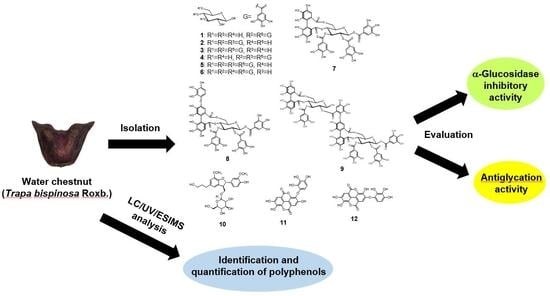
Characterization and Identification of Bioactive Polyphenols in the Trapabispinosa Roxb. Pericarp Extract
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation and Structural Elucidation of TBE-Derived Polyphenols and Related Compounds
2.2. α-Glucosidase Inhibitory Activity of the TBE-Derived Compounds
2.3. Antiglycation Effects of the TBE-Derived Compounds
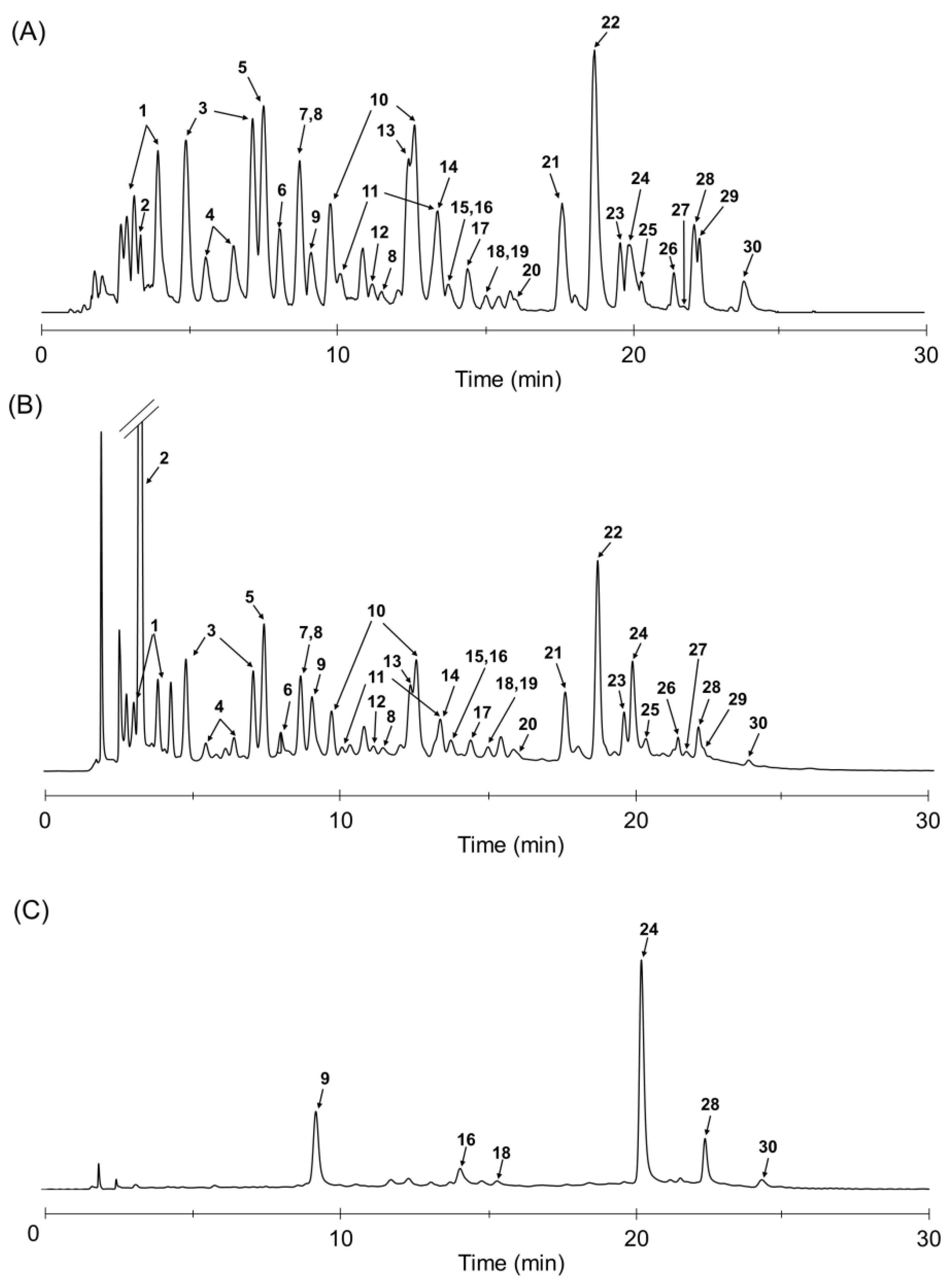
2.4. LC/UV/ESIMS Analysis of TBE
3. Materials and Methods
3.1. Chemicals
3.2. General Experimental Procedure
3.3. Extraction and Isolation
3.4. α-Glucosidase Inhibitory Activity
3.5. Inhibitory Effect on AGE-Formation
3.6. AGE-Derived Crosslink-Cleaving Effect
3.7. TBE Polyphenol Identification and Quantification by LC/UV/ESIMS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Takeshita, S.; Yagi, M.; Uemura, T.; Yamada, M.; Yonei, Y. Peel extract of water chestnut (Trapa bispinosa Roxb.) inhibits glycation, degrades α-dicarbonyl compound, and breaks advanced glycation end product crosslinks. Glycative Stress Res. 2015, 2, 72–79. [Google Scholar]
- Adkar, P.; Dongare, A.; Ambavade, S.; Bhaskar, V.H. Trapa bispinosa Roxb. A review on nutritional and pharmacological aspects. Adv. Pharmacol. Pharm. Sci. 2014, 2014, 959830. [Google Scholar] [CrossRef]
- Mann, S.; Gupta, D.; Gupta, V.; Gupta, R.K. Evaluation of nutritional, phytochemical and antioxidant potential of Trapa bispinosa Roxb. Fruits. Int. J. Pharm. Pharm. Sci. 2012, 4, 432–436. [Google Scholar]
- Xia, J.; Yang, C.; Wang, Y.; Yang, Y.; Yu, J. Antioxidant and antiproliferative activities of the leaf extracts from Trapa bispinosa and active components. S. Afr. J. Bot. 2017, 113, 377–381. [Google Scholar] [CrossRef]
- Saeed, M.; Nadeem, R.; Yousaf, M. Removal of industrial pollutant (Reactive Orange 122 dye) using environment-friendly sorbent Trapa bispinosa’s peel and fruit. Int. J. Environ. Sci. Technol. 2015, 12, 1223–1234. [Google Scholar] [CrossRef]
- Nonaka, G.; Matsumoto, Y.; Nishioka, I. Trapanin, a new hydrolyzable tannin from Trapa japonica FLEROV. Chem. Pharm. Bull. 1981, 29, 1184–1187. [Google Scholar] [CrossRef]
- Hatano, T.; Okonogi, A.; Yazaki, K.; Okuda, T. Trapanins A and B, oligomeric hydrolyzable tannins from Trapa japonica FLEROV. Chem. Pharm. Bull. 1990, 38, 2707–2711. [Google Scholar] [CrossRef]
- Kawabe, S.; Ganeko, N.; Ito, H. Ellagitannin dimers from the pericarps of Trapa japonica. Jpn. J. Pharmacog. 2017, 71, 53–54. [Google Scholar]
