The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site
Abstract
1. Introduction
2. Results
2.1. Synthesis of 2-Aminopyrrole Derivatives
2.2. Cytotoxic Activities of 2-Aminopyrroles in Epithelial Cancer Cell Lines
2.3. Molecular Docking of Pyrrole-Based Analogs Selected the Compounds Which Effectively Bound to a Colchicine-Binding Site in Tubulin
2.4. Structure–Activity Relationship (SAR) Analysis of CAs
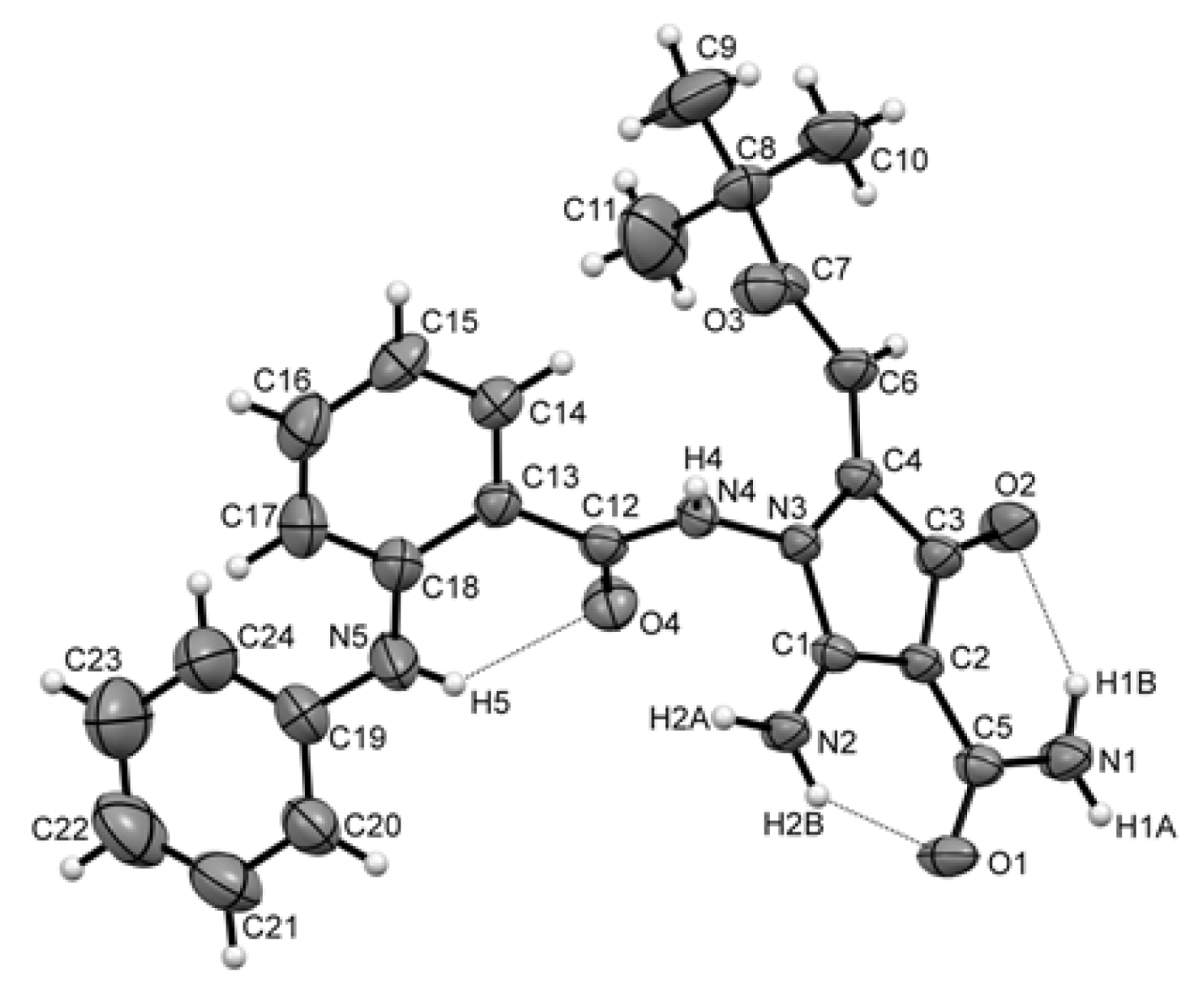
2.5. Synthesis, Spectrum Measurements and Basic Characteristics of CA-61 and -84
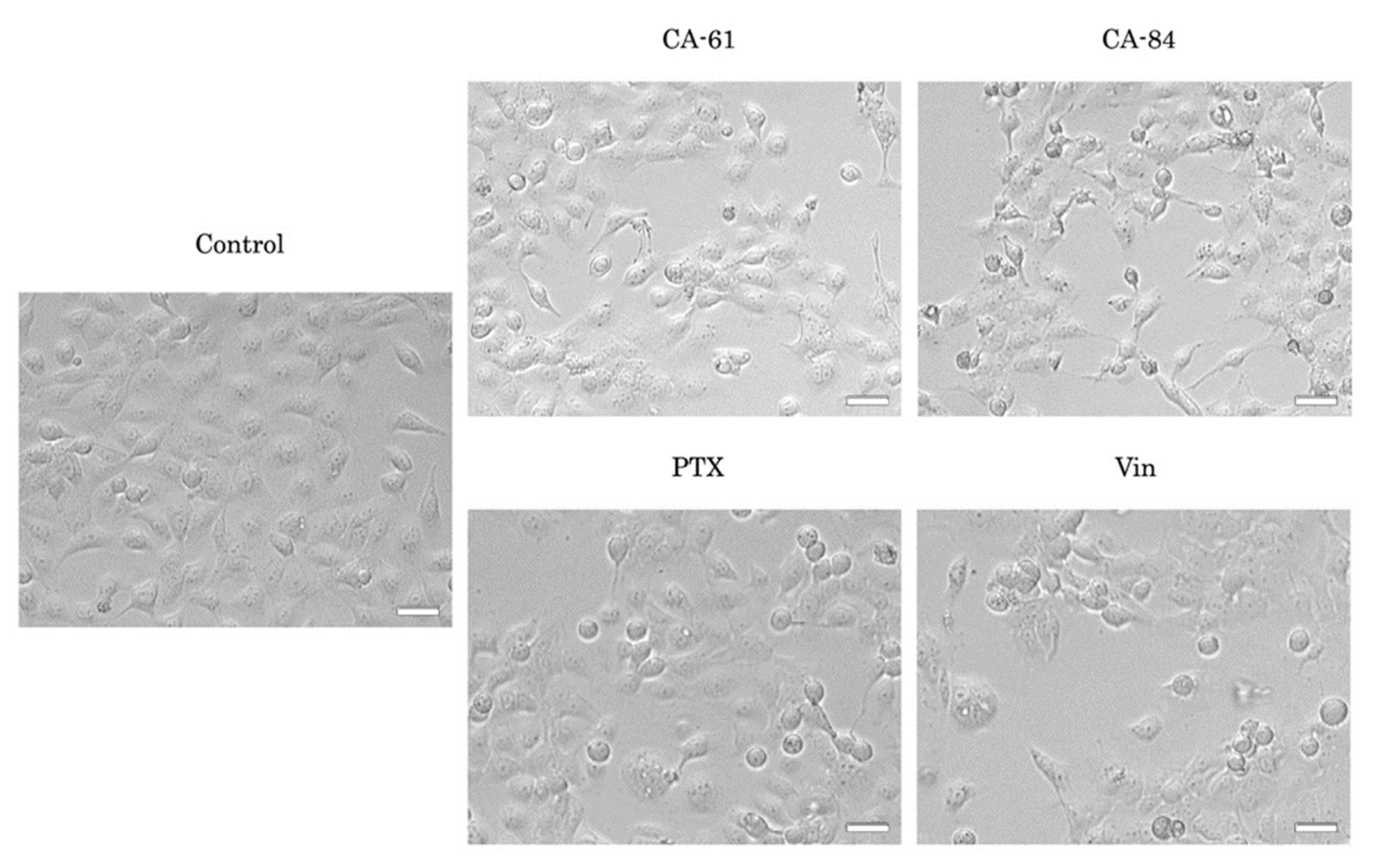
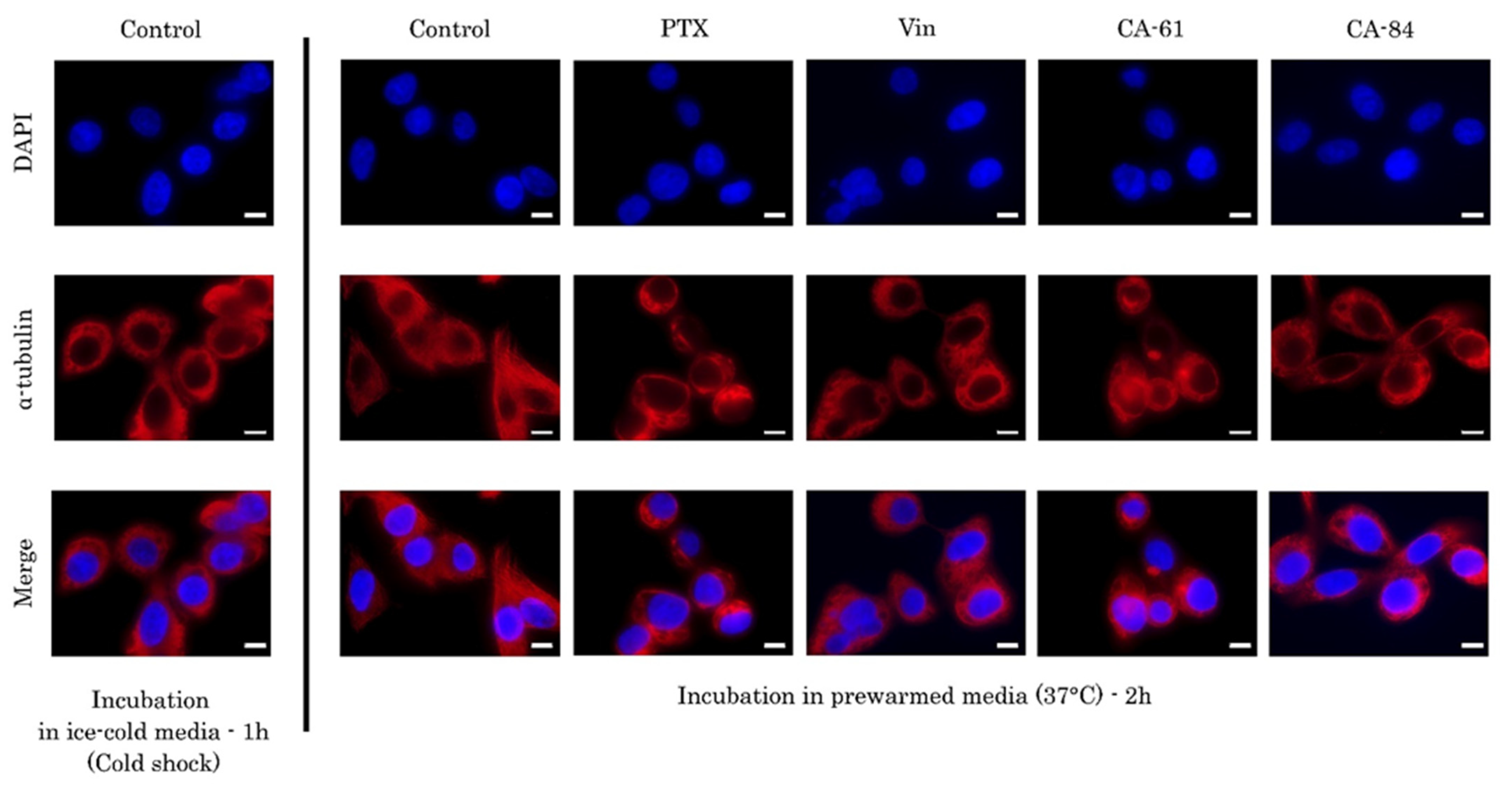
2.6. CAs Inhibit Tubulin Polymerization and Disrupt the Microtubule Network
2.7. CAs Effectively Reduce the Viability of the Epithelial Cancer Cell Lines In Vitro
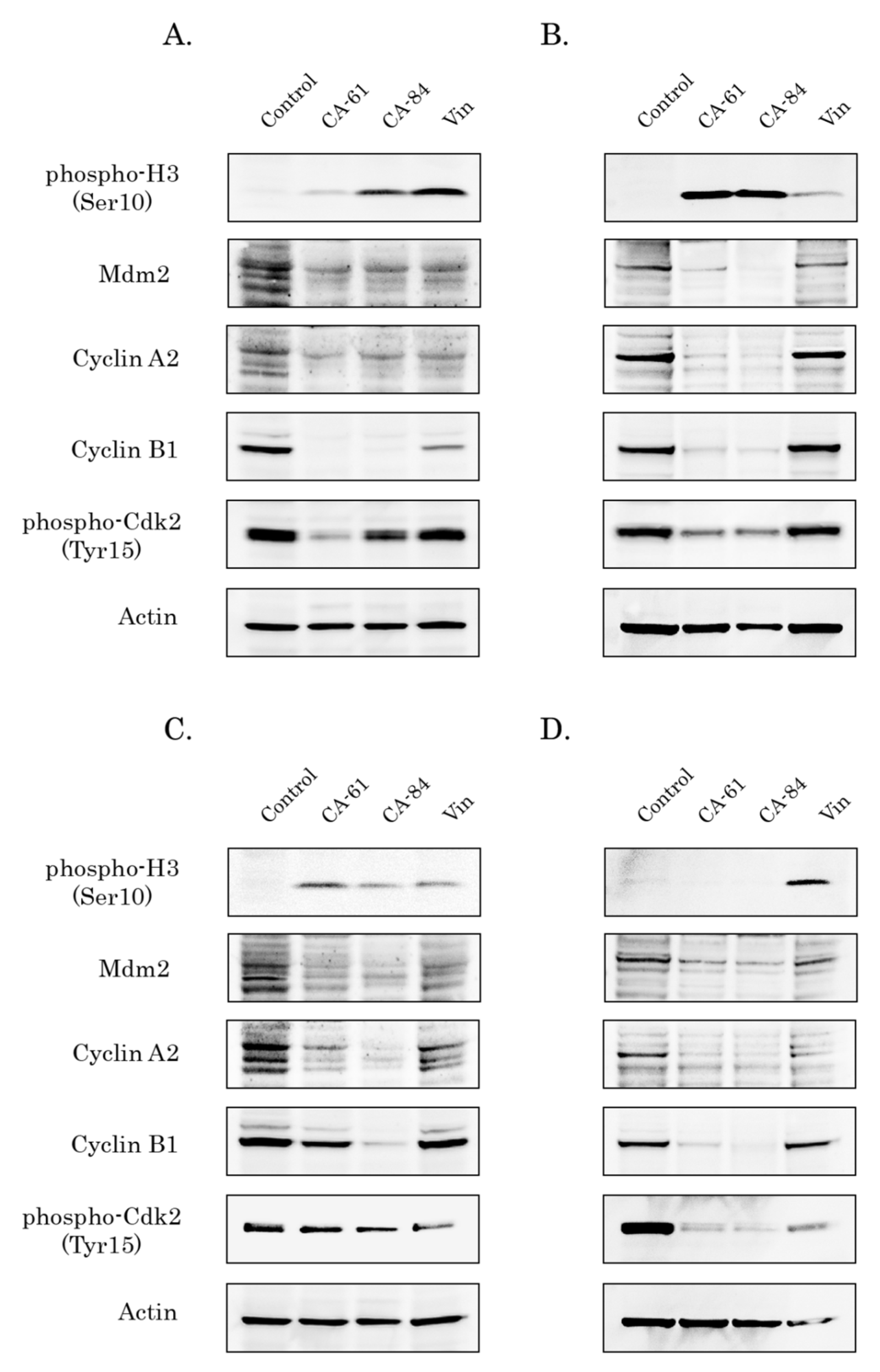
2.8. CA-61 and -84 Induce Cell Cycle Abnormalities and Enhance the Number of Cancer Cells in the G2/M Phase
2.9. CAs Induce Selective Accumulation of Cancer Cells in the M-Phase
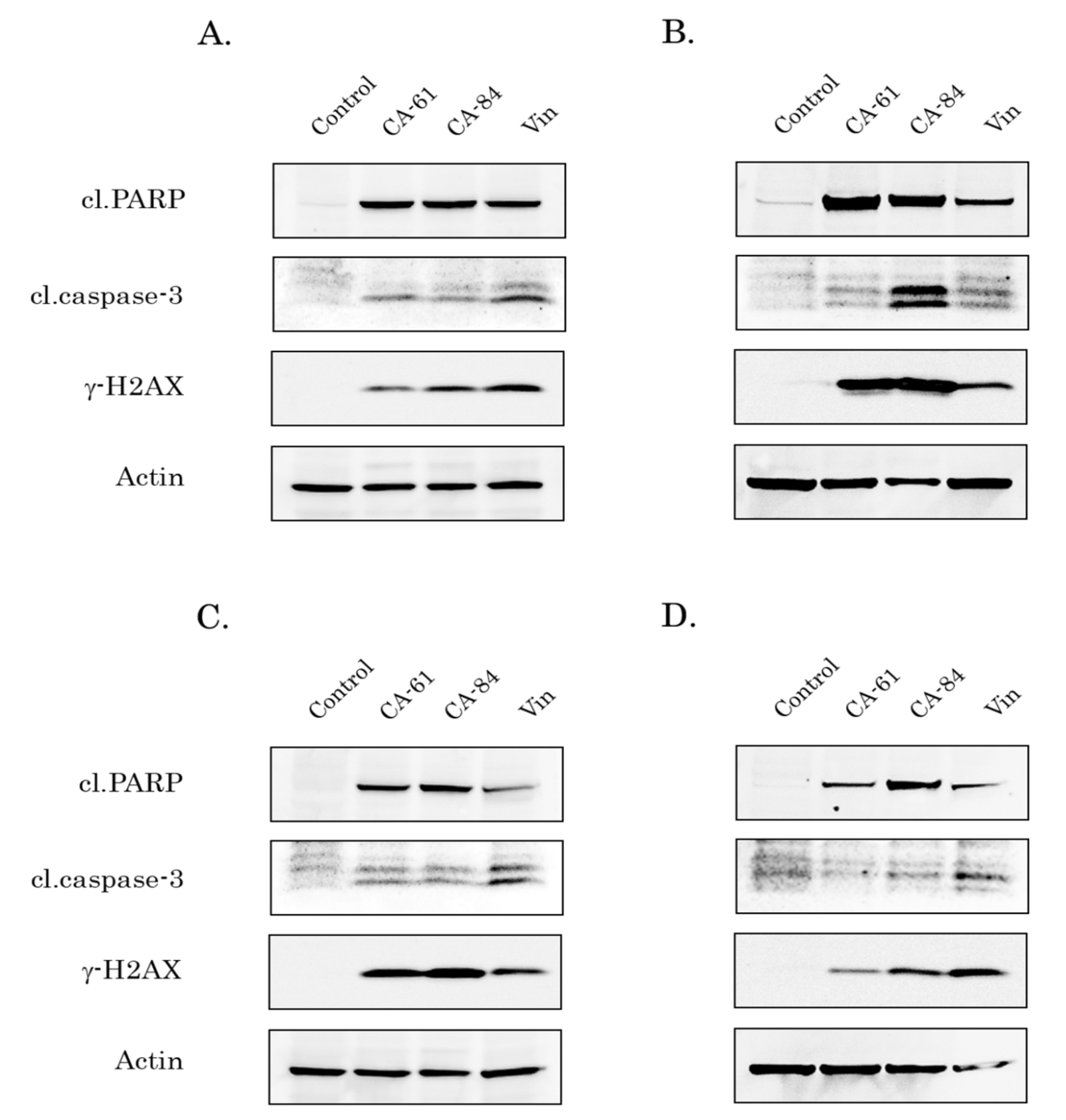
2.10. CAs Induce Apoptosis of Breast, Lung, and Prostate Cancer Cells
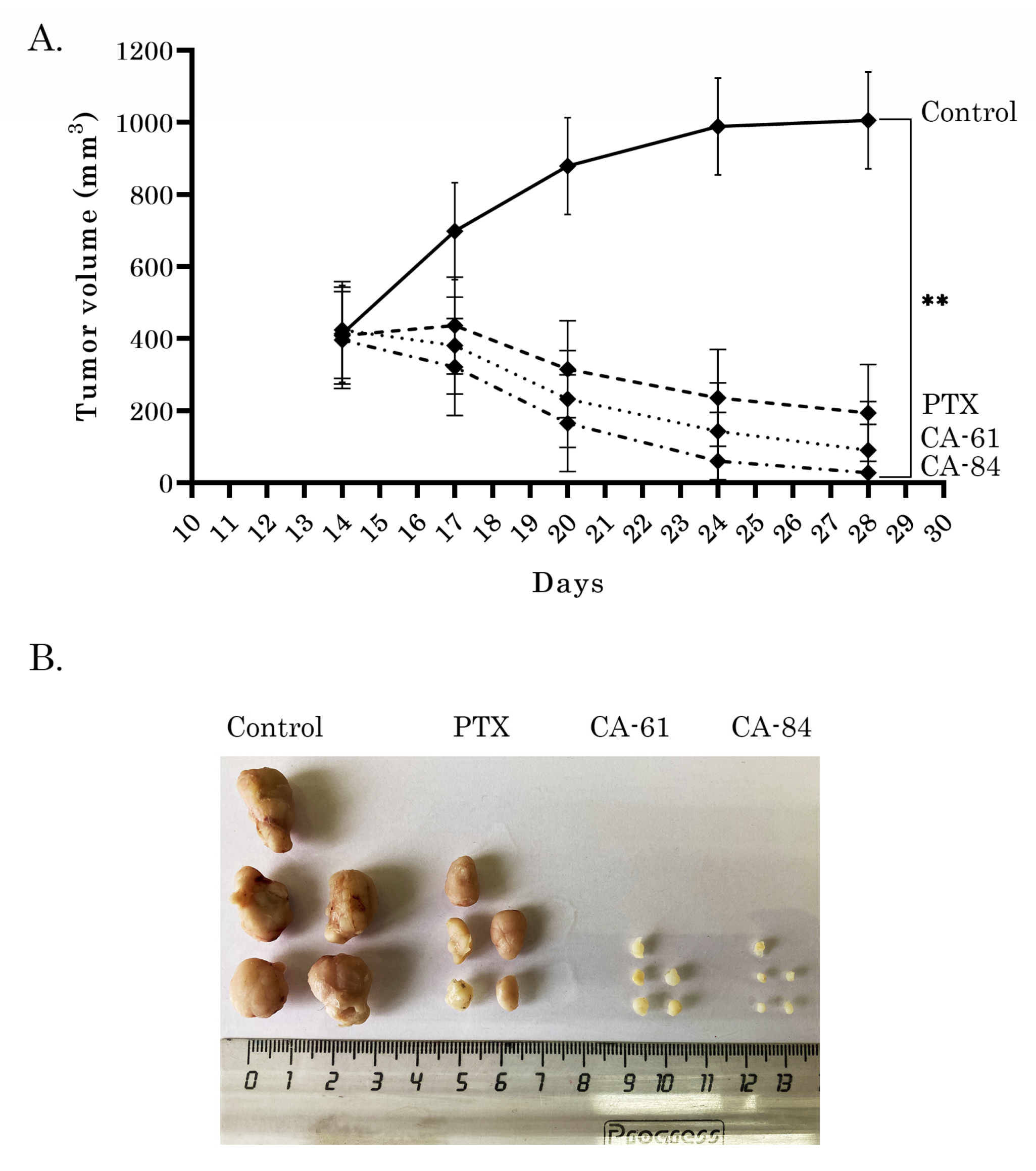
2.11. Anti-Tumor Activities of CAs in HCC1806 Xenografts
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Cell Lines and Culture Conditions
4.3. Antibodies
4.4. Cellular Survival MTS-Based Assay
4.5. Tubulin Polymerization Assay
4.6. Western Blotting
4.7. Immunofluorescence Staining
4.8. Flow Cytometry
4.9. Molecular Docking
4.10. Xenograft Studies Models
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Parker, A.L.; Kavallaris, M.; McCarroll, J.A. Microtubules and their role in cellular stress in cancer. Front. Oncol. 2014, 4, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Gigant, B.; Wang, C.; Ravelli, R.B.; Roussi, F.; Steinmetz, M.O.; Curmi, P.A.; Sobel, A.; Knossow, M. Structural basis for the regulation of tubulin by vinblastine. Nature 2005, 435, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.; Gigant, G.; Curmi, B.; Jourdain, P.A.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Y.; Wang, T.; Jiang, J.; Botting, C.H.; Liu, H.; Chen, Q.; Yang, J.; Naismith, J.H.; Zhu, X.; et al. Pironetin reacts covalently with cysteine-316 of α-tubulin to destabilize microtubule. Nat. Commun. 2016, 7, 12103. [Google Scholar] [CrossRef]
