Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide
Abstract
:1. Introduction
2. Results
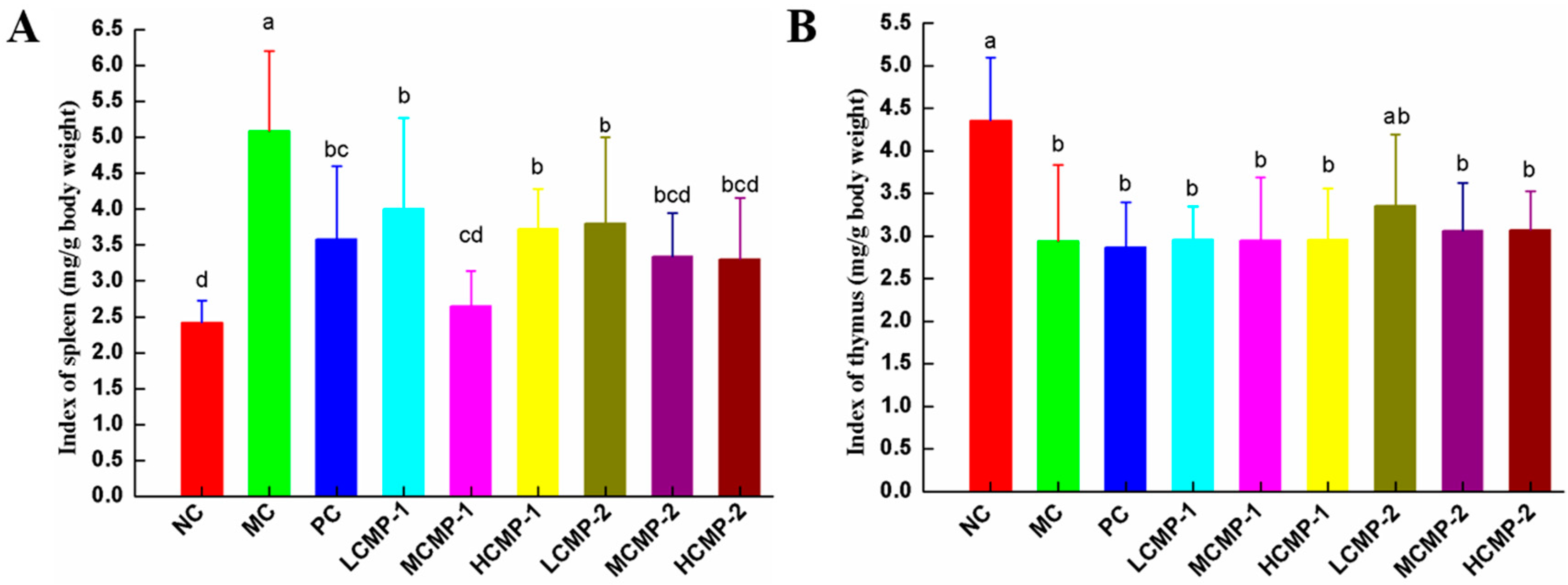
2.1. Effects of CMPs on Immune Organ Index
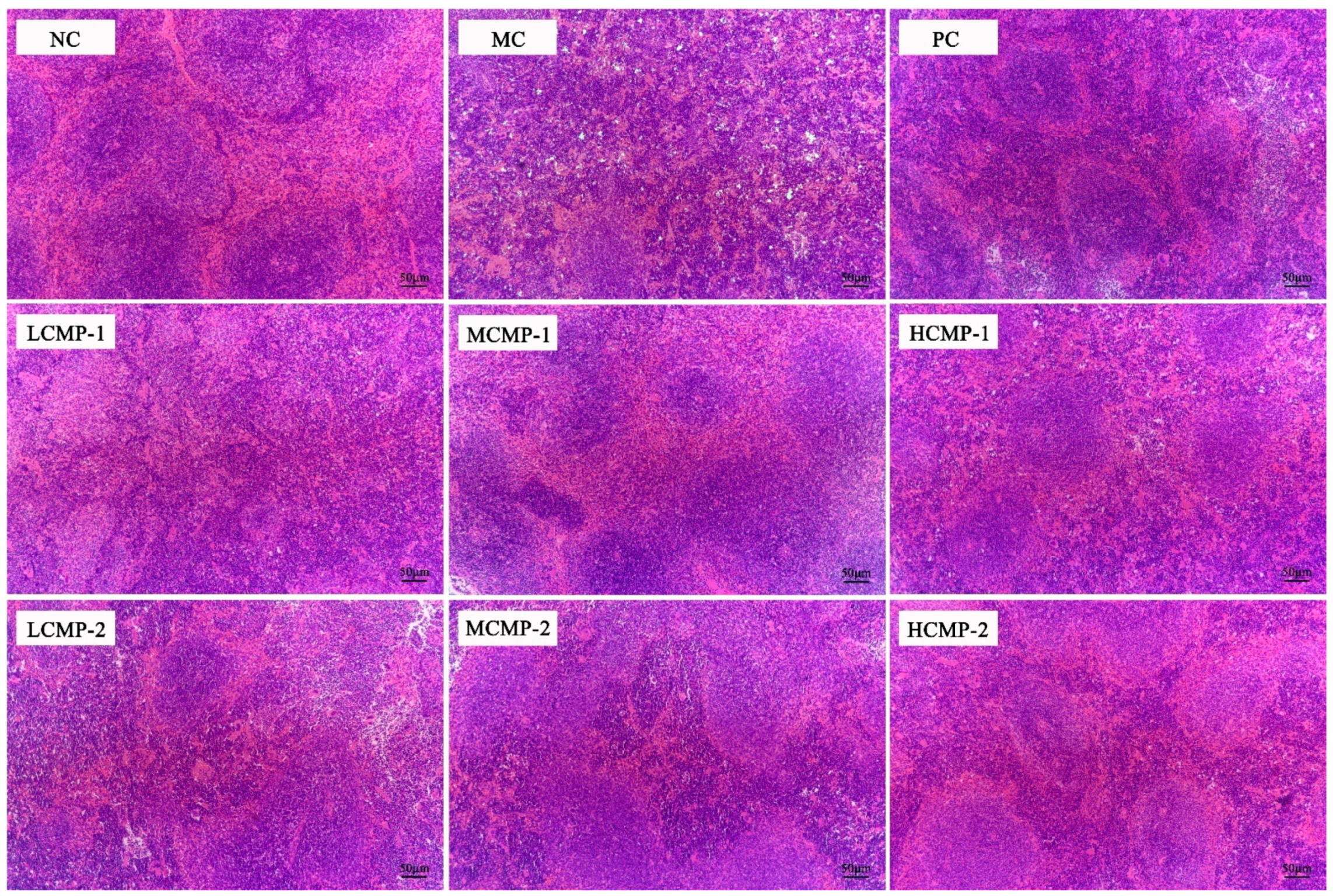
2.2. Effects of CMPs on Spleen Tissue Morphology of Mice
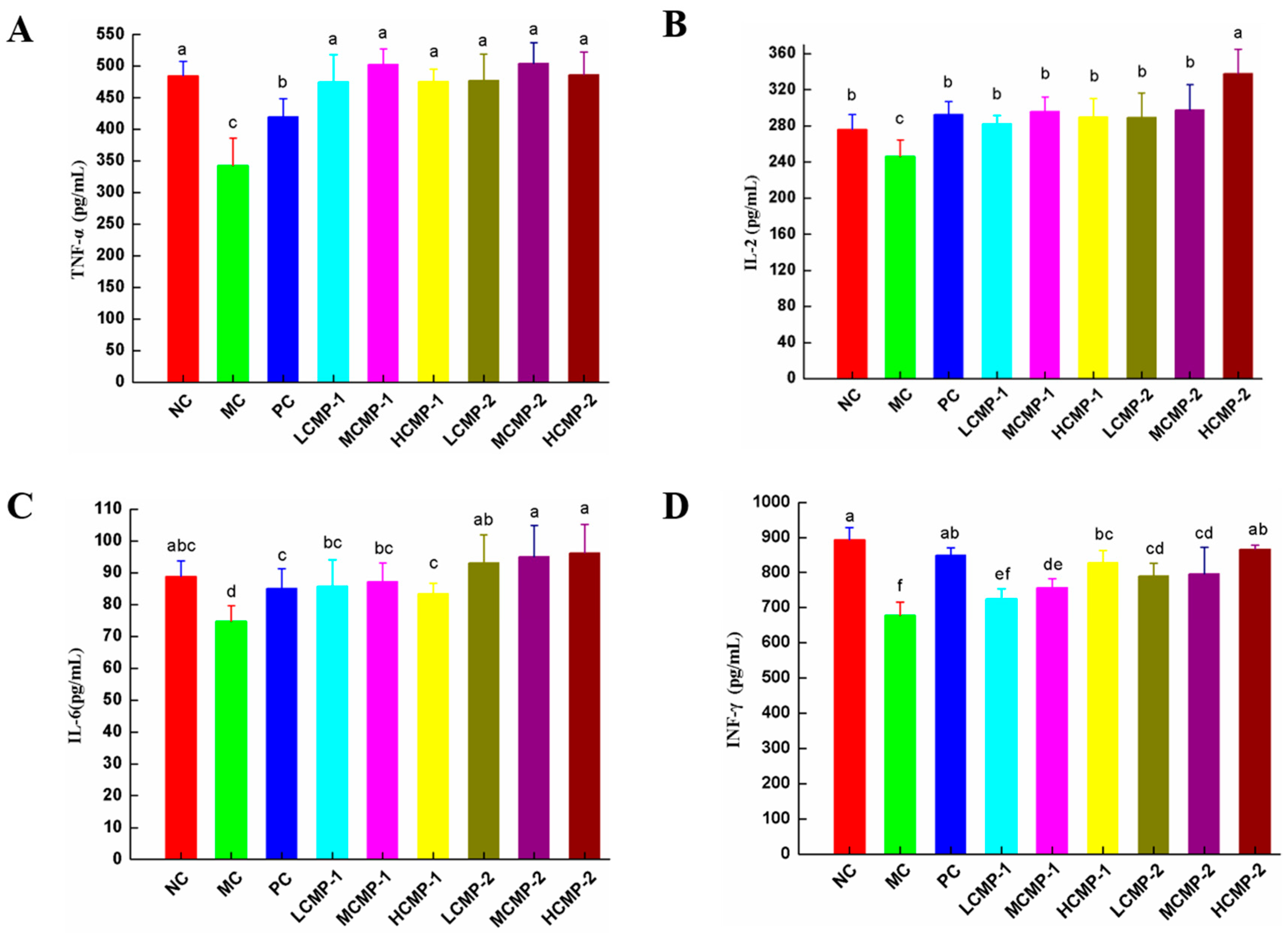
2.3. Effects of CMPs on Cytokine Production in Serum
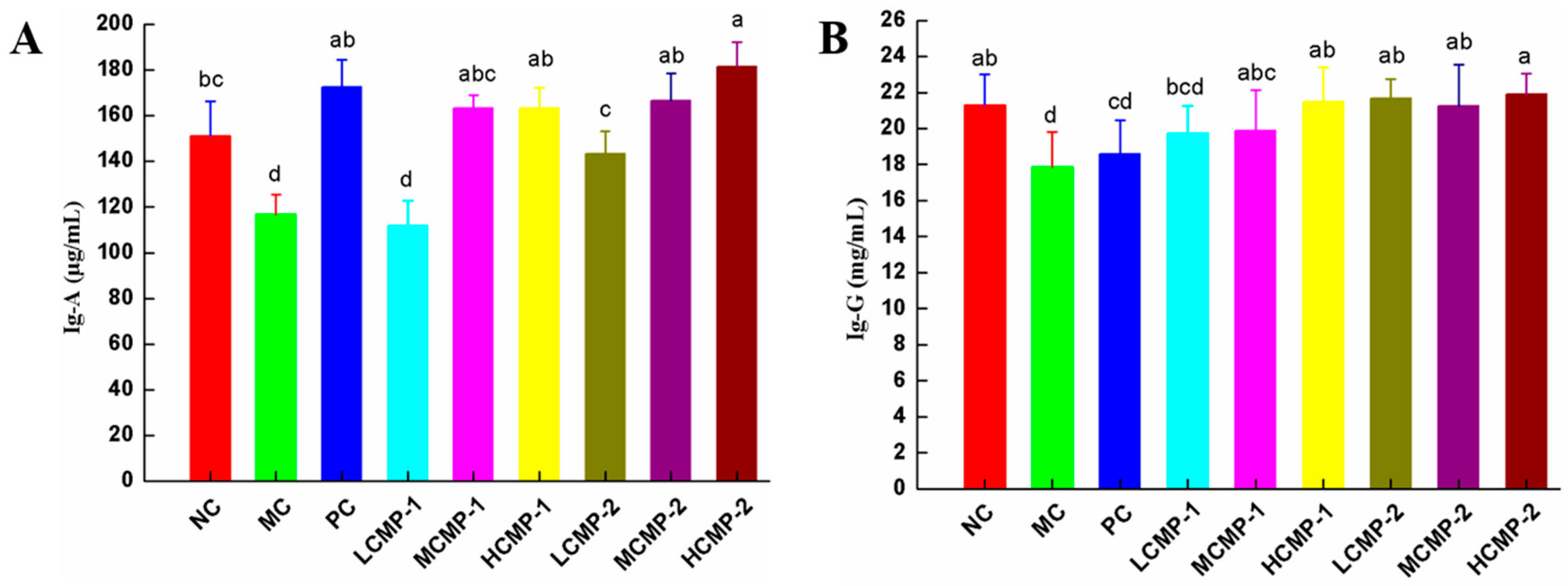
2.4. Effects of CMPs on the Levels of Immunoglobulin in Serum
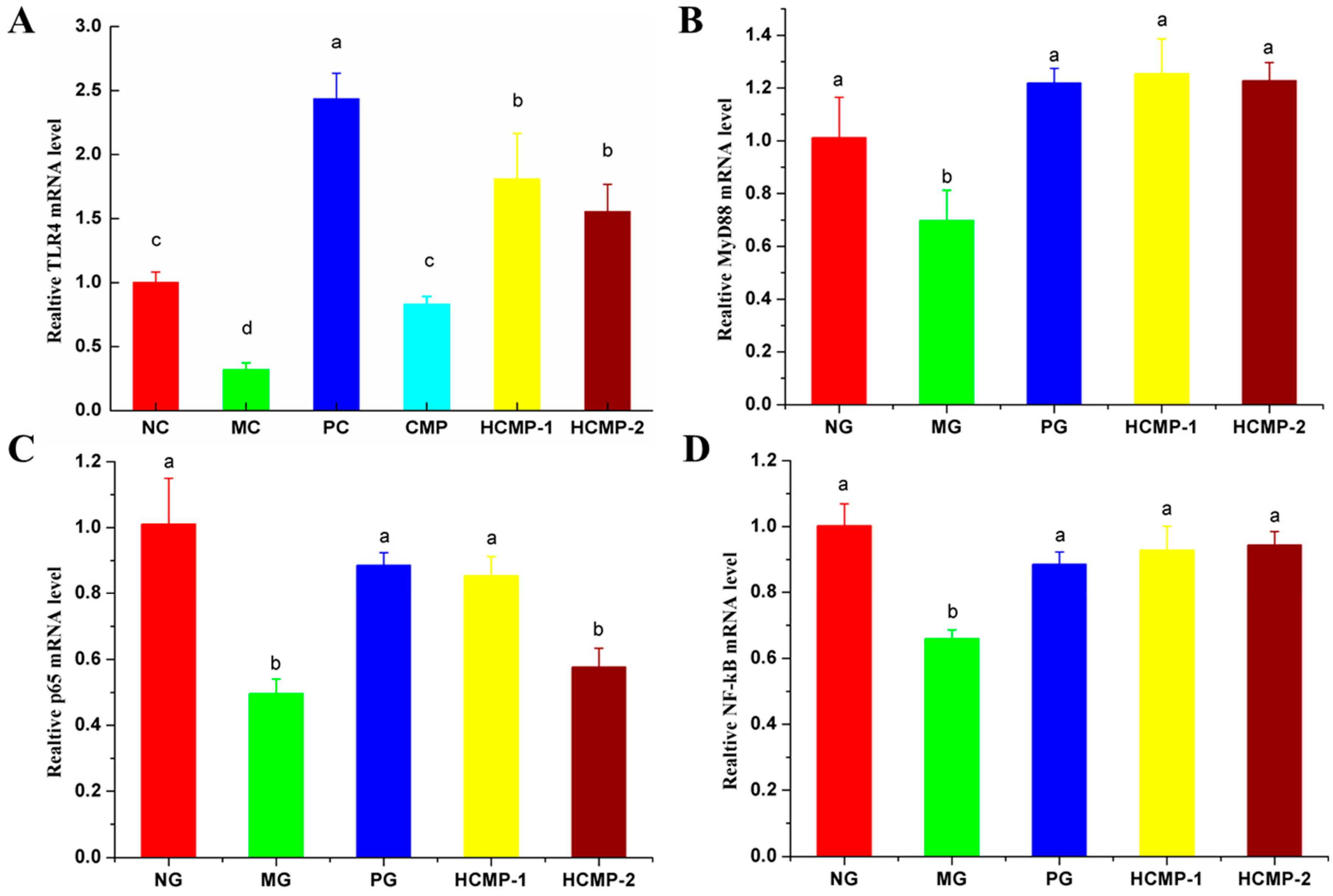
2.5. Effects of CMPs on Relative Gene Expression in Spleen
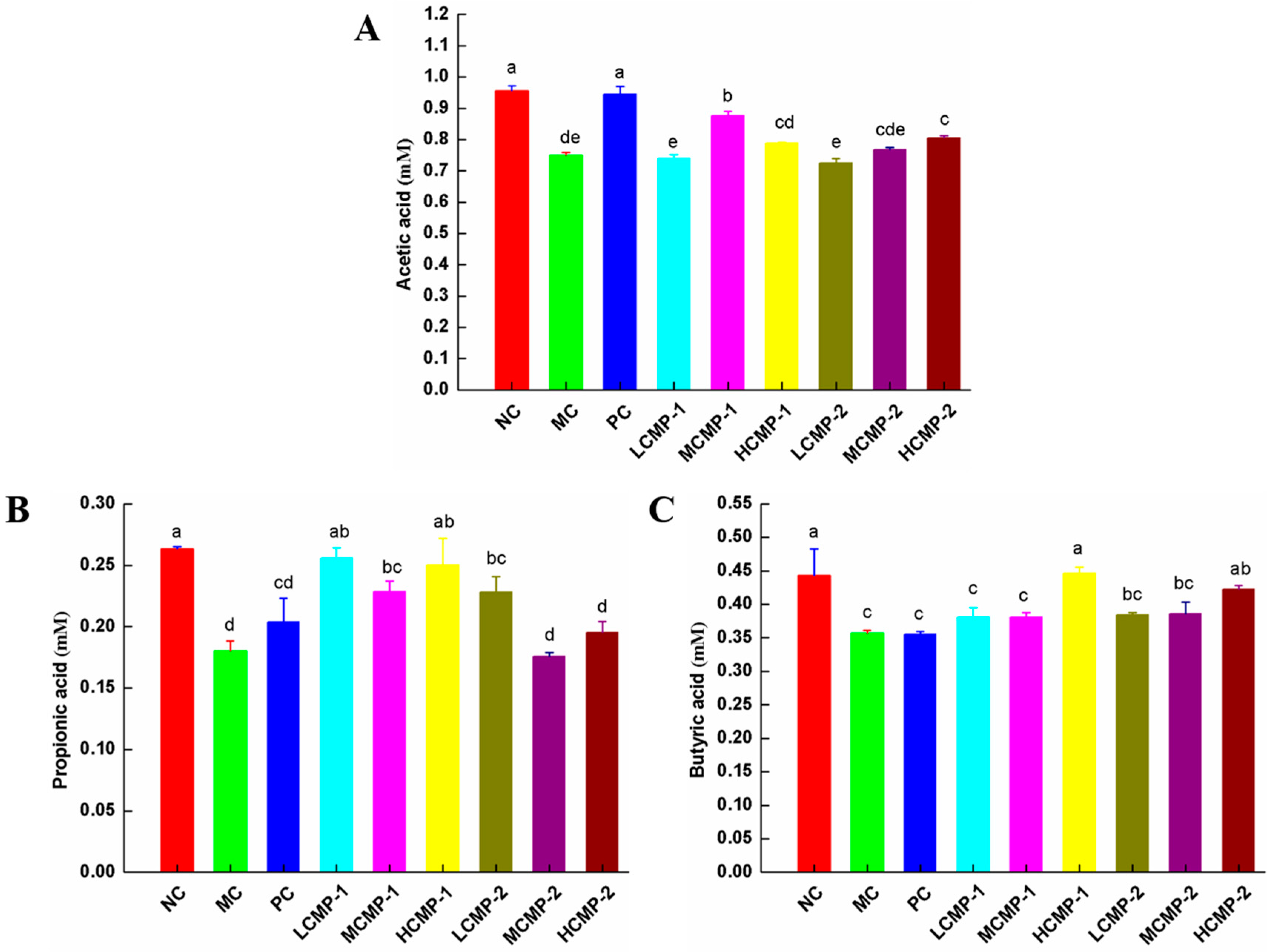
2.6. Effects of CMPs on SCFAs Production
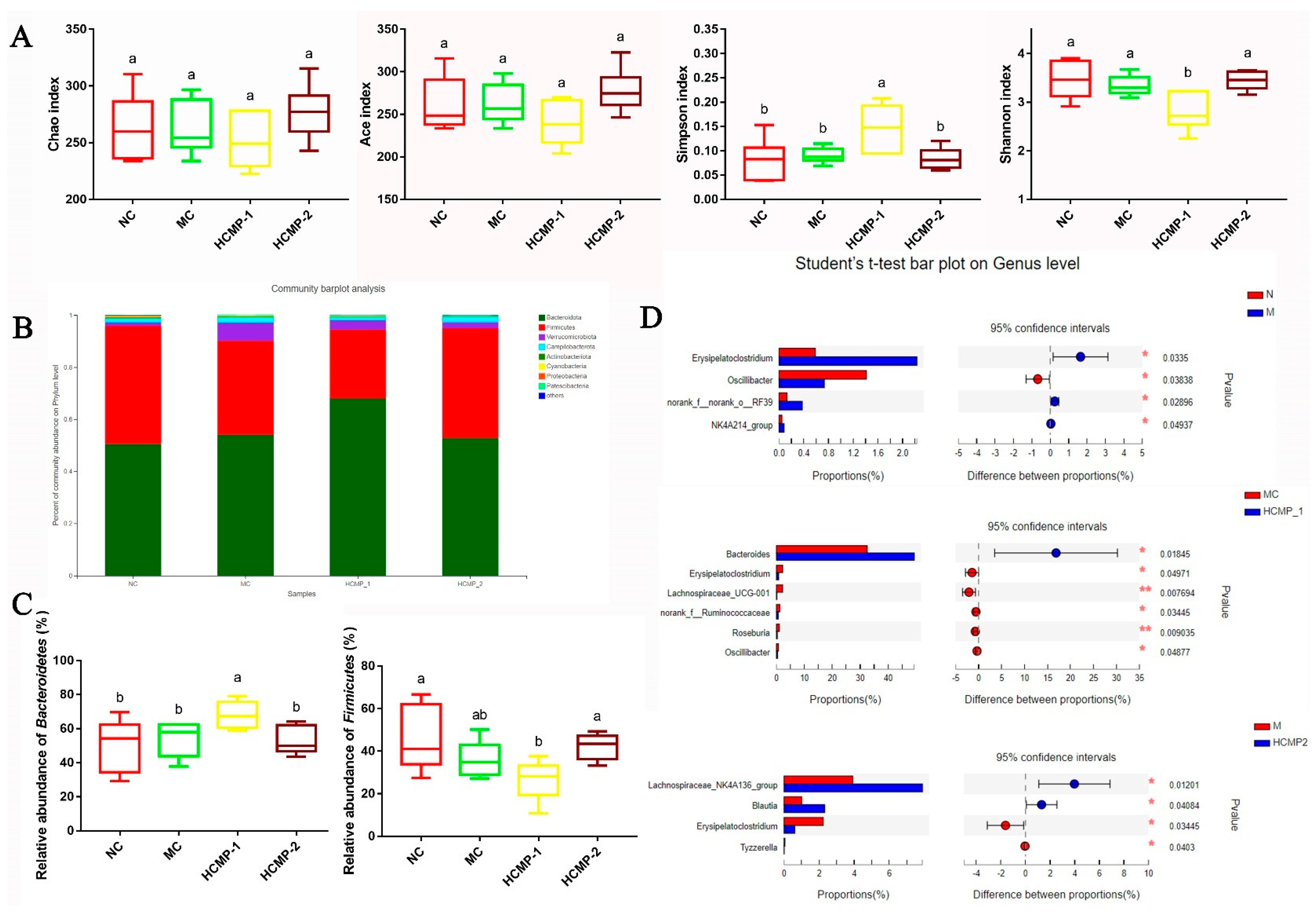
2.7. Effects of CMPs on the Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of CMP-1 and CMP-2
4.3. Animals and Experimental Design
4.4. Determination of Organ Index
4.5. Pathological Examination of Immune Ogran
4.6. Measurement of Serum Immunoglobulin and Cytokines
4.7. RNA Extraction and Real-Time Quantitative PCR
4.8. Determination of Contents of Short Chain Fatty Acids
4.9. Gut Microbiota Analysis
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Chen, D.; Chen, G.J.; Ding, Y.; Wan, P.; Peng, Y.J.; Chen, C.X.; Ye, H.; Zeng, X.X.; Ran, L.W. Polysaccharides from the flowers of tea (Camellia sinensis L.) modulate gut health and ameliorate cyclophosphamide-induced immunosuppression. J. Funct. Foods 2019, 61, 103470. [Google Scholar] [CrossRef]
