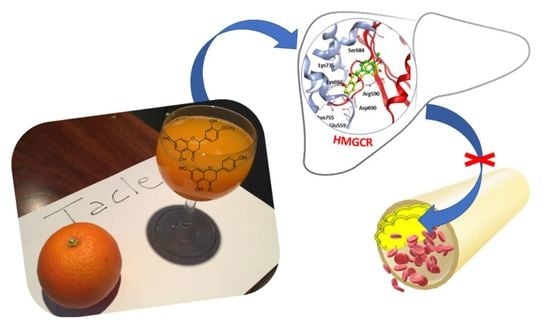
Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy
Abstract
:1. Introduction
2. Results and Discussion
2.1. In Vitro Studies
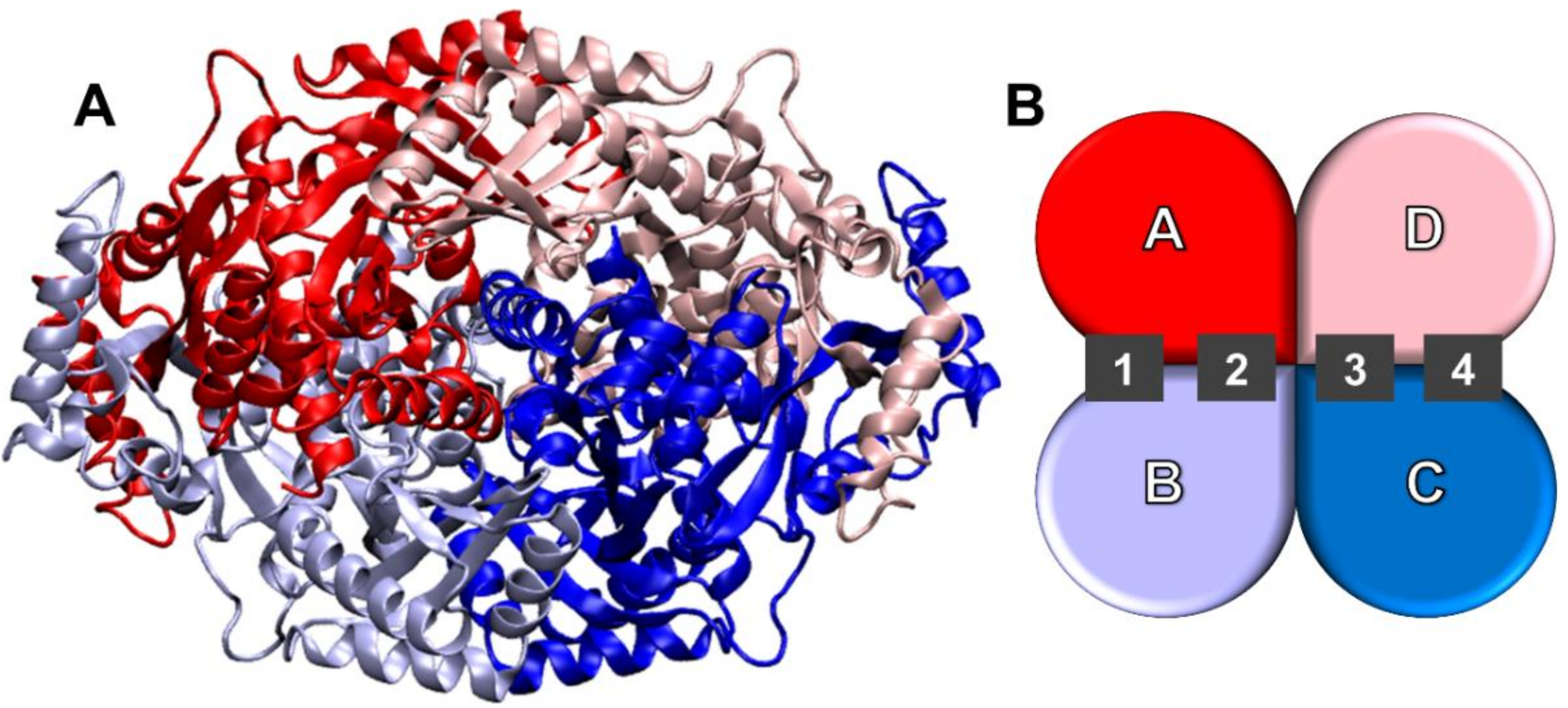
2.2. Molecular Docking
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material and Extraction Procedure
3.3. Total Polyphenol and Flavonoid Content Determination
3.3.1. Antioxidant Power of Polyphenols
3.3.2. Total Flavonoid Content
3.3.3. Flavanones Content
3.4. HMGCR Inhibition Assay
3.5. Statistical Analysis
3.6. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bhatnagar, D.; Soran, H.; Durrington, P.N. Hypercholesterolaemia and its management. BMJ 2008, 337, 503–508. [Google Scholar] [CrossRef] [Green Version]
- Adhyaru, B.B.; Jacobson, T.A. Safety and efficacy of statin therapy. Nat. Rev. Cardiol. 2018, 15, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Qi, F.; Wu, J.; Yin, G.; Hua, J.; Zhang, Q.; Qin, L. Red Yeast Rice: A systematic review of the traditional uses, chemistry, pharmacology, and quality control of an important Chinese folk medicine. Front. Pharmacol. 2019, 10, 1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosoglou, T.; Statkevich, P.; Johnson-Levonas, A.; Paolini, J.F.; Bergman, A.J.; Alton, K.B. Ezetimibe: A review of its metabolism, pharmacokinetics and drug interactions. Clin. Pharmacokinet. 2005, 44, 467–494. [Google Scholar] [CrossRef]
