Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Dryings of the Plants and Hydrodistillation for Obtaining Essential Oil Batches
2.3. Gas Chromatography-Flame Ionization Detector (GC-FID) Analyses
2.4. Identification of the Essential oil Constituents
2.5. In Vivo Hepatoprotective Assay
2.5.1. Experimental Animals
2.5.2. Acute Toxicity Studies
2.5.3. Animal Groups
2.5.4. Determination of Liver, Kidneys Functions, and Lipid Profile
2.6. Cell-proliferation Assays
Viability percentage and Selectivity Index:
2.7. Acetaminophen (AAP) Induced Hepatotoxicity in Hepg2 Cells
2.8. Measurement of Total Antioxidant Capacity (TAOxC)
2.9. Measurement of MDA for Lipid Peroxidation
2.10. Statistical Analysis
3. Results and Discussion
3.1. Sage Essential Oil Obtained from the Fresh Aerial Parts of the Plants and the Extended-Dried Plant Batches
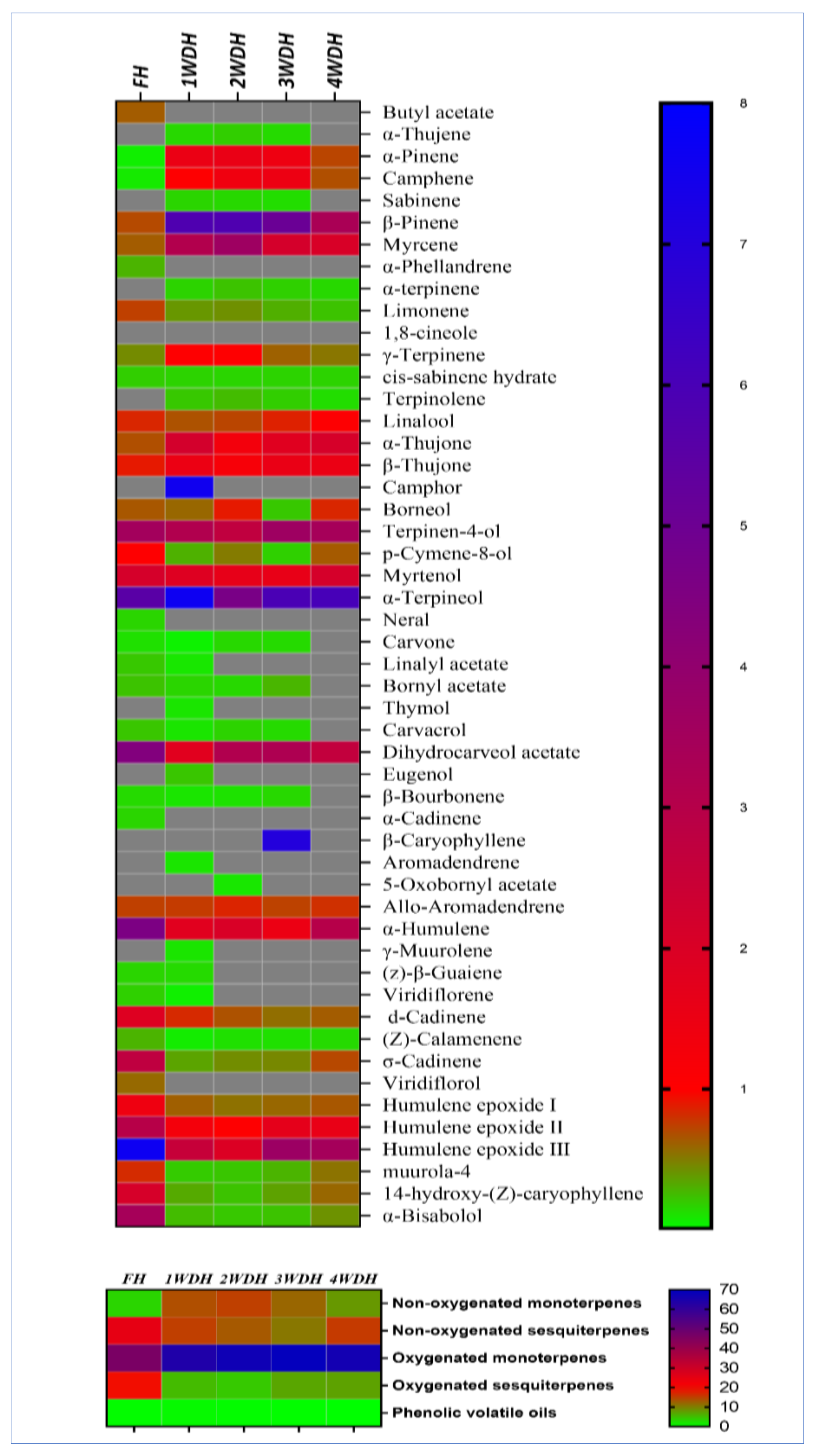
3.2. Componential Analysis of the Essential Oil Obtained from Different Batches
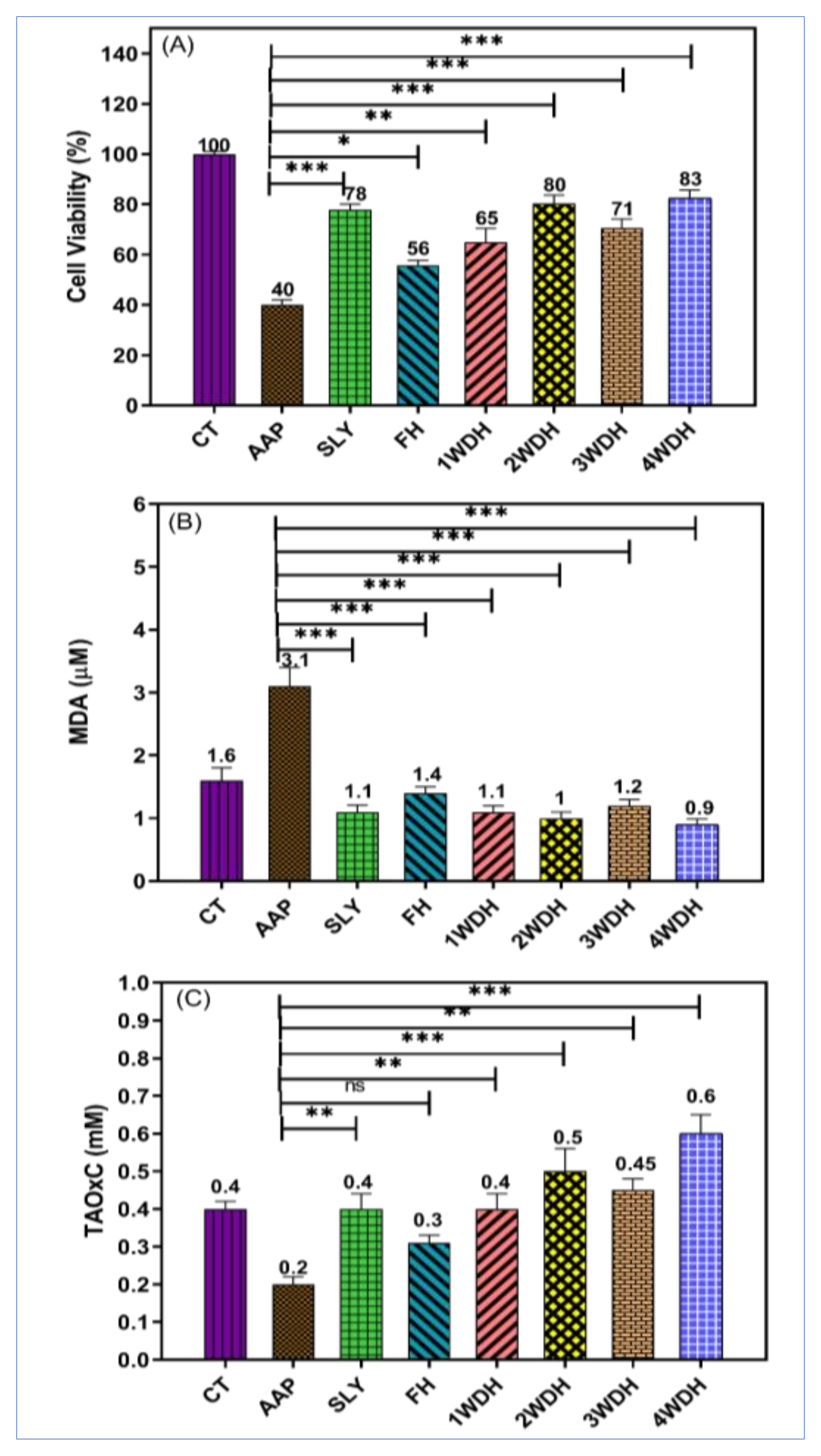
3.3. Hepatoprotective Effect of the Essential Oil Batches
3.3.1. In vivo Hepatoprotective Effect
3.3.2. In vitro Hepatoprotective Effects
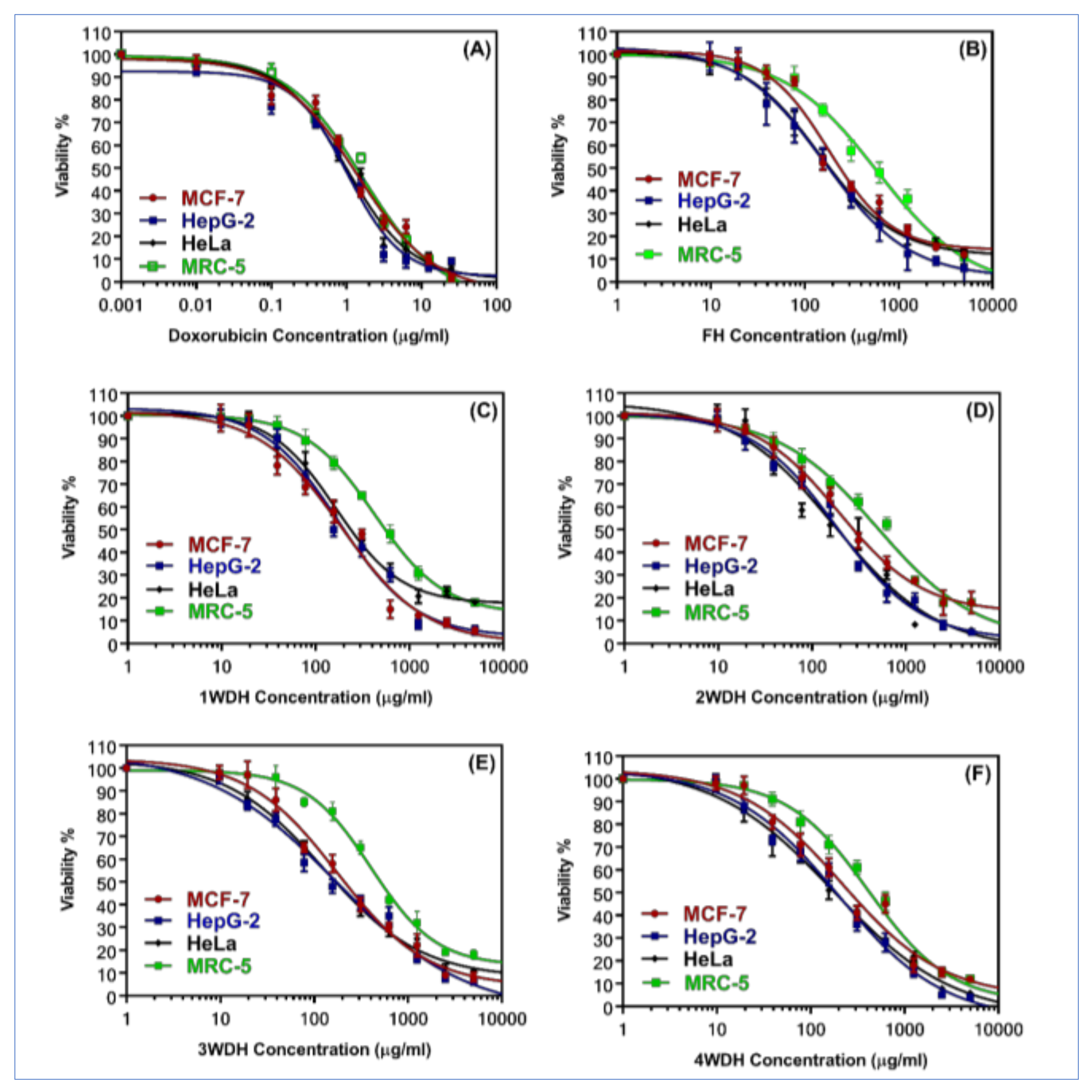
3.4. Anticancer Effects of Essential Oils Obtained from Different-Timed Drying Herbs Batches
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Leal, A.L.A.B. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar]
- Khedher, M.R.B.; Khedher, S.B.; Chaieb, I.; Tounsi, S.; Hammami, M. Chemical composition and biological activities of Salvia officinalis essential oil from Tunisia. EXCLI J. 2017, 16, 160. [Google Scholar] [PubMed]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar]
- Adams, M.; Gmünder, F.; Hamburger, M. Plants traditionally used in age-related brain disorders—A survey of ethnobotanical literature. J. Ethnopharmacol. 2007, 113, 363–381. [Google Scholar] [PubMed]
- Perry, E.K.; Pickering, A.T.; Wang, W.W.; Houghton, P.J.; Perry, N.S.L. Medicinal plants and Alzheimer’s disease: From ethnobotany to phytotherapy. J. Pharm. Pharmacol. 1999, 51, 527–534. [Google Scholar] [PubMed]
- Eidi, M.; Eidi, A.; Zamanizadeh, H. Effect of Salvia officinalis L. leaves on serum glucose and insulin in healthy and streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2005, 100, 310–313. [Google Scholar]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The chemotaxonomy of common Sage (Salvia officinalis) based on the volatile constituents. Medicines 2017, 4, 47. [Google Scholar]
- Perry, N.B.; Anderson, R.E.; Brennan, N.J.; Douglas, M.H.; Heaney, A.J.; McGimpsey, J.A.; Smallfield, B.M. Essential oils from Dalmatian Sage (Salvia officinalis L.): Variations among individuals, plant parts, seasons, and sites. J. Agric. Food Chem. 1999, 47, 2048–2054. [Google Scholar]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical composition and anticancer activity of essential oils of Mediterranean Sage (Salvia officinalis L.) grown in different environmental conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar]
