Facile Synthesis of Sulfonyl Chlorides/Bromides from Sulfonyl Hydrazides
Abstract
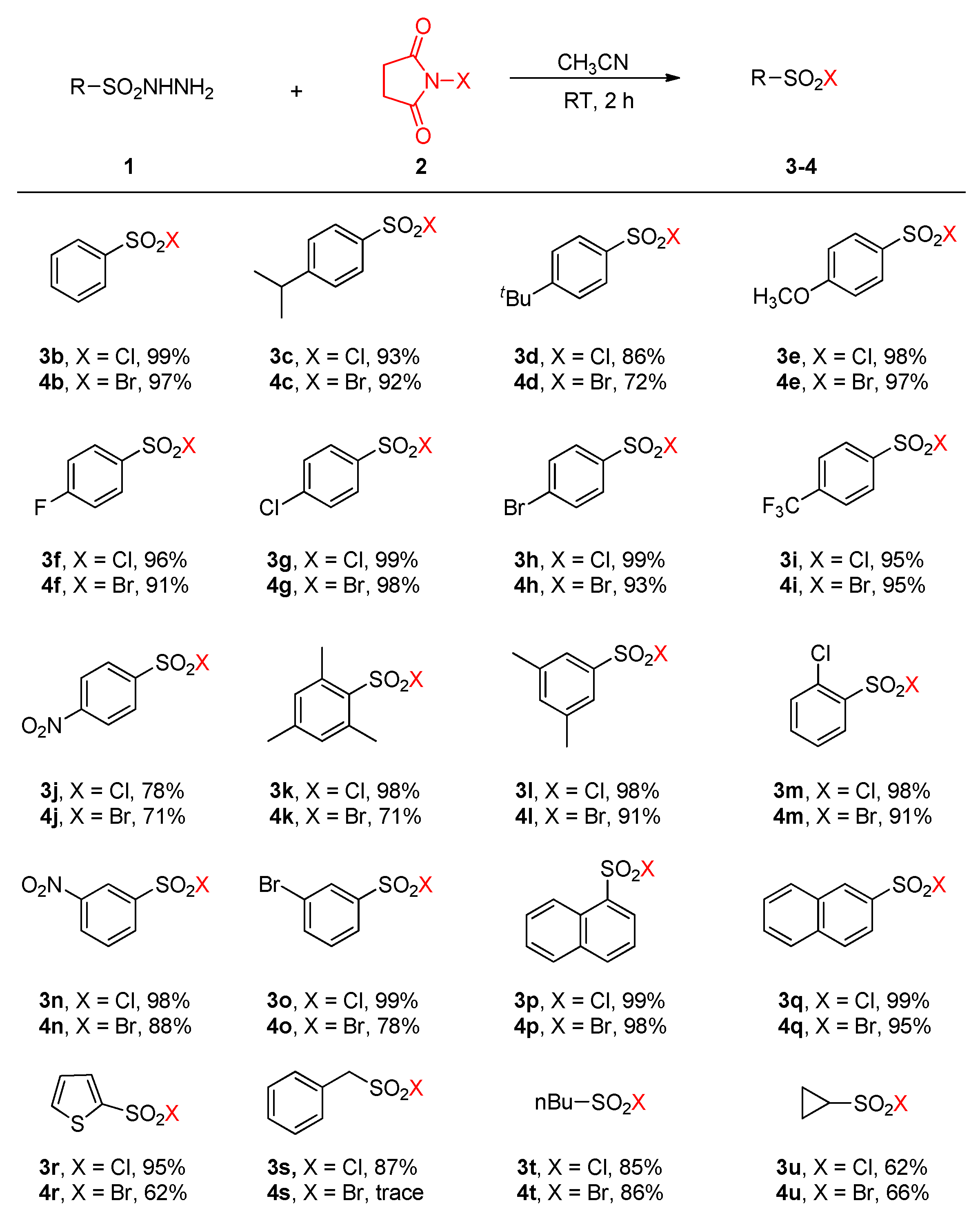
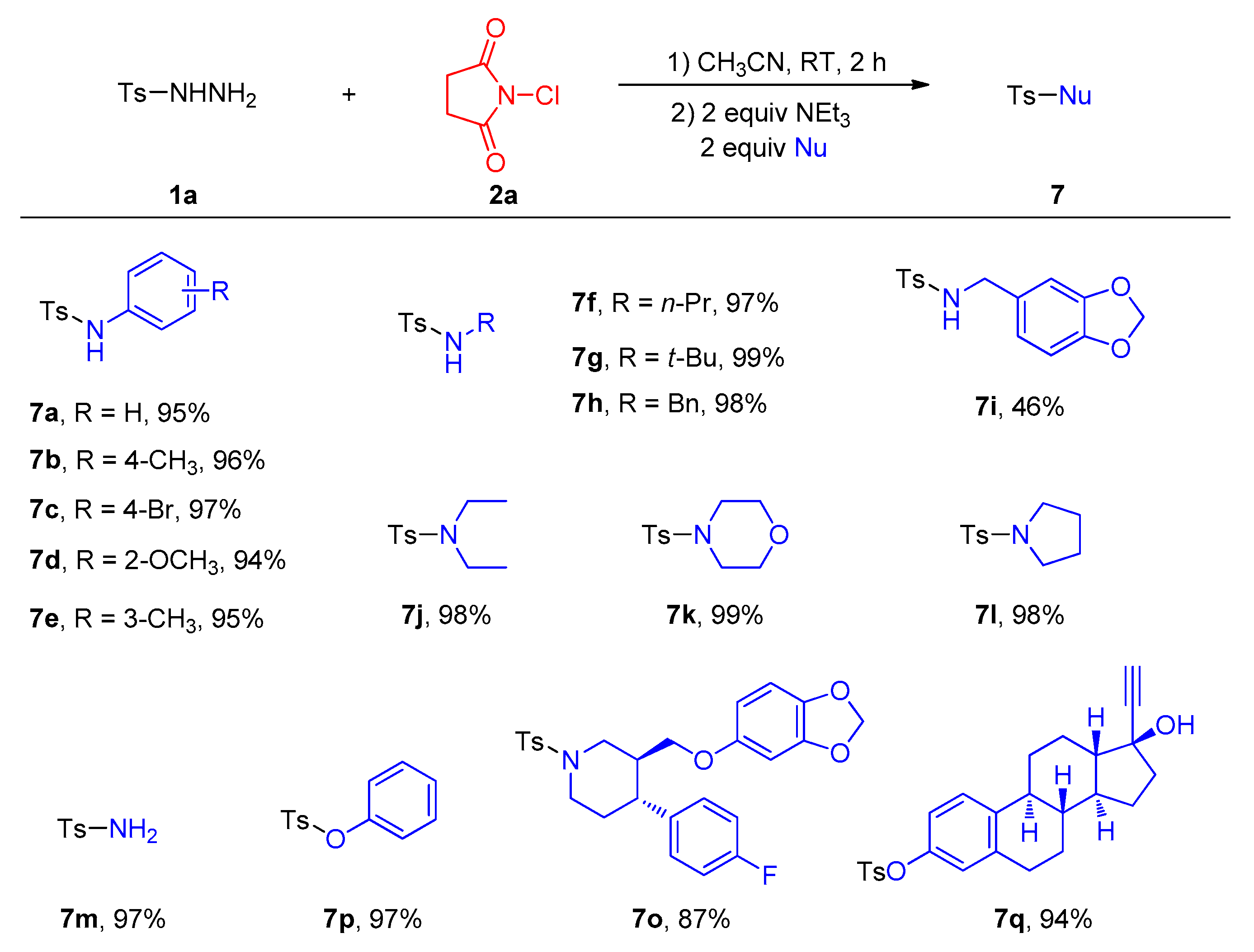
1. Introduction
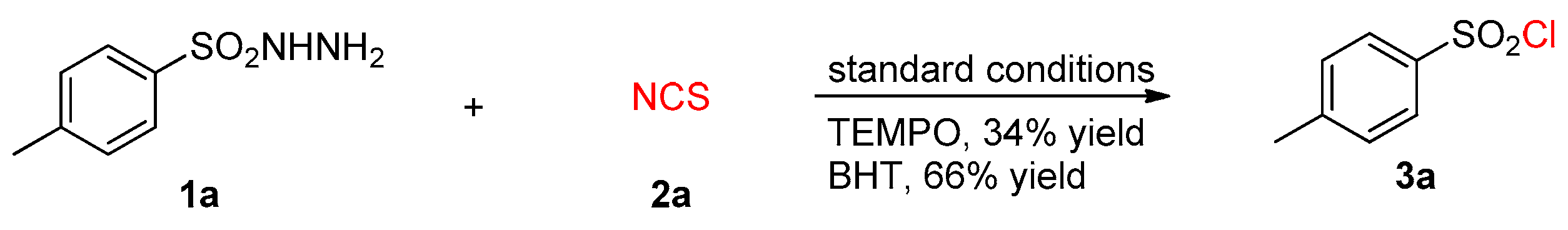
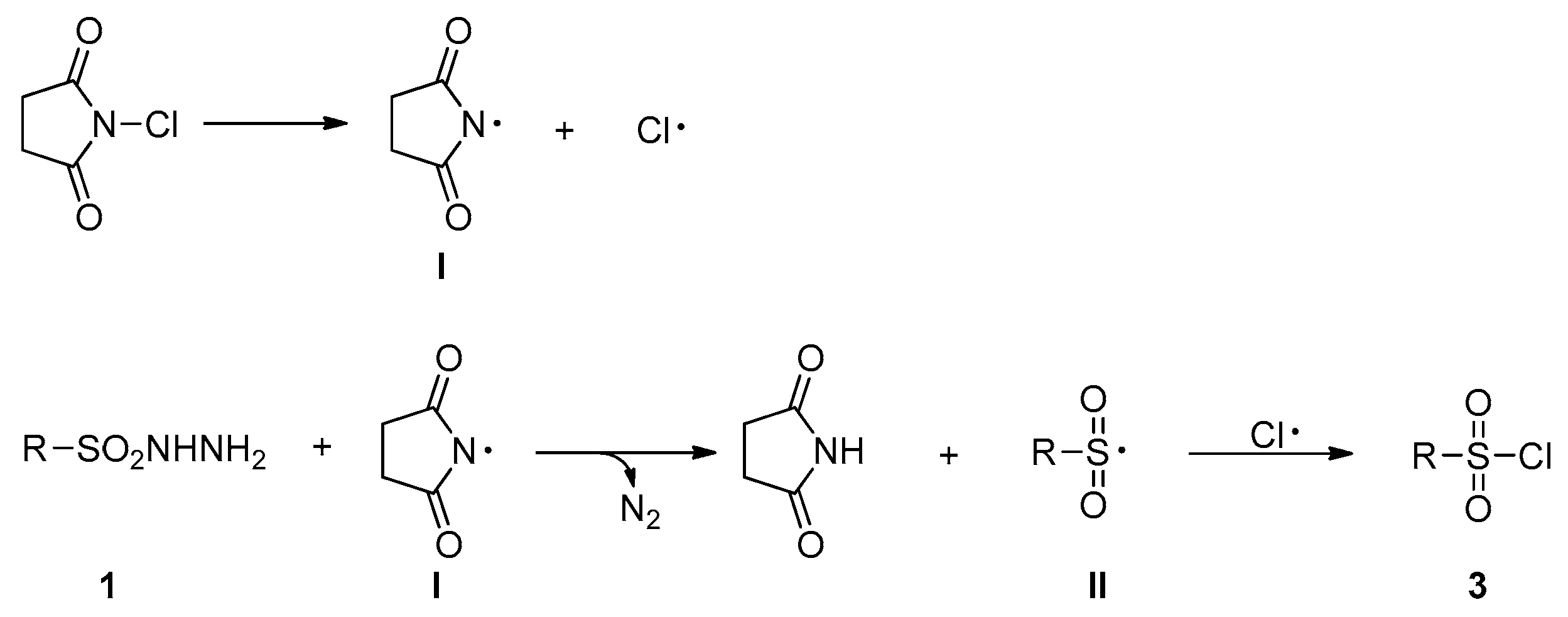
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for Synthesis of Sulfonyl Chloride 3 or Sulfonyl Bromide 4
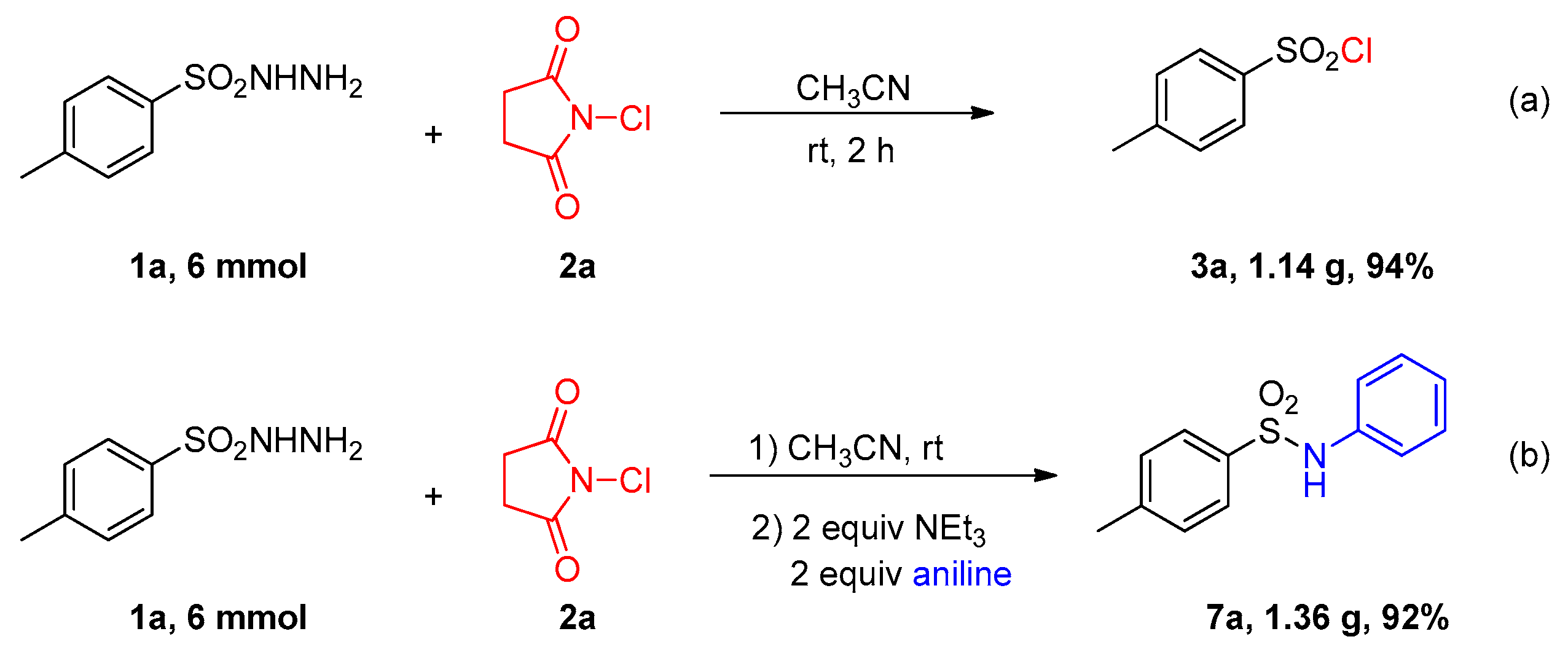
4.3. Large-Scale Reaction for the Synthesis of Sulfonyl Chloride 3a
4.4. General Procedure for One-Port Reaction with Nucleophile
4.5. Large-Scale Reaction for the Synthesis of 7a
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Milburn, R.R.; Snieckus, V. ortho-Anisylsulfonyl as a Protecting Group for Secondary Amines: Mild Ni0-Catalyzed Hydrodesulfonylation. Angew. Chem. Int. Ed. 2004, 43, 892–894. [Google Scholar] [CrossRef]
- Yan, J.; Li, J.; Cheng, D. Novel and Efficient Synthesis of 1-Iodoalkynes. Synlett 2007, 2007, 2442–2444. [Google Scholar] [CrossRef]
- Woolven, H.; González-Rodríguez, C.; Marco, I.; Thompson, A.L.; Willis, M.C. DABCO-Bis(sulfur dioxide), DABSO, as a Convenient Source of Sulfur Dioxide for Organic Synthesis: Utility in Sulfonamide and Sulfamide Preparation. Org. Lett. 2011, 13, 4876–4878. [Google Scholar] [CrossRef]
- Ho, D.K.H.; Chan, L.; Hooper, A.; Brennan, P.E. A general and mild two-step procedure for the synthesis of aryl and heteroaryl sulfonamides from the corresponding iodides. Tetrahedron Lett. 2011, 52, 820–823. [Google Scholar] [CrossRef]
