Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Profile of Steroids Treatment
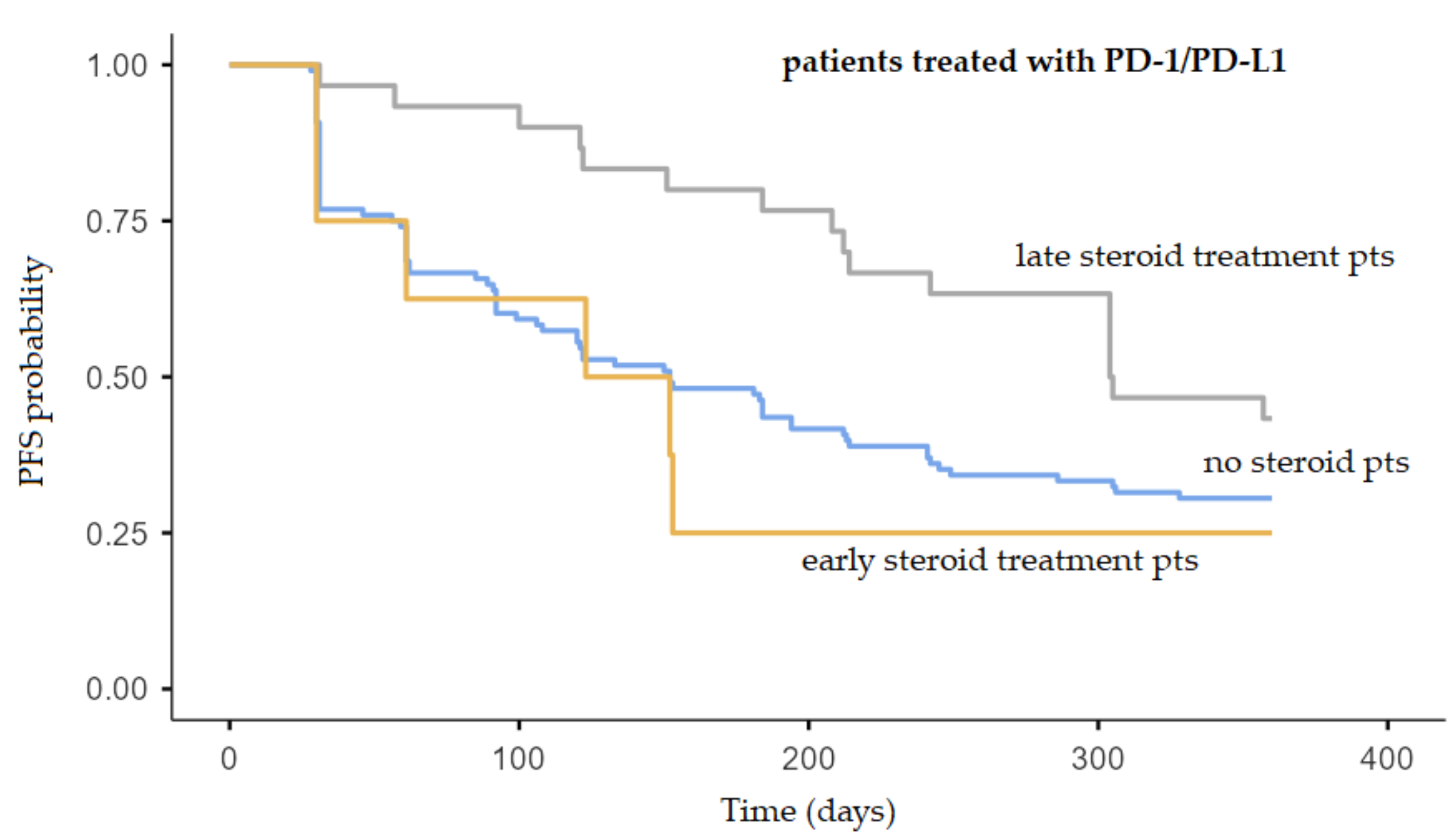
2.3. Relationship between Steroids Treatment and Patient Outcome
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Topalian, S.L. Targeting Immune Checkpoints in Cancer Therapy. JAMA 2017, 318, 1647–1648. [Google Scholar] [CrossRef] [PubMed]
- Lavacchi, D.; Pellegrini, E.; Palmieri, V.E.; Doni, L.; Mela, M.M.; Di Maida, F.; Amedei, A.; Pillozzi, S.; Carini, M.; Antonuzzo, L. Immune Checkpoint Inhibitors in the Treatment of Renal Cancer: Current State and Future Perspective. Int. J. Mol. Sci. 2020, 21, 4691. [Google Scholar] [CrossRef]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chávez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suárez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Prim. 2020, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Libert, C.; Dejager, L. How Steroids Steer T Cells. Cell Rep. 2014, 7, 938–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, M.; Meng, C.; Ivashkiv, L.B. Inhibition of IL-2-induced Jak-STAT signaling by glucocorticoids. Proc. Natl. Acad. Sci. USA 2000, 97, 9573–9578. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Oppenheim, J.J.; Winkler-Pickett, R.T.; Ortaldo, J.R.; Howard, O.M.Z. Glucocorticoid amplifies IL-2-dependent expansion of functional FoxP3+CD4+CD25+ T regulatory cellsin vivo and enhances their capacity to suppress EAE. Eur. J. Immunol. 2006, 36, 2139–2149. [Google Scholar] [CrossRef] [PubMed]
- Tetel, M.J.; De Vries, G.J.; Melcangi, R.C.; Panzica, G.; O’Mahony, S.M. Steroids, stress and the gut microbiome-brain axis. J. Neuroendocr. 2018, 30, e12548. [Google Scholar] [CrossRef] [PubMed]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Arbour, K.C.; Mezquita, L.; Long, N.; Rizvi, H.; Auclin, E.; Ni, A.; Martínez-Bernal, G.; Ferrara, R.; Lai, W.V.; Hendriks, L.E.L.; et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients with Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2018, 36, 2872–2878. [Google Scholar] [CrossRef]
- Larkin, J.; Minor, D.; D’Angelo, S.; Neyns, B.; Smylie, M.; Miller, W.H., Jr.; Gutzmer, R.; Linette, G.; Chmielowski, B.; Lao, C.D.; et al. Overall Survival in Patients with Advanced Melanoma Who Received Nivolumab Versus Investigator’s Choice Chemotherapy in CheckMate 037: A Randomized, Controlled, Open-Label Phase III Trial. J. Clin. Oncol. 2018, 36, 383–390. [Google Scholar] [CrossRef]
- Wolfe, F.; Caplan, L.; Michaud, K. Treatment for rheumatoid arthritis and the risk of hospitalization for pneumonia: Associations with prednisone, disease-modifying antirheumatic drugs, and anti–tumor necrosis factor therapy. Arthritis Rheum. 2006, 54, 628–634. [Google Scholar] [CrossRef]