- Bouajaj, S.; Benyamna, A.; Bouamama, H.; Romane, A.; Falconieri, D.; Piras, A.; Marongiu, B. Antibacterial, allelopathic and antioxidant activities of essential oil of Salvia officinalis L. growing wild in the Atlas Mountains of Morocco. Nat. Prod. Res. 2013, 27, 1673–1676. [Google Scholar] [PubMed]
- Reverchon, E.; Taddeo, R.; Porta, G.D. Extraction of Sage oil by supercritical C02: Influence of some process parameters. J. Supercrit. Fluids 1995, 8, 302–309. [Google Scholar]
- Glisic, S.; Ivanovic, J.; Ristic, M.; Skala, D. Extraction of Sage (Salvia officinalis L.) by supercritical CO2: Kinetic data, chemical composition and selectivity of diterpenes. J. Supercrit. Fluids 2010, 52, 62–70. [Google Scholar]
- Durling, N.E.; Catchpole, O.J.; Grey, J.B.; Webby, R.F.; Mitchell, K.A.; Foo, L.Y.; Perry, N.B. Extraction of phenolics and essential oil from dried Sage (Salvia officinalis) using ethanol-water mixtures. Food Chem. 2007, 101, 1417–1424. [Google Scholar]
- Sellami, I.H.; Rebey, I.B.; Sriti, J.; Rahali, F.Z.; Limam, F.; Marzouk, B. Drying Sage (Salvia officinalis L.) plants and its effects on content, chemical composition, and radical scavenging activity of the essential oil. Food Bioprocess Technol. 2012, 5, 2978–2989. [Google Scholar]
- Venskutonis, P.R. Effect of drying on the volatile constituents of thyme (Thymus vulgaris L.) and Sage (Salvia officinalis L.). Food Chem. 1997, 59, 219–227. [Google Scholar]
- Ibraliu, A.; Doko, A.; Hajdari, A.; Gruda, N.; Šatović, Z.; Karanfilova, I.C.; Stefkov, G. Essential oils chemical variability of seven populations of Salvia officinalis L. in North of Albania. Maced. J. Chem. Chem. Eng. 2020, 39, 31–39. [Google Scholar]
- Cvetkovikj, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S.; Satović, Z. Essential oils and chemical diversity of southeast European populations of Salvia officinalis L. Chem. Biodivers. 2015, 12, 1025–1039. [Google Scholar]
- Jug-Dujaković, M.; Ristić, M.; Pljevljakušić, D.; Dajić-Stevanović, Z.; Liber, Z.; Hančević, K.; Radić, T.; Šatović, Z. High diversity of indigenous populations of dalmatian Sage (Salvia officinalis L.) in essential-oil composition. Chem. Biodivers. 2012, 9, 2309–2323. [Google Scholar]
- Raal, A.; Orav, A.; Arak, E. Composition of the essential oil of Salvia officinalis L. from various European countries. Nat. Prod. Res. 2007, 21, 406–411. [Google Scholar]
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of extraction and drying method on chemical composition, and evaluation of antioxidant and antimicrobial activities of essential oils from Salvia officinalis L. J. Essent. Oil Bear. Plants 2019, 22, 717–727. [Google Scholar]
- Putievsky, E.; Ravid, U.; Dudai, N. The influence of season and harvest frequency on essential oil and herbal yields from a pure clone of Sage (Salvia officinalis) grown under cultivated conditions. J. Nat. Prod. 1986, 49, 326–329. [Google Scholar]
- Madrigal-Santillán, E.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Sumaya-Martínez, M.T.; Gutiérrez-Salinas, J.; Bautista, M.; Morales-González, Á.; y González-Rubio, M.G.-L.; Aguilar-Faisal, J.L.; Morales-González, J.A. Review of natural products with hepatoprotective effects. World J. Gastroenterol. WJG 2014, 20, 14787. [Google Scholar]