- Ray, K.K.; Bays, H.E.; Catapano, A.L.; Lalwani, N.D.; Bloedon, L.T.; Sterling, L.R.; Robinson, P.L.; Ballantyne, C.M. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N. Eng. J. Med. 2019, 380, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Landmesser, U.; Leiter, L.A.; Kallend, D.; Dufour, R.; Karakas, M.; Hall, T.; Troquay, R.P.T.; Turner, T.; Visseren, F.L.J.; et al. Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N. Engl. J. Med. 2017, 376, 1430–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonagh, M.; Peterson, K.; Holzhammer, B.; Fazio, S. A systematic review of PCSK9 inhibitors alirocumab and evolocumab. J. Manag. care Spec. Pharm. 2016, 22, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Selamoglu, Z.; Sener, B.; Kilic, M.; Kumar Jugran, A.; de Tommasi, N.; Sinisgalli, C.; Milella, L.; Rajkovic, J.; Flaviana, B.; et al. Berberis plants-drifting from farm to food applications, phytotherapy, and phytopharmacology. Foods 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talati, R.; Sobieraj, D.M.; Makanji, S.S.; Phung, O.J.; Coleman, C.I. The comparative efficacy of plant sterols and stanols on serum lipids: A systematic review and meta-analysis. J. Am. Diet. Assoc. 2010, 110, 719–726. [Google Scholar] [CrossRef]
- Roza, J.M.; Xian-Liu, Z.; Guthrie, N. Effect of citrus flavonoids and tocotrienols on serum cholesterol levels in hypercholesterolemic subjects. Altern. Ther. Health Med. 2007, 13, 44–48. [Google Scholar]
- Intrigliolo, F. Citrus Tree Named “Red Tacle”. U.S. Patents US20080189813P1, 7 August 2008. Available online: https://appft.uspto.gov/netacgi/nph-Parser?Sect1=PTO1&Sect2=HITOFF&p=1&u=/netahtml/PTO/srchnum.html&r=1&f=G&l=50&d=PG01&s1=20080189813.PGNR (accessed on 31 August 2021).