- Ngoc, T.M.; Hung, T.M.; Thuong, P.T.; Kim, J.C.; Choi, J.S.; Bae, K.; Hattori, M.; Choi, C.S.; Lee, J.S.; Min, B.S. Antioxidative activities of galloyl glucopyranosides from the stem-bark of Juglans mandshurica. Biosci. Biotechnol. Biochem. 2008, 72, 2158–2163. [Google Scholar] [CrossRef]
- Ko, S.Y.; Ko, H.A.; Chu, K.H.; Shieh, T.M.; Chi, T.C.; Chen, H.I.; Chang, W.C.; Chang, S.S. The possible mechanism of advanced glycation end products (AGEs) for Alzheimer’s disease. PLoS ONE 2015, 10, e0143345. [Google Scholar] [CrossRef]
- Luévano-Contreras, C.; Garay-Sevilla, M.E.; Wrobel, K.; Malacara, J.M.; Wrobel, K. Dietary advanced glycation end products restriction diminishes inflammation markers and oxidative stress in patients with type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2013, 52, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Takeshita, S.; Ishioka, Y.; Yagi, M.; Uemura, T.; Yamada, M.; Yonei, Y. The effects of water chestnut (Trapa bispinosa Roxb.) on the inhibition of glycometabolism and the improvement in postprandial blood glucose levels in humans. Glycative Stress Res. 2016, 3, 24–132. [Google Scholar]
- Kashiwada, Y.; Nonaka, G.; Nishioka, I. Tannins and related compounds. XXIII. Rhubarb (4): Isolation and structures of new classes of gallotannins. Chem. Pharm. Bull. 1984, 32, 3461–3470. [Google Scholar] [CrossRef]
- Okuda, T.; Hatano, T.; Yazaki, K.; Ogawa, N. Rugosin A, B, C and praecoxin A, tannin having a valoneoyl group. Chem. Pharm. Bull. 1982, 30, 4230–4233. [Google Scholar] [CrossRef]
- Ito, H.; Yamaguchi, K.; Kim, T.H.; Khennouf, S.; Gharzouli, K.; Yoshida, T. Dimeric and trimeric hydrolyzable tannins from Quercus coccifera and Quercus suber. J. Nat. Prod. 2002, 65, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Haddock, E.A.; Gupta, R.K.; Al-Shafi, S.M.K.; Haslam, E.; Magnolato, D. The metabolism of gallic acid and hexahydroxydiphenic acid in plants. Part 1. Introduction. Naturally occurring galloyl esters. J. Chem. Soc. Perkin Trans. 1982, 2515–2524. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannins of Casuarina and Stachyurus species. Part 1. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. 1983, 1765–1772. [Google Scholar] [CrossRef]
- Kato, E.; Uenishi, Y.; Inagaki, Y.; Kurokawa, M.; Kawabata, J. Isolation of rugosin A, B and related compounds as dipeptidyl peptidase-IV inhibitors from rose bud extract powder. Biosci. Biotechnol. Biochem. 2016, 80, 1–6. [Google Scholar] [CrossRef][Green Version]
