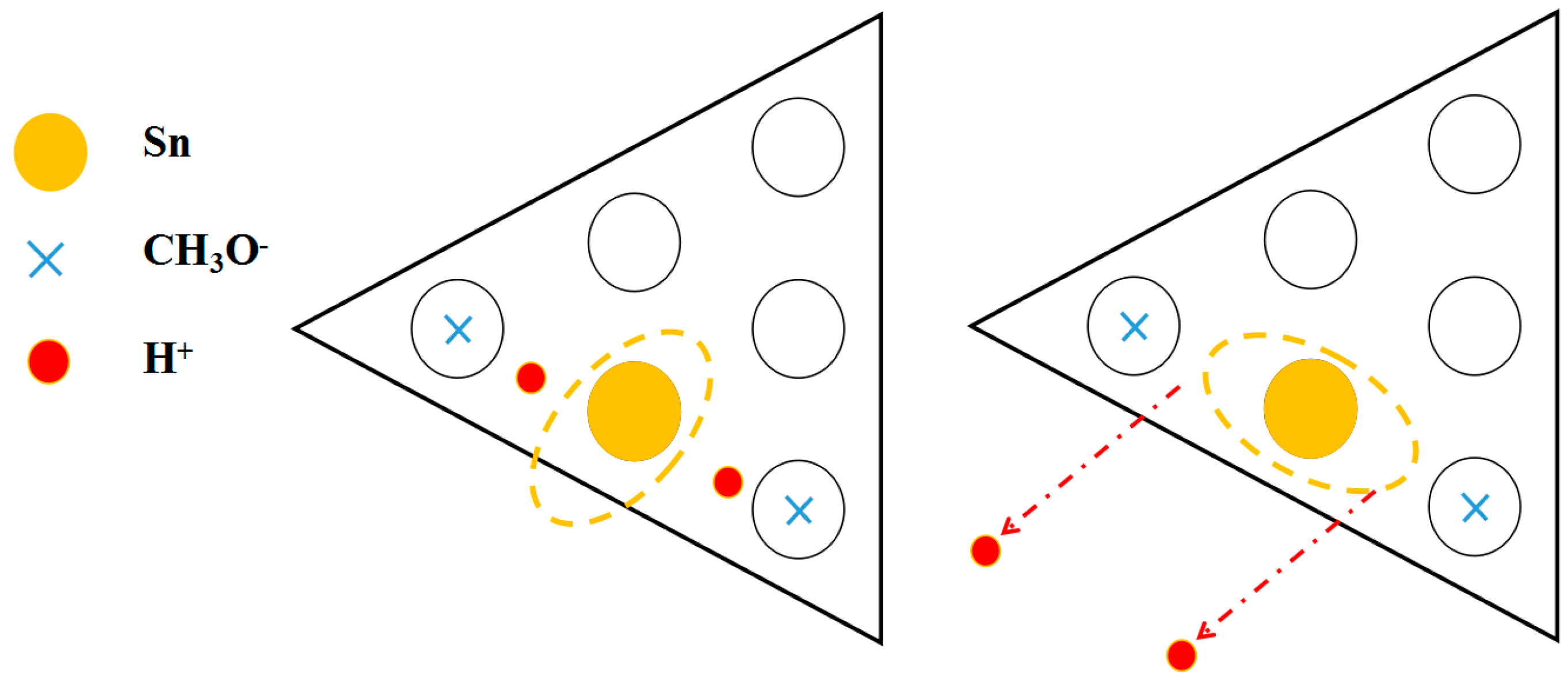
3.2. The Separation of CH3O– and H+ Adsorption
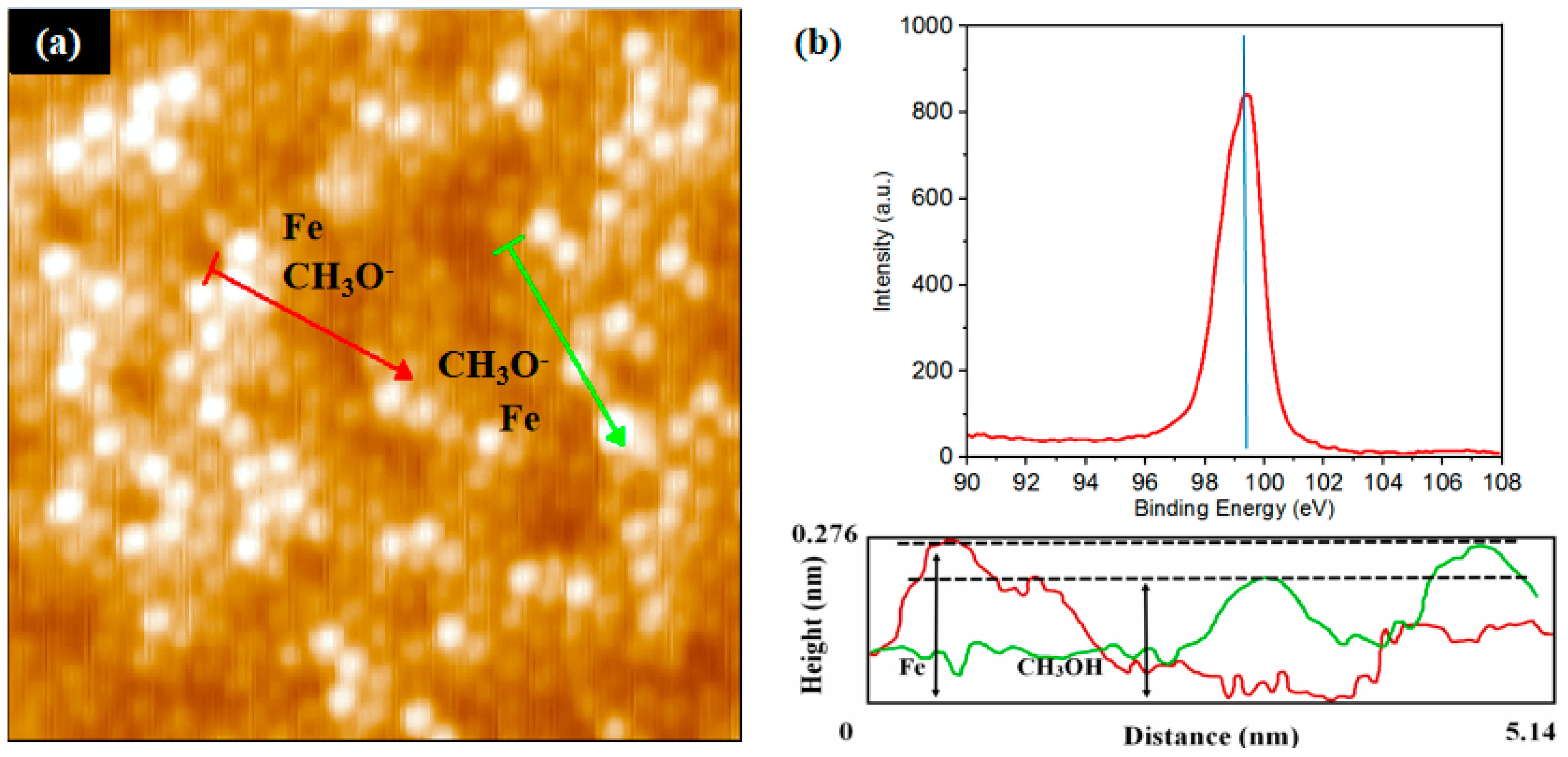
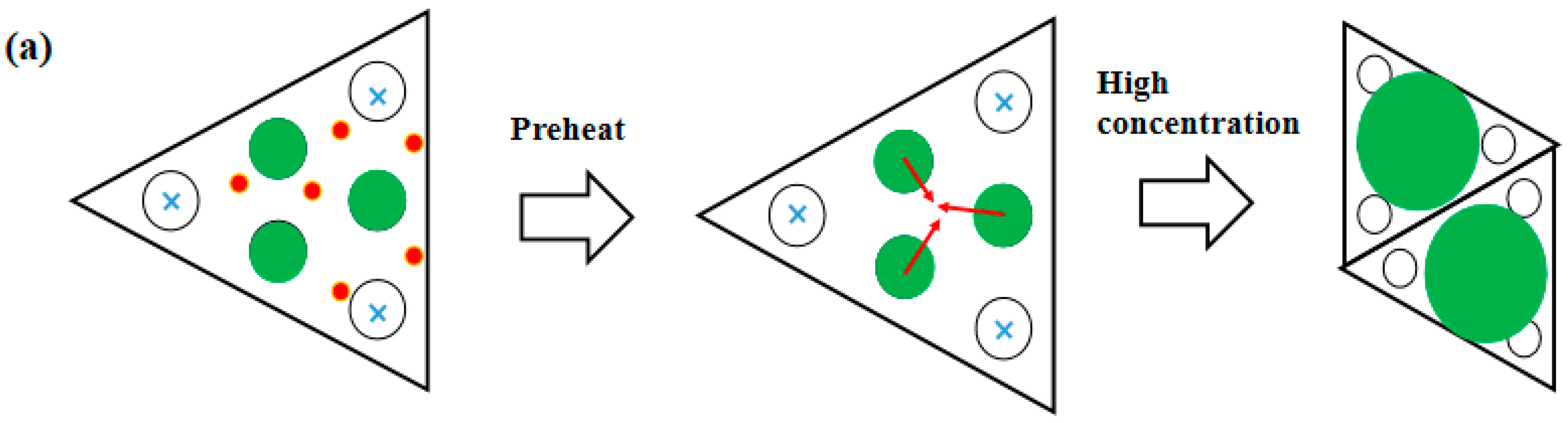
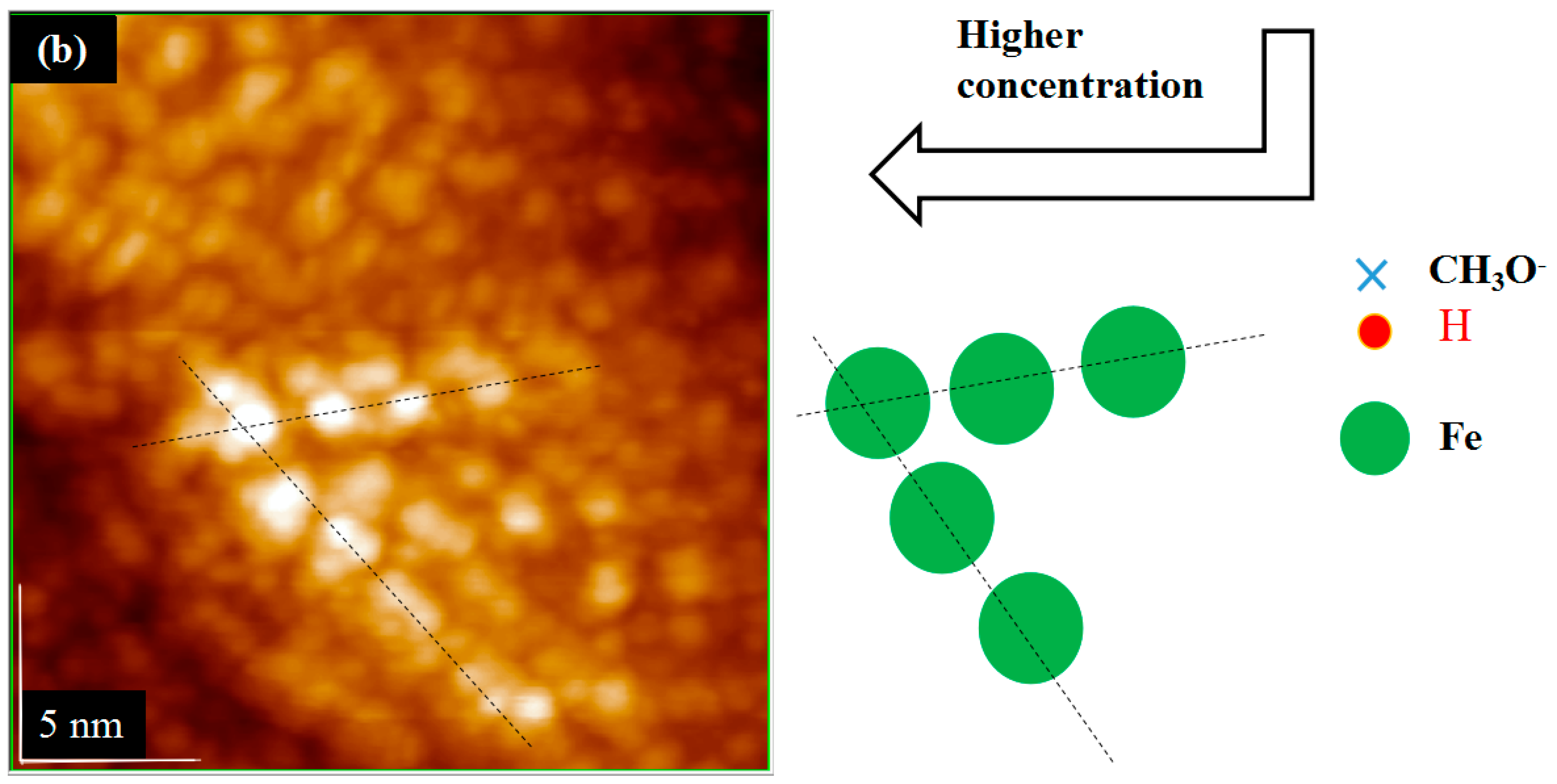
3.3. Derivation and Application of Dual Adsorption Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utecht, M.; Gaebel, T.; Klamroth, T. Desorption induced by low energy charge carriers on Si(111)-7 × 7: First principles molecular dynamics for benzene derivates. J. Comput. Chem. 2018, 39, 2517–2525. [Google Scholar] [CrossRef]
- Karbivska, L.; Karbivskii, V.L.; Romansky, A.A.; Kuznetsova, O.Y.; Teselko, P.; Artemyuk, V.A. Nanostructures of Cu, Au and In on the Silicon Single Crystal Surfaces at their Thermal Deposition. In Proceedings of the 2019 IEEE 39th International Conference on Electronics and Nanotechnology (ELNANO), Kyiv, Ukraine, 16–18 April 2019; pp. 214–219. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, K.; Yang, H.; Su, T.; Chan, C.T.; Loy, M.M.T.; Xiao, X. Experimental and Theoretical Investigation of Single Cu, Ag, and Au Atoms Adsorbed on Si (111)−(7 × 7). Phys. Rev. Lett. 2005, 94, 176104. [Google Scholar] [CrossRef] [Green Version]
- Xie, Z.X.; Tanaka, K.I. Tin atoms adsorbed on a Si(111)-(7 × 7) surface. Surf. Sci. 2001, 479, 26–32. [Google Scholar] [CrossRef]
