Chemical Evaluation, Antioxidant, Antiproliferative, Anti-Inflammatory and Antibacterial Activities of Organic Extract and Semi-Purified Fractions of the Adriatic Sea Fan, Eunicella cavolini
Abstract
:1. Introduction
2. Results and Discussion
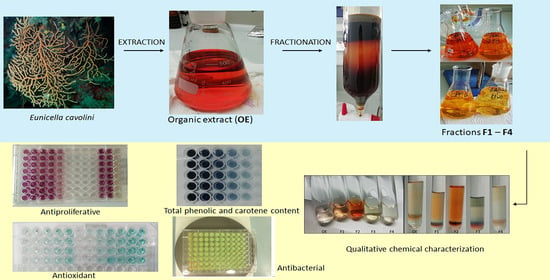
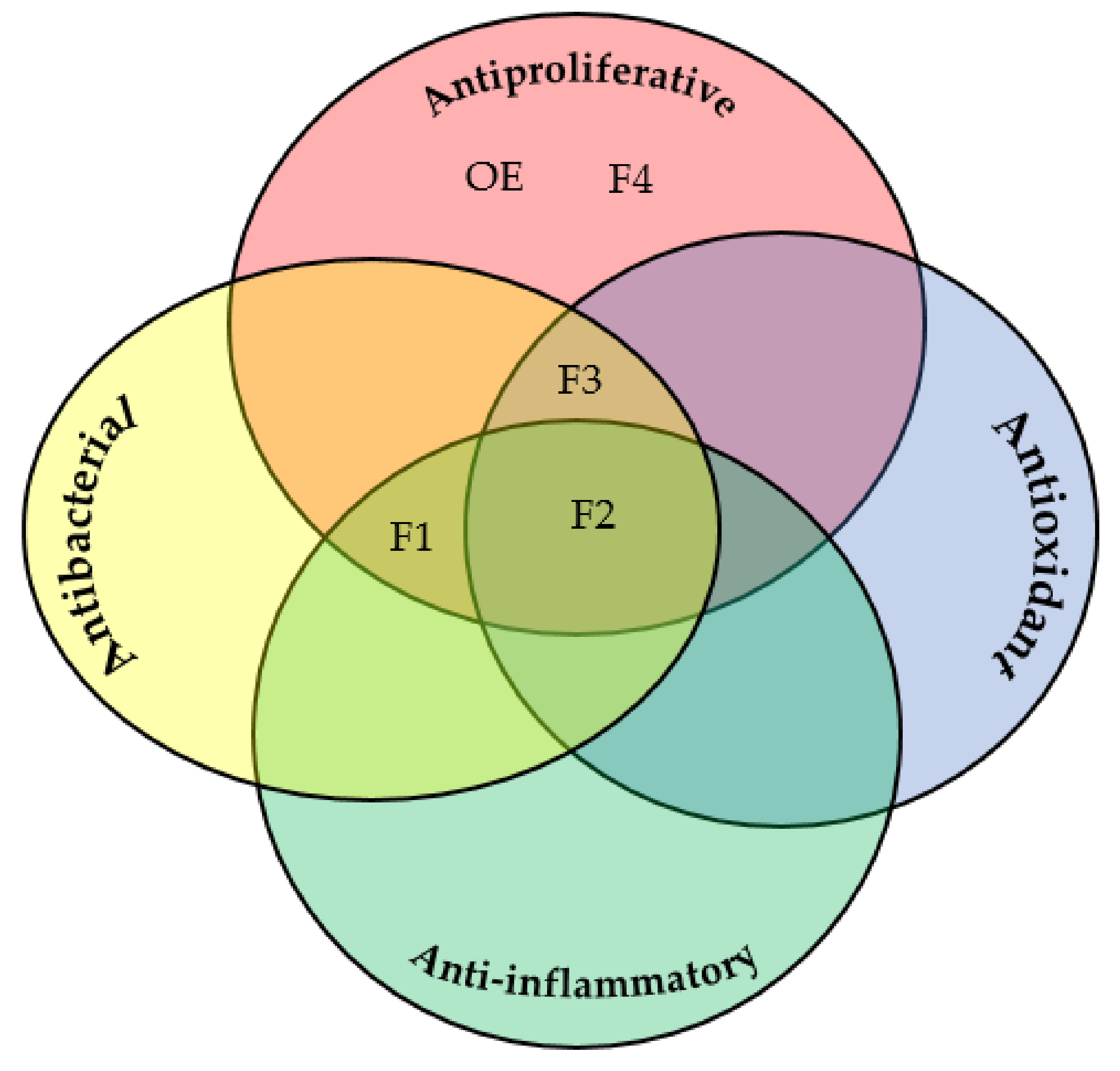
2.1. Extraction Yield, Qualitative and Quantitative Chemical Screening
2.2. Antioxidant Activities
2.3. In Vitro Anti-Inflammatory Activities
2.4. Evaluation of Antiproliferative Activity
2.5. Evaluation of Antibacterial Activity
3. Materials and Methods
3.1. Materials
3.2. Collection and Extraction of Animal Material
3.3. Qualitative Chemical Screening
- (a)
- Test for terpenoids (Salkowski test):
- (b)
- Test for saponins (froth test):
- (c)
- Test for sterols (Liebermann-Burchard’s test):
- (d)
- Test for alkaloids (Mayer’s test):
- (e)
- Test for phenols (Folin-Ciocalteu’s test):
- (f)
- Test for glycosides (Keller-Killiani test):
3.4. Total Phenolic Content and Total Carotene Content
- (a)
- Total phenolic content (TPC)
- (b)
- Total carotene content (TCC)
3.5. MS/MS Molecular Networking
3.6. Assessment of Antioxidant Activity
- (a)
- DPPH radical-scavenging activity:
- (b)
- ABTS radical-scavenging activity:
3.7. Assessment of In Vitro Anti-Inflammatory Activity
- (a)
- Inhibition of albumin denaturation
- (b)
- Inhibition of soybean lipoxygenase (sLOX)
3.8. Evaluation of In Vitro Antiproliferative Effect
3.9. Evaluation of Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cochrane, S.K.J.; Andersen, J.H.; Berg, T.; Blanchet, H.; Borja, A.; Carstensen, J.; Elliott, M.; Hummel, H.; Niquil, N.; Renaud, P.E. What is marine biodiversity? Towards common concepts and their implications for assessing biodiversity status. Front. Mar. Sci. 2016, 3, 248. [Google Scholar] [CrossRef]
- Heiskanen, A.S.; Berg, T.; Uusitalo, L.; Teixeira, H.; Bruhn, A.; Krause-Jensen, D.; Lynam, C.P.; Rossberg, A.G.; Korpinen, S.; Uyarra, M.C.; et al. Biodiversity in marine ecosystems-European developments toward robust assessments. Front. Mar. Sci. 2016, 3, 184. [Google Scholar] [CrossRef] [Green Version]
- Bangmei, X.; Abbott, I.A. Edible seaweeds of China and their place in the Chinese diet. Econ. Bot. 1987, 41, 341–353. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine Bioactive Compounds and Their Health Benefits: A Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Rocha, J.; Peixe, L.; Gomes, N.C.M.; Calado, R. Cnidarians as a source of new marine bioactive compounds—An overview of the last decade and future steps for bioprospecting. Mar. Drugs 2011, 9, 1860. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New Drugs from the Sea: Pro-Apoptotic Activity of Sponges and Algae Derived Compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Williams, D.E.; Andersen, R.J. Biologically active marine natural products and their molecular targets discovered using a chemical genetics approach. Nat. Prod. Rep. 2020, 37, 617–633. [Google Scholar] [CrossRef]
- Matulja, D.; Wittine, K.; Malatesti, N.; Laclef, S.; Turks, M.; Markovic, M.K.; Ambrožić, G.; Marković, D. Marine natural products with high anticancer activities. Curr. Med. Chem. 2020, 27, 1243–1307. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Pezzuto, J.M. Antioxidant marine products in cancer chemoprevention. Antioxidants Redox Signal. 2013, 19, 115–138. [Google Scholar] [CrossRef]
- Balakrishnan, D.; Kandasamy, D.; Nithyanand, P. A review on antioxidant activity of marine organisms. Int. J. ChemTech Res. 2014, 6, 3431–34363. [Google Scholar]
- Florean, C.; Dicato, M.; Diederich, M. Immune-modulating and anti-inflammatory marine compounds against cancer. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chen, Y.; Chan, W.Y. Marine natural products with anti-inflammatory activity. Appl. Microbiol. Biotechnol. 2016, 100, 1645–1666. [Google Scholar] [CrossRef]