- Prota, A.E.; Setter, J.; Waight, A.B.; Bargsten, K.; Murga, J.; Diaz, J.F.; Steinmetz, M.O. Pironetin binds covalently to αCys316 and perturbs a major loop and helix of α-tubulin to inhibit microtubule formation. J. Mol. Biol. 2016, 428, 2981–2988. [Google Scholar] [CrossRef]
- Steinmetz, M.O.; Prota, A.E. Microtubule-targeting agents: Strategies to hijack the cytoskeleton. Trends Cell. Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Fanale, D.; Bronte, G.; Passiglia, F.; Calo, V.; Castiglia, M.; Di Piazza, F.; Barraco, N.; Cangemi, A.; Catarella, M.T.; Insalaco, L.; et al. Stabilizing versus destabilizing the microtubules: A double-edge sword for an effective cancer treatment option? Anal. Cell. Pathol. 2015, 2015, 690916. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Tien, G.; Hernandez, A.H.; Plubrukarn, A.; Davidson, B.S. Laulimalide and isolaulimalide, new paclitaxel-like microtubule-stabilizing agents. Cancer Res. 1999, 59, 653–660. [Google Scholar]
- West, L.M.; Northcote, P.T.; Battershill, C.N. Peloruside A: A potent cytotoxic macrolide isolated from the New Zealand marine sponge Mycale sp. J. Org. Chem. 2000, 65, 445–449. [Google Scholar] [CrossRef] [PubMed]
- Prota, A.E.; Bargsten, K.; Northcote, P.T.; Marsh, M.; Altmann, K.H.; Miller, J.H.; Diaz, J.F.; Steinmetz, M.O. Structural basis of microtubule stabilization by laulimalide and peloruside A. Angew. Chem. Int. Ed. Engl. 2014, 53, 1621–1625. [Google Scholar] [CrossRef]
- Munshi, N.; Jeay, S.; Li, Y.; Chen, C.-R.; France, D.S.; Ashwell, M.A.; Hill, J.; Moussa, M.M.; Leggett, D.S.; Li, C.J. ARQ 197, a novel and selective inhibitor of the human c-met receptor tyrosine kinase with antitumor activity. Mol. Cancer Ther. 2010, 9, 1544–1553. [Google Scholar] [CrossRef]
- Katayama, R.; Aoyama, A.; Yamori, T.; Qi, J.; Oh-hara, T.; Song, Y.; Engelman, J.A.; Fujita, N. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res. 2013, 73, 3087–3096. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, A.; Katayama, R.; Oh-Hara, T.; Sato, S.; Okuno, Y.; Fujita, N. Tivantinib (ARQ 197) exhibits antitumor activity by directly interacting with tubulin and overcomes ABC transporter-mediated drug resistance. Mol. Cancer Ther. 2014, 13, 2978–2990. [Google Scholar] [CrossRef] [PubMed]