- Huang, L.; Shen, M.; Wu, T.; Yu, Y.; Yu, Q.; Chen, Y.; Xie, J. Mesona chinensis Benth polysaccharides protect against oxidative stress and immunosuppression in cyclophosphamide-treated mice via MAPKs signal transduction pathways. Int. J. Biol. Macromol. 2020, 152, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Chen, X.X.; Huang, L.X.; Yu, Q.; Chen, Y.; Xie, J.H. Sulfated Mesona chinensis Benth polysaccharide enhance the immunomodulatory activities of cyclophosphamide-treated mice. J. Funct. Foods 2021, 76, 104321. [Google Scholar] [CrossRef]
- Ahlmann, M.; Hempel, G. The effect of cyclophosphamide on the immune system: Implications for clinical cancer therapy. Cancer Chemother. Pharmacol. 2016, 78, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Diwanay, S.; Patki, P.; Patwardhan, B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extracts in experimental immune inflammation. J. Ethnopharmacol. 1999, 67, 27–35. [Google Scholar] [CrossRef]
- Patra, K.; Bose, S.; Sarkar, S.; Rakshit, J.; Jana, S.; Mukherjee, A.; Roy, A.; Mandal, D.P.; Bhattacharjee, S. Amelioration of cyclophosphamide induced myelosuppression and oxidative stress by cinnamic acid. Chem.-Biol. Interact. 2012, 195, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Zong, S.A.; Li, J.L.; Yang, L.; Huang, Q.L.; Ye, Z.Y.; Hou, G.H.; Meng, Y. Synergistic antitumor effect of polysaccharide from Lachnum sp in combination with cyclophosphamide in hepatocellular carcinoma. Carbohydr. Polym. Sci. Technol. Asp. Ind. Important Polysacch. 2018, 196, 33–46. [Google Scholar] [CrossRef]