- Choudhary, A.; Naughton, L.M.; Montánchez, I.; Dobson, A.D.W.; Rai, D.K. Current status and future prospects of Marine Natural Products (MNPs) as antimicrobials. Mar. Drugs 2017, 15, 272. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.S.; Rodríguez, A.D.; Taglialatela-Scafati, O.; Fusetani, N. Marine pharmacology in 2012–2013: Marine compounds with antibacterial, antidiabetic, antifungal, anti-inflammatory, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous systems, and other miscellaneous mechanisms of. Mar. Drugs 2017, 15, 273. [Google Scholar] [CrossRef] [PubMed]
- Wittine, K.; Saftić, L.; Peršurić, Ž.; Pavelić, S.K. Novel antiretroviral structures from marine organisms. Molecules 2019, 24, 3486. [Google Scholar] [CrossRef] [Green Version]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. In YOUMARES 9-The Oceans: Our Research, Our Future; Jungblut, S., Liebich, V., Bode-Dalby, M., Eds.; Springer International Publishing: New York City, NY, USA, 2020; pp. 159–180. [Google Scholar]
- Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2019, 36, 122–173. [Google Scholar] [CrossRef] [Green Version]
- Leal, M.C.; Calado, R.; Sheridan, C.; Alimonti, A.; Osinga, R. Coral aquaculture to support drug discovery. Trends Biotechnol. 2013, 31, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H. Bioactive Compounds from Marine Gorgonian Corals. Stud. Nat. Prod. Chem. 2012, 38, 325–351. [Google Scholar] [CrossRef]
- Changyun, W.; Haiyan, L.; Changlun, S.; Yanan, W.; Liang, L.; Huashi, G. Chemical defensive substances of soft corals and gorgonians. Acta Ecol. Sin. 2008, 28, 2320–2328. [Google Scholar] [CrossRef]
- Angiolillo, M.; Canese, S. Deep Gorgonians and Corals of the Mediterranean Sea. In Corals in a Changing World; Duque, C., Ed.; IntechOpen: Rijeka, Croatia, 2018; pp. 29–49. [Google Scholar]
- Weinbauer, M.G.; Velimirov, B. Comparative morphometry of fan-like colonies of three Mediterranean gorgonians (Cnidaria: Gorgonacea). Cah. Biol. Mar. 1998, 39, 41–49. [Google Scholar]
- Weinbauer, M.G.; Velimirov, B. Biomass and secondary production of the temperate gorgonian coral Eunicella cavolini (Coelenterata: Octocorallia). Mar. Ecol. Prog. Ser. 1995, 121, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, J.A.; Dueñas, L.F.; Rowley, S.J.; Gonzalez-Zapata, F.L.; Vergara, D.C.; Montaño-Salazar, S.M.; Calixto-Botía, I.; Gómez, C.E.; Abeytia, R.; Colin, P.L.; et al. Gorgonian Corals. In Mesophotic Coral Ecosystems; Loya, Y., Puglise, K.A., Bridge, T.C.L., Eds.; Springer: New York City, NY, USA, 2019; pp. 729–747. [Google Scholar]
- Turicchia, E.; Abbiati, M.; Sweet, M.; Ponti, M. Mass mortality hits gorgonian forests at Montecristo Island. Dis. Aquat. Organ. 2018, 131, 79–85. [Google Scholar] [CrossRef]
- Rubio, A.D.L.L.; De Figueroa, J.M.T.; Rodríguez, M.J.L.; Tocino, L.S. Mass mortality of Eunicella singularis (Anthozoa: Octocorallia) in the Chafarinas Islands (north Africa, western Mediterranean Sea). Rev. Biol. Mar. Oceanogr. 2018, 53, 285–290. [Google Scholar] [CrossRef] [Green Version]
- Carella, F.; Aceto, S.; Saggiomo, M.; Mangoni, O.; De Vico, G. Gorgonian disease outbreak in the Gulf of Naples: Pathology reveals cyanobacterial infection linked to elevated sea temperatures. Dis. Aquat. Organ. 2014, 111, 69–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sini, M.; Kipson, S.; Linares, C.; Koutsoubas, D.; Garrabou, J. The yellow gorgonian Eunicella cavolini: Demography and disturbance levels across the Mediterranean Sea. PLoS ONE 2015, 10, e0126253. [Google Scholar] [CrossRef] [Green Version]