- Abe, F.; Yamauchi, T. Lignans from Trachelospermum asiaticum (Tracheolospermum. II). Chem. Pharm. Bull. 1986, 34, 4340–4345. [Google Scholar] [CrossRef]
- Cui, C.B.; Zhao, Q.C.; Cai, B.; Yao, X.S.; Osadsa, H. Two new and four known polyphenolics obtained as new cell-cycle inhibitors from Rubus aleaefolius Poir. J. Asian Nat. Prod. Res. 2002, 4, 243–252. [Google Scholar] [CrossRef]
- Yang, S.W.; Zhou, B.N.; Wisse, J.H.; Evans, R.; van der Werff, H.; Miller, J.S.; Kingston, D.G. Three new ellagic acid derivatives from the bark of Eschweilera coriacea from the Suriname rainforest. J. Nat. Prod. 1998, 61, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Miwa, I.; Okuda, J.; Maeua, K.; Okuda, G. Mutarotase effect on colorimetric determination of blood glucose with β-d-glucose oxidase. Clin. Chim. Acta. 1972, 37, 538–540. [Google Scholar] [CrossRef]
- Li, S.; Iliefski, T.; Lundquist, K.; Wallis, A.F.A. Reassignment of relative stereochemistry at C-7 and C-8 in arylcoumaran neolignans. Phytochemistry 1997, 46, 929–934. [Google Scholar] [CrossRef]
- Yuen, M.S.M.; Xue, F.; Mak, T.C.W.; Wong, H.N.C. On the absolute structure of optically active neolignan containing a dihydrobenzo[b]furan skeleton. Tetrahedron 1998, 54, 12429–12444. [Google Scholar] [CrossRef]
- Ito, H.; Li, P.; Koreishi, M.; Nagatomo, A.; Nishida, N.; Yoshida, T. Ellagitannin oligomers and a neolignan from pomegranate arils and their inhibitory effects on the formation of advanced glycation end products. Food Chem. 2014, 152, 323–330. [Google Scholar] [CrossRef]
- Kim, T.; Ito, H.; Hayashi, K.; Hasegawa, T.; Machiguchi, T.; Yoshida, T. Aromatic constituents from the heartwood of Santalum album L. Chem. Pharm. Bull. 2005, 53, 641–644. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, H.B.; Lee, C.M.; Chang, H.M.; Wong, H.N.C. Compounds from Danshen. Part 7. Regioselective introduction of carbon-3 substituents to 5-alkyl-7-methoxy-2-phenylbenzo[b]furans: Synthesis of a novel adenosine A1 receptor ligand and its derivatives. J. Org. Chem. 1992, 57, 7248–7257. [Google Scholar] [CrossRef]
- Kato, N.; Kawabe, S.; Ganeko, N.; Yoshimura, M.; Amakura, Y.; Ito, H. Polyphenols from flowers of Magnolia coco and their anti-glycation effects. Biosci. Biotechnol. Biochem. 2017, 81, 1285–1288. [Google Scholar] [CrossRef]