- Mohammed, S.A.A.; Khan, R.A.; El-Readi, M.Z.; Emwas, A.-H.; Sioud, S.; Poulson, B.G.; Jaremko, M.; Eldeeb, H.M.; Al-Omar, M.S.; Mohammed, H.A. Suaeda vermiculata Aqueous-Ethanolic Extract-Based Mitigation of CCl4-Induced Hepatotoxicity in Rats, and HepG-2 and HepG-2/ADR Cell-Lines-Based Cytotoxicity Evaluations. Plants 2020, 9, 1291. [Google Scholar] [CrossRef]
- Eldeeb, H.M.; Mohammed, H.A.; Sajid, M.S.M.; Eltom, S.E.M.; Al-Omar, M.S.; Mobark, M.A.; Ahmed, A.S. Effect of Roasted Date Palm Rich Oil Extracts in Liver Protection and Antioxidant Restoration in CCl4-induced Hepato Toxicity in Rats. Int. J. Pharmacol. 2020, 16, 367. [Google Scholar] [CrossRef]
- Duthie, S.J.; Melvin, W.T.; Burke, M.D. Bromobenzene detoxification in the human liver-derived HepG-2 cell line. Xenobiotica 1994, 24, 265–279. [Google Scholar] [PubMed]
- Sassa, S.; Sugita, O.; Galbraith, R.A.; Kappas, A. Drug metabolism by the human hepatoma cell, Hep G2. Biochem. Biophys. Res. Commun. 1987, 143, 52–57. [Google Scholar]
- Bajt, M.L.; Knight, T.R.; Lemasters, J.J.; Jaeschke, H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: Protection by N-acetyl cysteine. Toxicol. Sci. 2004, 80, 343–349. [Google Scholar]
- Ingawale, D.K.; Mandlik, S.K.; Naik, S.R. Models of hepatotoxicity and the underlying cellular, biochemical and immunological mechanism (s): A critical discussion. Environ. Toxicol. Pharmacol. 2014, 37, 118–133. [Google Scholar] [PubMed]
- Banna, H.; Soliman, M.; Wabel, N. Hepatoprotective effects of Thymus and Salvia essential oils on paracetamol-induced toxicity in rats. J. Physiol. Pharmacol. Adv. 2013, 3, 41. [Google Scholar]
- Fahmy, M.A.; Diab, K.A.; Abdel-Samie, N.S.; Omara, E.A.; Hassan, Z.M. Carbon tetrachloride-induced hepato/renal toxicity in experimental mice: Antioxidant potential of Egyptian Salvia officinalis L essential oil. Environ. Sci. Pollut. Res. 2018, 25, 27858–27876. [Google Scholar]
- Koubaa, F.G.; Chaâbane, M.; Turki, M.; Ayadi, F.M.; El Feki, A. Antioxidant and hepatoprotective effects of Salvia officinalis essential oil against vanadium-induced oxidative stress and histological changes in the rat liver. Environ. Sci. Pollut. Res. 2021, 28, 11001–11015. [Google Scholar]
- Lima, C.F.; Carvalho, F.; Fernandes, E.; Bastos, M.D.L.; Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol. Vitr. 2004, 18, 457–465. [Google Scholar]
- Loizzo, M.R.; Tundis, R.; Menichini, F.; Saab, A.M.; Statti, G.A.; Menichini, F. Cytotoxic activity of essential oils from Labiatae and Lauraceae families against in vitro human tumor models. Anticancer Res. 2007, 27, 3293–3299. [Google Scholar] [PubMed]
- Foray, L.; Bertrand, C.; Pinguet, F.; Soulier, M.; Astre, C.; Marion, C.; Pélissier, Y.; Bessière, J.-M. In vitro cytotoxic activity of three essential oils from Salvia species. J. Essent. Oil Res. 1999, 11, 522–526. [Google Scholar]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Anticancer activity of Salvia officinalis essential oil against HNSCC cell line (UMSCC1). HNO 2011, 59, 1203–1208. [Google Scholar] [PubMed]