- Matulja, D.; Markovic, M.K.; Ambrožić, G.; Laclef, S.; Pavelić, S.K.; Marković, D. Secondary metabolites from gorgonian corals of the genus Eunicella: Structural characterizations, biological activities, and synthetic approaches. Molecules 2020, 25, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.E.F.; Moustafa, M.S.; El-Wahed, A.A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine natural products: A source of novel anticancer drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- BioProCro–Center of excellence for Marine Bioprospecting. Available online: http://bioprocro.zci.hr/ (accessed on 2 December 2020).
- WoRMS–World Register of Marine Species. Available online: http://www.marinespecies.org/aphia.php?p=stats (accessed on 27 March 2020).
- Ioannou, E.; Abdel-Razik, A.F.; Zervou, M.; Christofidis, D.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 5alpha,8alpha-Epidioxysterols from the gorgonian Eunicella cavolini and the ascidian Trididemnum inarmatum: Isolation and evaluation of their antiproliferative activity. Steroids 2009, 74, 73–80. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. 9,11-Secosterols with antiproliferative activity from the gorgonian Eunicella cavolini. Bioorganic Med. Chem. 2009, 17, 4537–4541. [Google Scholar] [CrossRef]
- Ioannou, E.; Abdel-Razik, A.F.; Alexi, X.; Vagias, C.; Alexis, M.N.; Roussis, V. Pregnanes with antiproliferative activity from the gorgonian Eunicella cavolini. Tetrahedron 2008, 64, 11797–11801. [Google Scholar] [CrossRef]
- Hall-Spencer, J.M.; Pike, J.; Munn, C.B. Diseases affect cold-water corals too: Eunicella verrucosa (Cnidaria: Gorgonacea) necrosis in SW England. Dis. Aquat. Organ. 2007, 76, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Heikoop, J.M.; Hickmott, D.D.; Risk, M.J.; Shearer, C.K.; Atudorei, V. Potential climate signals from the deep-sea gorgonian coral Primnoa resedaeformis. Hydrobiologia 2002, 471, 117–124. [Google Scholar] [CrossRef]
- Cimino, G.; De Rosa, S.; De Stefano, S. Antiviral agents from a gorgonian, Eunicella cavolini. Experientia 1984, 40, 339–340. [Google Scholar] [CrossRef]
- Cimino, G.; Desiderio, B.; De Stefano, S.; Sodano, G. Chemistry of mediterranean gorgonians. II. Pregna 4,20-dien-11α-ol-3-one acetate, a novel steroid from the gorgonian Eunicella cavolini. Experientia 1979, 35, 298–299. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Zibrowius, H.; Laurent, D.; Pietra, F. A novel type of a second epoxy bridge in eunicellane diterpenes: Isolation and characterization of massileunicellins A-C from the gorgonian Eunicella cavolinii. Helv. Chim. Acta 1999, 82, 1681–1689. [Google Scholar] [CrossRef]
- Mancini, I.; Guella, G.; Zibrowius, H.; Pietra, F. Configuration, conformation, and reactivity of highly functionalized eunicellane diterpenes isolated from the gorgonians Eunicella cavolinii and Eunicella singularis from Marseille. Helv. Chim. Acta 2000, 83, 1561–1575. [Google Scholar] [CrossRef]
- Edrada, R.A.; Wray, V.; Handayani, D.; Schupp, P.; Balbin-Oliveros, M.; Proksch, P. Structure-activity relationships of bioactive metabolites from some Indo-Pacific marine invertebrates. Stud. Nat. Prod. Chem. 2000, 21, 219–292. [Google Scholar] [CrossRef]
- Bracco, S.; Fumagalli, P.; Fusi, P.; Santambrogio, C.; Rolandi, V.; Brajkovic, A. Identification of the chromophores in Corallium rubrum gem quality corals by HPLC/UV, ESI-MS and 1H NMR spectroscopy. Period. di Mineral. 2016, 85, 83–93. [Google Scholar] [CrossRef]
- Weng, A.; Thakur, M.; Melzig, F.M.; Fuchs, H. Chemistry and pharmacology of saponins: Special focus on cytotoxic properties. Bot. Targets Ther. 2011, 1, 19–29. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from Medicinal Plants as Potential Anticancer Agents: Emerging Trends Towards Future Drugs. Curr. Med. Chem. 2018, 26, 2389–2406. [Google Scholar] [CrossRef]
- Thomas, N.V.; Kim, S.K. Potential pharmacological applications of polyphenolic derivatives from marine brown algae. Environ. Toxicol. Pharmacol. 2011, 32, 325–335. [Google Scholar] [CrossRef]