- Lee, D.; Williamson, C.L.; Chan, L.; Taylor, M.S. Regioselective, Borinic Acid-Catalyzed Monoacylation, Sulfonylation and Alkylation of Diols and Carbohydrates: Expansion of Substrate Scope and Mechanistic Studies. J. Am. Chem. Soc. 2012, 134, 8260–8267. [Google Scholar] [CrossRef] [PubMed]
- Mulina, O.M.; Ilovaisky, A.I.; Terent’ev, A.O. Oxidative Coupling with S–N Bond Formation. Eur. J. Org. Chem. 2018, 2018, 4648–4672. [Google Scholar] [CrossRef]
- Zhang, S.; Zeng, X.; Wei, Z.; Zhao, D.; Kang, T.; Zhang, W.; Yan, M.; Luo, M. Desulfitative Suzuki Cross-Couplings of Arylsulfonyl Chlorides and Boronic Acids Catalyzed by a Recyclable Polymer-Supported N-Heterocyclic Carbene-Palladium Complex Catalyst. Synlett 2006, 2006, 1891–1894. [Google Scholar] [CrossRef]
- Rao Volla, C.M.; Vogel, P. Iron-Catalyzed Desulfinylative C—C Cross-Coupling Reactions of Sulfonyl Chlorides with Grignard Reagents. Angew. Chem. Int. Ed. 2008, 47, 1305–1307. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhao, D.; Qin, X.; Lan, J.; You, J. Synthesis of di(hetero)aryl sulfides by directly using arylsulfonyl chlorides as a sulfur source. Chem. Commun. 2011, 47, 9188–9190. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Yu, Z. Transition-metal mediated carbon–sulfur bond activation and transformations. Chem. Soc. Rev. 2013, 42, 599–621. [Google Scholar] [CrossRef]
