Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review
Abstract
:1. Background
2. Wounds
- identification of processes involved in skin regeneration,
- disturbances in regenerative processes in chronic wounds,
- drug dosing systems enabling effective delivery of substances to the wound bed,
- development of materials that act as a scaffold for cell growth, and
- development of advanced dressings [3].
- superficial-not exceeding the subcutaneous tissue; and
2.1. Disorders of the Wound Healing Process
2.2. Wound Treatment
3. Propolis
3.1. Composition
3.2. Standarization and Quality Control
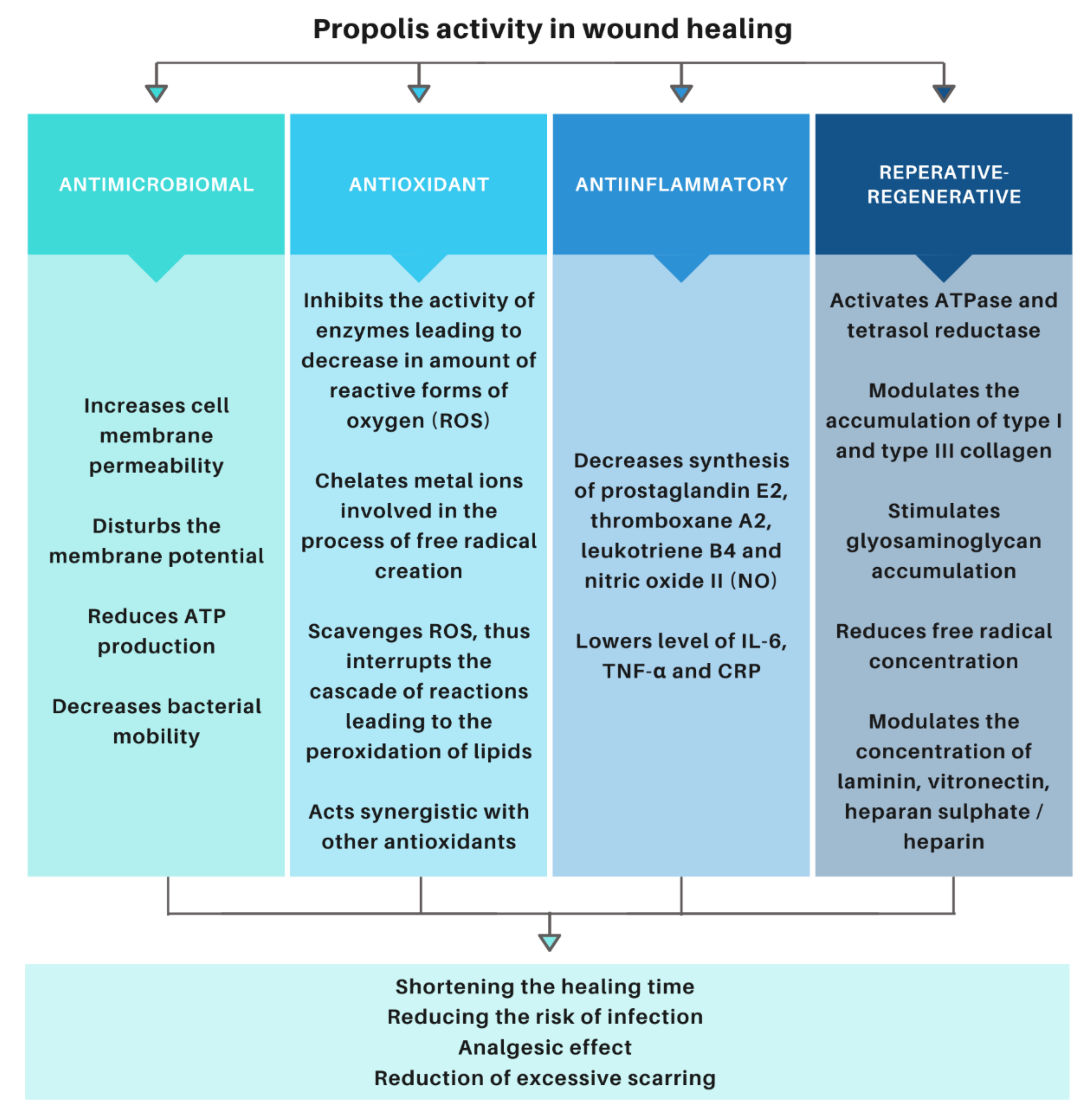
3.3. Activity
4. Biodegradable Polyesters
5. Electrospinning
5.1. Principle of the Method
5.2. Biomedical Applications of Eletrospinning Process
6. Conclusions
- The use of well-tested biodegradable polyesters makes the dressing biodegradable and biocompatible. The knowledge of the degradation processes and the release profiles of the drug substance allows for the selection of the appropriate material composition. The production of a dressing from a material that degrades and releases the active substance during wound healing enables its selection to avoid the necessity of dressing changes and supply of the active substance.
- Propolis is an apitherapeutic agent with documented antibacterial, antiviral, antifungal, antitumor, anti-inflammatory, analgesic, and repair and regenerative properties. The controlled release of propolis throughout the treatment period accelerates healing, reduces the risk of infection, and improves the cosmetic effect by reducing scar formation.
- Electrospinning is a method of producing nonwovens that mechanically protect the wound and mimics ECM, while allowing gas exchange within the wound. Moreover, it allows for the adaptation of the release profile and the time of complete degradation by modifying the production parameters.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, W.; Lu, K.J.; Yu, C.H.; Huang, Q.L.; Du, Y.-Z.Z. Nano-Drug Delivery Systems in Wound Treatment and Skin Regeneration. J. Nanobiotechnol. 2019, 17, 82. [Google Scholar] [CrossRef]
- Schreml, S.; Szeimies, R.M.; Prantl, L.; Landthaler, M.; Babilas, P. Wound Healing in the 21st Century. J. Am. Acad. Dermatol. 2010, 63, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Wound Care Market—Forecast to 2024. Available online: https://www.marketsandmarkets.com/Market-Reports/wound-care-market-371.html (accessed on 10 November 2020).
