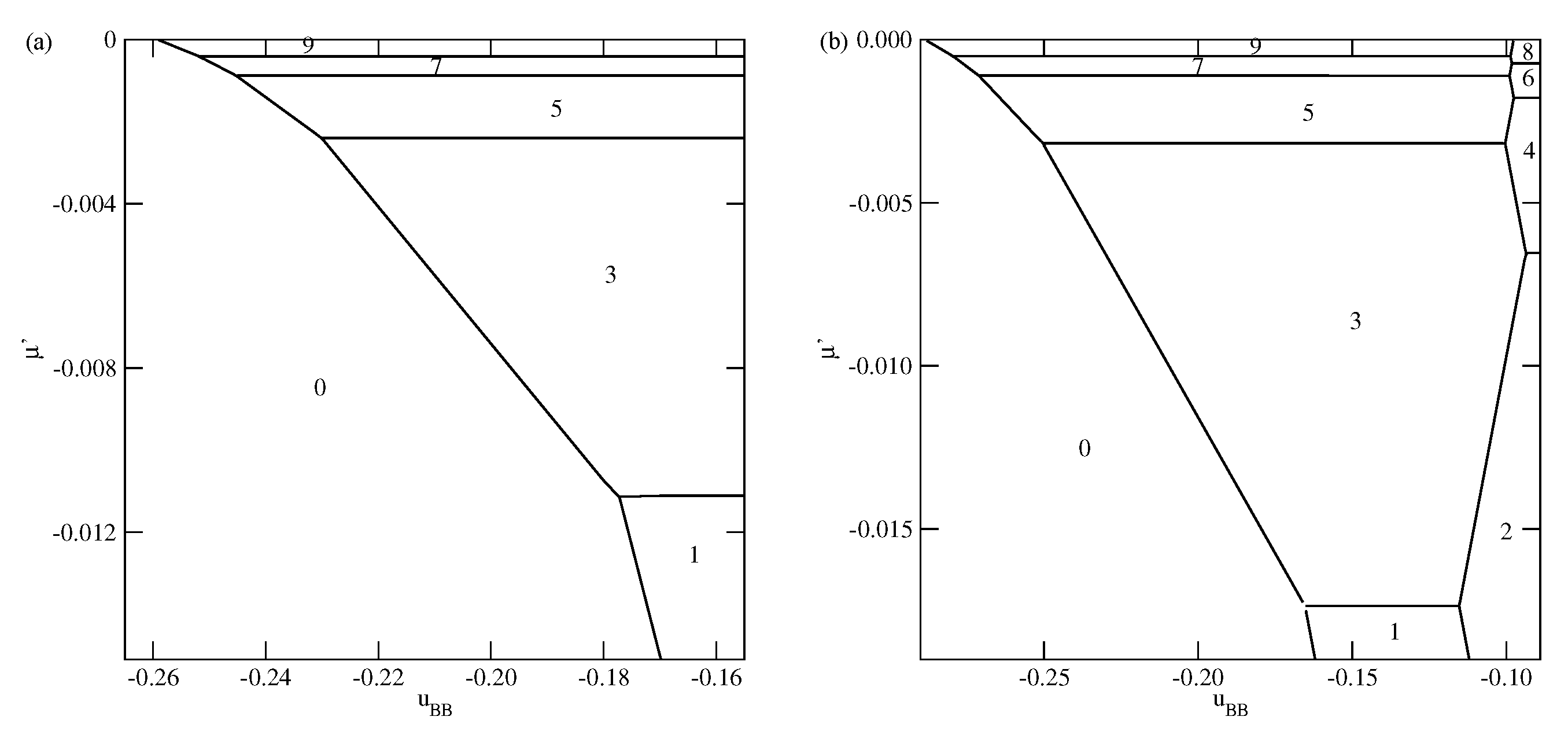1. Introduction
The formation of multilayer adsorbed films on solid substrates and wetting phenomena have been studied for several decades, both experimentally, with different theoretical approaches, and computer simulation methods. A comprehensive description of the results can be found in many books and review articles [
1,
2,
3,
4,
5,
6,
7,
8,
9]. Among the most frequently used theoretical models of adsorption are various lattice gas models [
3,
8,
10,
11,
12,
13,
14,
14]. Although such models are rather crude approximations to real systems, the emerging results resemble experimental results for multilayer adsorption quite well. The use of lattice models in studies of adsorption phenomena can be traced back to the beginning of the 20th century, when the Langmuir proposed his famous model of localized monolayer adsorption [
15]. Later, Brunauer, Emmett and Teller [
16] generalized the model of multilayer adsorption (BET model). A common feature of those early theoretical models was the negligence of attractive adsorbate–adsorbate interactions. Later, lattice models of multilayer adsorption involving attractive interactions between fluid particles were developed [
10,
13]. These models were applied to study adsorption of single component fluids and binary mixtures, using various mean-field theories [
10,
13,
14,
17,
18,
19], renormalization group methods [
20,
21] and computer simulations [
11,
13,
14,
22,
23,
24,
25,
26,
26].
From the collected experimental and theoretical results it was established that the scenarios of the adsorbed film development are primarily controlled by the relative strength of adsorbate–adsorbate and adsorbate–solid interactions [
12]. A comprehensive description of possible modes of the film growth was given already forty years ago by Pandit, Schick and Wortis [
12]. Using the lattice gas model, these authors demonstrated that three different regimes of the film formation can be singled out. The first, called the strong substrate regime, corresponds to the systems in which the adsorbate–solid interaction dominates, and the film thickness diverges upon the approach to the bulk coexistence. At low temperatures, the adsorption occurs via an infinite number of first-order layering transitions. These transitions terminate in critical points,
, where
n is the layer number, and
converges to the roughening transition temperature,
, when
. At higher temperatures the film thickness smoothly increases, and finally diverges, upon the approach to the bulk coexistence. The divergence of the film thickness implies a complete wetting to occur at any temperature down to zero. When the strength of the surface potential is lowered below a certain threshold value, the so-called intermediate substrate regime is entered. In this case, the wetting occurs only at finite temperatures, starting at the wetting temperature,
. Below
the film thickness remains finite at the bulk coexistence, and it diverges only at, and above,
. Within the intermediate substrate regime, three different subregions can be identified, determined by the relative values of the roughening and the wetting temperatures. When
, the layering transitions are still present, but occur only at finite temperatures. When
, the system may exhibit the prewetting transition, followed by the first-order wetting transition at the bulk coexistence, or only the critical (continuous) wetting transition. Finally, when the surface potential becomes weak enough, the weak substrate regime is met, with incomplete wetting at any temperature.
Experimental and theoretical studies confirmed the predictions stemming from the presented above classification, and revealed several new features [
9,
12,
23]. For example, it was shown that the layering transitions may involve a simultaneous condensation of more than one layer. However, the layers that condense together at low temperatures, may be separated by increasing entropy effects at higher temperatures, leading to the appearance of triple points [
12,
23]. In general, our understanding of multilayer films formation and wetting phenomena in systems with isotropic interactions is now quite advanced.
On the other hand, the effects of orientation dependent adsorbate–adsorbate interactions on the formation of multilayer films and wetting transitions have not been given much attention. A vast majority of experimental and theoretical studies has concentrated on adsorption of non-spherical molecules [
27,
28,
29,
30]. In such systems, the shape of adsorbate molecules gives rise to orientation-dependent interactions. Recently, experimental studies of the formation of multilayer structures by amphiphilic molecules have been reported [
31,
32,
33]. However, these multilayer structures are not formed via adsorption processes, but rather in the bulk, due to the nature of interparticle interactions in such systems.
Another interesting class of systems with strongly orientational interactions consists of colloidal patchy particles [
34,
35,
36] A special case of patchy particles, corresponds to Janus particles, in which there are only two regions of different chemical composition and physical properties [
35]. Self-assembly of Janus particles has been studied experimentally [
34,
37,
38] and with different theoretical tools [
39,
40], including computer simulations [
41,
42,
43]. Adsorption of Janus particles at the fluid-fluid interface has been theoretically studied by several authors [
44,
45,
46,
47,
48]. The only work dealing with adsorption of Janus particles at solid planar walls has been presented by Rosenthal and Klapp [
39], who used the density functional theory. We shall return to their findings later in
Section 5.
In this work, we consider a very simple lattice model of spherical Janus-like particles being in contact with solid substrates. Our main goal has been to elucidate the effects of orientation-dependent interactions on the structure of multilayer films, in systems characterized by different interaction energies between Janus particles and between Janus particles and the wall. In the case of a cubic lattice, each particle has been allowed to assume one of six orientations, and the interaction energy between a pair of neighboring particles has been assumed to depend on the degree to which the A and/or B halves overlap. In fact, the model is a generalization to three dimensions of the already considered two-dimensional model [
49].
The paper is organized as follows. In the next section, we present the model used here. Then, in
Section 3, we discuss its behavior in the ground state. In the following
Section 4, we briefly describe the Monte Carlo method used to study bulk and non-uniform systems. The results of Monte Carlo simulations and their discussion is given in the
Section 5. The paper concludes in
Section 6, where we summarize our findings and present final remarks.
2. The Model
We consider a fluid placed on a regular lattice consisting of sites arranged in D layers of sites each. The slab has been bounded on the top and the bottom by the same surfaces, being the source of the surface potential.