- Deghrigue, M.; Dellai, A.; Akremi, N.; Le Morvan, V.; Robert, J.; Bouraoui, A. Evaluation of antiproliferative and antioxidant activities of the organic extract and its polar fractions from the Mediterranean gorgonian Eunicella singularis. Environ. Toxicol. Pharmacol. 2013, 36, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Cvejic, J.; Tambutté, S.; Lotto, S.; Mikov, M.; Slacanin, I.; Allemand, D. Determination of canthaxanthin in the red coral (Corallium rubrum) from Marseille by HPLC combined with UV and MS detection. Mar. Biol. 2007, 152, 855–862. [Google Scholar] [CrossRef]
- Shahbudin, S.; Deny, S.; Zakirun, A.M.T.; Haziyamm, T.A.H.; Akbar John, B.; Taher, M. Antioxidant properties of soft coral Dendronephthya sp. Int. J. Pharmacol. 2011, 7, 263–267. [Google Scholar] [CrossRef]
- Mydlarz, L.D.; Harvell, C.D. Peroxidase activity and inducibility in the sea fan coral exposed to a fungal pathogen. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 54–62. [Google Scholar] [CrossRef]
- Yost, D.M.; Jones, R.J.; Mitchelmore, C.L. Alterations in dimethylsulfoniopropionate (DMSP) levels in the coral Montastraea franksi in response to copper exposure. Aquat. Toxicol. 2010, 98, 367–373. [Google Scholar] [CrossRef]
- Toledo-Hernández, C.; Ruiz-Diaz, C.P.; Díaz-Vázquez, L.M.; Santiago-Cárdenas, V.; Rosario-Berrios, D.N.; García-Almedina, D.M.; Roberson, L.M.; Ambiente, S.; Sam, M.; Juan, S.; et al. Comparison of chemical compounds associated with sclerites from healthy and diseased sea fan corals (Gorgonia ventalina). PeerJ 2017, 5, e3677. [Google Scholar] [CrossRef] [Green Version]
- Downs, C.A.; Fauth, J.E.; Halas, J.C.; Dustan, P.; Bemiss, J.; Woodley, C.M. Oxidative stress and seasonal coral bleaching. Free Radic. Biol. Med. 2002, 33, 533–543. [Google Scholar] [CrossRef]
- Griffin, S.P.; Bhagooli, R. Measuring antioxidant potential in corals using the FRAP assay. J. Exp. Mar. Biol. Ecol. 2004, 302, 201–211. [Google Scholar] [CrossRef]
- Sang, V.T.; Dat, T.T.H.; Vinh, L.B.; Cuong, L.C.V.; Oanh, P.T.T.; Ha, H.; Kim, Y.H.; Anh, H.L.T.; Yang, S.Y. Coral and coral-associated microorganisms: A prolific source of potential bioactive natural products. Mar. Drugs 2019, 17, 468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deghrigue, M.; Dellai, A.; Bouraoui, A.; Akremi, N.; Le Morvan, V.; Robert, J.; Bouraoui, A. In Vitro Antiproliferative and Antioxidant Activities of the Organic Extract and Its Semi-Purified Fractions from the Mediterranean Gorgonian Eunicella Singularis. Int. J. Pharm. Pharm. Sci. 2013, 5, 432–439. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—antioxidant properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Burgos, E.; Gomez-Serranillos, M.P. Terpene Compounds in Nature: A Review of Their Potential Antioxidant Activity. Curr. Med. Chem. 2012, 19, 5319–5341. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC technical report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef] [Green Version]
- San Miguel-Chávez, R. Phenolic Antioxidant Capacity: A Review of the State of the Art. In Phenolic Compounds—Biological Activity; Soto-Hernández, M., Palma-Tenango, M., García-Mateos, R., Eds.; IntechOpen: Rijeka, Croatia, 2017; pp. 59–74. [Google Scholar]
- Huang, D.; Boxin, O.U.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Deghrigue, M.; Festa, C.; Ghribi, L.; D’Auria, M.V.; De Marino, S.; Ben Jannet, H.; Bouraoui, A. Anti-inflammatory and analgesic activities with gastroprotective effect of semi-purified fractions and isolation of pure compounds from Mediterranean gorgonian Eunicella singularis. Asian Pac. J. Trop. Med. 2015, 8, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Deghrigue, M.; Festa, C.; Ghribi, L.; D’auria, M.V.; de Marino, S.; Ben Jannet, H.; Ben Said, R.; Bouraoui, A. Pharmacological evaluation of the semi-purified fractions from the soft coral Eunicella singularis and isolation of pure compounds. Daru J. Pharm. Sci. 2014, 22, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, F.; Spaziano, G.; Liparulo, A.; Roviezzo, F.; Nabavi, S.M.; Sureda, A.; Filosa, R.; D’Agostino, B. Recent advances in the search for novel 5-lipoxygenase inhibitors for the treatment of asthma. Eur. J. Med. Chem. 2018, 153, 65–72. [Google Scholar] [CrossRef]