- Herndon, D. Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323497428. [Google Scholar]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A Review on Wound Dressings with an Emphasis on Electrospun Nanofibrous Polymeric Bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Dystems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef] [PubMed]
- Percival, N.J. Classification of Wounds and Their Management. Surgery 2002, 20, 114–117. [Google Scholar] [CrossRef]
- Sarabahi, S.; Tiwari, V.K. Principles and Practice of Wound Care; Jaypee Brothers Medical Publishers (P) Ltd: New Delhi, India, 2012; ISBN 9789350258644. [Google Scholar]
- Fibak, J. Chirurgia; PZWL: Warszawa, Poland, 2010; ISBN 83-200-2012-3. [Google Scholar]
- Noszyczyk, W. Chirurgia; PZWL: Warszawa, Poland, 2007; ISBN 83-200-3120-6. [Google Scholar]
- Abazari, M.; Ghaffari, A.; Rashidzadeh, H.; Badeleh, S.M.; Maleki, Y. A Systematic Review on Classification, Identification, and Healing Process of Burn Wound Healing. Int. J. Low. Extrem. Wounds 2020. [Google Scholar] [CrossRef]
- Tiwari, V.K. Burn Wound: How It Differs from Other Wounds. Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Evers, L.H.; Bhavsar, D.; Mailänder, P. The Biology of Burn Injury. Exp. Dermatol. 2010, 19, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Broughton, G.; Janis, J.E.; Attinger, C.E. The Basic Science of Wound Healing. Plast. Reconstr. Surg. 2006, 117, 12S–34S. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic Wound Pathogenesis and Current Treatment Strategies: A Unifying Hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Liang, H.; Clarke, E.; Jackson, C.; Xue, M. Inflammation in Chronic Wounds. Int. J. Mol. Sci. 2016, 17, 2085. [Google Scholar] [CrossRef]
- Zielins, E.R.; Atashroo, D.A.; Maan, Z.N.; Duscher, D.; Walmsley, G.G.; Marecic, O.; Hu, M.; Senarath-Yapa, K.; McArdle, A.; Tevlin, R.; et al. Wound Healing: An Update. Regen. Med. 2014, 9, 817–830. [Google Scholar] [CrossRef] [PubMed]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME Concept: What Have We Learned in the Past 10 Years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef]
- Harries, R.L.; Bosanquet, D.C.; Harding, K.G. Wound Bed Preparation: TIME for an Update. Int. Wound J. 2016, 13, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Vivó, C.; Galeiras, R.; del Caz, M.D.P. Initial Evaluation and Management of the Critical Burn Patient. Med. Intensiva 2016, 40, 49–59. [Google Scholar] [CrossRef]
- Dhivya, S.; Vijaya Padma, V.; Santhini, E. Wound Dressings—A Review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Jawień, A.; Bartoszewicz, M.; Przondo-Mordarska, A.; Szewczyk, M.T.; Kaszuba, A.; Urbanek, T.; Staszkiewicz, W.; Sopata, M.; Kucharzewski, M.; Korzon-Burakowska, A.; et al. Wytyczne Postępowania Miejscowego i Ogólnego w Ranach Objętych Procesem Infekcji. Leczenie Ran 2012, 9, 59–75. [Google Scholar]
- Sopata, M.; Jawień, A.; Mrozikiewicz-Rakowska, B.; Augusewicz, Z.; Bakowska, M.; Samson, I.; Gabriel, M.; Grzela, T.; Karpiński, T.M.; Kuberka, I.; et al. Wytyczne Postępowania Miejscowego w Ranach Niezakażonych, Zagrożonych Infekcją Oraz Zakażonych-Przegląd Dostępnych Substancji Przeciwdrobnoustrojowych Stosowanych w Leczeniu Ran. Zalecania Polskiego Towarzystwa Leczenia Ran. Leczenie Ran 2020, 17, 151–184. [Google Scholar] [CrossRef]
- Lachapelle, J.-M.; Castel, O.; Casado, A.F.; Leroy, B.; Micali, G.; Tennstedt, D.; Lambert, J. Therapeutic Perspective Antiseptics in the Era of Bacterial Resistance: A Focus on Povidone Iodine. Clin. Pract. 2013, 10, 579–592. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Harding, K.; Téot, L.; Siebert, J. Effectiveness and Tissue Compatibility of a 12-Week Treatment of Chronic Venous Leg Ulcers with an Octenidine Based Antiseptic—A Randomized, Double-Blind Controlled Study. Int. Wound J. 2012, 9, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Ramos, P.; Komosinska-Vassev, K.; Stojko, J.; Pilawa, B. Positive Effect of Propolis on Free Radicals in Burn Wounds. Evid.-Based Complement. Altern. Med. 2013, 2013, 356737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, M.P.; Neto, A.I.; Correia, T.R.; Miguel, S.P.; Matsusaki, M.; Correia, I.J.; Mano, J.F. Bioinspired Multilayer Membranes as Potential Adhesive Patches for Skin Wound Healing. Biomater. Sci. 2018, 6, 1962–1975. [Google Scholar] [CrossRef] [Green Version]
- Dissemond, J.; Augustin, M.; Eming, S.A.; Goerge, T.; Horn, T.; Karrer, S.; Schumann, H.; Stücker, M. Modern Wound Care—Practical Aspects of Non-Interventional Topical Treatment of Patients with Chronic Wounds. J. Ger. Soc. Dermatol. 2014, 12, 541–554. [Google Scholar] [CrossRef]
- Canpolat, I.; Başa, A. Wound Healing and Current Treatment Techniques. Agric. Vet. Sci. 2017, 1, 180–184. [Google Scholar]