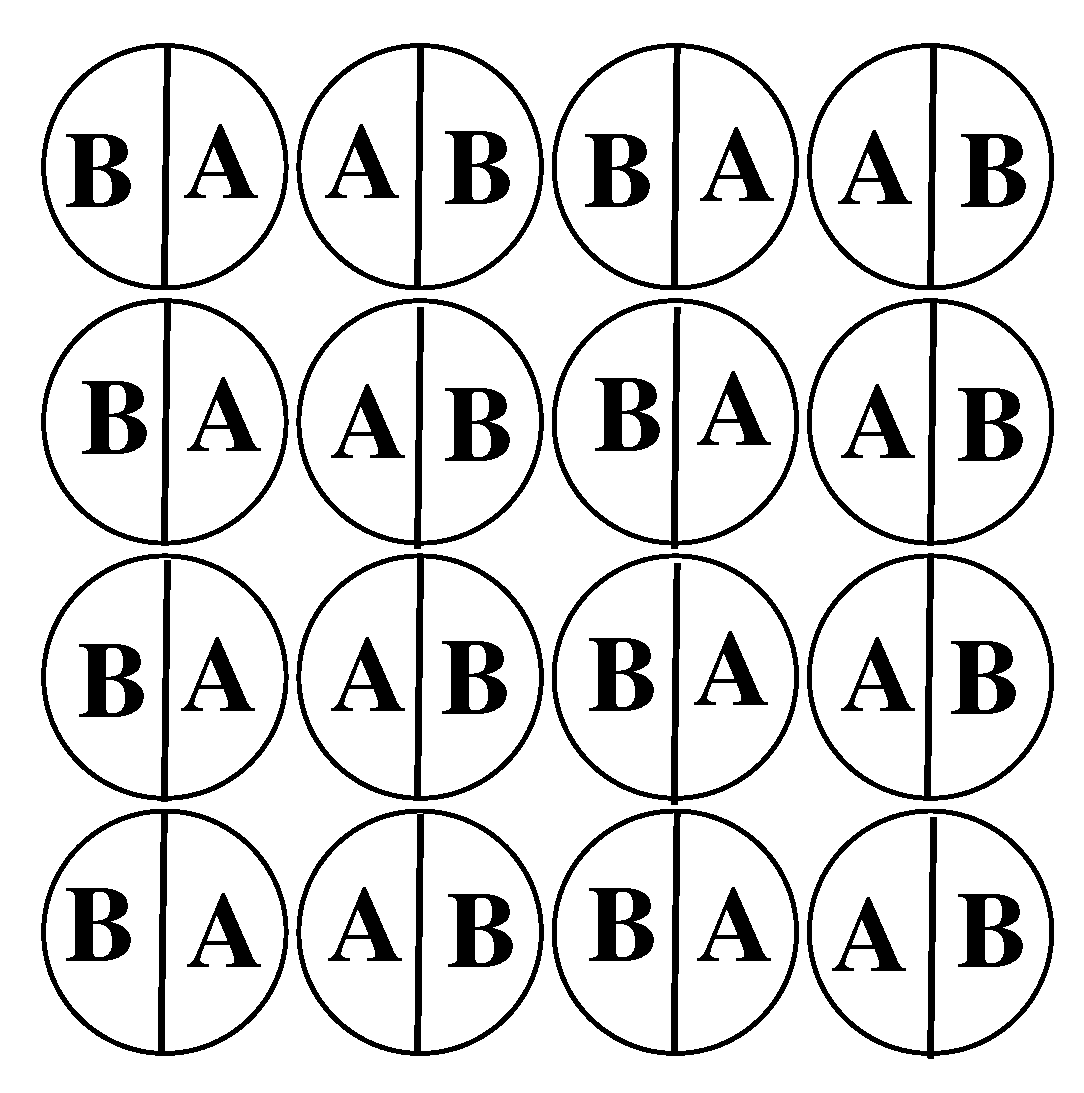
The fluid is assumed to consist of Janus-like particles with two halves, A and B. The interaction between a pair of particles is confined to the nearest neighbors only, and assumed to depend on the relative orientations of particles. Every particle can take on one of the six orientations, labeled by
k (
), as shown in
Figure 1. Throughout this paper, and for the reason to be explained later, we call the orientations 1,2,3 and 4 as the in-plane orientations, while the orientations 5 and 6 as the out-of-plane orientations.
Let
,
and
be the interaction energies corresponding to the relative orientations in which the AA, AB or BB halves face one another. Then, the interaction energy between a pair of particles located on neighboring sites can be written as
In the above, , and are the weights, determined by the degrees to which the AA, AB and BB regions overlap, and is the separation vector. In the case of a cubic lattice, there are six different separation vectors: , and .
Symmetry properties of the model imply that there are seven different values of
, summarized in
Table 1.
The surface external potential
, has been assumed to depend on the distance from the surface,
z, and on the orientation of the particle with respect to the surface,
k. Here, we assume that
has the following simple form:
with
with the above assumptions, the Hamiltonian of the model reads
In the above, , when the i-th site is occupied (empty), the first sum runs over all pairs of nearest neighbors, is just with the distance from the surface expressed in lattice spacings, and is the chemical potential.
Now, for the fixed
D and
L, the free energy of the system is given by
with
.
In principle, we are interested in the behavior of the model in the thermodynamic limit, when
. Under such conditions, the bulk and the surface excess free energies (per site) can be expressed as [
12,
23]
and
The factor 2 in the denominator of the last equation results from the assumed presence of two surfaces, at the bottom and at the top of the system.
Here we confine the discussion to the systems with
,
, and with different
. Thus, all inter-reactions are non-repulsive, but, in general, the BB attraction has been assumed to weaker than the AA attraction. This choice of parameters seems quite well suited to represent Janus-like particles [
50,
51,
52].
In order to show how the adsorption behavior is affected by the properties of the surface potential, we have considered systems with , and .
3. Ground State Properties
The bulk free energy
can be written as
with
,
and
being the bulk potential energy, entropy and density, respectively. Similarly, the surface excess free energy and
is given by
where
,
and
denote the surface excesses of potential energy, entropy and density, respectively.
In the ground state, the free energy lacks the entropic term, since
. In particular, the bulk free energy is given by
with
being the chemical potential at the coexistence between the dilute and condensed phases at
. Of course,
depends on the structure of the condensed phase. In the systems considered here, the condensed phase is well ordered, and built of layers consisting of the same sequence of rows of particles, with alternate orientations …AB-BA-AB-BA… (cf.
Figure 2).
The energy (per site) of this ordered structure at
is equal to
and the bulk coexistence is located at the chemical potential
.
Note that in the case of
,
, just like in the system with isotropic interactions [
23], since AB contacts do not appear at all, while the AA and BB pairs interact with the same energy.
In the ground state, the adsorbed film at the specified chemical potential is expected to consist of a certain number of occupied layers, with the surface excess free energy (per site) given by
In the above is the surface excess energy (per site) that depends on the particle orientations in all occupied layers, and . Of course, as long as we consider adsorption from a dilute (gas-like) phase, we need to take into account only the values of lower than zero.
The lowest energy of interparticle interactions in an individual layer is equal to
, and is reached for the configurations in which all particles assume the same out-of-plane orientation with their A or B halves down (
or 6), or when the layer consists of stripes, as those shown in
Figure 2, with two in-plane orientations,
and
or
and
.
In order to determine the energy of the film consisting of
n filled layers it is necessary to include the contributions due to the fluid-substrate interaction and the interactions between the particles in adjacent layers. Thus, the energy of the film can be written as
where
is the contribution to the film energy due to interaction between the particles in layers
and
l. In the considered here case of
,
takes on one of the values given in
Table 2.
In
Table 2, we have marked the configurations with in-plane orientations of particles as 1, since the interlayer energy does not depend on the orientations of stripes.
One should note that for any , the stacking 65 in neighboring layers is energetically most favorable. However, the actual stacking of orientations in multilayer films depends on the particular values of , and . An important parameter is . The case of corresponds to non-selective walls, while the cases with and correspond to selective walls.
At first we discuss the systems with
, with
. In general, there are three structures of the film with
n occupied layers possible. In the first, the particles in each layer have in-plane orientations, and the film energy is equal to
When the particles in the film assume out-of-plane orientations, the film energy for even and odd number of occupied layers is given by different expressions. As long as , the stacking that minimizes the energy is , due to strong interlayer attraction. Only in the case of the stacking as well as the stacking with in-plane orientations in all layers give the same film energy.
The potential energy of the film with an even number of layers is given by
while for the odd number of layers, the film energy is given by Equation (
14).
Having the film energies we can readily derive the expressions which give the chemical potential values at the transition points between the states of different numbers of occupied layers. In general, the following different layering transitions are possible:
and
In order to determine the actual sequence of transitions in a given system one needs to compare the excess free energies of the states with different numbers of occupied layers, since only the states that minimize the system free energy are stable.
Before we present the examples of ground state phase diagrams, let us note that the
transition is possible only for
, independently of
. However, the chemical potential at which this transition is located depends on the both
and
. For any
, the first layering transition involves a simultaneous condensation of two (or more) layers. In general, similarly to the systems systems with isotropic interactions [
12], the number of simultaneously condensing layers increases when
becomes lower, i.e., when the BB attraction becomes stronger. An ultimate limit of
transitions corresponds to
, and it determines the region of complete wetting at
. Assuming an infinite range of the surface potential, this limit corresponds to the following relation between
and
where
is the Riemann function [
53]. Whenever
is lower than
, the system exhibits a complete wetting at
. Thus, in the particular case of
, a complete wetting should occur even for very weakly attractive surfaces.