- Tripathi, J.K.; Markovich, G.; Goldfarb, I. Self-ordered magnetic α-FeSi2 nano-stripes on Si(111). Appl. Phys. Lett. 2013, 102, 251604. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.-I.; Xie, Z.-X.; Egawa, T.; Aramata, M. Dynamics of Sn and Zn atoms on a Si(111)-7 × 7 surface. J. Mol. Catal. A Chem. 2003, 199, 19–26. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Jiang, X.; Shimojo, M. Growth of nano-crystalline metal dots on the Si(111)-7 × 7 surface saturated with C2H5OH. Surf. Sci. 2007, 601, 5093–5097. [Google Scholar] [CrossRef]
- Ding, W.Y.; Ju, D.; Tanaka, K.I.; Komori, F. Formation of linearly linked Fe clusters on Si(111)-7 × 7-C2H5OH surface. Nanoscale Res. Lett. 2014, 9, 377. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ding, W.; Ju, D.; Tanaka, K.-I.; Komori, F. Study on Formation Process and Models of Linear Fe Cluster Structure on a Si(111)-7 × 7-CH3OH Surface. Materials 2018, 11, 1593. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.-I.; Nomoto, Y.; Xie, Z.-X. Dissociation mechanism of 2-propanol on a Si(111)-(7 × 7) surface studied by scanning tunneling microscopy. J. Chem. Phys. 2004, 120. [Google Scholar] [CrossRef]
- Tanaka, K.-I.; Xie, Z.-X. Adsorption kinetics and patterning of a Si(111)-7 × 7 surface by dissociation of methanol. J. Chem. Phys. 2005, 122, 054706. [Google Scholar] [CrossRef]
- Tanaka, K.I. Dynamic Chemical Processes on Solid Surfaces–Chemical Reactions and Catalysis; Springer: Singapore, 2017. [Google Scholar]
- Razado, I.C.; Zhang, H.M.; Uhrberg, R.I.G.; Hansson, G.V. STM study of site selective hydrogen adsorption on Si(111) 7 × 7. Phys. Rev. B 2005, 71, 235411. [Google Scholar] [CrossRef] [Green Version]