- Liang, S.; Jiang, L.; Yi, W.-b.; Wei, J. Copper-Catalyzed Vicinal Chloro-thiolation of Alkynes with Sulfonyl Chlorides. Org. Lett. 2018, 20, 7024–7028. [Google Scholar] [CrossRef]
- Chu, X.-Q.; Xie, T.; Wang, Y.-W.; Li, X.-R.; Rao, W.; Xu, H.; Shen, Z.-L. Synthesis of di(hetero)aryl sulfides by defluorinative sulfenylation of polyfluoroalkyl ketones with sodium sulfinates or arylsulfonyl chlorides. Chem. Commun. 2020, 56, 8699–8702. [Google Scholar] [CrossRef]
- Wei, J.; Liang, S.; Jiang, L.; Mumtaz, Y.; Yi, W.-B. Regioselective Chlorothiolation of Alkenes with Sulfonyl Chlorides. J. Org. Chem. 2020, 85, 977–984. [Google Scholar] [CrossRef]
- Brewster, J.H.; Ciotti, C.J. Dehydrations with Aromatic Sulfonyl Halides in Pyridine.1 A Convenient Method for the Preparation of Esters. J. Am. Chem. Soc. 1955, 77, 6214–6215. [Google Scholar] [CrossRef]
- Moore, J.D.; Herpel, R.H.; Lichtsinn, J.R.; Flynn, D.L.; Hanson, P.R. ROMP-Generated Oligomeric Sulfonyl Chlorides as Versatile Soluble Scavenging Agents. Org. Lett. 2003, 5, 105–107. [Google Scholar] [CrossRef]
- Werder, M.; Hauser, H.; Carreira, E.M. Carbohydrate Sulfonyl Chlorides for Simple, Convenient Access to Glycoconjugates. Org. Lett. 2005, 7, 1145–1148. [Google Scholar] [CrossRef] [PubMed]
- Cera, G.; Bazzoni, M.; Arduini, A.; Secchi, A. Ion-Pair Selective Conformational Rearrangement of Sulfonamide Calix[6]arene-Based Pseudorotaxanes. Org. Lett. 2020, 22, 3702–3705. [Google Scholar] [CrossRef] [PubMed]