- OECD. Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure; OECD Publishing: Paris, France, 2008; pp. 1–27. [Google Scholar]
- Machana, S.; Weerapreeyakul, N.; Barusrux, S.; Nonpunya, A.; Sripanidkulchai, B.; Thitimetharoch, T. Cytotoxic and apoptotic effects of six herbal plants against the human hepatocarcinoma (HepG-2) cell line. Chin. Med. 2011, 6, 39. [Google Scholar] [CrossRef] [Green Version]
- González, L.T.; Minsky, N.W.; Espinosa, L.E.M.; Aranda, R.S.; Meseguer, J.P.; Pérez, P.C. In vitro assessment of hepatoprotective agents against damage induced by acetaminophen and CCl4. BMC Complement. Altern. Med. 2017, 17, 39. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.; Aly, M.S.; Alsuqub, A.N.; Khan, R.A. Drying Induced Impact on Composition and Oil Quality of Rosemary Herb, Rosmarinus Officinalis Linn. Molecules 2020, 25, 2830. [Google Scholar] [CrossRef]
- Abu-Darwish, M.S.; Cabral, C.; Ferreira, I.V.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Al-Bdour, T.H.; Salgueiro, L. Essential oil of common Sage (Salvia officinalis L.) from Jordan: Assessment of safety in mammalian cells and its antifungal and anti-inflammatory potential. Biomed Res. Int. 2013, 2013, 538940. [Google Scholar]
- Abu-Darwish, M.S.; Al-Fraihat, A.H.; Al-Dalain, S.Y.A.; Afifi, F.M.U.; Al-Tabbal, J.A. Determination of Essential Oils and Heavy Metals Accumulation in Salvia officinalis Cultivated in three Intra-raw Spacing in Ash-Shoubak, Jordan. Int. J. Agric. Biol. 2011, 13, 981–985. [Google Scholar]
- Edris, A.E.; Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Slavchev, A. Chemical composition, antimicrobial activities, and olfactive evaluation of a Salvia officinalis L.(Sage) essential oil from Egypt. J. Essent. Oil Res. 2007, 19, 186–189. [Google Scholar]
- Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Drinking of Salvia officinalis tea increases CCl4-induced hepatotoxicity in mice. Food Chem. Toxicol. 2007, 45, 456–464. [Google Scholar] [PubMed] [Green Version]
- Amin, A.; Hamza, A.A. Hepatoprotective effects of Hibiscus, Rosmarinus and Salvia on azathioprine-induced toxicity in rats. Life Sci. 2005, 77, 266–278. [Google Scholar] [PubMed]
- Foruozandeh, H.; Vosughi Niri, M.; Kalantar, M.; Azadi, M.; Samadani, M. Protective Effect of Hydroalcoholic Extract of Salvia officinalis L. against Acute Liver Toxicity of Acetaminophen in Mice. Horiz. Med. Sci. 2016, 22, 185–191. [Google Scholar]
- Ray, S.D.; Mumaw, V.R.; Raje, R.R.; Fariss, M.W. Protection of acetaminophen-induced hepatocellular apoptosis and necrosis by cholesteryl hemisuccinate. J. Pharm. Exptl. Ther. 1996, 279, 1470–1483. [Google Scholar]
- Kamiyama, T.; Sato, C.; Liu, J.; Tajiri, K.; Miyakawa, H.; Marumo, F. Role of lipid peroxidation in acetaminophen-induced hepatotoxicity: Comparison with carbon tetrachloride. Toxicol. Lett. 1993, 66, 7–12. [Google Scholar]
- Salas, V.M.; Corcoran, G.B. Calcium-dependent DNA damage and adenosine 3′, 5′-cyclic monophosphate-independent glycogen phosphorylase activation in an in vitro model of acetaminophen-induced liver injury. Hepatology 1997, 25, 1432–1438. [Google Scholar]