- Demonty, I.; Lin, Y.; Zebregs, Y.E.M.P.; Vermeer, M.A.; van der Knaap, H.C.M.; Jäkel, M.; Trautwein, E.A. The Citrus flavonoids hesperidin and naringin do not affect serum cholesterol in moderately hypercholesterolemic men and women. J. Nutr. 2010, 140, 1615–1620. [Google Scholar] [CrossRef] [Green Version]
- Casacchia, T.; Occhiuzzi, M.A.; Grande, F.; Rizzuti, B.; Granieri, M.C.; Rocca, C.; Gattuso, A.; Garofalo, A.; Angelone, T.; Statti, G. A pilot study on the nutraceutical properties of the Citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J. Funct. Foods 2019, 52, 370–381. [Google Scholar] [CrossRef]
- Dewick, P.M. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2009; Chapter 4; pp. 166–177. [Google Scholar]
- Zhang, M.; Zhu, S.; Yang, W.; Huang, Q.; Ho, C.T. The biological fate and bioefficacy of citrus flavonoids: Bioavailability, biotransformation, and delivery systems. Food Funct. 2021, 12, 3307–3323. [Google Scholar] [CrossRef]
- Guo, X.; Li, K.; Guo, A.; Li, E. Intestinal absorption and distribution of naringin, hesperidin, and their metabolites in mice. J. Funct. Foods 2020, 74, 104158. [Google Scholar] [CrossRef]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Andrade, R.; Araujo-León, J.A.; Sánchez-Recillas, A.; Navarrete-Vazquez, G.; González-Sánchez, A.A.; Hidalgo-Figueroa, S.; Alonso-Castro, Á.J.; Aranda-González, I.; Hernández-Núñez, E.; Coral-Martínez, T.I.; et al. Toxicological screening of four bioactive citroflavonoids: In vitro, in vivo, and in silico approaches. Molecules 2020, 25, 5959. [Google Scholar] [CrossRef]
- Herranz-López., M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef]
- García, B.F.; Torres, A.; Macías, F.A. Synergy and other interactions between polymethoxyflavones from Citrus byproducts. Molecules 2015, 20, 20079–20106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Istvan, E.S.; Deisenhofer, J. The structure of the catalytic portion of human HMG-CoA reductase. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2000, 1529, 9–18. [Google Scholar] [CrossRef]
- Gesto, D.S.; Pereira, C.M.S.; Cerqueira, N.M.F.S.; Sousa, S.F. An atomic-level perspective of HMG-CoA-reductase: The target enzyme to treat hypercholesterolemia. Molecules 2020, 25, 3891. [Google Scholar] [CrossRef]
- Friesen, J.A.; Rodwell, V.W. The 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductases. Genome Biol. 2004, 5, 245. [Google Scholar] [CrossRef] [Green Version]
- Menichini, G.; Alfano, C.; Marrelli, M.; Toniolo, C.; Provenzano, E.; Statti, G.A.; Nicoletti, M.; Menichini, F.; Conforti, F. Hypericum perforatum L. subsp. perforatum induces inhibition of free radicals and enhanced phototoxicity in human melanoma cells under ultraviolet light. Cell Prolif. 2013, 46, 193–202. [Google Scholar] [CrossRef]
- Marrelli, M.; Conforti, F.; Araniti, F.; Casacchia, T.; Statti, G. Seasonal and environmental variability of non-cultivated edible Cichorioideae (Asteraceae). Plant Biosyst. 2018, 152, 759–766. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeerschd, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Grande, F.; Rizzuti, B.; Occhiuzzi, M.A.; Ioele, G.; Casacchia, T.; Gelmini, F.; Guzzi, R.; Garofalo, A.; Statti, G. Identification by molecular docking of homoisoflavones from Leopoldia comosa as ligands of estrogen receptors. Molecules 2018, 23, 894. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Concentrations | % Inhibition | ||||