- Ríos, J.L. Chemical Constituents and Pharmacological Properties of Poria cocos. Planta Medica 2011, 77, 681–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.L.; He, Y.L.; Zeng, P.J.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.H.; Zhang, L.J. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Niu, X.; Yu, J.; Xiao, X.; Li, W. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 2020, 80, 106173. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, X.; Li, S.; Qi, E.R.; Meng, J.; Ching Lam, K.Y.; Dong, X.; Xu, J.; Chen, H.; Zhao, Z. Qualitative and quantitative characterization of carbohydrate profiles in three different parts of Poria cocos. J. Pharm. Biomed. Anal. 2020, 179, 113009. [Google Scholar] [CrossRef]
- Shao, P.; Chen, X.; Sun, P. Chemical characterization, antioxidant and antitumor activity of sulfated polysaccharide from Sargassum horneri. Carbohydr. Polym. 2014, 105, 260–269. [Google Scholar] [CrossRef]
- Simsek, M.; Asiyanbi-Hammed, T.T.; Rasaq, N.; Hammed, A.M. Progress in Bioactive Polysaccharide-Derivatives: A Review. Food Rev. Int. 2021, 1–16. [Google Scholar] [CrossRef]
- Huang, R.; Shen, M.; Yu, Y.; Liu, X.; Xie, J. Physicochemical characterization and immunomodulatory activity of sulfated Chinese yam polysaccharide. Int. J. Biol. Macromol. 2020, 165, 635–644. [Google Scholar] [CrossRef]