- Osman, N.I.; Sidik, N.J.; Awal, A.; Adam, N.A.M.; Rezali, N.I. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. And total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult. Ethnopharmacol. 2016, 5, 343–349. [Google Scholar] [CrossRef]
- Williams, L.A.D.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (Immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals. West. Indian Med. J. 2008, 57, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Muthiyan, R.; Mahanta, N.; Nambikkairaj, B.; Immanuel, T.; De, A. Antioxidant and anti-inflammatory effects of a methanol extract from the marine sponge Hyrtios erectus. Pharmacogn. Mag. 2018, 14, 534–540. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; El Hilaly, J.; Ennassir, J.; Benlyas, M.; Alem, C.; Amarouch, M.Y.; Filali-Zegzouti, Y. Anti-inflammatory properties and phenolic profile of six Moroccan date fruit (Phoenix dactylifera L.) varieties. J. King Saud Univ.Sci. 2018, 30, 519–526. [Google Scholar] [CrossRef]
- Sircar, J.C.; Schwender, C.F.; Johnson, E.A. Soybean lipoxygenase inhibition by nonsteroidal antiinflammatory drugs. Prostaglandins 1983, 25, 393–396. [Google Scholar] [CrossRef]
- Wei, W.C.; Sung, P.J.; Duh, C.Y.; Chen, B.W.; Sheu, J.H.; Yang, N.S. Anti-inflammatory activities of natural products isolated from soft corals of Taiwan between 2008 and 2012. Mar. Drugs 2013, 11, 4083. [Google Scholar] [CrossRef] [Green Version]
- Lei, H. Diterpenoids of Gorgonian Corals: Chemistry and Bioactivity. Chem. Biodivers. 2016, 13, 345–365. [Google Scholar] [CrossRef]
- González, Y.; Torres-Mendoza, D.; Jones, G.E.; Fernandez, P.L. Marine Diterpenoids as Potential Anti-Inflammatory Agents. Mediat. Inflamm. 2015, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, C.R.M.; Bezerra, W.P.; Souto, J.T. Marine Alkaloids with Anti-Inflammatory Activity: Current Knowledge and Future Perspectives. Mar. Drugs 2020, 18, 147. [Google Scholar] [CrossRef] [Green Version]
- Jacquot, C.; McGinley, C.M.; Plata, E.; Holman, T.R.; Van Der Donk, W.A. Synthesis of 11-thialinoleic acid and 14-thialinoleic acid, inhibitors of soybean and human lipoxygenases. Org. Biomol. Chem. 2008, 6, 4242–4252. [Google Scholar] [CrossRef] [Green Version]
- Wyld, L.; Smith, O.; Lawry, J.; Reed, M.W.R.; Brown, N.J. Cell cycle phase influences tumour cell sensitivity to aminolaevulinic acid-induced photodynamic therapy in vitro. Br. J. Cancer 1998, 78, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Badisa, R.B.; Darling-Reed, S.F.; Joseph, P.; Cooperwood, J.S.; Latinwo, L.M.; Goodman, C.B. Selective Cytotoxic Activities of Two Novel Synthetic Drugs on Human Breast Carcinoma MCF-7 Cells. Anticancer Res. 2009, 29, 2993–2996. [Google Scholar]
- Rashidi, M.; Seghatoleslam, A.; Namavari, M.; Amiri, A.; Fahmidehkar, M.A.; Ramezani, A.; Eftekhar, E.; Hosseini, A.; Erfani, N.; Fakher, S. Selective cytotoxicity and apoptosis-induction of Cyrtopodion scabrum extract against digestive cancer cell lines. Int. J. Cancer Manag. 2017, 10, e8633. [Google Scholar] [CrossRef]
- Satomi, Y. Antitumor and Cancer-preventative Function of Fucoxanthin: A Marine Carotenoid. Anticancer Res. 2017, 37, 1557–1562. [Google Scholar] [CrossRef] [Green Version]
- Kim, K. Antimicrobial activity in gorgonian corals (Coelenterata, Octocorallia). Coral Reefs 1994, 13, 75–80. [Google Scholar] [CrossRef]