- Cera, G.; Balestri, D.; Bazzoni, M.; Marchiò, L.; Secchi, A.; Arduini, A. Trisulfonamide calix[6]arene-catalysed Michael addition to nitroalkenes. Org. Biomol. Chem. 2020, 18, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Sakai, K.; Kawamura, A.; Oisaki, K.; Kanai, M. Sulfonamides as new hydrogen atom transfer (HAT) catalysts for photoredox allylic and benzylic C–H arylations. Chem. Commun. 2018, 54, 3215–3218. [Google Scholar] [CrossRef]
- Cera, G.; Giovanardi, G.; Secchi, A.; Arduini, A. Merging Molecular Recognition and Gold(I) Catalysis with Triphoscalix[6]arene Ligands. Chem. Eur. J. 2021, 27, 10261–10266. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Soheilizad, M. A Novel, Practical Synthesis of Sulfonyl Chlorides from Thiol and Disulfide Derivatives. Synlett 2009, 2009, 2773–2776. [Google Scholar] [CrossRef]
- Bahrami, K.; Khodaei, M.M.; Khaledian, D. Synthesis of sulfonyl chlorides and thiosulfonates from H2O2–TiCl4. Tetrahedron Lett. 2012, 53, 354–358. [Google Scholar] [CrossRef]
- Wright, S.W.; Hallstrom, K.N. A Convenient Preparation of Heteroaryl Sulfonamides and Sulfonyl Fluorides from Heteroaryl Thiols. J. Org. Chem. 2006, 71, 1080–1084. [Google Scholar] [CrossRef] [PubMed]
- Prakash, G.K.S.; Mathew, T.; Panja, C.; Olah, G.A. Chlorotrimethylsilane−Nitrate Salts as Oxidants: Direct Oxidative Conversion of Thiols and Disulfides to Sulfonyl Chlorides. J. Org. Chem. 2007, 72, 5847–5850. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, K.; Khodaei, M.M.; Soheilizad, M. Direct Conversion of Thiols to Sulfonyl Chlorides and Sulfonamides. J. Org. Chem. 2009, 74, 9287–9291. [Google Scholar] [CrossRef]