- Ilenghoven, D. A Review of Wound Dressing Practices. Clin. Dermatol. Open Access J. 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Liu, H.; Li, Q.; Li, R.; Zhang, Y.; Liu, Y.; Shao, Y.; Wang, J. Selection of Appropriate Wound Dressing for Various Wounds. Front. Bioeng. Biotechnol. 2020, 8, 182. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, H.; Ubbink, D.T.; Goossens, A.; de Vos, R.; Legemate, D.A. Systematic Review of Dressings and Topical Agents for Surgical Wounds Healing by Secondary Intention. Br. J. Surg. 2005, 92, 665–672. [Google Scholar] [CrossRef]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1–26. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Lee, J.-S.; Chu, C.-S.; Chang, Y.-P.; Wang, Y.-J. Development of Two Alginate-Based Wound Dressings. J. Mater. Sci. Mater. Med. 2008, 19, 2503–2513. [Google Scholar] [CrossRef] [PubMed]
- Furtado, S.C.; Srinivasan, B.; Abraham, S. Wound Healing Concepts: Contemporary Practices and Future Perspectives. Int. J. Appl. Pharm. 2020, 12, 7–15. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachuau, L. Recent Developments in Novel Drug Delivery Systems for Wound Healing. Expert Opin. Drug Deliv. 2015, 12, 1895–1909. [Google Scholar] [CrossRef] [PubMed]
- Rieger, K.A.; Birch, N.P.; Schiffman, J.D. Designing Electrospun Nanofiber Mats to Promote Wound Healing—A Review. J. Mater. Chem. B 2013, 1, 4531–4541. [Google Scholar] [CrossRef] [Green Version]
- Shedoeva, A.; Leavesley, D.; Upton, Z.; Fan, C. Wound Healing and the Use of Medicinal Plants. Evid.-Based Complement. Altern. Med. 2019, 2019, 2684108. [Google Scholar] [CrossRef]
- Adomavičiūtė, E.; Pupkevičiūtė, S.; Juškaitė, V.; Žilius, M.; Stanys, S.; Pavilonis, A.; Briedis, V. Formation and Investigation of Electrospun PLA Materials with Propolis Extracts and Silver Nanoparticles for Biomedical Applications. J. Nanomater. 2017, 2017, 8612819. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and Functional Properties of Propolis (Bee Glue): A Review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef]
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292. [Google Scholar] [CrossRef]
- De Castro, S.L. Propolis: Biological and Pharmacological Activities. Ann. Rev. Biomed. Sci. 2001, 3, 49–83. [Google Scholar] [CrossRef]
- Olczyk, P.; Wisowski, G.; Komosinska-Vassev, K.; Stojko, J.; Klimek, K.; Olczyk, M.; Kozma, E.M. Propolis Modifies Collagen Types I and III Accumulation in the Matrix of Burnt Tissue. Evid.-Based. Complement. Alternat. Med. 2013, 2013, 423809. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Kozma, E.M.; Wisowski, G.; Stojko, J.; Klimek, K.; Olczyk, K. Propolis Modulates Vitronectin, Laminin, and Heparan Sulfate/Heparin Expression during Experimental Burn Healing. J. Zhejiang Univ. Sci. B 2012, 13, 932–941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Kozma, E.M. Propolis Induces Chondroitin/Dermatan Sulphate and Hyaluronic Acid Accumulation in the Skin of Burned Wound. Evid.-Based Complement. Altern. Med. 2013, 2013, 290675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Potential Role of Propolis in Wound Healing: Biological Properties and Therapeutic Activities. Biomed. Pharmacother. 2018, 98, 469–483. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.-P.P.; Wang, K.; Li, G.Q.; Hu, F.-L.L. Recent Advances in the Chemical Composition of Propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef] [Green Version]
- Monti, M.; Bertt, E.; Carminati, G.; Cusini, M. Occupational and Cosmetic Dermatitis from Propolis. Contact Dermat. 1983, 9, 163. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Rzepecka-Stojko, A.; Górecki, M.; Stojko, J.; Sosada, M.; Swierczek-Zieba, G. Structure and Antioxidant Activity of Polyphenols Derived from Propolis. Molecules 2014, 19, 78–101. [Google Scholar] [CrossRef] [Green Version]
- Kabała-Dzik, A.; Rzepecka-Stojko, A.; Kubina, R.; Iriti, M.; Wojtyczka, R.D.; Buszman, E.; Stojko, J. Flavonoids, Bioactive Components of Propolis, Exhibit Cytotoxic Activity and Induce Cell Cycle Arrest and Apoptosis in Human Breast Cancer Cells MDA-MB-231 and MCF-7-A Comparative Study. Cell. Mol. Biol. 2018, 64, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Maciejewicz, W. Isolation of Flavonoid Aglycones from Propolis by a Column Chromatography Method and Their Identification by GC-MS and TLC Methods. J. Liq. Chromatogr. Relat. Technol. 2006, 24, 1171–1179. [Google Scholar] [CrossRef]
- Bankova, V. Chemical Diversity of Propolis and the Problem of Standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M. Standardization and Quality Control of Propolis: A Brief Review. APOidea Riv. Ital. Apic. 2007, 1, 19–23. [Google Scholar]
- Banskota, A.H.; Tezuka, Y.; Kadota, S. Recent Progress in Pharmacological Research of Propolis. Phytother. Res. 2001, 15, 561–571. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Pietta, P.G.; Gardana, C.; Pietta, A.M. Analytical Methods for Quality Control of Propolis. Fitoterapia 2002, 73, S7–S20. [Google Scholar] [CrossRef]
- Galeotti, F.; Capitani, F.; Fachini, A.; Volpi, N. Recent Advances in Analytical Approaches for the Standardization and Quality of Polyphenols of Propolis. J. Med. Plants Res. 2019, 13, 487–500. [Google Scholar] [CrossRef]