- Cortellini, A.; Tucci, M.; Adamo, V.; Stucci, L.S.; Russo, A.; Tanda, E.T.; Spagnolo, F.; Rastelli, F.; Bisonni, R.; Santini, D.; et al. Integrated analysis of concomitant medications and oncological outcomes from PD-1/PD-L1 checkpoint inhibitors in clinical practice. J. Immunother. Cancer 2020, 8, e001361. [Google Scholar] [CrossRef]
- Lin, R.J.; Adelman, R.D.; Mehta, S.S. Dyspnea in Palliative Care: Expanding the Role of Corticosteroids. J. Palliat. Med. 2012, 15, 834–837. [Google Scholar] [CrossRef]
- Paulsen, Ø.; Klepstad, P.; Rosland, J.H.; Aass, N.; Albert, E.; Fayers, P.; Kaasa, S. Efficacy of Methylprednisolone on Pain, Fatigue, and Appetite Loss in Patients with Advanced Cancer Using Opioids: A Randomized, Placebo-Controlled, Double-Blind Trial. J. Clin. Oncol. 2014, 32, 3221–3228. [Google Scholar] [CrossRef] [PubMed]
- Ryken, T.C.; McDermott, M.; Robinson, P.D.; Ammirati, M.; Andrews, D.W.; Asher, A.L.; Burri, S.H.; Cobbs, C.S.; Gaspar, L.E.; Kondziolka, D.; et al. The role of steroids in the management of brain metastases: A systematic review and evidence-based clinical practice guideline. J. Neuro-Oncol. 2009, 96, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrelli, F.; Signorelli, D.; Ghidini, M.; Ghidini, A.; Pizzutilo, E.G.; Ruggieri, L.; Cabiddu, M.; Borgonovo, K.; Dognini, G.; Brighenti, M.; et al. Association of Steroids Use with Survival in Patients Treated with Immune Checkpoint Inhibitors: A Systematic Review and Meta-Analysis. Cancers 2020, 12, 546. [Google Scholar] [CrossRef] [Green Version]
- Fucà, G.; Galli, G.; Poggi, M.; Russo, G.L.; Proto, C.; Imbimbo, M.; Ferrara, R.; Zilembo, N.; Ganzinelli, M.; Sica, A.; et al. Modulation of peripheral blood immune cells by early use of steroids and its association with clinical outcomes in patients with metastatic non-small cell lung cancer treated with immune checkpoint inhibitors. ESMO Open 2019, 4, e000457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvat, T.; Adel, N.G.; Dang, T.-O.; Momtaz, P.; Postow, M.A.; Callahan, M.K.; Carvajal, R.D.; Dickson, M.A.; D’Angelo, S.P.; Woo, K.M.; et al. Immune-Related Adverse Events, Need for Systemic Immunosuppression, and Effects on Survival and Time to Treatment Failure in Patients with Melanoma Treated with Ipilimumab at Memorial Sloan Kettering Cancer Center. J. Clin. Oncol. 2015, 33, 3193–3198. [Google Scholar] [CrossRef] [Green Version]