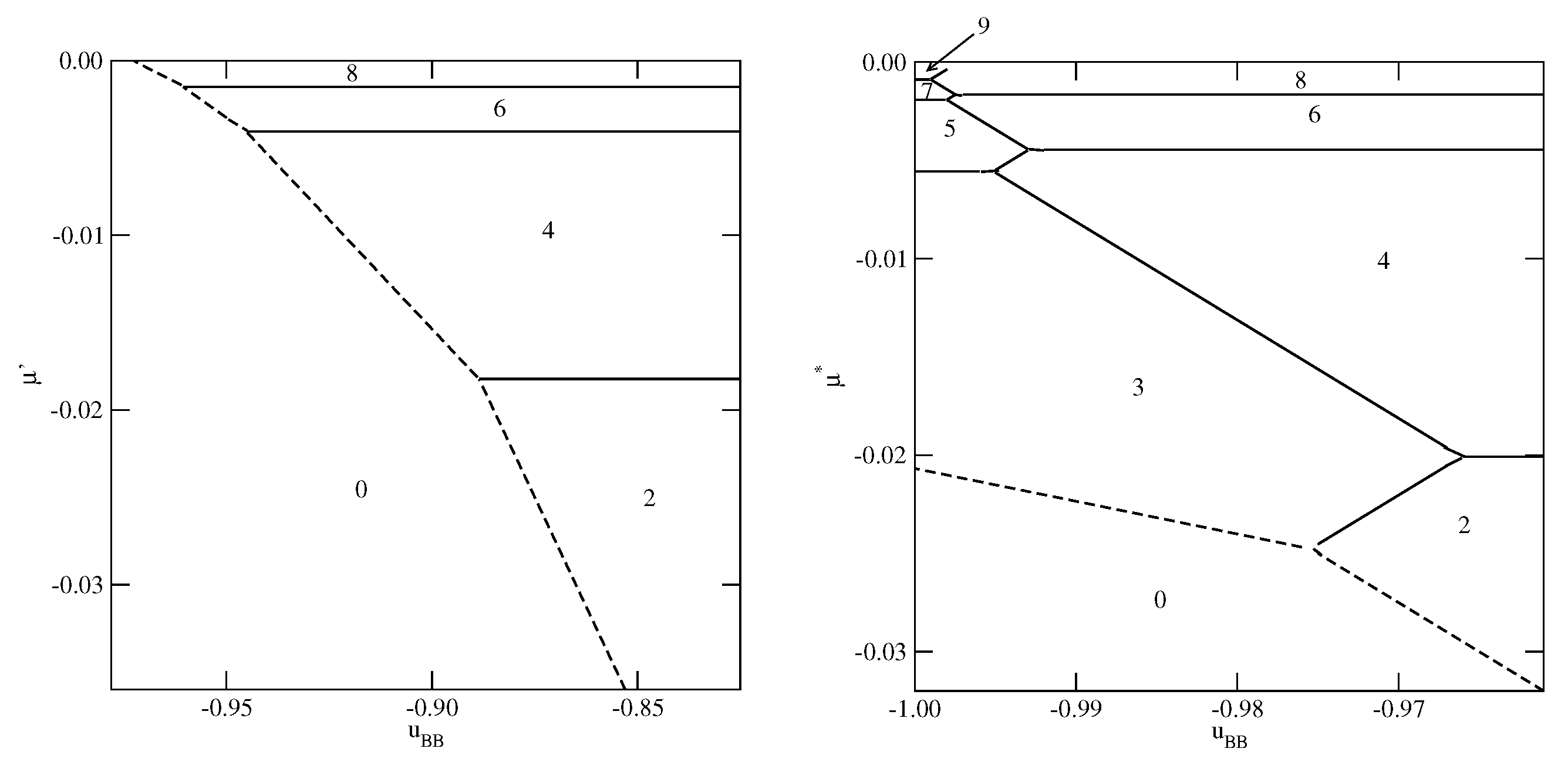
Figure 3,
Figure 4 and
Figure 5 present the examples of ground state phase diagrams, obtained for different values of
, and over the entire range of
between 0 and
. The phase diagrams for
(
Figure 3a) and
(
Figure 3b) are qualitatively the same, and demonstrate that independently of
, the film grows via a sequence of layering transitions leading to the films of only even numbers of occupied layers. These phase diagrams also demonstrate that the range of
allowing for the formation of wetting layers at
increases when the surface potential becomes stronger. In the case of
, it is confined to the values of
not lower than about
, while in the system with
, a complete wetting occurs over much wider range of
, between zero and about
. One should note that the reported here values of
, which delimits the regions of complete and incomplete wetting, are slightly different than predicted by Equation (
21), due to the assumed finite range of the surface potential. The results demonstrate also that the first layering transition,
, involves a simultaneous condensation of increasing number of layers when
becomes lower.
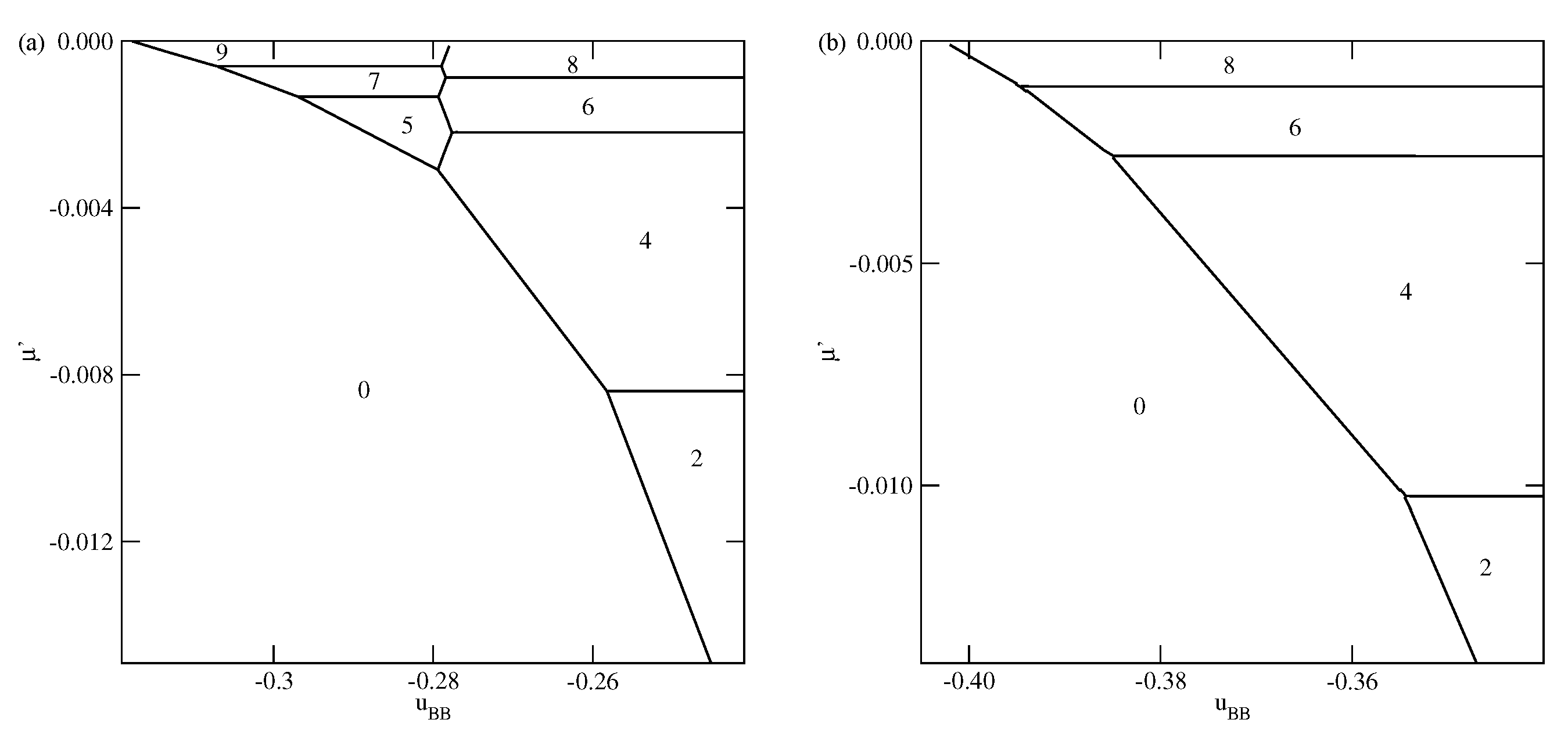
The phase behavior changes when the strength of the fluid–wall potential becomes high enough to ensure a complete wetting over the entire range of
, between 0 and
. In
Figure 4, we present two ground state phase diagrams, for
(
Figure 4a) and
(
Figure 4b).
In the systems with
, and
, the first layering transition leads to the formation of a bilayer, while for lower
, down to about
, a simultaneous condensation of four layers occurs. However, for still lower
, the first layering transition involves a simultaneous condensation of only three layers. When
approaches
, the formation of thicker films occur via an increasing number of
transitions. We recall that in the particular case of
, the film potential energy has the same value as the system with isotropic interactions, in which the film develops in a layer-by-layer mode [
12,
23]. When
(
Figure 4b), the first layering transition leads to the bilayer film, for any
between 0 and
. It results from the increased strength of the surface potential. The same was found in the isotropic system with
[
23]. Again for
approaching
, thick films develop via a sequence of
transitions.
As soon as
becomes lower than
, the first layering transition leads to the formation of a monolayer film, followed by the
transition, for any
(
Figure 5). However, the sequence of layering transition leading to the formation of thicker films, depends on
. Similarly as in the systems with
, the film with odd numbers of layers appears only when
is sufficiently close to
. For example, when
, the formation of films with odd numbers of filled layers is possible only when
is lower than about
. In the case of much stronger surface potential, with
, this region is only slightly wider and requires
to be lower than about
. However, for any
, the sequence of layering transitions
terminates at finite
, and a further film growth occurs via
transitions. Only when
the film thickness grows in a layer-by-layer mode.
In the case of selective surfaces with
, the first layer attains the lowest energy
when all particles have their B halves oriented towards the surface (
).
In the bilayer film, the 65 stacking of the energy equal to
is stable as long as
. Only for
, the stacking 66 of the energy
becomes stable. The stackings 55 and 56 can not appear at all.
The stable monolayer of the stacking 65 appears when .
The examples of ground phase diagrams for selected systems with
are presented in
Figure 6,
Figure 7 and
Figure 8.