- Feng, H.; Fan, J.; Lin, L.; Liu, Y.; Chai, D.; Yang, J. Immunomodulatory Effects of Phosphorylated Radix Cyathulae officinalis Polysaccharides in Immunosuppressed Mice. Molecules 2019, 24, 4150. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, L.; Cheung, P. Immunopotentiation and anti-tumor activity of carboxymethylated-sulfated beta-(1-->3)-d-glucan from Poria cocos. Int. Immunopharmacol. 2010, 10, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Madruga, L.; Sabino, R.M.; Santos, E.; Popat, K.C.; Kipper, M.J. Carboxymethyl-kappa-carrageenan: A study of biocompatibility, antioxidant and antibacterial activities. Int. J. Bio. Macromol. 2020, 152, 483–491. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, Y.; Mao, J. Carboxymethylated β-Glucan Derived from Poria cocos with Biological Activities. J. Agric. Food Chem. 2009, 57, 10913–10915. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, S.; Gao, L.; Wang, L.; Cao, L. Carboxymethyl pachyman (CMP) reduces intestinal mucositis and regulates the intestinal microflora in 5-fluorouracil-treated CT26 tumour-bearing mice. Food Funct. 2018, 9, 2695–2704. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yan, Y.; Zhou, W.; Chen, D.; Cao, Y. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- Meng, M.; Wang, H.Y.; Li, Z.B.; Guo, M.Z. Protective effects of polysaccharides from Cordyceps gunnii mycelia against cyclophosphamide-induced immunosuppression to TLR4/TRAF6/NF-κB signalling in BALB/c mice. Food Funct. 2019, 10, 3262. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tao, X.; Lin, Q.; Xing, Y.; Wang, F. Immunomodulatory activity of polysaccharides isolated from Strongylocentrotus nudus eggs. Int. Immunopharmacol. 2008, 8, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Jiao, L.L.; Zhang, X.; Tian, W.M.; Chen, S.; Zhang, L.P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar]