- Leighl, N.; Gandhi, L.; Hellmann, M.D.; Horn, L.; Ahn, M.-J.; Garon, E.B.; Hui, R.; Ramalingam, S.S.; Zhang, J.; Lubiniecki, G.; et al. Pembrolizumab for NSCLC: Immune-mediated adverse events and corticosteroid use. J. Thorac. Oncol. 2015, 10, S233. [Google Scholar]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Orillard, E.; Mansi, L.; Marabelle, A.; Scotte, F.; Lambotte, O.; Michot, J.-M. How to manage patients with corticosteroids in oncology in the era of immunotherapy? Eur. J. Cancer 2020, 141, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O.; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, A.; Kostine, M.; Barnetche, T.; Truchetet, M.-E.; Schaeverbeke, T. Immune related adverse events associated with anti-CTLA-4 antibodies: Systematic review and meta-analysis. BMC Med. 2015, 13, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maughan, B.L.; Bailey, E.; Gill, D.M.; Agarwal, N. Incidence of Immune-Related Adverse Events with Program Death Receptor-1- and Program Death Receptor-1 Ligand-Directed Therapies in Genitourinary Cancers. Front. Oncol. 2017, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front. Immunol. 2016, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.-K.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, R.; Luksik, A.S.; Garzon-Muvdi, T.; Hung, A.L.; Kim, E.S.; Wu, A.; Xia, Y.; Belcaid, Z.; Gorelick, N.; Choi, J.; et al. Contrasting impact of corticosteroids on anti-PD-1 immunotherapy efficacy for tumor histologies located within or outside the central nervous system. OncoImmunology 2018, 7, e1500108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenstein, S.; Ghias, K.; Krett, N.L.; Rosen, S.T. Mechanisms of glucocorticoid-mediated apoptosis in hematological malig-nancies. Clin. Cancer Res. 2002, 8, 1681–1694. [Google Scholar] [PubMed]
- Xing, K.; Gu, B.; Zhang, P.; Wu, X. Dexamethasone enhances programmed cell death 1 (PD-1) expression during T cell activation: An insight into the optimum application of glucocorticoids in anti-cancer therapy. BMC Immunol. 2015, 16, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, S.C.; Pennell, N.A. Early Use of Systemic Corticosteroids in Patients with Advanced NSCLC Treated with Nivolumab. J. Thorac. Oncol. 2018, 13, 1771–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciuti, B.; Dahlberg, S.E.; Adeni, A.; Sholl, L.M.; Nishino, M.; Awad, M.M. Immune Checkpoint Inhibitor Outcomes for Patients with Non–Small-Cell Lung Cancer Receiving Baseline Corticosteroids for Palliative Versus Nonpalliative Indications. J. Clin. Oncol. 2019, 37, 1927–1934. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, A.; Mezquita, L.; Auclin, E.; Blanc-Durand, F.; Riudavets, M.; Caramella, C.; Martinez, G.; Benitez, J.C.; Martín-Romano, P.; El-Amarti, L.; et al. Impact of Intercurrent Introduction of Steroids on Clinical Outcomes in Advanced Non-Small-Cell Lung Cancer (NSCLC) Patients under Immune-Checkpoint Inhibitors (ICI). Cancers 2020, 12, 2827. [Google Scholar] [CrossRef] [PubMed]