- Sun, S.; He, J.; Liu, M.; Yin, G.; Zhang, X. A Great Concern Regarding the Authenticity Identification and Quality Control of Chinese Propolis and Brazilian Green Propolis. J. Food Nutr. Res. 2019, 7, 725–735. [Google Scholar] [CrossRef]
- Bridi, R.; Montenegro, G.; Nuñez-Quijada, G.; Giordano, A.; Fernanda Morán-Romero, M.; Jara-Pezoa, I.; Speisky, H.; Atala, E.; López-Alarcón, C. International Regulations of Propolis Quality: Required Assays Do Not Necessarily Reflect Their Polyphenolic-Related In Vitro Activities. J. Food Sci. 2015, 80, C1188–C1195. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Antioxidant Activity of Food Constituents: An Overview. Arch. Toxicol. 2011, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of Ethanol/Water Solvents on Phenolic Profiles and Antioxidant Properties of Beijing Propolis Extracts. Evid.-Based Complement. Altern. Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [Green Version]
- Bonamigo, T.; Campos, J.F.; Oliveira, A.S.; Torquato, H.F.V.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; de Picoli Souza, K.; Dos Santos, E.L. Antioxidant and Cytotoxic Activity of Propolis of Plebeia Droryana and Apis Mellifera (Hymenoptera, Apidae) from the Brazilian Cerrado Biome. PLoS ONE 2017, 12, e0183983. [Google Scholar] [CrossRef] [Green Version]
- Bonamigo, T.; Campos, J.F.; Alfredo, T.M.; Balestieri, J.B.P.; Cardoso, C.A.L.; Paredes-Gamero, E.J.; De Picoli Souza, K.; Dos Santos, E.L. Antioxidant, Cytotoxic, and Toxic Activities of Propolis from Two Native Bees in Brazil: Scaptotrigona Depilis and Melipona Quadrifasciata Anthidioides. Oxid. Med. Cell. Longev. 2017, 2017, 1038153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittencourt, M.; Ribeiro, P.; Franco, R.; Hilhorst, H.; de Castro, R.; Fernandez, L. Metabolite Profiling, Antioxidant and Antibacterial Activities of Brazilian Propolis: Use of Correlation and Multivariate Analyses to Identify Potential Bioactive Compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.K.S.; Denadai, M.; de Oliveira, C.S.; Nunes, M.L.; Narain, N. Evaluation of Bioactive Compounds Potential and Antioxidant Activity of Brown, Green and Red Propolis from Brazilian Northeast Region. Food Res. Int. 2017, 101, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shen, X.; Chen, J.; Jiang, X.; Hu, F. Identification of Free Radical Scavengers from Brazilian Green Propolis Using Off-Line HPLC-DPPH Assay and LC-MS. J. Food Sci. 2017, 82, 1602–1607. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Bugaj, M.; Juszczak, L. Phenolic Composition and Antioxidant Activity of Propolis from Various Regions of Poland. Nat. Prod. Res. 2015, 29, 416–422. [Google Scholar] [CrossRef]
- Song, Y.S.; Park, E.H.; Hur, G.M.; Ryu, Y.S.; Lee, Y.S.; Lee, J.Y.; Kim, Y.M.; Jin, C. Caffeic Acid Phenethyl Ester Inhibits Nitric Oxide Synthase Gene Expression and Enzyme Activity. Cancer Lett. 2002, 175, 53–61. [Google Scholar] [CrossRef]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular Mechanisms Underlying the in Vitro Anti-Inflammatory Effects of a Flavonoid-Rich Ethanol Extract from Chinese Propolis (Poplar Type). Evid.-Based Complement. Altern. Med. 2013, 2013, 127672. [Google Scholar] [CrossRef] [Green Version]
- Alp, H.; Aytekin, I.; Atakisi, O.; Hatipoglu, N.K.; Basarali, K.; Ogun, M.; Buyukbas, S.; Altintas, L.; Ekici, H.; Alp, A. The Effects of Caffeic Acid Phenethyl Ester and Ellagic Acid on the Levels of Malondialdehyde, Reduced Glutathione and Nitric Oxide in the Lung, Liver and Kidney Tissues in Acute Diazinon Toxicity in Rats. J. Anim. Vet. Adv. 2011, 10, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- De Moura, S.A.L.; Negri, G.; Salatino, A.; da Cunha Lima, L.D.; Dourado, L.P.A.; Mendes, J.B.; Andrade, S.P.; Ferreira, M.A.N.D.; Cara, D.C. Aqueous Extract of Brazilian Green Propolis: Primary Components, Evaluation of Inflammation and Wound Healing by Using Subcutaneous Implanted Sponges. Evid.-Based. Complement. Alternat. Med. 2011, 2011, 748283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, A.; Ligresti, A.; Longo, R.; Russo, A.; Borrelli, F.; Sautebin, L. The Inhibitory Effect of Propolis and Caffeic Acid Phenethyl Ester on Cyclooxygenase Activity in J774 Macrophages. Phytomedicine 2002, 9, 530–535. [Google Scholar] [CrossRef] [Green Version]
- Ignatova, M.; Rashkov, I.; Manolova, N. Drug-Loaded Electrospun Materials in Wound -Dressing Applications and in Local Cancer Treatment. Expert Opin. Drug Deliv. 2013, 10, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M. Apitherapeutics and Phage-Loaded Nanofibers as Wound Dressings with Enhanced Wound Healing and Antibacterial Activity. Nanomedicine 2017, 12, 2055–2067. [Google Scholar] [CrossRef] [PubMed]