- Gumireddy, K.; Reddy, M.V.R.; Cosenza, S.C.; Nathan, R.B.; Baker, S.J.; Papathi, N.; Jiang, J.; Holland, J.; Reddy, E.P. ON01910, a non-ATP-competitive small molecule inhibitor of Plk1, is a potent anticancer agent. Cancer Cell. 2005, 7, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Jost, M.; Chen, Y.; Gilbert, L.A.; Horlbeck, M.A.; Krenning, L.; Menchon, G.; Rai, A.; Cho, M.Y.; Stern, J.J.; Prota, A.E.; et al. Combined CRISPRi/a-based chemical genetic screens reveal that rigosertib is a microtubule-destabilizing agent. Mol. Cell. 2017, 68, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Hong, S.; Hong, S. Nocodazole is a high-affinity ligand for the cancer-related kinases ABL, c-KIT, BRAF, and MEK. ChemMedChem 2012, 7, 53–56. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, X.; Li, Y.; Guo, Y.; Wang, J.; Li, Y.; Shen, B.; Sun, D.; Zhang, J. Nocodazole increases the ERK activity to enhance MKP-1 expression which inhibits p38 activation induced by TNF-α. Mol. Cell. Biochem. 2012, 364, 373–380. [Google Scholar] [CrossRef]
- Tanabe, K. Microtubule depolymerization by kinase Inhibitors: Unexpected findings of dual Inhibitors. Int. J. Mol. Sci. 2017, 18, 2508. [Google Scholar] [CrossRef]
- Ramirez-Rios, S.; Michallet, S.; Peris, L.; Barette, C.; Rabat, C.; Feng, Y.; Fauvarque, M.O.; Andrieux, A.; Sadoul, K.; Lafanechère, L. A New Quantitative Cell-Based Assay Reveals Unexpected Microtubule Stabilizing Activity of Certain Kinase Inhibitors, Clinically Approved or in the Process of Approval. Front. Pharmacol. 2020, 11, 543. [Google Scholar] [CrossRef]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
- Mechetner, E.; Kyshtoobayeva, A.; Zonis, S.; Kim, H.; Stroup, R.; Garcia, R.; Parker, R.J.; Fruehauf, J.P. Levels of multidrug resistance (MDR1) P-glycoprotein expressing by human breast cancer correlate with in vitro resistance to taxol and doxorubicin. Clin. Cancer Res. 1998, 4, 389–398. [Google Scholar]
- Kavallaris, M.; Kuo, D.Y.; Burkhart, C.A.; Regl, D.L.; Norris, M.D.; Haber, M.; Horwitz, S.B. Taxol-resistant epithelial ovarian tumors are associated with altered expression of specific beta-tubulin isotypes. J. Clin. Investig. 1997, 100, 1282–1293. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Zykova, S.S.; Boĭchuk, S.V.; Galembikova, A.R.; Ramazanov, B.R.; Mustafin, I.G.; Igidov, N.M.; Odegova, T.F. 3-Hydroxy-1,5-diaryl-4-pivaloyl-2,5-dihydro-2-pyrrolones induce the mitotic exit failure and cell death in tumor cells in vitro. Tsitologiia 2014, 56, 439–442. [Google Scholar]