- Kim, K.; Kim, P.D.; Alker, A.P.; Harvell, C.D. Chemical resistance of gorgonian corals against fungal infections. Mar. Biol. 2000, 137, 393–401. [Google Scholar] [CrossRef]
- Kim, K.; Harvell, C.D.; Kim, P.D.; Smith, G.W.; Merkel, S.M. Fungal disease resistance of caribbean sea fan corals (Gorgonia spp.). Mar. Biol. 2000, 136, 259–267. [Google Scholar] [CrossRef]
- Jensen, P.R.; Harvell, C.D.; Wirtz, K.; Fenical, W. Antimicrobial activity of extracts of Caribbean gorgonian corals. Mar. Biol. 1996, 125, 411–419. [Google Scholar] [CrossRef]
- Alker, A.P.; Smith, G.W.; Kim, K. Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals. Hydrobiologia 2001, 460, 105–111. [Google Scholar] [CrossRef]
- Alker, A.P.; Kim, K.; Dube, D.H.; Harvell, C.D. Localized induction of a generalized response against multiple biotic agents in Caribbean sea fans. Coral Reefs 2004, 23, 397–405. [Google Scholar] [CrossRef]
- Liang, L.F.; Wang, X.J.; Zhang, H.Y.; Liu, H.L.; Li, J.; Lan, L.F.; Zhang, W.; Guo, Y.W. Bioactive polyhydroxylated steroids from the Hainan soft coral Sinularia depressa Tixier-Durivault. Bioorganic Med. Chem. Lett. 2013, 23, 1334–1337. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.; Arif, C.; Ferrier-Pagès, C.; Zoccola, D.; Aranda, M.; Voolstra, C.R. Bacteria of the genus Endozoicomonas dominate the microbiome of the Mediterranean gorgonian coral Eunicella cavolini. Mar. Ecol. Prog. Ser. 2013, 479, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Bally, M.; Garrabou, J. Thermodependent bacterial pathogens and mass mortalities in temperate benthic communities: A new case of emerging disease linked to climate change. Glob. Chang. Biol. 2007, 13, 2078–2088. [Google Scholar] [CrossRef]
- Baier, R.E.; Meyer, A.E. Aspects of Bioadhesion. In Fundamentals of Adhesion; Lee, L.H., Ed.; Springer: New York City, NY, USA, 1991; pp. 407–425. [Google Scholar]
- Hunt, L.R.; Smith, S.M.; Downum, K.R.; Mydlarz, L.D. Microbial regulation in gorgonian corals. Mar. Drugs 2012, 10, 1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, X.; Sun, X.; Wang, S.; Xu, Y. Fucoxanthin Isolated from Undaria pinnatifida Can Interact with Escherichia coli and lactobacilli in the Intestine and Inhibit the Growth of Pathogenic Bacteria. J. Ocean. Univ. China 2019, 18, 926–932. [Google Scholar] [CrossRef]
- Samorì, C.; Costantini, F.; Galletti, P.; Tagliavini, E.; Abbiati, M. Inter- and Intraspecific Variability of Nitrogenated Compounds in Gorgonian Corals via Application of a Fast One-Step Analytical Protocol. Chem. Biodivers. 2018, 15, e1700449. [Google Scholar] [CrossRef]
- Kodzius, R.; Gojobori, T. Marine metagenomics as a source for bioprospecting. Mar. Genom. 2015, 24, 21–30. [Google Scholar] [CrossRef] [Green Version]
- Edeoga, H.O.; Okwu, D.E.; Mbaebie, B.O. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005, 4, 685–688. [Google Scholar] [CrossRef]
- Taga, M.S.; Miller, E.E.; Pratt, D.E. Chia seeds as a source of natural lipid antioxidants. J. Am. Oil Chem. Soc. 1984, 61, 928–931. [Google Scholar] [CrossRef]
- Ghorai, N.; Chakraborty, S.; Gucchait, S.; Saha, S.K.; Biswas, S. Estimation of total Terpenoids concentration in plant tissues using a monoterpene, Linalool as standard reagent. Protoc. Exch. 2012, 5, 1038. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with GNPS. Nat. Biotechnol. 2017, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Noh, J.H.; Lee, S.; Choi, J.S.; Suh, H.; Chung, H.Y.; Song, Y.O.; Choi, W.C. The first total synthesis of 2,3,6-tribromo-4,5-dihydroxybenzyl methyl ether (TDB) and its antioxidant activity. Bull. Korean Chem. Soc. 2002, 23, 661–662. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Oso, B.; Karigidi, K. Inhibitory action of dried leaf of Cassia alata (Linn.) Roxb against lipoxygenase activity and nitric oxide generation. Sci. Agropecu. 2019, 10, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Gazivoda, T.; Raić-Malić, S.; Krištafor, V.; Makuc, D.; Plavec, J.; Bratulić, S.; Kraljević-Pavelić, S.; Pavelić, K.; Naesens, L.; Andrei, G.; et al. Synthesis, cytostatic and anti-HIV evaluations of the new unsaturated acyclic C-5 pyrimidine nucleoside analogues. Bioorganic Med. Chem. 2008, 16, 5624–5634. [Google Scholar] [CrossRef] [PubMed]