- Stojko, J.; Juszko-Piekut, M.; Rzepecka-Stojko, A.; Stojko, R.; Moździerz, A.; Olczyk, D.; Romaniuk, D.; Kasprzak, M.; Morawiec, T. Application of the Preparation Sepropol—in the Bedsore Prophylaxis and Treatment. Polish J. Environ. Stud. 2007, 16, 609–611. [Google Scholar]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial Action of Propolis and Some of Its Components: The Effects on Growth, Membrane Potential and Motility of Bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Kubina, R.; Kabała-Dzik, A.; Bułdak, R.J. Aktywność Przeciwdrobnoustrojowa Etanolowego Ekstraktu Propolisu. Ann. Acad. Medicae Silesiensis 2012, 66, 39–48. [Google Scholar]
- Wojtyczka, R.D.; Kȩpa, M.; Idzik, D.; Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Wasik, T.J. In Vitro Antimicrobial Activity of Ethanolic Extract of Polish Propolis against Biofilm Forming Staphylococcus Epidermidis Strains. Evid.-Based Complement. Altern. Med. 2013, 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Kabała-Dzik, A.; Szaflarska-Stojko, E.; Wojtyczka, R.D.; Stojko, A.; Stojko, R.; Pacha, J. Comparative Studies on the Antimicrobial Activity of Propolis Balm and Silver Sulphadiazine Applied to Burn Wounds in Pigs. Bull. Vet. Inst. Pulawy 2003, 47, 541–545. [Google Scholar]
- Nasser, S.; Mabrouk, A.; Maher, A. Colonization of Burn Wounds in Ain Shams University Burn Unit. Burns 2003, 29, 229–233. [Google Scholar] [CrossRef]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of Topical Administration of Propolis in Chronic Periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Nascimento, A.P.; Bueno, P.C.P.; de Oliveira Lima Leite Vaz, M.M.; Marchetti, J.M. Propolis Standardized Extract (EPP-AF®), an Innovative Chemically and Biologically Reproducible Pharmaceutical Compound for Treating Wounds. Int. J. Biol. Sci. 2012, 8, 512–521. [Google Scholar] [CrossRef]
- Barud, H.D.S.; De Araújo Júnior, A.M.; Saska, S.; Mestieri, L.B.; Campos, J.A.D.B.; De Freitas, R.M.; Ferreira, N.U.; Nascimento, A.P.; Miguel, F.G.; de Oliveira Lima Leite Vaz, M.M.; et al. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid.-Based Complement. Altern. Med. 2013, 2013, 703024. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.T.; Abo-Salem, O.M.; Osman, A. The Influence of Egyptian Propolis on Induced Burn Wound Healing in Diabetic Rats; Antibacterial Mechanism. Sci. J. Med. Clin. Trials 2011, 11, 1–8. [Google Scholar]
- Hozzein, W.N.; Badr, G.; Al Ghamdi, A.A.; Sayed, A.; Al-Waili, N.S.; Garraud, O. Topical Application of Propolis Enhances Cutaneous Wound Healing by Promoting TGF-Beta/Smad-Mediated Collagen Production in a Streptozotocin-Induced Type I Diabetic Mouse Model. Cell. Physiol. Biochem. 2015, 37, 940–954. [Google Scholar] [CrossRef]
- McLennan, S.V.; Bonner, J.; Milne, S.; Lo, L.; Charlton, A.; Kurup, S.; Jia, J.; Yue, D.K.; Twigg, S.M. The Anti-Inflammatory Agent Propolis Improves Wound Healing in a Rodent Model of Experimental Diabetes. Wound Repair Regen. 2008, 16, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Wisowski, G.; Mencner, L.; Stojko, J.; Kozma, E.M. Propolis Modulates Fibronectin Expression in the Matrix of Thermal Injury. Biomed Res. Int. 2014, 2014, 748101. [Google Scholar] [CrossRef] [PubMed]
- Olczyk, P.; Ramos, P.; Bernaś, M.; Komosinska-Vassev, K.; Stojko, J.; Pilawa, B. Application of Electron Paramagnetic Resonance Spectroscopy to Comparative Examination of Different Groups of Free Radicals in Thermal Injuries Treated with Propolis and Silver Sulphadiazine. Evid.-Based Complement. Altern. Med. 2013, 2013, 851940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Olczyk, K. Propolis-Chemical Composition, Properties and Application. Farm. Pol. 2007, 63, 1102–1107. [Google Scholar]
- Barroso, P.R.; Lopes-Rocha, R.; Pereira, E.M.F.; Marinho, S.A.; de Miranda, J.L.; Lima, N.L.; Verli, F.D. Effect of Propolis on Mast Cells in Wound Healing. Inflammopharmacology 2011, 20, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Pu, L.; Wei, J.; Li, J.; Wu, J.; Xin, Z.; Gao, W.; Guo, C. Brazilian Green Propolis Improves Antioxidant Function in Patients with Type 2 Diabetes Mellitus. Int. J. Environ. Res. Public Health 2016, 13, 498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mujica, V.; Orrego, R.; Pérez, J.; Romero, P.; Ovalle, P.; Zúñiga-Hernández, J.; Arredondo, M.; Leiva, E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 4272940. [Google Scholar] [CrossRef] [Green Version]
- Mujica, V.; Orrego, R.; Fuentealba, R.; Leiva, E.; Zúñiga-Hernández, J. Clinical Study Propolis as an Adjuvant in the Healing of Human Diabetic Foot Wounds Receiving Care in the Diagnostic and Treatment Centre from the Regional Hospital of Talca. J. Diabetes Res. 2019, 2019, 2507578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.H.; Lee, M.Y.; Chung, Y.-J.; Rhee, C.-K.; Lee, S.J. Effect of Topical Propolis on Wound Healing Process After Tonsillectomy: Randomized Controlled Study. Clin. Exp. Otorhinolaryngol. 2018, 11, 146. [Google Scholar] [CrossRef]