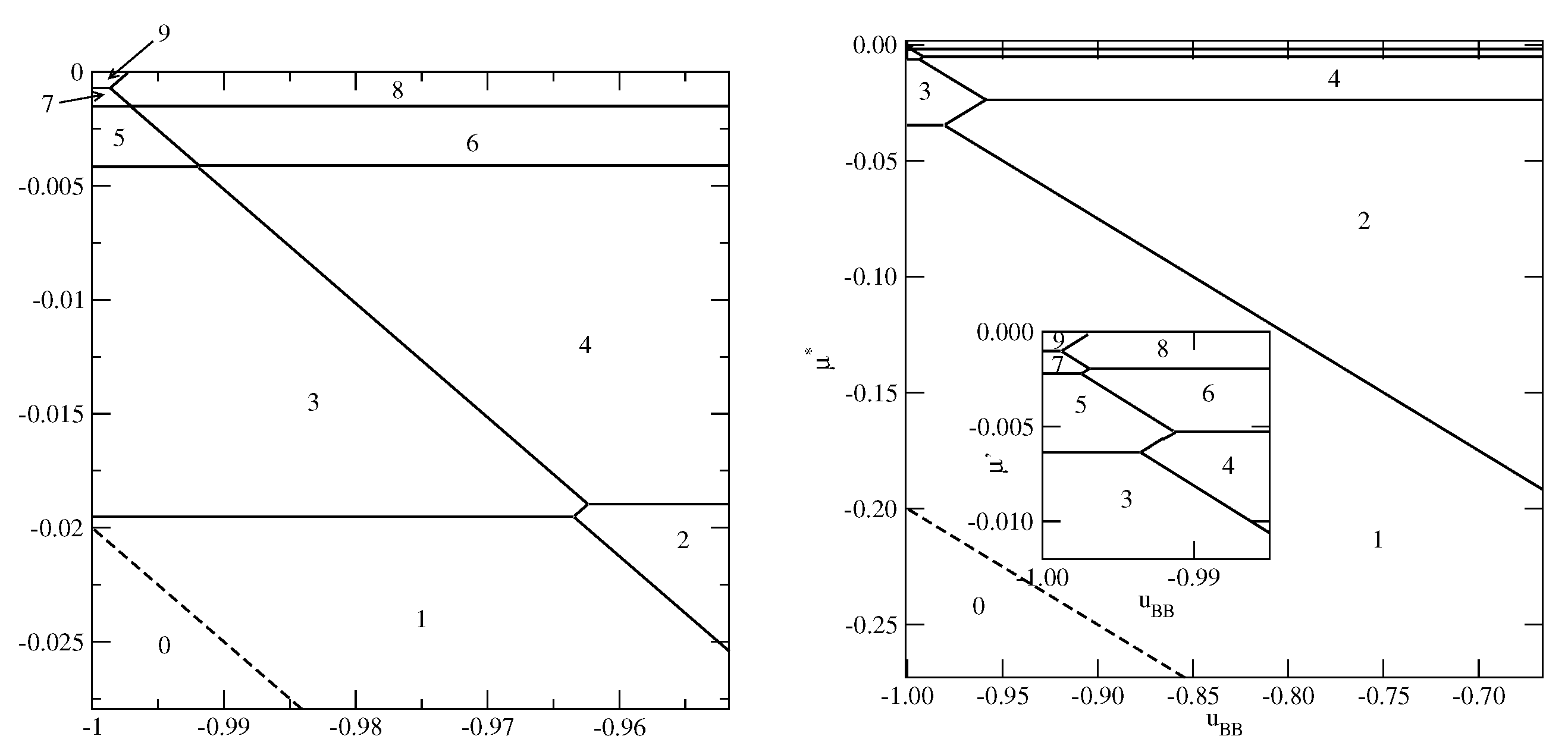
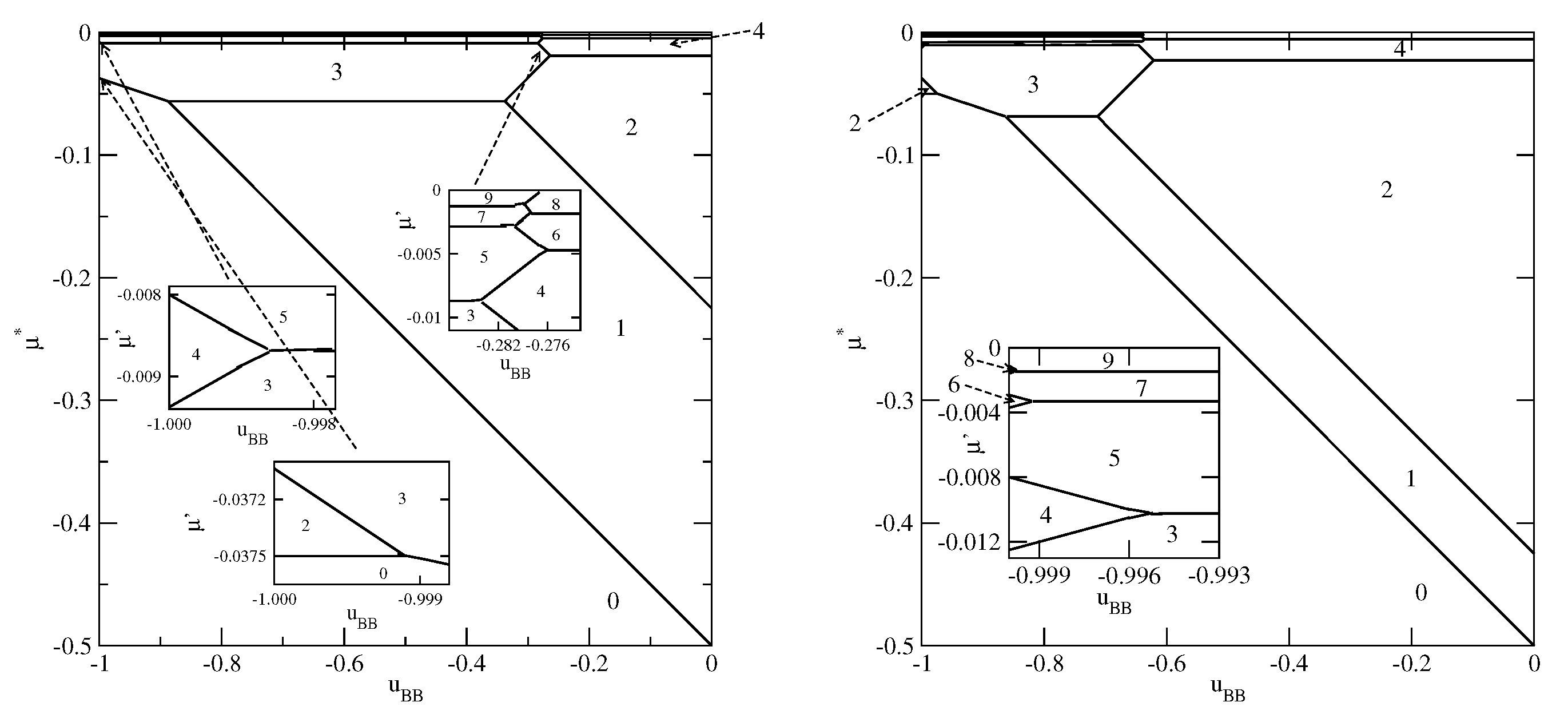
Figure 6 presents the phase diagrams for two systems, with
and
(left panel) and
(right panel), in which monolayer films do not appear.
The systems with exhibit a complete wetting only when greater than about and shows the presence of only even numbers of occupied layers, with the stacking (). On the other hand, when , the system exhibits a complete wetting for any , down to , and shows the formation of films with only even numbers of layers for . When the BB attraction becomes stronger () the films with odd numbers of layers are also present. The first layering transition involves a simultaneous condensation of three layers. For sufficiently close to only odd numbers of layers appear, and the stacking remains the same (). This behavior is different from that observed in the systems with , for which the film was found to grow in a layer-by-layer mode only when .
Figure 7 presents two examples of phase diagrams demonstrating that when
becomes even slightly lower than
, or when
becomes lower than
a monolayer film is stable over the entire range of
.
By changing the strengths of the surface potential felt by the A and B halves, different scenarios of the film development can be observed. As an example, we show in
Figure 8 the phase diagram for the system with
and
.
The behavior in the region of close to is quite the same as in other systems with sufficiently large . On the other hand, for close to zero a certain number of transitions occurs. Only when the film becomes sufficiently thick, it develops via the transitions, with even values of n.
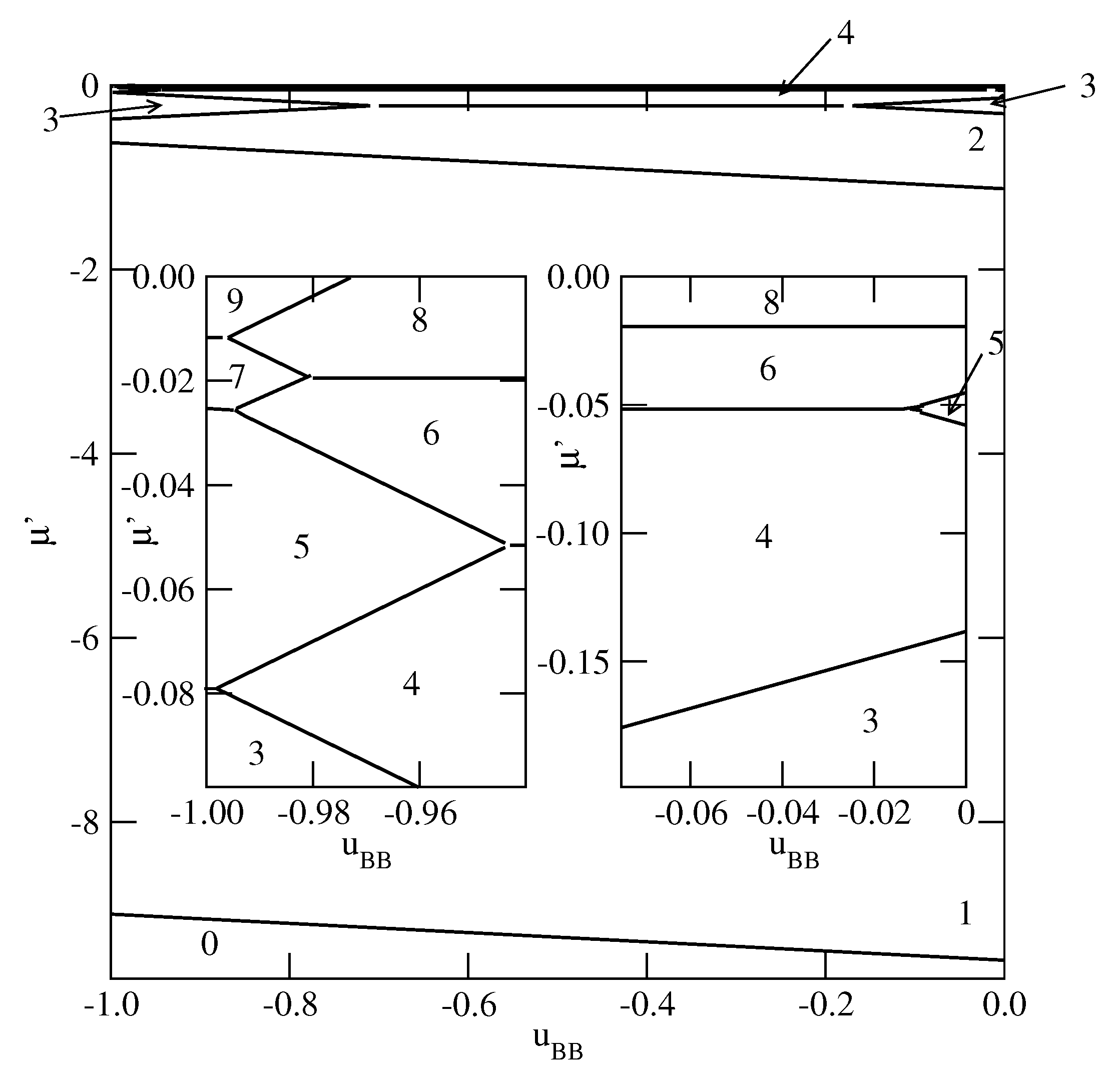
Finally we present the results for selective surfaces with
. The most important difference is that for
the formation of stable monolayer films may occur for considerably weaker surface fields. Since the monolayer reaches the lowest energy when the particles assume the orientation 5, while the bilayer has the lowest energy for the stacking 65, the stable monolayer appears whenever
. In
Figure 9 and
Figure 10, we present a series of ground state phase diagrams obtained for the systems with
and different
, between
and
.