- Madabhushi, S.; Jillella, R.; Sriramoju, V.; Singh, R. Oxyhalogenation of thiols and disulfides into sulfonyl chlorides/bromides using oxone-KX (X = Cl or Br) in water. Green Chem. 2014, 16, 3125–3131. [Google Scholar] [CrossRef]
- Jereb, M.; Hribernik, L. Conversion of thiols into sulfonyl halogenides under aerobic and metal-free conditions. Green Chem. 2017, 19, 2286–2295. [Google Scholar] [CrossRef]
- Parnian, R.; Soleimani, E.; Bahrami, K. A Practical Method for the Preparation of Sulfonyl Chlorides and Sulfonamides from Thiols using H2O2-TAPC Reagent System. ChemistrySelect 2019, 4, 8554–8557. [Google Scholar] [CrossRef]
- Kataoka, T.; Iwama, T.; Setta, T.; Takagi, A. Preparation of Sulfonamides from Sodium Sulfonates: Ph3P·Br2 and Ph3P·Cl2 as a Mild Halogenating Reagent for Sulfonyl Bromides and Sulfonyl Chlorides. Synthesis 1998, 1998, 423–426. [Google Scholar] [CrossRef][Green Version]
- Blotny, G. A new, mild preparation of sulfonyl chlorides. Tetrahedron Lett. 2003, 44, 1499–1501. [Google Scholar] [CrossRef]
- Chantarasriwong, O.; Jang, D.O.; Chavasiri, W. A practical and efficient method for the preparation of sulfonamides utilizing Cl3CCN/PPh3. Tetrahedron Lett. 2006, 47, 7489–7492. [Google Scholar] [CrossRef]
- Bahrami, K. TAPC-Promoted Synthesis of Sulfonyl Chlorides from Sulfonic Acids. Synlett 2011, 2011, 2671–2674. [Google Scholar] [CrossRef]