- Drakaki, A.; Dhillon, P.K.; Wakelee, H.; Chui, S.Y.; Shim, J.; Kent, M.; Degaonkar, V.; Hoang, T.; McNally, V.; Luhn, P.; et al. Association of baseline systemic corticosteroid use with overall survival and time to next treatment in patients receiving immune checkpoint inhibitor therapy in real-world US oncology practice for advanced non-small cell lung cancer, melanoma, or urothelial carcinoma. OncoImmunology 2020, 9, 1824645. [Google Scholar] [CrossRef]
- Paderi, A.; Giorgione, R.; Giommoni, E.; Mela, M.; Rossi, V.; Doni, L.; Minervini, A.; Carini, M.; Pillozzi, S.; Antonuzzo, L. Association between Immune Related Adverse Events and Outcome in Patients with Metastatic Renal Cell Carcinoma Treated with Immune Checkpoint Inhibitors. Cancers 2021, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Seymour, L.; Litière, S.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1–Standardisation and disease-specific adaptations: Perspectives from the RECIST Working Group. Eur. J. Cancer 2016, 62, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seymour, L.; Bogaerts, J.; Perrone, A.; Ford, R.; Schwartz, L.H.; Mandrekar, S.; Lin, N.U.; Litière, S.; Dancey, J.; Chen, A.; et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017, 18, e143–e152, Erratum in: 2019, 20, e242. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | No. of Patients (n = 146) | |
|---|---|---|
| Sex | ||
| Male | 98 | 67.1% |
| Female | 48 | 32.9% |
| Age, years | ||
| Average | 67 | |
| Median | 70 | |
| Range | 27–91 | |
| Tumor | ||
| NSCLC | 67 | 45.9% |
| Melanoma | 46 | 31.5% |
| RCC | 33 | 22.6% |
| Therapy line | ||
| 1 | 63 | 43.2% |
| 2 | 70 | 47.9% |
| 3 | 10 | 6.8% |
| 4 | 3 | 2.1% |
| Immune checkpoint inhibitors | ||
| Nivolumab | 93 | 63.7% |
| Pembrolizumab | 42 | 28.8% |
| Atezolizumab | 11 | 7.5% |
| Outcome | ||
| CR | 9 | 6.2% |
| PR | 19 | 13.0% |
| SD | 43 | 29.5% |
| PD | 75 | 51.4% |
| Steroid treatment during immunotherapy | ||
| Yes | 41 | 28.1% |
| No | 105 | 71.9% |
| Characteristics of Steroids Treatment and irAEs | No. of Patients (n = 41) | |
|---|---|---|
| Cumulative dose of steroid | ||
| <500 mg prednisone or equivalent | 25 | 61.0% |
| >500 mg prednisone or equivalent | 16 | 39.0% |
| Dose of steroid mg/kg | ||
| Prednisone 0.5–1 mg/kg/day (or equivalent of methylprednisolone) | 25 | 61.0% |