- Freitag, A.F.; Cardia, G.F.E.; Da Rocha, B.A.; Aguiar, R.P.; Silva-Comar, F.M.D.S.; Spironello, R.A.; Grespan, R.; Caparroz-Assef, S.M.; Bersani-Amado, C.A.; Cuman, R.K.N. Hepatoprotective effect of silymarin (Silybum marianum) on hepatotoxicity induced by acetaminophen in spontaneously hypertensive rats. Evid.-Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [Green Version]
- El-Hosseiny, L.S.; Alqurashy, N.N.; Sheweita, S. Oxidative stress alleviation by Sage essential oil in co-amoxiclav induced hepatotoxicity in rats. Int. J. Biomed. Sci. IJBS 2016, 12, 71. [Google Scholar]
- Shahrzad, K.; Mahya, N.; Fatemeh, T.B.; Maryam, K.; Mohammadreza, F.B.; Jahromy, M.H. Hepatoprotective and antioxidant effects of Salvia officinalis L. hydroalcoholic extract in male rats. Chin. Med. 2014, 2014, 47465. [Google Scholar]
- Parsai, A.; Eidi, M.; Sadeghipour, A. Hepatoprotective effect of Sage (Salvia officinalis L.) Leaves hydro-methanolic extract against Aspergillus parasiticus aflatoxin-induced liver damage in male rats. Bull. Pharm. Res 2014, 4, 129–132. [Google Scholar]
- Elshibani, F.; Alshalmani, S.; Mohammed, H.A. Pituranthos tortuosus Essential Oil from Libya: Season Effect on the Composition and Antioxidant Activity. J. Essent. Oil Bear. Plants 2020, 23, 1095–1104. [Google Scholar]
- Rašković, A.; Milanović, I.; Pavlović, N.; Ćebović, T.; Vukmirović, S.; Mikov, M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014, 14, 225. [Google Scholar]
- Eidi, A.; Eidi, M. Antidiabetic effects of Sage (Salvia officinalis L.) leaves in normal and streptozotocin-induced diabetic rats. Diabetes Metab. Syndr. Clin. Res. Rev. 2009, 3, 40–44. [Google Scholar]
- Cover, C.; Mansouri, A.; Knight, T.R.; Bajt, M.L.; Lemasters, J.J.; Pessayre, D.; Jaeschke, H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005, 315, 879–887. [Google Scholar] [PubMed] [Green Version]
- Lin, J.; Schyschka, L.; Mühl-Benninghaus, R.; Neumann, J.; Hao, L.; Nussler, N.; Dooley, S.; Liu, L.; Stöckle, U.; Nussler, A.K. Comparative analysis of phase I and II enzyme activities in 5 hepatic cell lines identifies Huh-7 and HCC-T cells with the highest potential to study drug metabolism. Arch. Toxicol. 2012, 86, 87–95. [Google Scholar] [PubMed]
- Shahneh, F.Z.; Baradaran, B.; Orangi, M.; Zamani, F. In vitro cytotoxic activity of four plants used in Persian traditional medicine. Adv. Pharm. Bull. 2013, 3, 453. [Google Scholar]

| Periods of Drying | Fresh Weight | Weight after Drying | Essential Oil (mg) | % Yields * |
|---|---|---|---|---|
| Fresh Herb (FH) | 400 g | 400 g | 631 ± 8.05 | 0.16 |
| 1WDH | 131 g | 923 ± 6.34 | 0.23 | |
| 2WDH | 111 g | 1102 ± 15.58 | 0.28 | |
| 3WDH | 107 g | 944 ± 5.73 | 0.24 | |
| 4WDH | 107 g | 702 ± 9.10 | 0.18 |
| No. | Components | KIexp. | KIrep. | FH | 1WDH | 2WDH | 3WDH | 4WDH |
|---|---|---|---|---|---|---|---|---|
| 1 | Butyl acetate | 815 | 817 | 0.64 ± 0.09 | ||||
| 2 | α-Thujene | 930 | 932 | 0.16 ± 0.02 | 0.20 ± 0.01 | 0.15 ± 0.01 | ||