- Pandya, R.; Murashima, T.; Tedeschi, L.; Barrett, A.G.M. Facile One-Pot Synthesis of Aromatic and Heteroaromatic Sulfonamides. J. Org. Chem. 2003, 68, 8274–8276. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cuevas, C.; Perez-Arrieta, C.; Polindara-García, L.A.; Lujan-Montelongo, J.A. Sulfonyl halide synthesis by thiol oxyhalogenation using NBS/NCS—iPrOH. Tetrahedron Lett. 2017, 58, 2244–2247. [Google Scholar] [CrossRef]
- Gómez-Palomino, A.; Cornella, J. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4. Angew. Chem. Int. Ed. 2019, 58, 18235–18239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, K.; Zhang, L.; Yang, W.; Huang, D. Sulfonyl Hydrazides in Organic Synthesis: A Review of Recent Studies. Adv. Synth. Catal. 2020, 362, 3516–3541. [Google Scholar] [CrossRef]
- Poshkus, A.C.; Herweh, J.E.; Magnotta, F.A. The Synthesis of Aromatic Sulfonyl Bromides from Sulfonylhydrazides. J. Org. Chem. 1963, 28, 2766–2769. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.-D.; Chen, G.-P.; Wei, W.-T.; Guo, Z. Transition-Metal-Free Synthesis of Thiosulfonates through Radical Coupling Reaction. Synlett 2018, 29, 2076–2080. [Google Scholar] [CrossRef]
- Zhu, Y.-L.; Jiang, B.; Hao, W.-J.; Wang, A.-F.; Qiu, J.-K.; Wei, P.; Wang, D.-C.; Li, G.; Tu, S.-J. A new cascade halosulfonylation of 1,7-enynes toward 3,4-dihydroquinolin-2(1H)-ones via sulfonyl radical-triggered addition/6-exo-dig cyclization. Chem. Commun. 2016, 52, 1907–1910. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, X.; Cheng, X.; Xuan, J. Arylsulfonyl Radical Triggered 1,6-Enyne Cyclization: Synthesis of γ-Lactams Containing Alkenyl C–X Bonds. Org. Lett. 2018, 20, 449–452. [Google Scholar] [CrossRef]