- Boichuk, S.; Galembikova, A.; Zykova, S.; Ramazanov, B.; Khusnutdinov, R.; Dunaev, P.; Khaibullina, S.; Lombardi, V. Ethyl-2-amino-pyrrole-3-carboxylates are novel potent anticancer agents that affect tubulin polymerization, induce G2/M cell-cycle arrest, and effectively inhibit soft tissue cancer cell growth in vitro. Anti-Cancer Drugs 2016, 27, 620–634. [Google Scholar] [CrossRef]
- Boichuk, S.; Galembikova, A.; Dunaev, P.; Micheeva, E.; Novikova, M.; Khromova, N.; Kopnin, P. Ethyl-2-amino-pyrrole-3-carboxylates are active against imatinib-resistant gastrointestinal stromal tumors in vitro and in vivo. Anti-Cancer Drugs 2019, 30, 475–484. [Google Scholar] [CrossRef]
- Carta, D.; Bortolozzi, R.; Sturlese, M.; Salmaso, V.; Hamel, E.; Basso, G.; Calderan, L.; Quintieri, L.; Moro, S.; Viola, G.; et al. Synthesis, structure-activity relationships and biological evaluation of 7-phenyl-pyrroloquinolinone 3-amide derivatives as potent antimitotic agents. Eur. J. Med. Chem. 2017, 127, 643–660. [Google Scholar] [CrossRef]
- Brindisi, M.; Ulivieri, C.; Alfano, G.; Gemma, S.; de Asís Balaguer, F.; Khan, T.; Grillo, A.; Chemi, G.; Menchon, G.; Prota, A.E.; et al. Structure-activity relationships, biological evaluation and structural studies of novel pyrrolonaphthoxazepines as antitumor agents. Eur. J. Med. Chem. 2019, 162, 290–320. [Google Scholar] [CrossRef] [PubMed]
- Kharitonova, S.S.; Igidov, N.M.; Zakhmatov, A.V.; Rubtsov, A.E. Chemistry of iminofurans VIII. Recyclization of 5-aryl-3-arylimino-3 H-furan-2-ones by the action of cyanoacetic acid derivatives. Russ. J. Org. Chem. 2013, 49, 243–252. [Google Scholar] [CrossRef]
- Igidov, N.M.; Zakhmatov, A.V.; Rubtsov, A.E. Chemistry of iminofurans. XIII. Recyclization of 4-arylamino-2-tert-butyl-5-oxo-2,5-dihydrofuran-2-yl acetates with ethyl cyanoacetate. Russ. J. Org. Chem. 2016, 52, 974–977. [Google Scholar] [CrossRef]
- Zykova, S.; KIzimova, I.; Syutkina, A.; Toksarova, Y.; Igidov, N.; Ibishov, D.; Boichuk, S.; Dunaev, P.; Galembikova, A.; Korochkina, R. Synthesis and cytostatic activity of (E)-ethyl-2-amino-5-(3,3-dimethyl-4-oxobutyliden-4-oxo-1-(2-phenylaminobenzamido)-4,5-dihydro-1Hpyrrol-3-carboxylate. Pharm. Chem. J. 2020, 53, 895–898. [Google Scholar] [CrossRef]