- Kim, J.I.; Pant, H.R.; Sim, H.-J.J.; Lee, K.M.; Kim, C.S. Electrospun Propolis/Polyurethane Composite Nanofibers for Biomedical Applications. Mater. Sci. Eng. C 2014, 44, 52–57. [Google Scholar] [CrossRef]
- Sutjarittangtham, K.; Sanpa, S.; Tunkasiri, T.; Chantawannakul, P.; Intatha, U.; Eitssayeam, S. Bactericidal Effects of Propolis/Polylactic Acid (PLA) Nanofibres Obtained via Electrospinning. J. Apic. Res. 2014, 53, 109–115. [Google Scholar] [CrossRef]
- Dobrzynski, P.; Li, S.; Kasperczyk, J.; Bero, M.; Gasc, F.; Vert, M. Structure-Property Relationships of Copolymers Obtained by Ring-Opening Polymerization of Glycolide and ε-Caprolactone. Part 1. Synthesis and Characterization. Biomacromolecules 2005, 6, 483–488. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [Green Version]
- Cingolani, A.; Casalini, T.; Caimi, S.; Klaue, A.; Sponchioni, M.; Rossi, F.; Perale, G. A Methodologic Approach for the Selection of Bio-Resorbable Polymers in the Development of Medical Devices: The Case of Poly(l-Lactide-Co-ε-Caprolactone). Polymers 2018, 10, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef] [Green Version]
- Kasperczyk, J.; Jelonek, K.; Dobrzyñski, P.; Jarz, B. The Influence of Copolymer Chain Microstructure on Cyclosporine a (CyA) and Sirolimus Prolonged and Sustained Release from PLA/TMC and PLA/PCL Matrices. J. Control. Release 2006, 116, 6–8. [Google Scholar] [CrossRef]
- Dobrzyński, P.; Kasperczyk, J.; Janeczek, H.; Bero, M. Synthesis of Biodegradable Copolymers with the Use of Low Toxic Zirconium Compounds. 1. Copolymerization of Glycolide with L-Lactide Initiated by Zr(Acac)4. Macromolecules 2001, 34, 5090–5098. [Google Scholar] [CrossRef]
- Dobrzynski, P. Synthesis of Biodegradable Copolymers with Low-Toxicity Zirconium Compounds. III. Synthesis and Chain-Microstructure Analysis of Terpolymer Obtained from L-Lactide, Glycolide, and ε-Caprolactone Initiated by Zirconium(IV) Acetylacetonate. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3129–3143. [Google Scholar] [CrossRef]
- Kasperczyk, J. Microstructural Analysis of Poly[(l,l-Lactide)-Co-(Glycolide)] by 1H and 13C n.m.r. Spectroscopy. Polymer 1996, 37, 201–203. [Google Scholar] [CrossRef]
- Kasperczyk, J.E. Microstructure Analysis of Poly(Lactic Acid) Obtained by Lithium Tert-Butoxide as Initiator. Macromolecules 2002, 28, 3937–3939. [Google Scholar] [CrossRef]
- Maciejowska, J.; Kasperczyk, J.; Dobrzyñski, P.; Bero, M. The Influence of Chain Microstructure on Hydrolytic Degradation of Glycolide/Lactide Copolymers Used in Drug Delivery Systems. J. Control. Release 2006, 116, e6–e8. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, K.; Kasperczyk, J.; Li, S.; Dobrzynski, P.; Jarzabek, B. Controlled Poly(l-Lactide-Co-Trimethylene Carbonate) Delivery System of Cyclosporine A and Rapamycine—The Effect of Copolymer Chain Microstructure on Drug Release Rate. Int. J. Pharm. 2011, 414, 203–209. [Google Scholar] [CrossRef]
- Orchel, A.; Jelonek, K.; Kasperczyk, J.; Dobrzynski, P.; Marcinkowski, A.; Pamula, E.; Orchel, J.; Bielecki, I.; Kulczycka, A. The Influence of Chain Microstructure of Biodegradable Copolyesters Obtained with Low-Toxic Zirconium Initiator to in Vitro Biocompatibility. Biomed Res. Int. 2013, 2013, 176946. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Jaworska-Kik, M.; Musiał-Kulik, M.; Marcinkowski, A.; Szewczenko, J.; Kajzer, W.; Pastusiak, M.; Kasperczyk, J. Development of Antibacterial, Ciprofloxacin-Eluting Biodegradable Coatings on Ti6Al7Nb Implants to Prevent Peri-Implant Infections. J. Biomed. Mater. Res. Part A 2020, 108, 1006–1015. [Google Scholar] [CrossRef]
- Jelonek, K.; Jaworska, J.; Pastusiak, M.; Sobota, M.; Włodarczyk, J.; Karpeta-Jarzabek, P.; Kaczmarczyk, B.; Kasperczyk, J.; Dobrzyński, P. Effect of Vascular Scaffold Composition on Release of Sirolimus. Eur. J. Pharm. Biopharm. 2018, 132, 41–49. [Google Scholar] [CrossRef]
- Rim, N.G.; Shin, C.S.; Shin, H. Current Approaches to Electrospun Nanofibers for Tissue Engineering. Biomed. Mater. 2013, 8, 14102. [Google Scholar] [CrossRef]
- Fredenberg, S.; Wahlgren, M.; Reslow, M.; Axelsson, A. The Mechanisms of Drug Release in Poly(Lactic-Co-Glycolic Acid)-Based Drug Delivery Systems—A Review. Int. J. Pharm. 2011, 415, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Hirenkumar, M.; Steven, S. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2012, 3, 1377–1397. [Google Scholar] [CrossRef]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A Unique Polymer for Drug Delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Chereddy, K.K.; Vandermeulen, G.; Préat, V. PLGA Based Drug Delivery Systems: Promising Carriers for Wound Healing Activity. Wound Repair Regen. 2016, 24, 223–236. [Google Scholar] [CrossRef]
- Fernández, J.; Etxeberria, A.; Sarasua, J.R. Synthesis, Structure and Properties of Poly(L-Lactide-Co-ε-Caprolactone) Statistical Copolymers. J. Mech. Behav. Biomed. Mater. 2012, 9, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Musiał-Kulik, M.; Włodarczyk, J.; Stojko, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Kasperczyk, J. Bioresorbable, Electrospun Nonwoven for Delayed and Prolonged Release of Temozolomide and Nimorazole. Eur. J. Pharm. Biopharm. 2021, 161, 29–36. [Google Scholar] [CrossRef]