- He, X.; Wang, X.; Fang, J.; Chang, Y.; Ning, N.; Guo, H.; Huang, L.; Huang, X.; Zhao, Z. Structures, biological activities, and industrial applications of the polysaccharides from Hericium erinaceus (Lion’s Mane) mushroom: A review. Int. J. Biol. Macromol. 2017, 97, 228–237. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, C.-p.; Zhang, Y.; Li, Y.; Sun, J.-r. Immunomodulatory and antioxidant effects of pomegranate peel polysaccharides on immunosuppressed mice. Int. J. Biol. Macromol. 2019, 137, 504–511. [Google Scholar]
- Fekete, A. Anti-cyclic citrullinated peptide antibody isotypes in rheumatoid arthritis: Association with disease duration, rheumatoid factor production and the presence of shared epitope. Clin. Exp. Rheumatol. 2008, 26, 253–260. [Google Scholar]
- Donaldson, G.P.; Ladinsky, M.S.; Yu, K.B.; Sanders, J.G.; Yoo, B.B.; Chou, W.C.; Conner, M.E.; Earl, A.M.; Knight, R.; Bjorkman, P.J. Gut microbiota utilize immunoglobulin A for mucosal colonization. Science 2018, 360, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Mebius, R.E.; Kraal, G. Structure and function of the spleen. Nat. Rev. Immunol. 2005, 5, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Ansel, K.M.; Ngo, V.N.; Hyman, P.L.; Luther, S.A.; Forster, R.; Sedgwick, J.D.; Browning, J.L.; Lipp, M.; Cyster, J.G. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature 2000, 406, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Carvalho, T.; Kearney, J.F. Development and selection of marginal zone B cells. Immunol. Rev. 2004, 197, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Attanavanich, K.; Kearney, J.F. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J. Immunol. 2004, 172, 803–811. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Nie, S.P.; Wang, J.Q.; Huang, D.F.; Li, W.J.; Xie, M.Y. Molecular mechanism underlying chemoprotective effects of Ganoderma atrum polysaccharide in cyclophosphamide-induced immunosuppressed mice. J. Funct. Foods 2015, 15, 52–60. [Google Scholar] [CrossRef]
- Min, T.; Sun, J.; Yang, Y.; Wang, H.X.; Wang, L.M. Microanalysis, Pharmacokinetics and Tissue Distribution of Polysaccharide-Protein Complexes from Longan Pulp in Mice. Int. J. Mol. Sci. 2015, 16, 24403–24416. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Dong, B.; Fe Ng, Z.; Yu, S.; Bao, Y. A study on immunomodulatory mechanism of Polysaccharopeptide mediated by TLR4 signaling pathway. BMC Immunol. 2015, 16, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campolo, M.; Paterniti, I.; Siracusa, R.; Filippone, A.; Esposito, E.; Cuzzocrea, S. TLR4 absence reduces neuroinflammation and inflammasome activation in Parkinson’s diseases in vivo model. Brain Behav. Immun. 2019, 76, 236–247. [Google Scholar] [CrossRef]