- Yin, Z.; Zhang, W.; Feng, F.; Hang, Y.; Kang, W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness. 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Yun, X.; Ken, N.; Pangzhen, Z.; Robyn, D.W.; Shuibao, S.; Hsi-Yang, T.; Zijian, L.; Zhongxiang, F. In vitro α-glucosidase and α-amylase inhibitory activities of free and bound phenolic extracts from the bran and kernel fractions of five sorghum grain genotypes. Foods 2020, 9, 1301. [Google Scholar]
- Brownlee, M.; Vlassara, H.; Kooney, A.; Ulrich, P.; Cerami, A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science 1986, 232, 1629–1632. [Google Scholar] [CrossRef] [PubMed]
- Yeh, W.J.; Hsia, S.M.; Lee, W.H.; Wu, C.H. Polyphenols with antiglycation activity and mechanisms of action: A review of recent findings. J. Food Drug Anal. 2017, 25, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Takabe, W.; Mitsuhashi, R.; Parengkuan, L.; Yagi, M.; Yonei, Y. Cleaving effect of melatonin on crosslinks in advanced glycation end products. Glycative Stress Res. 2016, 3, 38–43. [Google Scholar]
- Huang, H.C.; Chao, C.L.; Liaw, C.C.; Hwang, S.Y.; Kuo, Y.H.; Chang, T.C.H.; Chen, C.J.; Kuo, Y.H. Hypoglycemic constituents isolated from Trapa natans L. pericarps. J. Agric. Food Chem. 2016, 64, 3794–3803. [Google Scholar] [CrossRef] [PubMed]
- Shirataki, Y.; Yoshida, S.; Toda, S. Dibenzo-α-pyrons in fluits of Trapa natans. Natural Med. 2000, 54, 160. [Google Scholar]
- Nawwar, M.A.M.; Souleman, A.M.A. 3,4,8,9,10-Pentahydroxy-dibenzo[b,d]pyran-6-one from Tamarix nilotica. Phytochemistry 1984, 23, 2966–2967. [Google Scholar] [CrossRef]
- Amakura, Y.; Kawada, K.; Hatano, T.; Okuda, T. Four new hydrolyzable tannins and an acylated flavonol glycoside from Euphorbia maculata. Can. J. Chem. 1996, 75, 727–733. [Google Scholar] [CrossRef]
- Amakura, Y.; Yoshida, T. Tannins and related polyphenols of euphorbiaceous Plants. XIV. Euphorbin I, a new dimeric hydrolyzable tannin from Euphorbia watanabei. Chem. Pharm. Bull. 1996, 44, 1293–1297. [Google Scholar] [CrossRef]
- Yoshida, T.; Itoh, H.; Matsunaga, S.; Tanaka, R.; Okuda, T. Tannins and related polyphenols of Euphorbiaceous plants. IX. Hydrolyzable tannins with 1C4 glucose core from Phyllanthus flexuosus Muell. Arg. Chem. Pharm. Bull. 1992, 40, 53–60. [Google Scholar] [CrossRef]