- Jaworska, J.; Jelonek, K.; Sobota, M.; Kasperczyk, J.; Dobrzynski, P.; Musial-Kulik, M.; Smola-Dmochowska, A.; Janeczek, H.; Jarzabek, B. Shape-Memory Bioresorbable Terpolymer Composite with Antirestenotic Drug. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Turek, A.; Borecka, A.; Janeczek, H.; Sobota, M.; Kasperczyk, J. Formulation of Delivery Systems with Risperidone Based on Biodegradable Terpolymers. Int. J. Pharm. 2018, 548, 159–172. [Google Scholar] [CrossRef]
- Gębarowska, K.; Kasperczyk, J.; Dobrzyński, P.; Scandola, M.; Zini, E.; Li, S. NMR Analysis of the Chain Microstructure of Biodegradable Terpolymers with Shape Memory Properties. Eur. Polym. J. 2011, 47, 1315–1327. [Google Scholar] [CrossRef]
- Jaros, A.; Gębarowska, K.; Stojko, J.; Kasperczyk, J.; Smola, A. Badania Mechanizmu Degradacji in vivo Terpolimerów z Pamięcią Kształtu. Eng. Biomater. 2011, 14, 106–108. [Google Scholar]
- Persano, L.; Camposeo, A.; Tekmen, C.; Pisignano, D. Industrial Upscaling of Electrospinning and Applications of Polymer Nanofibers: A Review. Macromol. Mater. Eng. 2013, 298, 504–520. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Poly(Vinyl Alcohol)-Based Nanofibrous Electrospun Scaffolds for Tissue Engineering Applications. Polymers 2020, 12, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Bowlin, G.L. Electrospinning Jets and Nanofibrous Structures. Biomicrofluidics 2011, 5, 13405. [Google Scholar] [CrossRef] [Green Version]
- Haider, A.; Haider, S.; Kang, I.K. A Comprehensive Review Summarizing the Effect of Electrospinning Parameters and Potential Applications of Nanofibers in Biomedical and Biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of Polymeric Nanofibers for Drug Delivery Applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Asawahame, C.; Sutjarittangtham, K.; Eitssayeam, S.; Tragoolpua, Y.; Sirithunyalug, B.; Sirithunyalug, J. Antibacterial Activity and Inhibition of Adherence of Streptococcus Mutans by Propolis Electrospun Fibers. AAPS PharmSciTech 2015, 16, 182. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Maleki, H.; Samadian, H.; Doostan, M.; Khorramizadeh, M. Antibacterial and Antioxidant Assessment of Cellulose Acetate/Polycaprolactone Nanofibrous Mats Impregnated with Propolis. Int. J. Biol. Macromol. 2019, 140, 1260–1268. [Google Scholar] [CrossRef]
- Razavizadeh, B.M.; Niazmand, R. Characterization of Polyamide-6/ Propolis Blended Electrospun Fibers. Heliyon 2020, 6, 12004. [Google Scholar] [CrossRef] [PubMed]
- Zeighampour, F.; Alihosseini, F.; Morshed, M.; Rahimi, A.A. Comparison of Prolonged Antibacterial Activity and Release Profile of Propolis-Incorporated PVA Nanofibrous Mat, Microfibrous Mat, and Film. J. Appl. Polym. Sci. 2018, 135, 45794. [Google Scholar] [CrossRef]
- Moradkhannejhad, L.; Abdouss, M.; Nikfarjam, N.; Mazinani, S.; Heydari, V. Electrospinning of Zein/Propolis Nanofibers; Antimicrobial Properties and Morphology Investigation. J. Mater. Sci. Mater. Med. 2018, 29, 2958–2964. [Google Scholar] [CrossRef] [PubMed]
- Adomavičiūtė, E.; Stanys, S.; Žilius, M.; Briedis, V. Formation and Analysis of Electrospun Nonwoven Mats from Bicomponent PVA/Aqueous Propolis Nano-Microfibres. Fibres Text. East. Eur. 2015, 23, 35–41. [Google Scholar] [CrossRef]
- Ulag, S.; Ilhan, E.; Demirhan, R.; Sahin, A.; Yilmaz, B.; Aksu, B.; Sengor, M.; Ficai, D.; Titu, A.; Ficai, A.; et al. Propolis-Based Nanofiber Patches to Repair Corneal Microbial Keratitis. Molecules 2021, 26, 2577. [Google Scholar] [CrossRef]
- Eskandarinia, A.; Kefayat, A.; Gharakhloo, M.; Agheb, M.; Khodabakhshi, D.; Khorshidi, M.; Sheikhmoradi, V.; Rafienia, M.; Salehi, H. A Propolis Enriched Polyurethane-Hyaluronic Acid Nanofibrous Wound Dressing with Remarkable Antibacterial and Wound Healing Activities. Int. J. Biol. Macromol. 2020, 149, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, O.M.; Mignon, A.; Iacob, A.T.; Simionescu, N.; Confederat, L.G.; Tuchilus, C.; Profire, L. New Hyaluronic Acid/Polyethylene Oxide-Based Electrospun Nanofibers: Design, Characterization and In Vitro Biological Evaluation. Polymer 2021, 13, 1291. [Google Scholar] [CrossRef]
- Rebia, R.A.; Sadon, N.S.; Tanaka, T. Natural Antibacterial Reagents (Centella, Propolis, and Hinokitiol) Loaded into Poly[(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyhexanoate] Composite Nanofibers for Biomedical Applications. Nanomaterials 2019, 9, 1665. [Google Scholar] [CrossRef] [Green Version]
- Stojko, M.; Włodarczyk, J.; Sobota, M.; Karpeta-Jarząbek, P.; Pastusiak, M.; Janeczek, H.; Dobrzyński, P.; Starczynowska, G.; Orchel, A.; Stojko, J.; et al. Biodegradable Electrospun Nonwovens Releasing Propolis as a Promising Dressing Material for Burn Wound Treatment. Pharmaceutics 2020, 12, 883. [Google Scholar] [CrossRef]