- Meng, X.Y.; Zhang, H.X.; Mezei, M.; Cui, M. Molecular docking: A powerful approach for structure-based drug discovery. Curr. Comput. Aided Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular docking and structure-based drug design strategies. Molecules 2015, 20, 3384–3421. [Google Scholar] [CrossRef]
- Prota, A.E.; Danel, F.; Bachmann, F.; Bargsten, K.; Buey, R.M.; Pohlmann, J.; Reinelt, S.; Lane, H.; Steinmetz, M.O. The novel microtubule-destabilizing drug BAL27862 binds to the colchicine site of tubulin with distinct effects on microtubule organization. J. Mol. Biol. 2014, 426, 1848–1860. [Google Scholar] [CrossRef] [PubMed]
- Kizimova, I.A.; Igidov, N.M.; Kiselev, M.A.; Dmitriev, M.V.; Chashchina, S.V.; Syutkina, A.I. Synthesis of new derivatives of 2-aminopyrroles by reaction of 3-acylhydrazones 2,3 -furandiones with CH-nucleophiles. Russ. J. Gen. Chem. 2020, 90, 182–186. [Google Scholar] [CrossRef]
- Distefano, M.; Scambia, G.; Ferlini, C.; Gallo, D.; De Vincenzo, R.; Filippini, P.; Riva, A.; Bombardelli, E.; Mancuso, S. Antitumor activity of paclitaxel (taxol) analogues on MDR-positive human cancer cells. Anticancer Drug Des. 1998, 13, 489–499. [Google Scholar] [PubMed]
- Horwitz, S.B.; Cohen, D.; Rao, S.; Ringel, I.; Shen, H.J.; Yang, C.P. Taxol: Mechanisms of action and resistance. J. Natl. Cancer Inst. Monogr. 1993, 15, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pirol, Ş.C.; Çalışkan, B.; Durmaz, I.; Atalay, R.; Banoglu, E. Synthesis and preliminary mechanistic evaluation of 5-(p-tolyl)-1-(quinolin-2-yl)pyrazole-3-carboxylic acid amides with potent anti-proliferative activity on human cancer cell lines. Eur. J. Med. Chem. 2014, 87, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.; Lu, Q.; Wang, X.; Sun, R.; Jin, Z.; Zhan, X.; Hu, J.; Wan, D.C.; Hu, C. Discovery of 4,5-dihydro-1H-thieno[2′,3′:2,3]thiepino [4,5-c]pyrazole-3-carboxamide derivatives as the potential epidermal growth factor receptors for tyrosine kinase inhibitors. Molecules 2018, 23, 1980. [Google Scholar] [CrossRef]
- Lin, T.; Li, J.; Liu, L.; Li, Y.; Jiang, H.; Chen, K.; Xu, P.; Luo, C.; Zhou, B. Design, synthesis, and biological evaluation of 4-benzoylamino-1H-pyrazole-3-carboxamide derivatives as potent CDK2 inhibitors. Eur. J. Med. Chem. 2021, 215, 113281. [Google Scholar] [CrossRef]