- Hatano, T.; Ogawa, N.; Kira, R.; Yasuhira, T.; Okuda, T. Tannins of cornaceous plants. I. Cornusiins A, B and C, dimeric monomeric and trimeric hydrolyzable tannins from Cornus officinalis, and orientation of valoneoyl group in related tannins. Chem. Pharm. Bull. 1989, 37, 2083–2090. [Google Scholar] [CrossRef]
- Ito, H.; Miki, K.; Yoshida, T. Elaeagnatins A-G, C-glucosidic ellagitannins from Elaeagnus umbellate. Chem. Pharm. Bull. 1999, 47, 536–542. [Google Scholar] [CrossRef]
- Niemetz, R.; Gross, G.G. Gallotannin biosynthesis: Purification of β-glucogallin: 1,2,3,4,6-pentagalloyl-β-d-glucose galloyltransferase from sumac leaves. Phytochemistry 1998, 2, 327–332. [Google Scholar] [CrossRef]
- Shimozu, Y.; Kuroda, T.; Tsuchiya, T.; Hatano, T. Structures and antibacterial properties of isorugosins H–J, oligomeric ellagitannins from liquidambar formosana with characteristic bridging groups between sugar moieties. J. Nat. Prod. 2017, 80, 2723–2733. [Google Scholar] [CrossRef]
- Yoshida, T.; Amakura, Y.; Liu, Y.Z.; Okuda, T. Tannins and related polyphenols of euphorbiaceous Plants. XI.Three new hydrolyzable tannins and a polyphenol glucoside from Euphorbia humifusa. Chem. Pharm. Bull. 1994, 42, 1803–1807. [Google Scholar] [CrossRef]
- Yagi, K.; Goto, K.; Nanjo, F. Identification of a major polyphenol and polyphenolic composition in leaves of Camellia irrawadiensis. Chem. Pharm. Bull. 2009, 11, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Iguchi, A.; Hatano, T. Identification of urinary and intestinal bacterial metabolites of ellagitannin geraniin in rats. J. Agric. Food Chem. 2008, 56, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Kirino, A.; Takasuka, Y.; Nishi, A.; Kawabe, S.; Kirino, A.; Takasuka, Y.; Nishi, A.; Kawabe, S.; Yamashita, H.; Kimoto, M.; et al. Analysis and functionality of major polyphenolic components of Plygonum cuspidatum (Itadori). J. Nutr. Sci. Vitaminol. 2012, 58, 278–286. [Google Scholar] [CrossRef]


| Compound | Inhibitory Effects on α-Glucosidase Activity |
|---|---|
| IC50 (μM) | |
| Gallic acid [8] | >100 |
| Ellagic acid | >100 |
| 1,2,3-Tri-O-galloyl-β-d-glucose (2) [8] | >100 |
| 1,2,6-Tri-O-galloyl-β-d-glucose (3) [8] | >100 |
| 1,2,3,6-Tetra-O-galloyl-β-d-glucose (5) [8] | >100 |
| 1,2,4,6-Tetra-O-galloyl-β-d-glucose (6) | >100 |
| 1,2,3,4,6-Penta-O-galloyl-β-d-glucose | 59.0 ± 0.4 |
| Tellimagrandin II (7) [8] | >100 |
| Decarboxylated rugosin A (8) | 20.7 ± 0.1 |
| Camptothin B (9) | >100 |
| Compound 10 | >100 |
| Compound 13 | >100 |
| Compound 14 | >100 |