- Guo, M.; Meng, M.; Zhao, J.; Wang, X.; Wang, C. Immunomodulatory effects of the polysaccharide from Craterellus cornucopioides via activating the TLR4-NFkappaB signaling pathway in peritoneal macrophages of BALB/c mice. Int. J. Biol. Macromol. 2020, 160, 871–879. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, R.; Wu, X.; Liu, X.; Wu, Y. Dynamic digestion of tamarind seed polysaccharide: Indigestibility in gastrointestinal simulations and gut microbiota changes in vitro. Carbohydr. Polym. 2020, 239, 116194. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, X.; Zhao, Z.; Pi, X.; Meng, Y.; Fei, D.; Liu, D.; Wang, X. Effect of chitooligosaccharides on human gut microbiota and antiglycation. Carbohydr. Polym. 2020, 242, 116413. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Xu, S.; Qiu, M.; Zhang, Q.; Wu, J.; Huimin, X.; Chen, J. Chain structure and immunomodulatory activity of a fructosylated chondroitin from an engineered Escherichia coli K4. Int. J. Biol. Macromol. 2019, 133, 702–711. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Ling, C. Water-soluble yeast beta-glucan fractions with different molecular weights: Extraction and separation by acidolysis assisted-size exclusion chromatography and their association with proliferative activity. Int. J. Biol. Macromol. 2019, 123, 269–279. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Y.; Feng, X.; Ibrahim, S.A.; Huang, W. Structure characterization and in vitro immunomodulatory activities of carboxymethyl pachymaran. Int. J. Biol. Macromol. 2021, 178, 94–103. [Google Scholar] [CrossRef]