- Che, H.; Che, G.; Zhou, P.; Liu, C.; Dong, H.; Li, C.; Song, N.; Li, C. Nitrogen doped carbon ribbons modified g-C3N4 for markedly enhanced photocatalytic H2-production in visible to near-infrared region. Chem. Eng. J. 2020, 382, 122870. [Google Scholar] [CrossRef]
- Lyu, J.; Kudiiarov, V.; Lider, A. An Overview of the Recent Progress in Modifications of Carbon Nanotubes for Hydrogen Adsorption. Nanomaterials 2020, 10, 255. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ding, W.; Ju, D.; Tanaka, K.-I.; Komori, F. Formation process and mechanism of iron-nitride compounds on Si(111)-7 × 7-CH3OH surface. Chem. Phys. Lett. 2018, 703, 17–22. [Google Scholar] [CrossRef]
- Yang, H.; Hattori, K. Initial Adsorption of Fe on an Ethanol-Saturated Si(111)7 × 7 Surface: Statistical Analysis in Scanning Tunneling Microscopy. J. Phys. Soc. Jpn. 2018, 87. [Google Scholar] [CrossRef]
- Cahangirov, S.; Topsakal, M.; Akturk, E.; Sahin, H.; Ciraci, S. Two- and One-Dimensional Honeycomb Structures of Silicon and Germanium. Phys. Rev. Lett. 2009, 102, 236804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharimani, N.; Nysten, B. Hybrid gate dielectrics: A comparative study between polyvinyl alcohol/SiO2 nanocomposite and pure polyvinyl alcohol thin-film transistors. Bull. Mater. Sci. 2019, 42, 26. [Google Scholar] [CrossRef] [Green Version]