- Komosinska-Vassev, K.; Olczyk, P.; Kasperczyk, J.; Pilawa, B.; Krzyminiewski, R.; Dobosz, B.; Ramos, P.; Stojko, J.; Stojko, M.; Ivanova, D.; et al. EPR Spectroscopic Examination of Different Types of Paramagnetic Centers in the Blood in the Course of Burn Healing. Oxid. Med. Cell. Longev. 2019, 2019, 7506274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olczyk, P.; Komosinska-Vassev, K.; Krzyminiewski, R.; Kasperczyk, J.; Ramos, P.; Dobosz, B.; Batoryna, O.; Stojko, J.; Stojko, M.; Ivanova, D.; et al. The Estimation of Blood Paramagnetic Center Changes during Burns Management with Biodegradable Propolis-Nanofiber Dressing. Oxid. Med. Cell. Longev. 2020, 2020, 3675603. [Google Scholar] [CrossRef] [PubMed]
| Type of Wound | Description |
|---|---|
| Minor | Partial-thickness < 10% TBSA of children 1 and elderly |
| Partial-thickness < 15% TBSA of adults | |
| Full-thickness < 2% TBSA | |
| Moderate | Partial-thickness 10–20% TBSA of children and elderly |
| Partial-thickness 15–25% TBSA of adults | |
| Full-thickness 2–10% TBSA | |
| Major | Partial-thickness > 20% TBSA of children and elderly |
| Partial-thickness > 25% TBSA of adults | |
| Full-thickness > 10% TBSA | |
| Burns in critical areas 2 | |
| Complicated burns 3 |
| Material | Propolis Content | Application | Scope of Research | Ref. |
|---|---|---|---|---|
| Polyvinylpyrrolidone (PVP) | 5% (w/v) | Mouth-dissolving dosage form and an anticariogenic agent in the oral cavity | Fiber morphology (SEM) Antibacterial activity Contact-angle Disintegration/Dissolving Time | [135] |
| Cellulose acetate (CA), Polycaprolactone (PCL)/Cellulose acetate (CA) | Wound healing | Fiber morphology (SEM) Water absorption Contact-angle ATR-FTIR analysis Antioxidant assay Antibacterial activity | [136] | |
| Polyamide-6 (PA-6) | 20%, 30%, 40%, 50% (w/w) | Medicine and food industry | Thermal analysis (TGA, DSC) FTIR analysis Fiber morphology (FESEM) XRD analysis Drug release Antioxidant assay | [137] |
| Honey/polyvinyl alcohol (PVA) / chitosan (HPCS) 30:7:3.5 | 10% (w/w) | Wound dressing | FTIR analysis Fiber morphology (FESEM) Antibacterial activity In vivo wound-healing Histological examination Cell viability Cell proliferation | [79] |
| Polyvinyl alcohol (PVA) | 5%, 10%, 20%, 40%, 60% (w/w) | Wound dressing | Fiber morphology (SEM) FTIR analysis XRD analysis Loading efficiency Drug release Water absorption Weight loss Antibacterial activity | [138] |
| Zein | 5%, 10%, 15%, 20%, 25%, 30% 35%, 40% (w/w) | Wound dressing | Fiber morphology (SEM) Antibacterial activity FTIR analysis | [139] |
| Polyurethane (PU) | 5%, 10%, 30% (w/w) | Wound dressing, tissue engineering | Fiber morphology (FESEM) FTIR analysis Mechanical properties Contact-angle Antibacterial activity Cell viability | [101] |
| Polyvinyl alcohol (PVA) | 10%, 30%, 50% (w/w) | Controlled delivery system | Fiber morphology (SEM) Drug release | [140] |
| Polyvinyl alcohol (PVA) / Gelatin (Gel) 13%:0.5% (w/w) | 3%, 5% (w/w) | Corneal patches | Fiber morphology (SEM) Thermal analysis (DSC) Antibacterial activity Mechanical properties Drug release Cell viability Contact-angle | [141] |
| Polyurethane (PU) / Hyaluronic acid (HA) 1:1 | 0.5%, 1%, 2% (w/w) | Wound dressing | FTIR analysis Thermal analysis (TGA) Fiber morphology (SEM) Mechanical properties Contact-angle Water absorption Drug release Cell viability Cell morphology In vivo wound-healing Histological examination | [142] |
| Poly(ethylene oxide) (PEO) / hyaluronic acid (HA) | 7% (w/w) | Wound dressing | FTIR analysis Fiber morphology (SEM) Water-vapor transmission rate Antioxidant assay Cell viability Antibacterial activity | [143] |
| Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) (PHBH) | 1%, 5%, 7% (v/v) | Wound dressing | Fiber morphology (SEM) FTIR analysis XRD analysis Mechanical properties Drug release Antibacterial activity | [144] |
| Polylactide (PLA) | 10%, 20% (w/w) | Wound dressing | Fiber morphology (SEM) FTIR analysis Contact-angle Drug release Antimicrobial activity Cell viability | [43] |
| Poly(lactide-co-glycolide) (PLGA) | 5%, 10% (w/w) | Wound dressing | Fiber morphology (SEM) Water absorption Weight loss Changes in polymer composition (NMR) Drug release In vivo wound-healing | [145] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules 2021, 26, 5701. https://doi.org/10.3390/molecules26185701
Stojko M, Wolny D, Włodarczyk J. Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules. 2021; 26(18):5701. https://doi.org/10.3390/molecules26185701
Chicago/Turabian StyleStojko, Mateusz, Daniel Wolny, and Jakub Włodarczyk. 2021. "Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review" Molecules 26, no. 18: 5701. https://doi.org/10.3390/molecules26185701
APA StyleStojko, M., Wolny, D., & Włodarczyk, J. (2021). Nonwoven Releasing Propolis as a Potential New Wound Healing Method—A Review. Molecules, 26(18), 5701. https://doi.org/10.3390/molecules26185701