|---|---|---|---|---|---|
| Tacle Extract | Naringin | Hesperidin | Naringenin | Hesperetin | |
| 500 µg/mL | 55.5 ± 1.1 * | 17.3 ± 1.1 * | 43.2 ± 1.6 * | 53.8 ± 1.5 * | 55.7 ± 3.0 * |
| 400 µg/mL | 46.7 ± 0.9 * | 8.3 ± 1.1 ** | 30.9 ± 2.7 * | 36.9 ± 1.8 * | 23.2 ± 2.2 * |
| 300 µg/mL | 29.6 ± 2.1 * | - | 12.2 ± 0.6 * | 13.2 ± 1.5 * | 13.9 ± 1.4 * |
| 200 µg/mL | 21.4 ± 0.9 * | - | - | - | - |
| 100 µg/mL | 12.3 ± 1.7 * | - | - | - | - |
| Protein Structure | Binding Energy * (kcal/mol) | |||
| PDB Code | Crystallographic Ligands | Ligand Re-Docking | Naringenin | Hesperetin |
| 1HW8 | mevastatin | −6.53 ± 0.22 | −7.65 ± 0.58 | −7.57 ± 0.15 |
| 1HW9 | simvastatin | −6.59 ± 0.27 | −7.70 ± 0.05 | −7.60 ± 0.14 |
| 1HWI | fluvastatin | −7.88 ± 0.38 | −7.67 ± 0.15 | −7.55 ± 0.13 |
| 1HWJ | cerivastatin | −7.21 ± 0.12 | −7.80 ± 0.24 | −7.45 ± 0.17 |
| 1HWK | atorvastatin | −9.11 ± 0.04 | −7.62 ± 0.09 | −7.42 ± 0.05 |
| 1HWL | rosuvastatin | −7.57 ± 0.04 | −7.77 ± 0.05 | −7.47 ± 0.09 |
| PDB Entry | Site | Naringenin | Hesperetin |
|---|---|---|---|
| 1HW8 | 1 | Lys735 B | Ser684 A, Lys735 B |
| 2 | Lys735 A | Glu559 A, Lys735 A | |
| 3 | Lys735 D | Glu559 D, Glu665 C, Glu665 C, Lys735 D, Lys691 C | |
| 4 | Lys735 C | Lys735 C | |
| 1HW9 | 1 | Lys735 B, Asn755 B | Ser684 A, Lys735 B |
| 2 | Glu559 A, Lys735 A, Ser684 B, Lys691 B | Glu559 A, Lys735 A, Ser684 B, Lys691 B | |
| 3 | Glu665 C, Lys735 D | Glu665 C, Ser684 C, Lys735 D | |
| 4 | Glu665 D, Lys735 C | Lys735 C, Ser684 D, Asn755 C | |
| 1HWI | 1 | Lys735 B, Asn755b | Glu559 B, Ser684 A, Lys735 B |
| 2 | Ala751 A, Glu665 B, Lys735 A | Glu665 B, Lys735 A | |
| 3 | Ala751 D, Ser684 C, Lys735 D | Ala751 D, Ser684 C, Lys735 D | |
| 4 | Lys735 C, Ser661 D | Lys735 C | |
| 1HWJ | 1 | Lys735 B | Lys735 B |
| 2 | Lys735 A | Lys735 A, Ser684 B | |
| 3 | Lys735 D | Lys735 D | |
| 4 | Glu665 D, Lys735 C | Glu559 C, Lys735 C, Lys691 D | |
| 1HWK | 1 | Glu665 A, Lys735 B | Ser684 A, Lys735 B |
| 2 | Glu559 A, Glu665 B, Lys735 A, Lys691 B | Glu665 B, Lys735 A, Ser684 B | |
| 3 | Ala751 D, Lys735 D, Asn755 D | Lys735 D | |
| 4 | Glu665 D, Lys735 C | Glu559 C, Lys735 C, Lys691 D | |
| 1HWL | 1 | Ser684 A, Lys735 B | Glu559 B, Ser684 A, Lys735 B |
| 2 | Lys735 A, Ser684 B | Lys735 A, Ser684 B | |
| 3 | Ser684 C, Lys735 D | Lys735 D | |
| 4 | Lys735 C, Ser684 D | Glu559 C, Glu665 D, Lys735 C, Ser684 D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grande, F.; Occhiuzzi, M.A.; Perri, M.R.; Ioele, G.; Rizzuti, B.; Statti, G.; Garofalo, A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules 2021, 26, 5718. https://doi.org/10.3390/molecules26185718
Grande F, Occhiuzzi MA, Perri MR, Ioele G, Rizzuti B, Statti G, Garofalo A. Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules. 2021; 26(18):5718. https://doi.org/10.3390/molecules26185718
Chicago/Turabian StyleGrande, Fedora, Maria Antonietta Occhiuzzi, Maria Rosaria Perri, Giuseppina Ioele, Bruno Rizzuti, Giancarlo Statti, and Antonio Garofalo. 2021. "Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy" Molecules 26, no. 18: 5718. https://doi.org/10.3390/molecules26185718
APA StyleGrande, F., Occhiuzzi, M. A., Perri, M. R., Ioele, G., Rizzuti, B., Statti, G., & Garofalo, A. (2021). Polyphenols from Citrus Tacle® Extract Endowed with HMGCR Inhibitory Activity: An Antihypercholesterolemia Natural Remedy. Molecules, 26(18), 5718. https://doi.org/10.3390/molecules26185718