- Yasuda, Y.; Arakawa, T.; Nawata, Y.; Shimada, S.; Oishi, S.; Fujii, N.; Nishimura, S.; Hattori, A.; Kakeya, H. Design, synthesis, and structure-activity relationships of 1-ethylpyrazole- 3-carboxamide compounds as novel hypoxia-inducible factor (HIF)-1 inhibitors. Bioorg. Med. Chem. 2015, 23, 1776–1787. [Google Scholar] [CrossRef]
- Gul, H.I.; Mete, E.; Eren, S.E.; Sakagami, H.; Yamali, C.; Supuran, C.T. Designing, synthesis and bioactivities of 4-[3-(4-hydroxyphenyl)-5-aryl-4,5-dihydro-pyrazol-1-yl]benzenesulfonamides. J. Enzyme Inhib. Med. Chem. 2017, 32, 169–175. [Google Scholar] [CrossRef][Green Version]
- Gul, H.I.; Yamali, C.; Bulbuller, M.; Kirmizibayrak, P.B.; Gul, M.; Angeli, A.; Bua, S.; Supuran, C.T. Anticancer effects of new dibenzenesulfonamides by inducing apoptosis and autophagy pathways and their carbonic anhydrase inhibitory effects on hCA I, hCA II, hCA IX, hCA XII isoenzymes. Bioorg. Chem. 2018, 78, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Gul, H.I.; Yamali, C.; Sakagami, H.; Angeli, A.; Leitans, J.; Kazaks, A.; Tars, K.; Ozgun, D.O.; Supuran, C.T. New anticancer drug candidates sulfonamides as selective hCA IX or hCA XII inhibitors. Bioorg. Chem. 2018, 77, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Yamali, C.; Sakagami, H.; Uesawa, Y.; Kurosaki, K.; Satoh, K.; Masuda, Y.; Yokose, S.; Ece, A.; Bua, S.; Angeli, A.; et al. Comprehensive study on potent and selective carbonic anhydrase inhibitors: Synthesis, bioactivities and molecular modelling studies of 4-(3-(2-arylidenehydrazine-1-carbonyl)-5-(thiophen-2-yl)-1H-pyrazole-1-yl) benzenesulfonamides. Eur. J. Med. Chem. 2021, 217, 113351. [Google Scholar] [CrossRef] [PubMed]
- Mooberry, S.L.; Weiderhold, K.N.; Dakshanamurthy, S.; Hamel, E.; Banner, E.J.; Kharlamova, A.; Hempel, J.; Gupton, J.T.; Brown, M.L. Identification and characterization of a new tubulin-binding tetrasubstituted brominated pyrrole. Mol. Pharmacol. 2007, 72, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Da, C.; Telang, N.; Barelli, P.; Jia, X.; Gupton, J.T.; Mooberry, S.L.; Kellogg, G.E. Pyrrole-Based Antitubulin Agents: Two Distinct Binding Modalities Are Predicted for C-2 Analogues in the Colchicine Site. ACS Med. Chem. Lett. 2012, 3, 53–57. [Google Scholar] [CrossRef]
- Romagnoli, R.; Oliva, P.; Salvador, M.K.; Manfredini, S.; Padroni, C.; Brancale, A.; Ferla, S.; Hamel, E.; Ronca, R.; Maccarinelli, F.; et al. A facile synthesis of diaryl pyrroles led to the discovery of potent colchicine site antimitotic agents. Eur. J. Med. Chem. 2021, 214, 113229. [Google Scholar] [CrossRef]