- CLSI. M07-A10 Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Tenth Edition; CLSI: Wayne, PA, USA, 2015. [Google Scholar]
- Ritz, C.; Baty, F.; Streibig, J.C.; Gerhard, D. Dose-response analysis using R. PLoS ONE 2015, 10, e0146021. [Google Scholar] [CrossRef] [Green Version]
| Solvents | Sample Abbreviation | Extraction/ Purification Yield (%) * | TPC (mg GAE/g Sample) | TCC (mg β-CA/g Sample) |
|---|---|---|---|---|
| DCM/MeOH = 3/1 | OE | 1.38 | 2.69 ± 0.01 | 4.93 ± 0.05 |
| c-HEX/EA = 3/1 | F1 | 0.73 (52.86) | 1.05 ± 0.09 | 13.30 ± 1.26 |
| c-HEX/EA = 1/1 | F2 | 0.13 (9.61) | 1.21 ± 0.17 | 16.29 ± 1.48 |
| EA | F3 | 0.13 (9.58) | 1.70 ± 0.06 | 23.11 ± 2.48 |
| EA/MeOH = 4/1 | F4 | 0.24 (17.13) | 3.28 ± 0.04 | 10.62 ± 0.97 |
| Sample | Saponins | Sterols | Alkaloids | Terpenoids | Glycosides | Phenols |
|---|---|---|---|---|---|---|
| OE | − | + | + | + | + | + |
| F1 | − | + | + | + | + | + |
| F2 | − | + | + | + | + | + |
| F3 | + | + | + | + | + | + |
| F4 | + | − | + | + | + | + |
| Compound Name | Structure | Class | Exact Mass | |
|---|---|---|---|---|
| 1 | (2E,4E)-5-{(1S,2R,4aS,5S,8S,8aS)-2-[(2E)-2-Buten-2-yl]-5-hydroxy-3,8-dimethyl-1,2,4a,5,6,7,8,8a-octahydro-1-naphthalenyl}-2,4-pentadienoic acid Phomopsidin) | 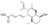 | Fatty acyls | 330.219 |
| 2 | (3bS,5aS,7R,8R,10aR,10bS)-3b,4,5,6,7,8,9,10,10a,10b-Decahydro-7-hydroxy-10b-methyl-5a,8-Methano-5aH-cyclohepta(5,6)naphtho(2,1-b)furan-7-methanol (Kahweol) | 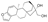 | Terpenoids | 314.188 |
| 3 | (5Z,8Z,11Z,14Z)-17,18-Epoxy-5,8,11,14-icosatetraenoic acid | 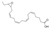 | Fatty acids | 318.22 |
| 4 | (2S,4S)-Icos-19-ene-1,2,4-triol | 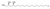 | Fatty acyls | 328.297 |
| 5 | 5-[2-(Furan-3-yl)ethyl]-8-hydroxy-5,6,8a-trimethyl-3,4,4a,6,7,8-hexahydronaphthalene-1-carboxylic acid * | 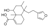 | Prenol lipids | 332.198 |
| 6 | 3-Hydroxy-4-(2,6,7-trihydroxy-6-methylheptan-2-yl)benzoic acid * | 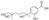 | Prenol lipids | 298.142 |
| 7 | (Z)-4-Oxo-1-(4-(5-oxotetrahydrofuran-2-yl)butyl)-5-(pent-2-en-1-yl acetate) * |  | Fatty Acyls | 348.194 |
| 8 | 5-[2-(3-Furyl)ethyl]-8a-(hydroxymethyl)-5,6-dimethyl-3,4,4a,5,6,7,8,8a-octahydro-1-naphthalenecarboxylic acid * | 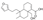 | Prenol lipids Diterpenoids | 332.199 |
| 9 | Abieta-8(14),9(11),12-triene-7,18-diol | 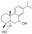 | Prenol lipids Diterpenoids | 302.225 |
| 10 | N-[4-[1-[2-(6-methylpyridin-2-yl)ethyl]piperidine-4-carbonyl]phenyl]methanesulfonamide | 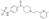 | Alkaloids | 401.177 |
| 11 | 2’,4’,6’-Trihydroxydihydrochalcone | 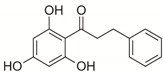 | Phenylpropanoids and polyketides | 258.089 |
| 12 | (3S,6R,6aS,7S)-6,6a,7,8-Tetrahydroxy-3-methyl-3,4,5,6,7,12a-hexahydro-2H-benzo[a]anthracene-1,12-dione | 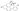 | Benzenoids Tetralins | 344.125 |
| 13 | Methyl (1S,15S,18S,19S,20S)-18-hydroxy-1,3,11,12,14,15,16,17, 18,19,20,21-dodecahydroyohimban-19-carboxylate (Rauwolscine) | 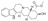 | Alkaloids | 354.450 |
| 14 | (9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-1,2,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one | 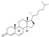 | Steroids and steroid derivatives | 382.324 |