When
only odd numbers of occupied layers are stable, and the complete wetting at
occurs only for
greater than about
. Independently of the film thickness, all particles in the first layer assume the same orientation with
, i.e., with their A halves pointing towards the surface. Each of the subsequent layering transition adds two layers with the orientations 6 and 5, due to strong interlayer attraction. Since the assumed here strengths of the fluid–wall interactions are rather small, a gradual decrease of
causes the first layering transition to involve a simultaneous condensation of an increasing number of layers. When
, the ground state behavior becomes different (see
Figure 9b). Namely, the films with even numbers of occupied layers appear only for sufficiently weak attractive BB interaction. It should be noted that the
transition is preserved, and the formation of a bilayer involves the reorientation of particles in the first layer from
to
. In the second layer all particles assume the orientation with
, since this stacking is energetically favorable due to strong interlayer attraction. When the BB interaction becomes strong enough, multilayer films with only odd numbers of filled layers appear. Over a very narrow range of
, between the regimes with even and odd numbers of occupied layers, the films grow in a layer-by-layer mode.
In the case of
, the phase behavior changes, since the
transition does not appear at all. Instead, a series of
transitions, with even and odd
n, occurs (see
Figure 10a). The formation of thicker films occurs in a similar way as in the systems with
, but the crossover between the regimes in which even and odd numbers of layers are stable is shifted towards considerably lower
, close to
.
In the system with
only the films with even numbers of occupied layers appear (see
Figure 10b).
The observed in
Figure 9 and
Figure 10 changes in the ground state behavior, can be easily understood by taking into account a competition between the contributions to the film energy resulting from the fluid–wall and interlayer interactions. When
becomes closer to
, the interlayer attraction dominates, but its role becomes lower when the BB attraction increases.
When
becomes strong enough to ensure a compete wetting over the entire range of
, different scenarios of the film development appear. We present explicit results for the systems with
and different values of
. For
between 0.0 and about
, the films consist of odd numbers of layers only, while for
between about
and about
, the behavior is qualitatively the same as shown in
Figure 7. As soon as
becomes equal to
, or assume still lower values, the layer-by-layer growth occurs, but only for
very close to
(see
Figure 11). This is illustrated by the insets to
Figure 11. It is evident that in the systems with
, the layer-by-layer growth occurs over a wider region of
than in the systems with
. When
becomes closer to
, the ground state behavior gradually approaches that found for non-selective walls.
4. Monte Carlo Simulations
We have used a standard Monte Carlo simulation method in the grand canonical ensemble [
54,
55], to study the bulk behavior of the model and the formation of multilayer films.
In the case of bulk systems, the cubic simulation cell of the size with
, and with periodic boundary conditions applied in all three directions, has been used. It has been found that the chosen system size was sufficient to estimate the phase diagrams, by measuring the isotherms at different temperatures. In some cases, we have used larger systems with
and 40. The quantities recorded, included the system density
the densities of differently oriented particles
the average potential energy per site
the heat capacity
and the density susceptibility
In order to obtain reliable results at any state point, determined by the temperature and the chemical potential, the system had to be well equilibrated. This has been achieved by performing runs using – Monte Carlo steps. Each Monte Carlo step involved 10 L attempts to either create a particle of randomly chosen orientation in a randomly chosen position, or to annihilate one of also randomly chosen particles.
In order to determine the behavior of closed packed bulk systems, we have used Monte Carlo method in the canonical ensemble. In this case, the only possible move involved the change of orientation of randomly chosen particle.
On the other hand, in the study of adsorption phenomena the system was a slab consisting of
sites. The width of the slab has been chosen as equal to
. With the assumed cut-off distance of the surface potential,
, the system interior with
z between 15 and 45, was not affected by the surface potential and could be considered as having the properties of the bulk. The linear dimension of each layer was set to
, and we have applied periodic boundary conditions to each layer. The basic recorded quantities were the surface excess density
and the surface excesses of differently oriented particles
In the above, and , are the total layer density and the layer density of particles with the k-th orientation. The values of and were obtained by averaging the corresponding layer densities for l between 15 and 45. We have also recorded the total density profiles, , and the density profiles of differently oriented particles .
Since the strongest fluctuations occur in the layers rather close to the surface, we have used the preferential sampling of the surface region. Namely, the first 10 layers adjacent to the surfaces, at the bottom and at the top of the simulation cell, have been sampled 10 times more often than the system interior, consisting of the layers between 15 and 45. The layers 11–14 and 46–49, have been sampled 5 times more frequently than the system interior. One should note that fluctuations deep in the bulk are considerably smaller than in the surface region, in particular, at low temperatures. Significant fluctuations in the bulk occur at the temperatures close to the critical point. However, the considered here bulk systems do not exhibit critical points, as it is shown below in
Section 5.1.
6. Final Remarks
In this work, we have discussed the formation of multilayer films by Janus-like particles on non-selective and selective walls using a simple lattice model. The interaction between a pair of particles has been limited to the first nearest neighbors, and assumed to depend on their mutual orientations. In particular, we have assumed that
and
. The ground state calculations have shown that the phase behavior strongly depends on the strengths of surface potential felt by the A and B halves of Janus particles, as well as on the interaction energy between their B halves (
). It has been shown that in the particular case of
, a complete wetting should occur even for very weakly attractive surfaces. On the other hand, for negative
(attractive BB interaction), a complete wetting at
was found to occur only for sufficiently strongly attractive fluid–wall interactions. This agrees with the previous results obtained for the systems with isotropic interactions [
12], which demonstrated that the formation of multilayer films and wetting behavior is determined by the relative strengths of fluid-fluid and fluid-wall interactions.
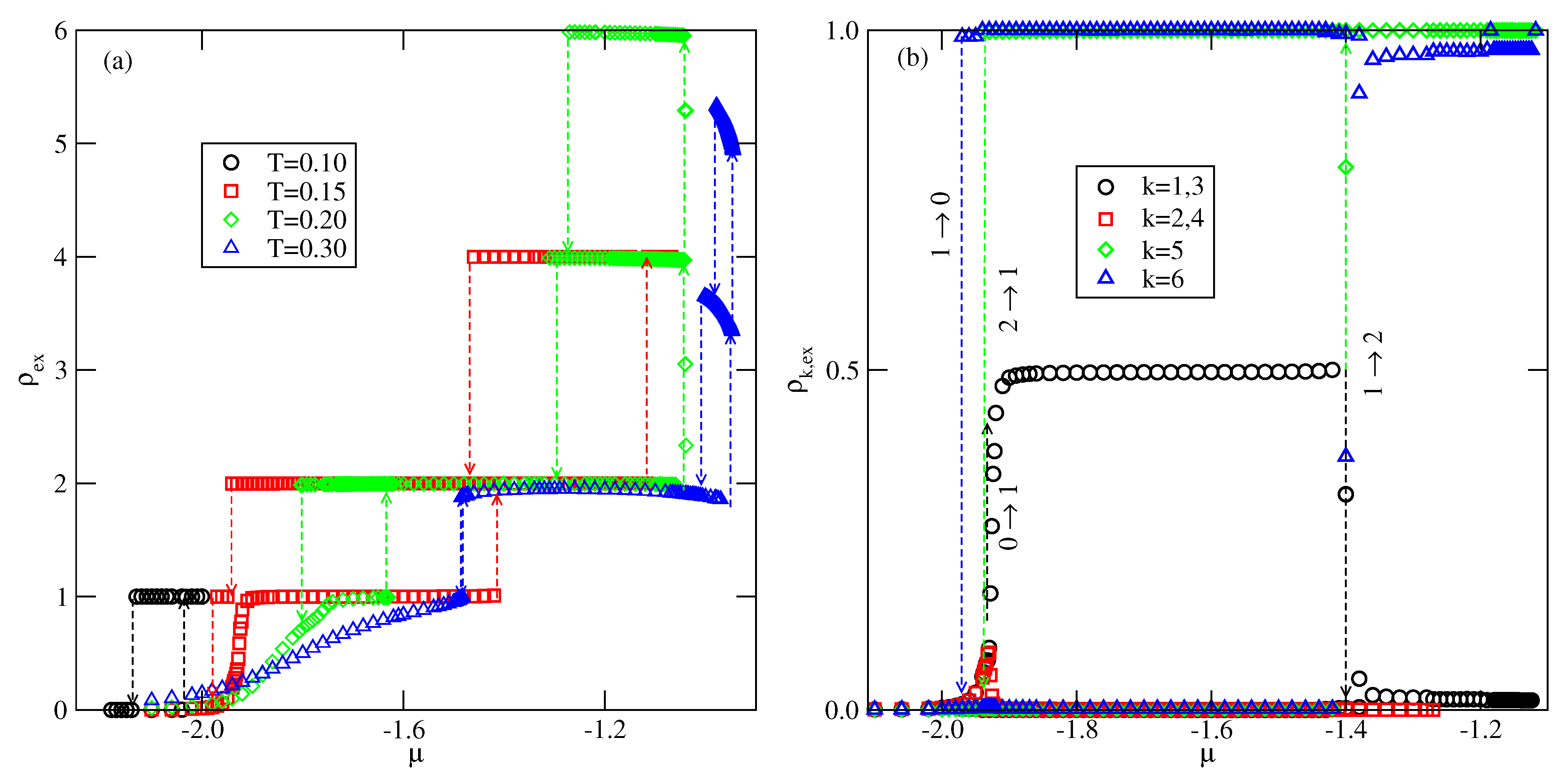
We have estimated bulk phase diagrams for several system characterized by different
between 0 and
. It has been shown that all exhibit qualitatively the same behavior. In particular, a high stability of the ordered high density phase (cf.
Figure 2) suppresses the existence of a fluid, and hence the phase diagrams have a swan neck shape. It has been also demonstrated than at
, the bulk phase undergoes a discontinuous (first-order) order order-disorder transition.