| 3 | α-Pinene | 938 | 939 | 0.07 ± 0.02 A | 1.54 ± 0.14 B | 1.57 ± 0.06 B | 1.46 ± 0.05 B | 0.73 ± 0.08 B |
| 4 | Camphene | 954 | 950 | 0.09 ± 0.02 A | 1.01 ± 0.05 B | 1.40 ± 0.08 B | 1.49 ± 0.08 B | 0.69 ± 0.05 B |
| 5 | Sabinene | 977 | 976 | 0.17 ± 0.02 | 0.16 ± 0.01 | 0.14 ± 0.01 | ||
| 6 | β-Pinene | 983 | 980 | 0.71 ± 0.08 A | 5.81 ± 0.56 B | 5.82 ± 0.24 B | 5.08 ± 0.22 B | 3.33 ± 0.13 B |
| 7 | Myrcene | 992 | 992 | 0.64 ± 0.08 A | 3.10 ± 0.23 B | 3.64 ± 0.17 B | 2.17 ± 0.05 B | 2.05 ± 0.05 B |
| 8 | α-Phellandrene | 1001 | 1008 | 0.30 ± 0.04 | ||||
| 9 | α-terpinene | 1021 | 1018 | 0.18 ± 0.03 | 0.24 ± 0.02 | 0.19 ± 0.00 | 0.16 ± 0.01 | |
| 10 | Limonene | 1034 | 1033 | 0.75 ± 0.07 A | 0.41 ± 0.03 B | 0.44 ± 0.03 B | 0.32 ± 0.01 B | 0.24 ± 0.02 B |
| 11 | 1,8-Cineole | 1040 | 1039 | 16.70 ± 1.62 A | 35.70 ± 1.49 B | 38.70 ± 0.49 B | 37.90 ± 0.92 B | 33.21 ± 0.15 B |
| 12 | γ-Terpinene | 1063 | 1064 | 0.46 ± 0.04 A | 1.01 ± 0.04 B | 1.02 ± 0.06 B | 0.62 ± 0.02 A | 0.54 ± 0.03 A |
| 13 | cis-Sabinene hydrate | 1074 | 1076 | 0.20 ± 0.02 A | 0.18 ± 0.01 A | 0.17 ± 0.01 A | 0.18 ± 0.01 A | 0.18 ± 0.01 A |
| 14 | Terpinolene | 1094 | 1089 | 0.22 ± 0.00 | 0.27 ± 0.02 | 0.20 ± 0.01 | 0.14 ± 0.02 | |
| 15 | Linalool | 1104 | 1104 | 0.85 ± 0.06 A | 0.68 ± 0.03 A | 0.73 ± 0.11 A | 0.87 ± 0.03 A | 1.03 ± 0.06 A |
| 16 | α-Thujone | 1112 | 1117 | 0.69 ± 0.05 A | 2.20 ± 0.04 B | 1.32 ± 0.03 B | 1.83 ± 0.05 B | 2.12 ± 0.03 B |
| 17 | β-Thujone | 1124 | 1127 | 0.99 ± 0.06 A | 1.51 ± 0.03 B | 1.21 ± 0.02 A | 1.56 ± 0.03 B | 1.54 ± 0.05 B |
| 18 | Camphor | 1155 | 1150 | 8.32 ± 0.53 A | 7.56 ± 0.08 A | 10.71 ± 0.15 B | 11.50 ± 0.24 B | 12.09 ± 0.06 B |
| 19 | Borneol | 1170 | 1170 | 0.66 ± 0.04 A | 0.60 ± 0.01 A | 0.90 ± 0.02 A | 0.22 ± 0.00 A | 0.85 ± 0.02 A |
| 20 | Тerpinen-4-ol | 1177 | 1178 | 3.50 ± 0.13 A | 3.13 ± 0.06 A | 2.71 ± 0.05 A | 3.66 ± 0.05 A | 3.42 ± 0.07 A |
| 21 | p-Cymene-8-ol | 1185 | 1183 | 1.02 ± 0.05 A | 0.31 ± 0.01 B | 0.52 ± 0.01 B | 0.19 ± 0.01 B | 0.65 ± 0.01 B |
| 22 | Myrtenol | 1188 | 1194 | 2.13 ± 0.09 A | 1.88 ± 0.05 B | 1.65 ± 0.01 B | 1.62 ± 0.02 B | 2.13 ± 0.04 A |
| 23 | α-Terpineol | 1204 | 1199 | 5.53 ± 0.15 A | 7.64 ± 0.22 A | 4.67 ± 0.02 A | 5.96 ± 0.07 A | 6.03 ± 0.07 A |
| 24 | Neral | 1236 | 1238 | 0.17 ± 0.01 | ||||
| 25 | Carvone | 1257 | 1258 | 0.13 ± 0.01 A | 0.06 ± 0.02 A | 0.16 ± 0.01 A | 0.15 ± 0.00 A | |
| 26 | Linalyl acetate | 1261 | 1259 | 0.22 ± 0.03 A | 0.10 ± 0.02 A | |||
| 27 | Bornyl acetate | 1293 | 1288 | 0.24 ± 0.04 A | 0.17 ± 0.02 A | 0.16 ± 0.02 A | 0.29 ± 0.00 A | |
| 28 | Thymol | 1305 | 1293 | 0.11 ± 0.01 | ||||
| 29 | Carvacrol | 1324 | 1309 | 0.23 ± 0.01 A | 0.11 ± 0.00 A | 0.18 ± 0.00 A | 0.15 ± 0.04 A | |
| 30 | Dihydrocarveol acetate | 1357 | 1347 | 4.42 ± 0.17 A | 1.82 ± 0.17 B | 3.12 ± 0.05 B | 3.21 ± 0.02 B | 2.67 ± 0.06 B |
| 31 | Eugenol | 1378 | 1359 | 0.23 ± 0.01 | ||||
| 32 | β-Bourbonene | 1385 | 1384 | 0.15 ± 0.02 A | 0.11 ± 0.03 A | 0.12 ± 0.01 A | 0.16 ± 0.03 A | |
| 33 | α-Cadinene | 1424 | 0.17 ± 0.03 | |||||