| 15 | 1-[2,4-Dihydroxy-6-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxyphenyl]-3-(4-methoxyphenyl)propan-1-one |  | Flavonoid glycosides | 450.153 |
| Sample | IC50 of DPPH Scavenging Activity (µg/mL) | IC50 of ABTS Scavenging Activity (µg/mL) |
|---|---|---|
| OE | 963.67 ± 80.07 | 395.84 ± 24.25 |
| F1 | 896.22 ± 39.36 | >1000 |
| F2 | 984.68 ± 24.35 | 282.68 ± 23.77 |
| F3 | 767.09 ± 11.57 | 157.16 ± 10.83 |
| F4 | >1000 | >1000 |
| Trolox | <62.5 | <62.5 |
| Sample | IC50 (µg/mL) | |
|---|---|---|
| Inhibition of Albumin Denaturation | Inhibition of Soybean Lipoxygenase | |
| OE | 319.61 ± 7.85 | 812.19 ± 85.89 |
| F1 | 331.65 ± 28.30 | 254.49 ± 49.17 |
| F2 | 198.70 ± 28.77 | 364.35 ± 23.42 |
| F3 | 458.83 ± 27.99 | 370.49 ± 17.02 |
| F4 | >500 | >1000 |
| Indomethacin | 90.33 ± 9.50 | 34.57 ± 9.12 |
| Sample | IC50 (µg/mL) | ||||
|---|---|---|---|---|---|
| SW620 | CFPAC-1 | MCF-7 | HepG2 | HFF-1 | |
| OE | 13.14 ± 5.02 | 22.69 ± 4.14 | 36.20 ± 3.87 | 30.11 ± 1.44 | 6.48 ± 0.12 |
| F1 | 158.78 ± 86.47 | 73.03 ± 14.63 | 67.01 ± 5.42 | 231.18 ± 46.13 | 207.80 ± 21.60 |
| F2 | 0.82 ± 0.14 | 5.61 ± 2.11 | 5.21 ± 1.03 | 2.58 ± 0.57 | 0.39 ± 0.03 |
| F3 | 5.73 ± 2.97 | 7.93 ± 0.12 | 14.63 ± 9.41 | 6.48 ± 1.91 | 6.95 ± 1.96 |
| F4 | 18.76 ± 2.61 | 48.80 ± 1.12 | 42.76 ± 5.96 | 37.66 ± 3.47 | 9.97 ± 1.55 |
| 5-FU | 0.83 ± 0.03 | 0.84 ± 0.09 | 0.01 ± 0.001 | 7.18 ± 1.95 | 0.12 ± 0.02 * |
| Sample | SI | |||
|---|---|---|---|---|
| SW620 | CFPAC-1 | MCF-7 | HepG2 | |
| OE | 0.49 | 0.29 | 0.18 | 0.22 |
| F1 | 1.3 | 2.85 | 3.10 | 0.90 |
| F2 | 0.48 | 0.07 | 0.07 | 0.15 |
| F3 | 1.21 | 0.88 | 0.48 | 1.07 |
| F4 | 0.53 | 0.20 | 0.23 | 0.26 |
| 5-FU | 0.14 | 0.14 | 12 | 0.02 |
| Sample | EC50 (µg/mL) | ||
|---|---|---|---|
| Escherichia coli | Pseudomonas aeruginosa | Staphylococcus aureus | |
| OE | >1000 | >1000 | 514 ± 114 |
| F1 | 422 ± 87 | 523 ± 42 | 417 ± 46 |
| F2 | 255 ± 176 | >1000 | 175 ± 25 |
| F3 | 389 ± 135 | >1000 | 171 ± 60 |
| F4 | 797 ± 176 | >1000 | >1000 |
| Chloramphenicol | 0.75 ± 0.09 | - * | 1.50 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matulja, D.; Grbčić, P.; Bojanić, K.; Topić-Popović, N.; Čož-Rakovac, R.; Laclef, S.; Šmuc, T.; Jović, O.; Marković, D.; Pavelić, S.K. Chemical Evaluation, Antioxidant, Antiproliferative, Anti-Inflammatory and Antibacterial Activities of Organic Extract and Semi-Purified Fractions of the Adriatic Sea Fan, Eunicella cavolini. Molecules 2021, 26, 5751. https://doi.org/10.3390/molecules26195751
Matulja D, Grbčić P, Bojanić K, Topić-Popović N, Čož-Rakovac R, Laclef S, Šmuc T, Jović O, Marković D, Pavelić SK. Chemical Evaluation, Antioxidant, Antiproliferative, Anti-Inflammatory and Antibacterial Activities of Organic Extract and Semi-Purified Fractions of the Adriatic Sea Fan, Eunicella cavolini. Molecules. 2021; 26(19):5751. https://doi.org/10.3390/molecules26195751
Chicago/Turabian StyleMatulja, Dario, Petra Grbčić, Krunoslav Bojanić, Natalija Topić-Popović, Rozelindra Čož-Rakovac, Sylvain Laclef, Tomislav Šmuc, Ozren Jović, Dean Marković, and Sandra Kraljević Pavelić. 2021. "Chemical Evaluation, Antioxidant, Antiproliferative, Anti-Inflammatory and Antibacterial Activities of Organic Extract and Semi-Purified Fractions of the Adriatic Sea Fan, Eunicella cavolini" Molecules 26, no. 19: 5751. https://doi.org/10.3390/molecules26195751
APA StyleMatulja, D., Grbčić, P., Bojanić, K., Topić-Popović, N., Čož-Rakovac, R., Laclef, S., Šmuc, T., Jović, O., Marković, D., & Pavelić, S. K. (2021). Chemical Evaluation, Antioxidant, Antiproliferative, Anti-Inflammatory and Antibacterial Activities of Organic Extract and Semi-Purified Fractions of the Adriatic Sea Fan, Eunicella cavolini. Molecules, 26(19), 5751. https://doi.org/10.3390/molecules26195751