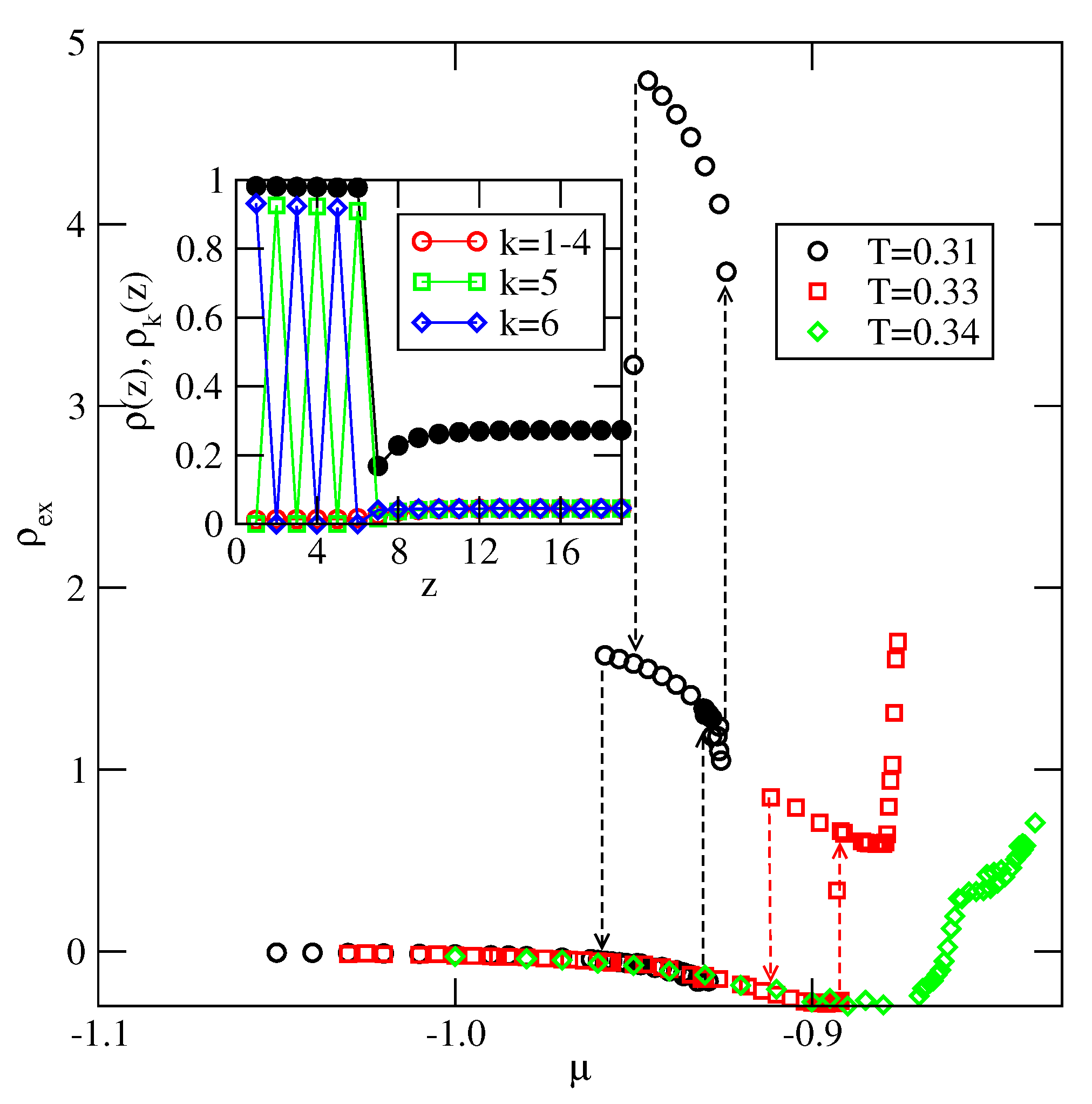
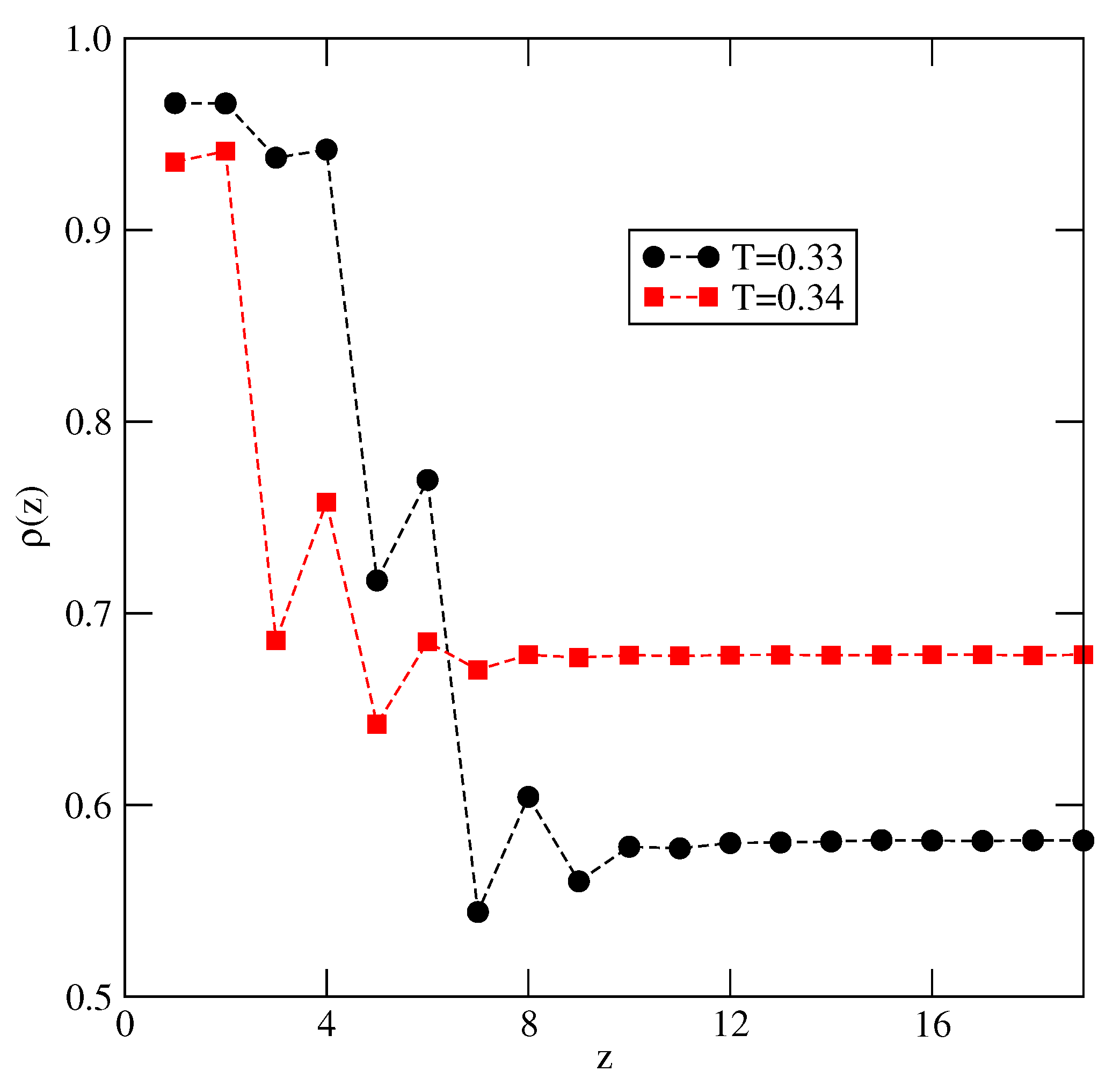
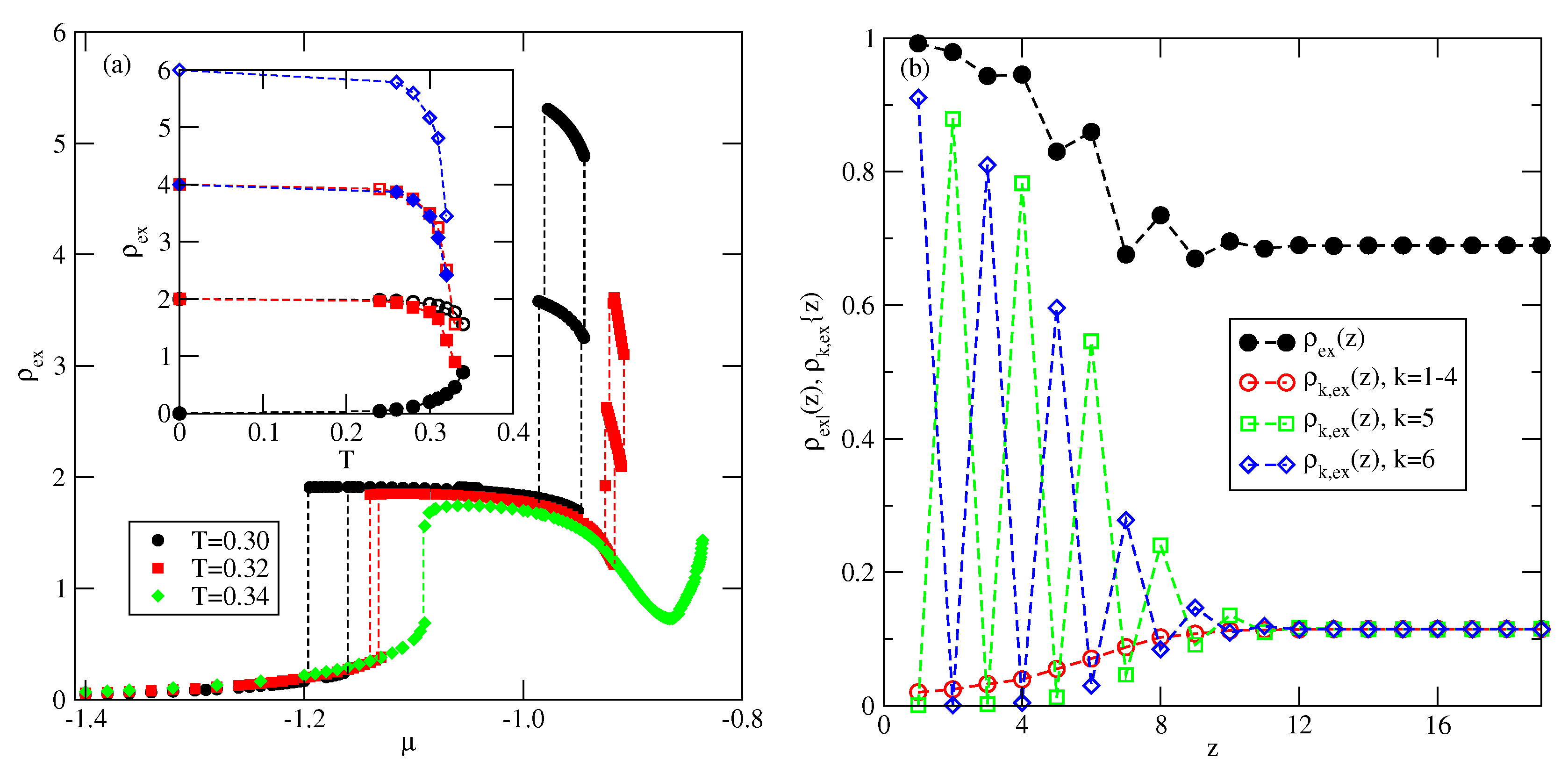
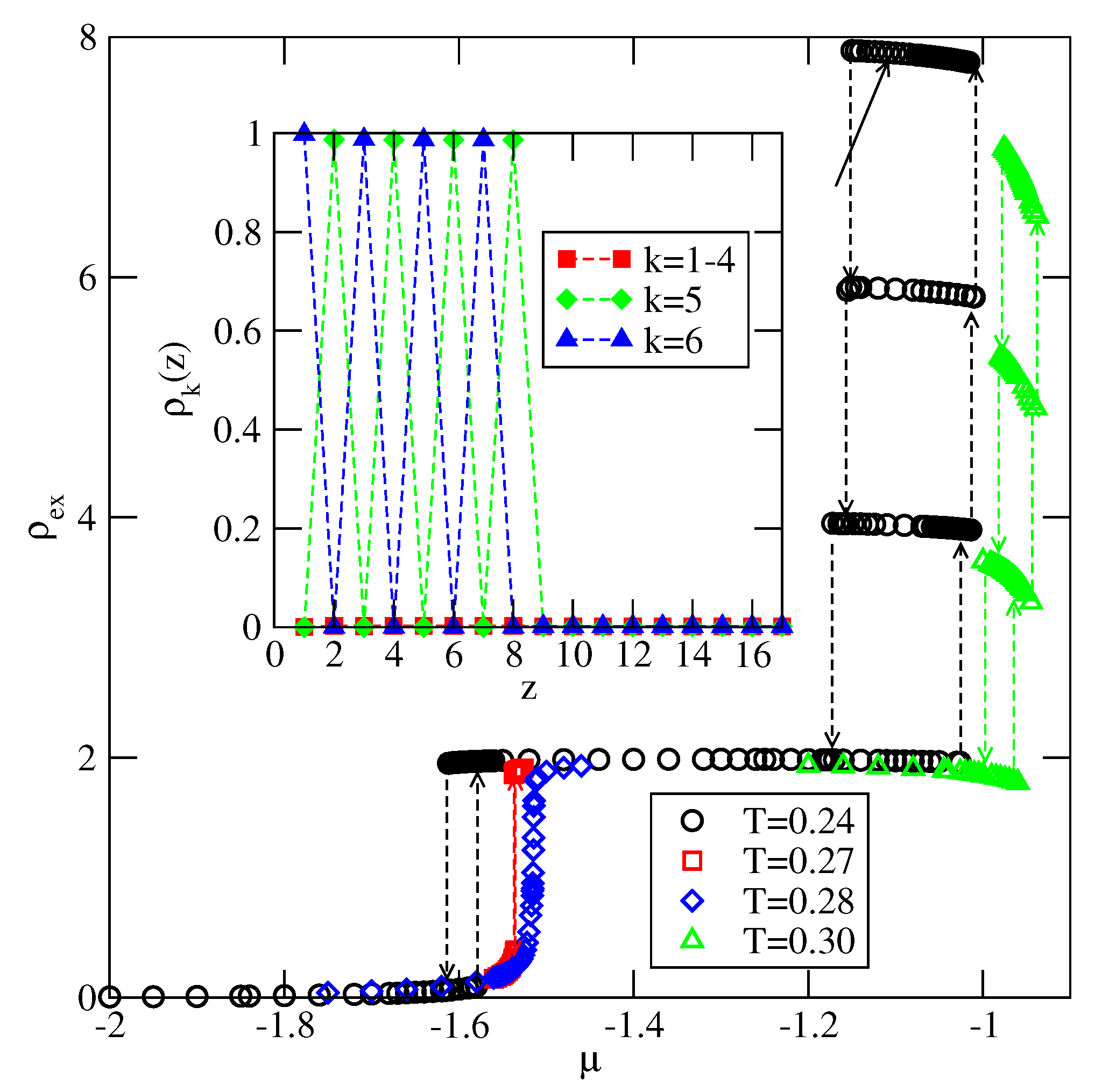
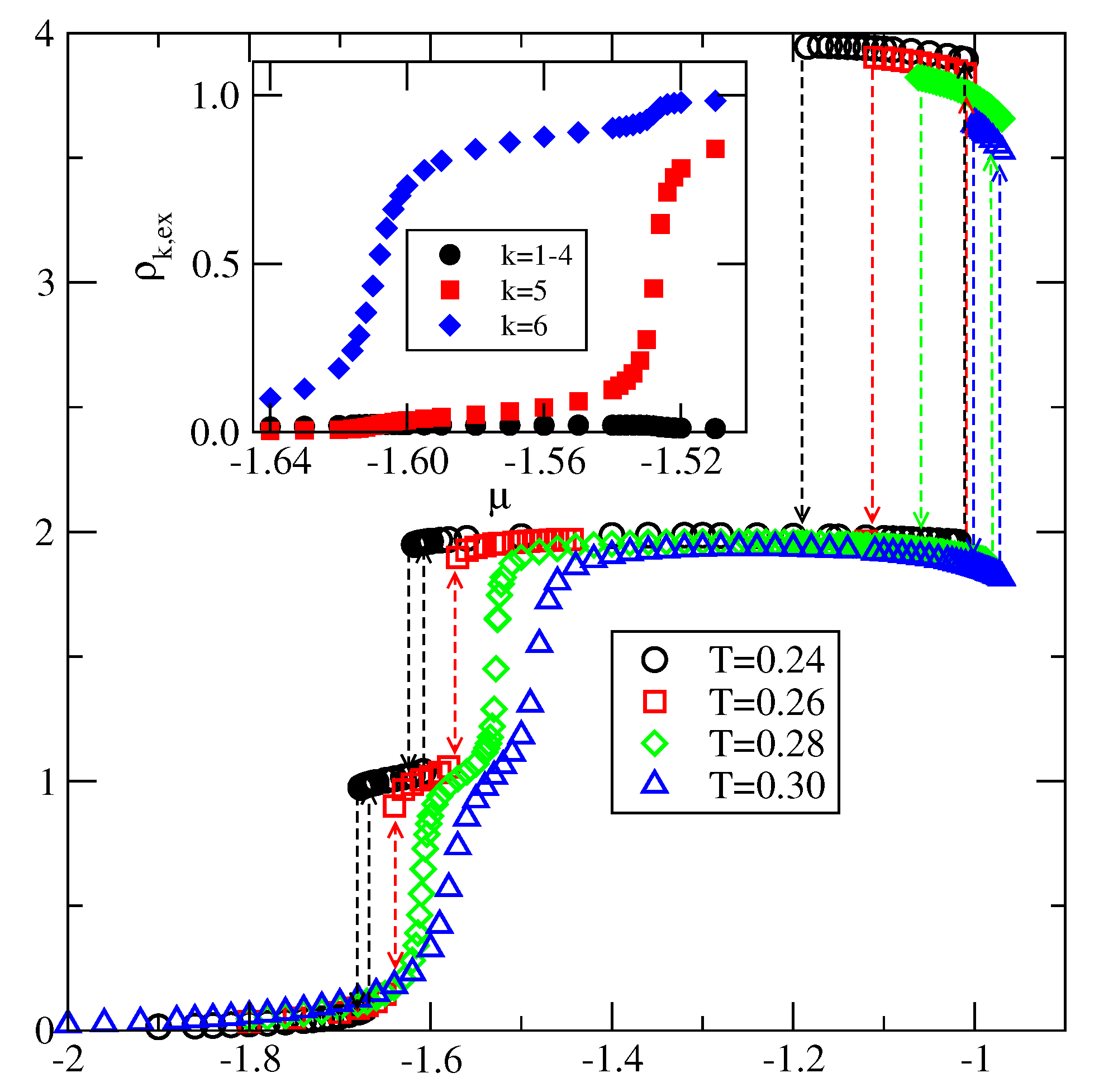
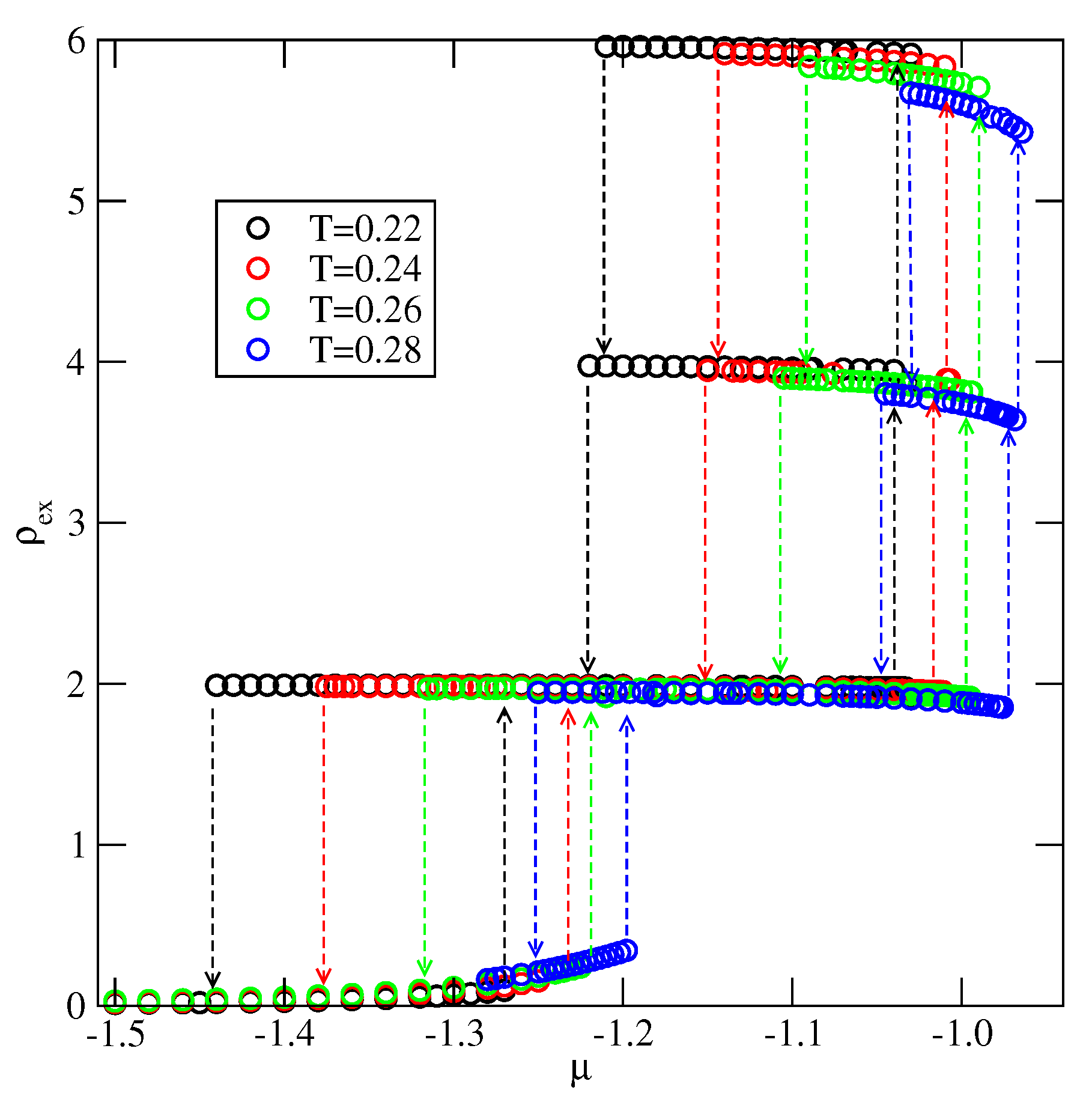
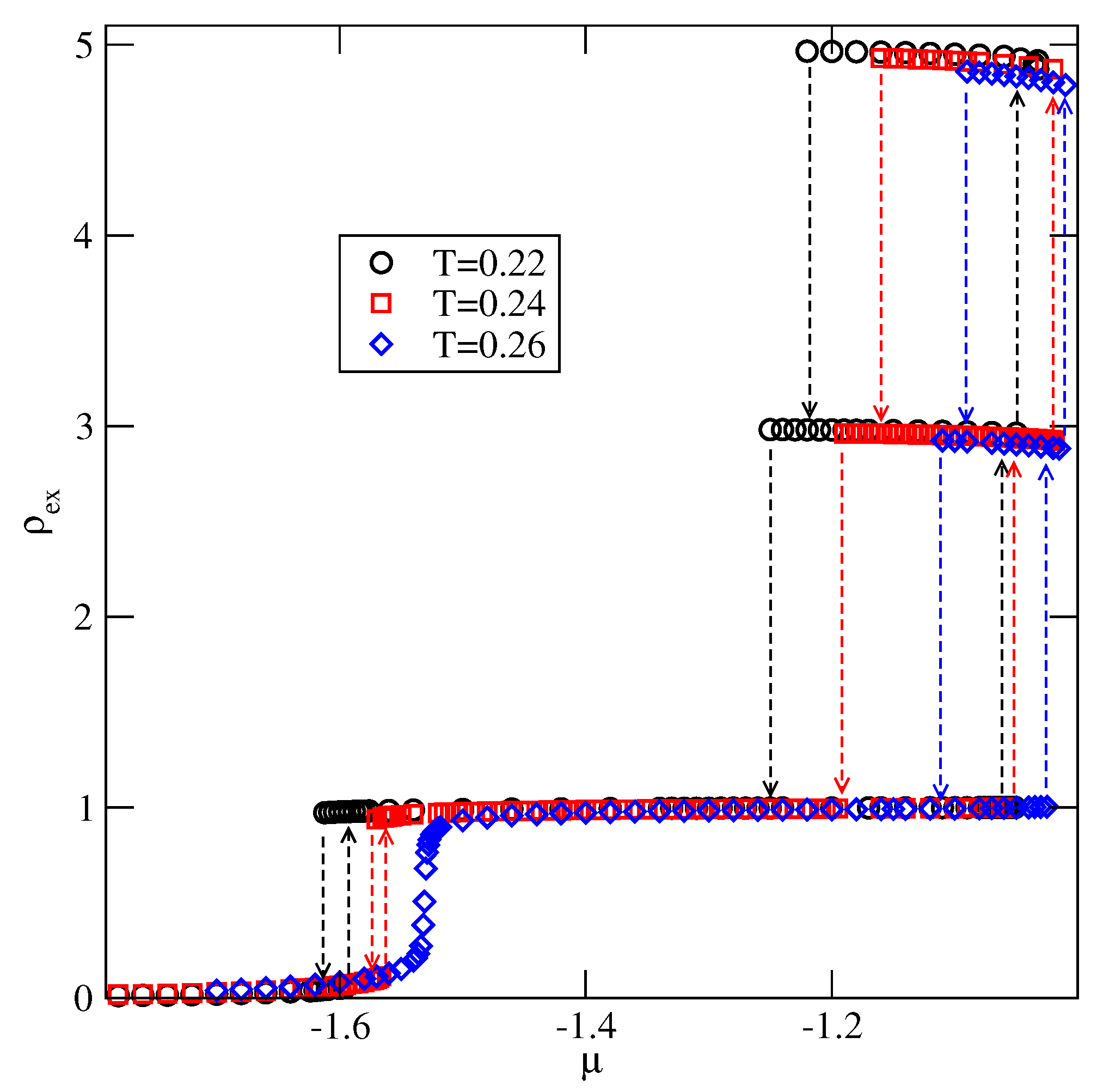
Explicit results for nonuniform systems at finite temperatures, also obtained via Monte Carlo simulations in the grand-canonical ensemble, have been presented only for the systems with . We have confirmed that a complete wetting occurs even for very weakly attractive surface. In general, the formation of multilayer films has been shown to agree very well with the results of ground state calculations. At low