| 34 | β-Caryophyllene | 1439 | 1426 | 13.88 ± 0.63 A | 10.63 ± 0.54 B | 8.73 ± 0.17 B | 7.02 ± 0.04 B | 10.14 ± 0.10 B |
| 35 | Aromadendrene | 1444 | 1440 | 0.11 ± 0.01 | ||||
| 36 | 5-Oxobornyl acetate | 1452 | 1484 | 0.10 ± 0.00 | ||||
| 37 | α-Humulene ne | 1456 | 1456 | 0.75 ± 0.04 A | 0.77 ± 0.08 A | 0.86 ± 0.02 A | 0.74 ± 0.02 A | 0.81 ± 0.02 A |
| 38 | Allo-Aromadendrene | 1471 | 1462 | 4.58 ± 0.21 A | 1.80 ± 0.09 B | 2.04 ± 0.05 B | 1.52 ± 0.01 B | 2.99 ± 0.03 B |
| 39 | γ-Muurolene | 1478 | 1477 | 0.11 ± 0.01 | ||||
| 40 | (z)-β-Guaiene | 1489 | 1490 | 0.17 ± 0.04 A | 0.15 A | |||
| 41 | Viridiflorene | 1497 | 1494 | 0.19 ± 0.02 A | 0.07 ± 0.01 B | |||
| 42 | γ-Cadinene | 1513 | 1513 | 1.89 ± 0.10 A | 0.84 ± 0.05 B | 0.68 ± 0.02 B | 0.57 ± 0.01B | 0.64 ± 0.02 B |
| 43 | (Z)-Calamenene | 1529 | 1526 | 0.30 ± 0.04 A | 0.08 ± 0.00 B | 0.13 ± 0.03 B | 0.13 ± 0.01 B | 0.15 ± 0.01 B |
| 44 | δ-Cadinene | 1537 | 1531 | 2.74 ± 0.24 A | 0.36 ± 0.02 B | 0.45 ± 0.02 B | 0.47 ± 0.02 B | 0.72 ± 0.03 B |
| 45 | UD | 1543 | 1.00 ± 0.14 | |||||
| 46 | Viridiflorol | 1595 | 1590 | 0.59 ± 0.19 | ||||
| 47 | Humulene epoxide I | 1599 | 1596 | 1.43 ± 0.07 A | 0.63 ± 0.03 B | 0.56 ± 0.02 B | 0.60 ± 0.02 B | 0.66 ± 0.01 B |
| 48 | Humulene epoxide II | 1605 | 1600 | 2.96 ± 0.18 A | 1.28 ± 0.07 B | 1.01 ± 0.04 B | 1.71 ± 0.03 B | 1.59 ± 0.06 B |
| 49 | Humulene epoxide III | 1616 | 1615 | 7.62 ± 0.25 A | 2.54 ± 0.14 B | 1.94 ± 0.06 B | 3.70 ± 0.11 B | 3.46 ± 0.08 B |
| 50 | muurola-4,10(14)-dien-1-b-ol | 1632 | 1625 | 0.83 ± 0.11 A | 0.21 ± 0.01 B | 0.23 ± 0.02 B | 0.30 ± 0.01 B | 0.55 ± 0.02 B |
| 51 | UD | 1656 | 0.35 ± 0.04 | |||||
| 52 | 14-hydroxy-(Z)-caryophyllene | 1660 | 1667 | 2.10 ± 0.16 A | 0.34 ± 0.12 B | 0.24 ± 0.02 B | 0.37 ± 0.10 B | 0.60 ± 0.03 B |
| 53 | UD | 1667 | 0.79 ± 0.08 | |||||
| 54 | α-Bisabolol | 1675 | 1683 | 3.43 ± 0.36 A | 0.27 ± 0.01 B | 0.22 ± 0.02 B | 0.24 ± 0.02 B | 0.43 ± 0.01 B |
| 55 | UD | 1693 | 2.09 ± 0.06 A | 0.53 ± 0.03 B | 0.37 ± 0.01 B | 0.61 ± 0.04 B | 0.62 ± 0.03 B | |
| Identified components | 46 | 46 | 40 | 39 | 33 | |||
| Total Yields % | 98.3 ± 2.39 | 98.43 ± 1.82 | 99.37 ± 0.18 | 99.26 ± 0.67 | 97.14 ± 0.35 | |||
| Non-oxygenated monoterpenes | 3.22 | 13.79 | 14.93 | 12.0 | 8.06 | |||
| Non-oxygenated sesquiterpenes | 24.82 | 15.03 | 13.01 | 10.61 | 15.45 | |||
| Oxygenated monoterpenes | 45.57 | 63.36 | 66.66 | 68.96 | 65.74 | |||
| Oxygenated sesquiterpenes | 18.96 | 5.27 | 4.2 | 6.92 | 7.29 | |||
| Phenolics constituents | 0.23 | 0.45 | 0.18 | 0.15 | 0 | |||
| Test | AST IU/L | ALT IU/L | ALP IU/L | Total Protein gm/dL |
|---|---|---|---|---|
| Control group | 93.32 ± 41.39 | 28.82 ± 2.731 | 111.3 ± 11.11 | 7.903 ± 0.28 |
| AAP group | 202.6 ± 36.60 a | 59.56 ± 21.55 a | 260.5 ± 40.72 a | 4.133 ± 0.195 a |
| AAP + FH | 132.2 ± 14.95 b | 46.55 ± 17.25 b | 103.0 ± 4.29 b | 8.597 ± 0.22 b |
| AAP + 1WDH | 123.9 ± 6.671 b | 25.89 ± 18.27 b | 138.2 ± 15.32 b | 8.670 ± 0.81 b |
| AAP + 2WDH | 122.4 ± 15.13 b | 23.75 ± 3.064 b | 144.6 ± 19.29 b | 8.810 ± 0.61 b |
| AAP + 3WDH | 139.2 ± 31.64 b | 25.89 ± 3.397 b | 102.0 ± 1.08 b | 9.210 ± 0.20 b,c |