| Rubuphenol (11) | >100 |
| Eschweilenol A (12) | >100 |
| Cornusiin G [8] | 6.3 ± 0.1 |
| Acarbose | 4.0 ± 0.1 |
| Compound | Inhibitory Effects on AGE Formation | Crosslink-Cleaving Activities | |
|---|---|---|---|
| IC50 (μM) | |||
| Glucose | Fructose | Relative Ratio * | |
| Gallic acid | 14.7 ± 2.0 | 27.0 ± 2.6 | 720.1 ± 54.1 |
| Ellagic acid | 1.8 ± 0.1 | 2.5 ± 0.1 | 11.4 ± 0.1 |
| 2,6-Di-O-galloyl-β-d-glucose (1) | 1.5 ± 0.1 | 3.3 ± 0.0 | 77.4 ± 4.0 |
| 1,2,3-Tri-O-galloyl-β-d-glucose (2) | 0.4 ± 0.0 | 0.4 ± 0.0 | 190.5 ± 2.5 |
| 1,2,6-Tri-O-galloyl-β-d-glucose (3) | 0.3 ± 0.0 | 0.4 ± 0.0 | 146.6 ± 12.6 |
| 2,3,6-Tri-O-galloyl-β-d-glucose (4) | 0.3 ± 0.0 | 1.0 ± 0.0 | N.T. |
| 1,2,3,6-Tetra-O-galloyl-β-d-glucose (5) | 0.3 ± 0.0 | 0.3 ± 0.0 | 209.0 ± 33.7 |
| 1,2,4,6-Tetra-O-galloyl-β-d-glucose (6) | 0.3 ± 0.0 | 0.3 ± 0.0 | 159.6 ± 12.7 |
| 1,2,3,4,6-Penta-O-galloyl-β-d-glucose | 0.2 ± 0.0 | 0.2 ± 0.0 | 97.9 ± 2.1 |
| Tellimagrandin II (7) | 0.2 ± 0.0 | 0.3 ± 0.0 | 230.8 ± 12.2 |
| Decarboxylated rugosin A (8) | 0.3 ± 0.0 | 0.3 ± 0.0 | 233.0 ± 5.8 |
| Camptothin B (9) | 0.1 ± 0.0 | 0.2 ± 0.0 | 180.6 ± 4.2 |
| Compound 10 | >500 | >1000 | 16.7 ± 1.4 |
| Compound 13 | >500 | >1000 | 17.2 ± 1.1 |
| Compound 14 | >500 | >1000 | 0.5 ± 1.1 |
| Rubuphenol (11) | 2.4 ± 0.0 | 4.7 ± 0.3 | 514.8 ± 11.2 |
| Eschweilenol A (12) | 2.4 ± 0.0 | 2.9 ± 0.1 | 484.5 ± 12.5 |
| Aminoguanidine | 258.9 ± 6.8 | 801.0 ± 17.7 | N.T. |
| N-Phenacylthiazolium bromide (PTB) | N.T. | N.T. | 100 |
| Peak No. | Compound | tR (min) | MS (m/z) | Content (mg/g of Dry Weight) |
|---|---|---|---|---|
| 1 | 2,3-Di-O-galloyl-β-d-glucose | 3.18, 3.94 | 483 [M − H]− | 15.5 ± 0.2 |
| 2 | Gallic acid | 3.23 | 339 [2M − H]− | 32.2 ± 0.1 |
| 3 | 2,6-Di-O-galloyl-β-d-glucose (1) | 4.90, 7.52 | 483 [M − H]− | N.T. |
| 4 | 3,6-Di-O-galloyl-β-d-glucose | 5.55, 6.58 | 483 [M − H]− | 3.9 ± 0.0 |
| 5 | 1,6-Di-O-galloyl-β-d-glucose | 7.53 | 483 [M − H]− | 16.8 ± 1.2 |
| 6 | Digalloyl glucose | 8.07 | 483 [M − H]− | N.T. |
| 7 | 1,2,3-Tri-O-galloyl-β-d-glucose (2) | 8.75 | 635 [M − H]− | 4.8 ± 0.0 |
| 8 | 3,4,6-Tri-O-galloyl-β-d-glucose | 8.75, 11.6 | 635 [M − H]− | 3.4 ± 0.1 |
| 9 | Brevifolincarboxylic acid | 9.22 | 291 [M − H]− | 1.7 ± 0.1 |
| 10 | 2,3,6-Tri-O-galloyl-β-d-glucose (4) | 9.86, 12.6 | 635 [M − H]− | N.T. |
| 11 | 2,4,6-Tri-O-galloyl-β-d-glucose | 10.2, 13.5 | 635 [M − H]− | 0.2 ± 0.1 |
| 12 | Trigalloyl glucose | 11.3 | 635 [M − H]− | N.T. |