- Galenko, E.; Kaminskiy, N.; Novikov, M.; Khlebnikov, A. Synthesis of Water-Soluble α-Aminopyrroles, 1-(2-Amino-1H-pyrrol-3-yl)pyridinium Chlorides. Russ. J. Gen. Chem. 2021, 91, 1424–1428. [Google Scholar] [CrossRef]
- Huang, G.S.; Lopez-Barcons, L.; Freeze, B.S.; Smith, A.B., 3rd; Goldberg, G.L.; Horwitz, S.B.; McDaid, H.M. Potentiation of taxol efficacy and by dis-codermolide in ovarian carcinoma xenograft-bearing mice. Clin. Cancer Res. 2006, 12, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Abel, R.; Zhu, K.; Cao, Y.; Zhao, S.; Friesner, R.A. The VSGB 2.0 model: A next generation energy model for high resolution protein structure modeling. Proteins 2011, 79, 2794–2812. [Google Scholar] [CrossRef] [PubMed]

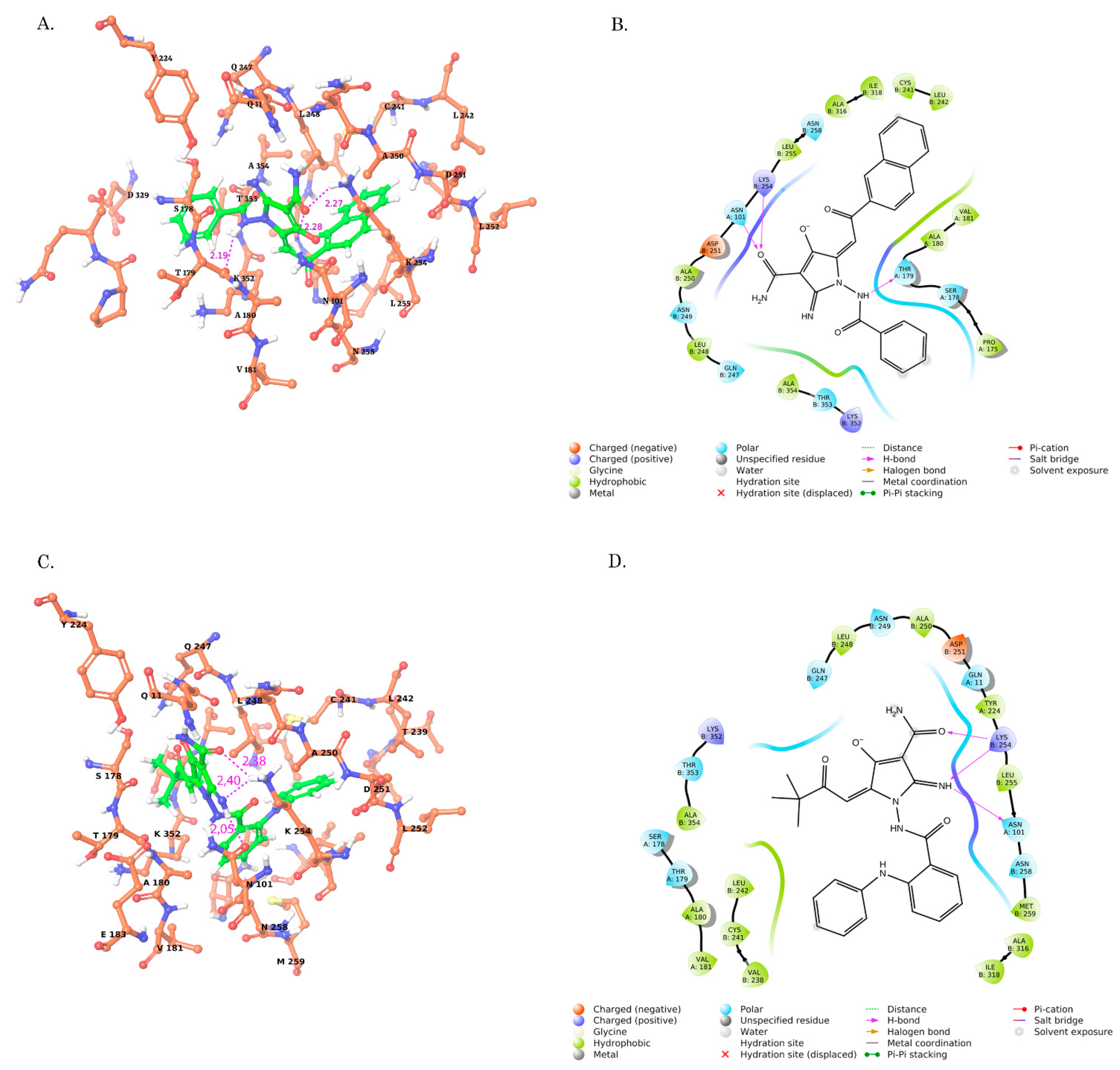






| Compound | Glide Score XP (kcal/mol) | Glide Score XP (kcal/mol) | MM-GBSA ΔGbind (kcal/mol) | Ligand Strain Energy | H-Bonds Interactions D(A,B)-A[X] | Hydrophobic Interaction (A,B) |
|---|---|---|---|---|---|---|
| CA-84 | −9.903 | −7.703 | −16.82 | 11.22 | OD1 (ASN A:101)-H13 [2.05] HZ3 (LYS B:254)-O3 [2.38] HZ3 (LYS B:254)-N4 [2.40] | VAL B:238 LEU B:248 CYS B:241 LEU B:242 MET B:259 ALA B:250 ALA B:354 ALA A:180 VAL A:181 |
| CA-61 | −9.390 | −7.420 | −16.60 | 12.95 | HZ3 (LYS B:254)-O2 [2.53] HD22 (ASN A:101)-O2 [2.05] O (THR A:179)-H12 [2.29] | LEU B:248 ALA B:250 CYS B:241 LEU B:242 LEU B:255 ALA B:354 ALA B:316 ILE B:318 |
| CA-166 | −8.334 | −7.009 | −14.79 | 15.5 | HD22 (ASN A:101)-O31 [2.41] NZ (LYS B:254)-O31 [2. 59] H (THR B:353)-O12 [2.09] | VAL B:238 LEU B:248 CYS B:241 LEU B:242 MET B:259 ALA B:250 ALA B:354 ALA A:180 VAL A:181 |
| CA-59 | −7.365 | −6.359 | −13.31 | 18.9 | NZ (LYS B:254)-O31 [2. 78] H (THR B:353) -O12 [2.34] | LEU B:248 LEU B:255 ALA B:354 ILE A: 171 ALA B:250 VAL A:177 ALA A:180 VAL A:181 TYR A:224 |
| CA-1489I | −8.823 | −7.961 | −14.63 | 28.7 | HZ3 (LYS B:254)-O1 [2.20] HE22 (GNL A:11)-O3 [2.24] O (GNL B:247)-H3 [2.60] Halogen bound H (THR B:353) -Br23 [2.20] | LEU B:248 ALA B:250 LEU B:255 ALA B:354 ILE A: 171 VAL A:177 ALA A:180 VAL A:181 TYR A:224 |
| CA-1488I | −7.125 | −6.895 | −10.24 | 13.41 | HZ3 (LYS B:254)-O1 [2.18] O (GNL B:247)-H3 [2.63] Halogen bound H (THR B:353) -Br23 [2.22] | ALA A:180 VAL A:181 ALA B:354 ILE B:318 CYS B:241 MET B:259 LEU B:255 ALA B:316 ALA B:317 |