- Shen, C.Y.; Yang, L.; Jiang, J.G.; Zheng, C.Y.; Zhu, W. Immune enhancement effects and extraction optimization of polysaccharides from Citrus aurantium L. var. amara Engl. Food Funct. 2017, 8, 796–807. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Xu, X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT 2020, 125, 109260. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Chen, D.; Ran, L.; Mi, J.; Lu, L.; Jing, B.; Li, X.; Zeng, X.; Cao, Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019, 10, 3671–3683. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Y.; Ai, C.; Song, S.; Chen, X. Caulerpa lentillifera polysaccharides enhance the immunostimulatory activity in immunosuppressed mice in correlation with modulating gut microbiota. Food Funct. 2019, 10, 4315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide. Molecules 2021, 26, 5733. https://doi.org/10.3390/molecules26195733
Liu F, Zhang L, Feng X, Ibrahim SA, Huang W, Liu Y. Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide. Molecules. 2021; 26(19):5733. https://doi.org/10.3390/molecules26195733
Chicago/Turabian StyleLiu, Feng, Lijia Zhang, Xi Feng, Salam A. Ibrahim, Wen Huang, and Ying Liu. 2021. "Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide" Molecules 26, no. 19: 5733. https://doi.org/10.3390/molecules26195733
APA StyleLiu, F., Zhang, L., Feng, X., Ibrahim, S. A., Huang, W., & Liu, Y. (2021). Immunomodulatory Activity of Carboxymethyl Pachymaran on Immunosuppressed Mice Induced by Cyclophosphamide. Molecules, 26(19), 5733. https://doi.org/10.3390/molecules26195733