| Prednisone >1 mg/kg/day (or equivalent of methylprednisolone) | 16 | 39.0% |
| Molecule | ||
| Prednisone | 33 | 80.5% |
| Methylprednisolone | 8 | 19.5% |
| Duration of treatment | ||
| <14 days | 21 | 51.2% |
| >14 days | 20 | 48.8% |
| Type of irAEs treated with steroids | ||
| Pneumonitis | 8 | 19.5% |
| Colitis | 9 | 22.0% |
| Skin reactions | 9 | 22.0% |
| Endocrine-related events | 9 | 22.0% |
| Rheumatologic events | 5 | 12.2% |
| Hepatitis | 1 | 2.4% |
| irAEs grade (CTCAE v 4.0) | ||
| Non-serious (CTCAE grade 2) | 31 | 75.6% |
| Serious (CTCAE grade 3–4) | 10 | 24.4% |
| Patients who discontinued ICIs due to toxicity | ||
| ICI discontinued | 5 | 12.1% |
| ICI continued | 36 | 87.8% |
| Time of steroid treatment | ||
| Early steroid treatment (first 30 days of immunotherapy) | 9 | 22.0% |
| Late steroid treatment (after 30 days of immunotherapy) | 32 | 78.0% |
| irAEs Type | |||||||
|---|---|---|---|---|---|---|---|
| Onset | Pulmonary | Colitis | Hepatitis | Cutaneous | Rheumatologic | Endocrine | Total |
| Late | 4 (13.3%) | 8 (26.7%) | 1 (3.3%) | 5 (16.7%) | 3 (10.0%) | 9 (30.0%) | 30 (100%) |
| Early | 4 (36.4%) | 1 (9.0%) | 0 (0.0%) | 4 (36.4%) | 2 (18.2%) | 0 (0.0%) | 11 (100%) |
| Total | 8 (19.6%) | 9 (21.9%) | 1 (2.4%) | 9 (21.9%) | 5 (12.3%) | 9 (21.9%) | 41 (100%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paderi, A.; Gambale, E.; Botteri, C.; Giorgione, R.; Lavacchi, D.; Brugia, M.; Mazzoni, F.; Giommoni, E.; Bormioli, S.; Amedei, A.; et al. Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis. Molecules 2021, 26, 5789. https://doi.org/10.3390/molecules26195789
Paderi A, Gambale E, Botteri C, Giorgione R, Lavacchi D, Brugia M, Mazzoni F, Giommoni E, Bormioli S, Amedei A, et al. Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis. Molecules. 2021; 26(19):5789. https://doi.org/10.3390/molecules26195789
Chicago/Turabian StylePaderi, Agnese, Elisabetta Gambale, Cristina Botteri, Roberta Giorgione, Daniele Lavacchi, Marco Brugia, Francesca Mazzoni, Elisa Giommoni, Susanna Bormioli, Amedeo Amedei, and et al. 2021. "Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis" Molecules 26, no. 19: 5789. https://doi.org/10.3390/molecules26195789
APA StylePaderi, A., Gambale, E., Botteri, C., Giorgione, R., Lavacchi, D., Brugia, M., Mazzoni, F., Giommoni, E., Bormioli, S., Amedei, A., Pillozzi, S., Matucci Cerinic, M., & Antonuzzo, L. (2021). Association of Systemic Steroid Treatment and Outcome in Patients Treated with Immune Checkpoint Inhibitors: A Real-World Analysis. Molecules, 26(19), 5789. https://doi.org/10.3390/molecules26195789