- Botas, A.; Leitao, J.P.; Falcao, B.P.; Wiesinger, M.; Eckmann, F.; Teixeira, J.P.; Wingers, H.; Stutzmann, M.; Ferreira, R.A.S.; Rui, N.P. Silicon nanoparticle films infilled with al 2 o 3 using atomic layer deposition for photosensor, light emission, and photovoltaic applications. ACS Appl. Energ. Mater. 2020, 3, 5033–5044. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, L. Iron triad (Fe, Co, Ni) nanomaterials: Structural design, functionalization and their applications. Chem. Soc. Rev. 2015, 44, 6697–6707. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, I.; Pradeep, T. Atomically Precise Clusters of Noble Metals: Emerging Link between Atoms and Nanoparticles. Chem. Rev. 2017, 117, 8208–8271. [Google Scholar] [CrossRef]
- Chizh, K.V.; Arapkina, L.V.; Stavrovsky, D.B.; Gaiduk, P.I.; Yuryev, V.A. Diffusion of hydrogen atoms in silicon layers deposited from molecular beams on dielectric substrates. Mater. Sci. Semicond. Process. 2019, 99, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Brunner, J.; Baburin, I.A.; Sturm, S.; Kvashnina, K.; Rossberg, A.; Pietsch, T.; Andreev, S.; Sturm, E.; Cölfen, H. Self-assembled magnetite mesocrystalline films: Toward structural evolution from 2D to 3D superlattices. Adv. Mater. Interfaces 2017, 4, 1600431. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Miyamachi, T.; Nakashima, S.; Kawamura, N.; Takagi, Y.; Uozumi, M.; Antonov, V.N.; Yokoyama, T.; Ernst, A.; Komori, F. Thickness-dependent electronic and magnetic properties of γ′Fe4N atomic layers on Cu(001). Phys. Rev. B 2017, 95, 224417. [Google Scholar] [CrossRef] [Green Version]