| CA-1348I | −6.448 | −3.446 | −6.20 | 31.4 | OD1 (ASN A:101)-H31 [2.26] HZ3 (LYS B:254)-N11 [2.72] | CYS B:241 MET B:259 LEU B:255 ALA B:354 ILE A: 171 VAL A:177 ALA A:180 VAL A:181 TYR A:224 |
| BAL27862 | −12.434 | −10.987 | −32.60 | 12.4 | O (TYR B:202)-H46 [1.96] O (VAL B:239)-H45 [2.51] O (THR A:179)-H36 [2.76] HZ2 (LYS B:352)-N9 [2.00] | LEU B:248 LEU B:255 CYS B:241 ALA B:354 TYR B:202 VAL B:238 VAL B:351 ILE B: 347 PRO B:348 |
| Cell Line | CA-61 (µM) | CA-84 (µM) |
|---|---|---|
| BJ tert | 4.0 ± 0.5 | 4.2 ± 0.4 |
| HCC1806 | 8.4 ± 0.3 | 7.2 ± 0.6 |
| MDA-MB-231 | 3.2 ± 0.3 | 4.8 ± 0.6 |
| H1299 | 2.8 ± 0.3 | 3.1 ± 0.5 |
| PC-3 | 7.0 ± 0.4 | 6.8 ± 0.3 |
| G0/G1 | S | G2/M | |
|---|---|---|---|
| Control | 37.04 ± 2.17 | 18.80 (18.5–18.9) | 42.26 ± 3.03 |
| CA-61 | 22.84 ± 2.35 ** | 23.38 ± 2.68 * | 51.26 ± 3.65 * |
| CA-84 | 24.10 (24–24.5) ** | 23.14 ± 0.69 * | 68.30 (67.6–69)* |
| PTX | 1.90 ± 0.85 ** | 20.40 ± 2.47 | 68.24 ± 1.80 * |
| Vin | 30.48 ± 1.54 ** | 19.56 ± 0.74 | 47.12 ± 1.34 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boichuk, S.; Galembikova, A.; Syuzov, K.; Dunaev, P.; Bikinieva, F.; Aukhadieva, A.; Zykova, S.; Igidov, N.; Gankova, K.; Novikova, M.; et al. The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site. Molecules 2021, 26, 5780. https://doi.org/10.3390/molecules26195780
Boichuk S, Galembikova A, Syuzov K, Dunaev P, Bikinieva F, Aukhadieva A, Zykova S, Igidov N, Gankova K, Novikova M, et al. The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site. Molecules. 2021; 26(19):5780. https://doi.org/10.3390/molecules26195780
Chicago/Turabian StyleBoichuk, Sergei, Aigul Galembikova, Kirill Syuzov, Pavel Dunaev, Firuza Bikinieva, Aida Aukhadieva, Svetlana Zykova, Nazim Igidov, Ksenia Gankova, Maria Novikova, and et al. 2021. "The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site" Molecules 26, no. 19: 5780. https://doi.org/10.3390/molecules26195780
APA StyleBoichuk, S., Galembikova, A., Syuzov, K., Dunaev, P., Bikinieva, F., Aukhadieva, A., Zykova, S., Igidov, N., Gankova, K., Novikova, M., & Kopnin, P. (2021). The Design, Synthesis, and Biological Activities of Pyrrole-Based Carboxamides: The Novel Tubulin Inhibitors Targeting the Colchicine-Binding Site. Molecules, 26(19), 5780. https://doi.org/10.3390/molecules26195780