| AAP + 4WDH | 141.9 ± 18.35 b | 20.40 ± 5.143 b,d | 114.4 ± 8.46 b | 9.035 ± 0.25 b,c |
| AAP + silymarin | 132.4 ± 23.30 b | 36.10 ± 9.336 b | 118.7 ± 9.90 b | 7.910 ± 0.25 b |
| p-value | 0.001 ** | 0.001 ** | 0.001 ** | 0.001 ** |
| Test (Unit/L) | Cholesterol mg/dL | Triglycerides mg/dL | Urea mg/dL | Creatinine mg/dL |
|---|---|---|---|---|
| Control group | 86.87 ± 10.37 | 137.4 ± 2.117 | 34.28 ± 8.15 | 0.62 ± 0.33 |
| AAP group | 119.3 ± 37.25 a | 160.1 ± 18.81 a | 52.28 ± 6.61 a | 1.43 ± 0.39 a |
| AAP + FH | 73.20 ± 8.39 b | 130.3 ± 2.20 b | 23.23 ± 17.57 b | 0.92 ± 0.06 b |
| AAP + 1WDH | 84.66 ± 6.99 b | 141.8 ± 7.91 b | 42.66 ± 20.69 | 0.76 ± 0.21 b |
| AAP + 2WDH | 85.89 ± 10.45 b | 162.0 ± 1.69 | 47.75 ± 5.383 | 0.54 ± 0.37 b |
| AAP + 3WDH | 84.10 ± 14.47 b | 137.1 ± 7.12 b | 41.29 ± 12.38 | 0.86 ± 0.16 b |
| AAP + 4WDH | 79.13 ± 11.53 b | 138.3 ± 7.42 b | 39.46 ± 11.00 | 0.91 ± 0.08 b |
| AAP + silymarin | 75.45 ± 6.54 b | 152.0 ± 19.56 b | 46.13 ± 19.91 | 0.89 ± 0.09 b |
| p-value | 0.001 *** | 0.001*** | 0.01 ** | 0.001 *** |
| Groups | MCF-7 | HepG-2 | HeLa | MRC-5 | |||
|---|---|---|---|---|---|---|---|
| IC50 ± SD | SI | IC50 ± SD | SI | IC50 ± SD | SI | IC50 ± SD | |
| DOX | 1.4 ± 0.1 | 1.1 | 1.04 ± 0.1 | 1.5 | 1.02 ± 0.1 | 1.6 | 1.6 ± 0.1 |
| FH | 181.3 ± 18.3 | 3.3 | 161.7 ± 15.3 | 3.7 | 142.1 ± 12.4 | 4.2 | 596.5 ± 20 |
| 1WDH | 189.3 ± 17.45 | 2.2 | 179.12 ± 16.5 | 2.4 | 164.1 ± 15.5 | 2,6 | 425.6 ± 30.2 |
| 2WDH | 194.1 ± 20.1 | 2.6 | 182.4 ± 14.2 | 2.7 | 174.7 ± 13.6 | 2.9 | 499.4 ± 32 |
| 3WDH | 176.3 ± 16.5 | 2.3 | 174.9 ± 13.2 | 2.3 | 127.5 ± 11.4 | 3.2 | 405.2 ± 18.3 |
| 4WDH | 215.7 ± 18.4 | 1.9 | 195.4 ± 20 | 2.1 | 176.2 ± 18 | 2.4 | 414.7 ± 27.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, H.A.; Eldeeb, H.M.; Khan, R.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Sajid, M.S.M.; Aly, M.S.A.; Ahmad, A.M.; Abdellatif, A.A.H.; Eid, S.Y.; et al. Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules 2021, 26, 5757. https://doi.org/10.3390/molecules26195757
Mohammed HA, Eldeeb HM, Khan RA, Al-Omar MS, Mohammed SAA, Sajid MSM, Aly MSA, Ahmad AM, Abdellatif AAH, Eid SY, et al. Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules. 2021; 26(19):5757. https://doi.org/10.3390/molecules26195757
Chicago/Turabian StyleMohammed, Hamdoon A., Hussein M. Eldeeb, Riaz A. Khan, Mohsen S. Al-Omar, Salman A. A. Mohammed, Mohammed S. M. Sajid, Mohamed S. A. Aly, Adel M. Ahmad, Ahmed A. H. Abdellatif, Safaa Yehia Eid, and et al. 2021. "Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis" Molecules 26, no. 19: 5757. https://doi.org/10.3390/molecules26195757
APA StyleMohammed, H. A., Eldeeb, H. M., Khan, R. A., Al-Omar, M. S., Mohammed, S. A. A., Sajid, M. S. M., Aly, M. S. A., Ahmad, A. M., Abdellatif, A. A. H., Eid, S. Y., & El-Readi, M. Z. (2021). Sage, Salvia officinalis L., Constituents, Hepatoprotective Activity, and Cytotoxicity Evaluations of the Essential Oils Obtained from Fresh and Differently Timed Dried Herbs: A Comparative Analysis. Molecules, 26(19), 5757. https://doi.org/10.3390/molecules26195757