| 13 | 1,2,6-Tri-O-galloyl-β-d-glucose (3) | 12.5 | 635 [M − H]− | 4.1 ± 0.5 |
| 14 | 1,3,6-Tri-O-galloyl-β-d-glucose | 13.5 | 635 [M − H]− | 1.8 ± 0.7 |
| 15 | 1,2-Di-O-galloyl-4,6-hexahydroxydiphenoyl-β-d-glucose | 13.8 | 785 [M − H]− | 0.9 ± 0.0 |
| 16 | Valoneic acid dilactone | 13.9 | 469 [M − H]− | 1.8 ± 0.2 |
| 17 | Trigalloyl glucose | 14.5 | 635 [M − H]− | N.T. |
| 18 | Urolithin M5 | 15.1 | 275 [M − H]− | 1.4 ± 0.4 |
| 19 | 1,4,6-Tri-O-galloyl-β-d-glucose | 15.1 | 635 [M − H]− | 0.6 ± 0.0 |
| 20 | Camptothin B (9) | 16.2 | 860 [M − 2H]2− | N.T. |
| 21 | Tellimagrandin II (7) | 17.7 | 937 [M − H]− | 5.7 ± 0.0 |
| 22 | 1,2,3,6-Tetra-O-galloyl-β-d-glucose (5) | 18.8 | 787 [M − H]− | 13.3 ± 0.0 |
| 23 | 1,2,4,6-Tetra-O-galloyl-β-d-glucose (6) | 19.7 | 787 [M − H]− | 1.5 ± 0.0 |
| 24 | Ellagic acid | 19.9 | 301 [M − H]− | 6.9 ± 0.1 |
| 25 | Decarboxylated rugosin A (8) | 20.3 | 1061 [M − H]− | 2.4 ± 0.0 |
| 26 | 1,2,3,4,6-Penta-O-galloyl-β-d-glucose | 21.5 | 939 [M − H]− | 0.7 ± 0.0 |
| 27 | Cornusiin G | 21.7 | 861 [M − 2H]2− | 0.3 ± 0.0 |
| 28 | Rubuphenol (11) | 22.2 | 425 [M − H]− | 4.3 ± 0.1 |
| 29 | Compound 10 | 22.1 | 521 [M − H]− | 1.3 ± 0.1 |
| 30 | Eschweilenol A (12) | 24.0 | 425 [M − H]− | 0.9 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwaoka, Y.; Suzuki, S.; Kato, N.; Hayakawa, C.; Kawabe, S.; Ganeko, N.; Uemura, T.; Ito, H. Characterization and Identification of Bioactive Polyphenols in the Trapabispinosa Roxb. Pericarp Extract. Molecules 2021, 26, 5802. https://doi.org/10.3390/molecules26195802
Iwaoka Y, Suzuki S, Kato N, Hayakawa C, Kawabe S, Ganeko N, Uemura T, Ito H. Characterization and Identification of Bioactive Polyphenols in the Trapabispinosa Roxb. Pericarp Extract. Molecules. 2021; 26(19):5802. https://doi.org/10.3390/molecules26195802
Chicago/Turabian StyleIwaoka, Yuji, Shoichi Suzuki, Nana Kato, Chisa Hayakawa, Satoko Kawabe, Natsuki Ganeko, Tomohiro Uemura, and Hideyuki Ito. 2021. "Characterization and Identification of Bioactive Polyphenols in the Trapabispinosa Roxb. Pericarp Extract" Molecules 26, no. 19: 5802. https://doi.org/10.3390/molecules26195802
APA StyleIwaoka, Y., Suzuki, S., Kato, N., Hayakawa, C., Kawabe, S., Ganeko, N., Uemura, T., & Ito, H. (2021). Characterization and Identification of Bioactive Polyphenols in the Trapabispinosa Roxb. Pericarp Extract. Molecules, 26(19), 5802. https://doi.org/10.3390/molecules26195802