temperatures, the systems with , and with , develop via a sequence of layering transitions. Only when the surface potential becomes strong enough the first layering transition leads to the monolayer film, but a further grow of the adsorbed layer involves only even numbers of occupied layers.
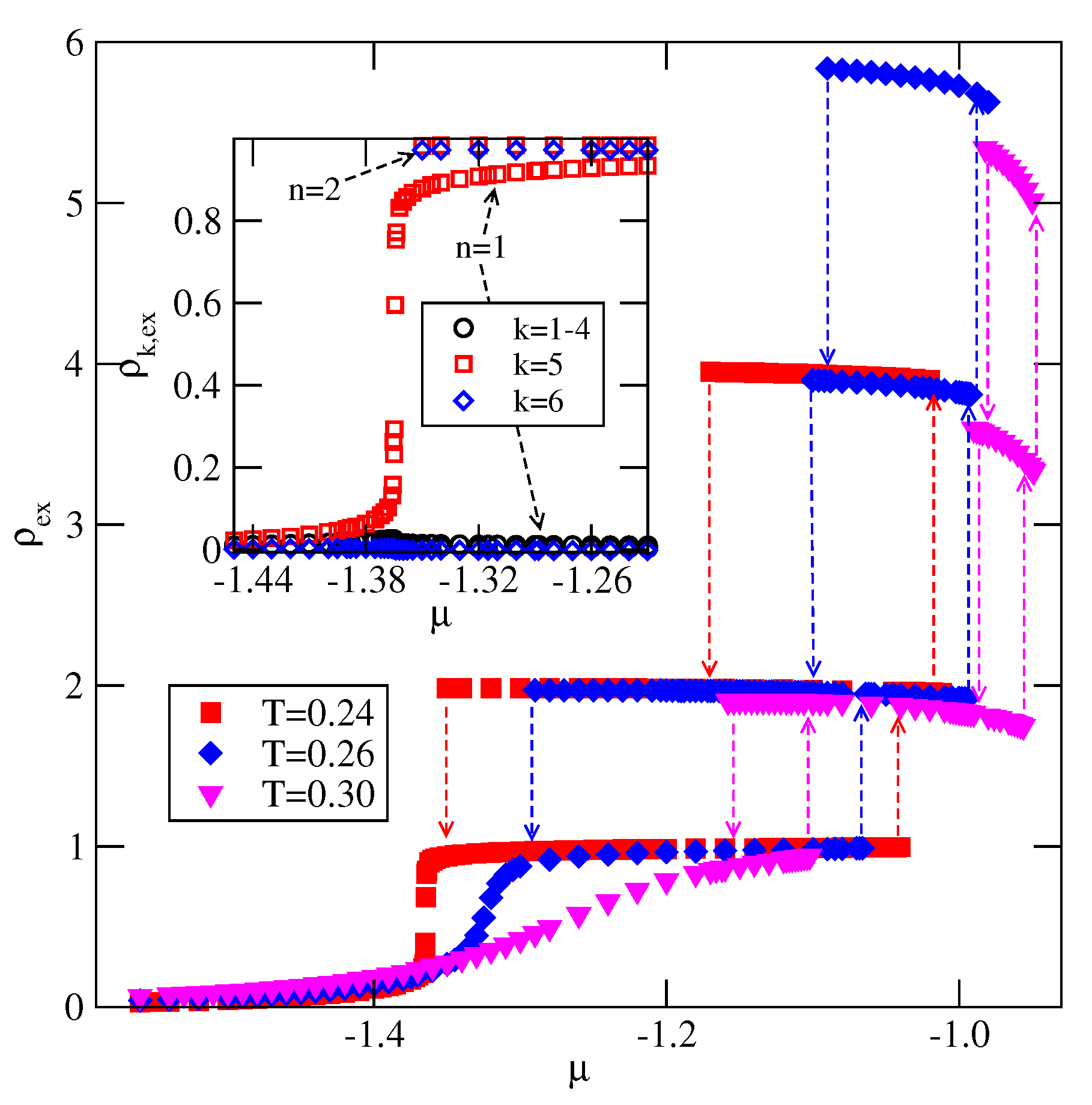
When , a sequence of layering transitions and the stacking of multilayer films have been found to depend on and on . For larger than about the film grows via a sequence of transitions, while for lower values of , the first layering transition leads to the monolayer film. What happens next depends on . For smaller than about , the monolayer undergoes the transition to the bilayer, and a further film development involves transitions. When is larger than about , the multilayer films consist of only odd numbers of layers.
The formation of multilayer films and. in particular, wetting behavior is expected to change when the BB interaction becomes attractive (). Already the ground state behavior have shown that when the strength of attractive BB interaction increases, a complete wetting occurs at only when the fluid-surface interactions ar sufficiently strong. It is expected that in the systems with weaker surface potential, a complete wetting occurs at, and above, a certain wetting temperature, which depends on , and . These problems will be considered in our future paper.