- Alam, K.; Disseler, S.M.; Ratcliff, W.D.; Borchers, J.A.; Ponce-Pérez, R.; Cocoletzi, G.H.; Takeuchi, N.; Foley, A.; Richard, A.; Ingram, D.C.; et al. Structural and magnetic phase transitions in chromium nitride thin films grown by rf nitrogen plasma molecular beam epitaxy. Phys. Rev. B 2017, 96, 104433. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wang, J.; Ding, W.; Gong, Y.; Chen, H.; Ju, D. Exploring the Dual Characteristics of CH3OH Adsorption to Metal Atomic Structures on Si (111)-7 × 7 Surface. Molecules 2021, 26, 5824. https://doi.org/10.3390/molecules26195824
Li W, Wang J, Ding W, Gong Y, Chen H, Ju D. Exploring the Dual Characteristics of CH3OH Adsorption to Metal Atomic Structures on Si (111)-7 × 7 Surface. Molecules. 2021; 26(19):5824. https://doi.org/10.3390/molecules26195824
Chicago/Turabian StyleLi, Wenxin, Jiawen Wang, Wanyu Ding, Youping Gong, Huipeng Chen, and Dongying Ju. 2021. "Exploring the Dual Characteristics of CH3OH Adsorption to Metal Atomic Structures on Si (111)-7 × 7 Surface" Molecules 26, no. 19: 5824. https://doi.org/10.3390/molecules26195824
APA StyleLi, W., Wang, J., Ding, W., Gong, Y., Chen, H., & Ju, D. (2021). Exploring the Dual Characteristics of CH3OH Adsorption to Metal Atomic Structures on Si (111)-7 × 7 Surface. Molecules, 26(19), 5824. https://doi.org/10.3390/molecules26195824