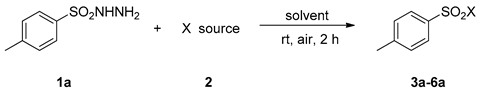
| Entry | X Source | Solvent | Product | Yield (%) b |
|---|---|---|---|---|
| 1 | MgCl2 | CH3CN | 3a | N.R. |
| 2 | NaCl | CH3CN | 3a | N.R. |
| 3 | CaCl2 | CH3CN | 3a | N.R. |
| 4 | ZnCl2 | CH3CN | 3a | N.R. |
| 5 | HCl | CH3CN | 3a | N.R. |
| 6 | CuCl | CH3CN | 3a | 38 |
| 7 | FeCl3 | CH3CN | 3a | 8 |
| 8 | SOCl2 | CH3CN | 3a | trace |
| 9 | PCl5 | CH3CN | 3a | 65 |
| 10 | NCS | CH3CN | 3a | 99 |
| 11 | NCS | CH2Cl2 | 3a | 91 |
| 12 | NCS | EtOAc | 3a | 94 |
| 13 | NCS | DME | 3a | 87 |
| 14 | NCS | THF | 3a | 56 |
| 15 | NCS | DCE | 3a | 58 |
| 16 | NCS | Dioxane | 3a | 75 |
| 17 | NBS | CH3CN | 4a | 87 |
| 18 | NIS | CH3CN | 5a | N.R. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.; Xu, S.; Shen, F.; Xu, C.; Wang, K.; Wang, Z.; Liu, L. Facile Synthesis of Sulfonyl Chlorides/Bromides from Sulfonyl Hydrazides. Molecules 2021, 26, 5551. https://doi.org/10.3390/molecules26185551
Chen R, Xu S, Shen F, Xu C, Wang K, Wang Z, Liu L. Facile Synthesis of Sulfonyl Chlorides/Bromides from Sulfonyl Hydrazides. Molecules. 2021; 26(18):5551. https://doi.org/10.3390/molecules26185551
Chicago/Turabian StyleChen, Rongxiang, Shaohong Xu, Fumin Shen, Canran Xu, Kaikai Wang, Zhanyong Wang, and Lantao Liu. 2021. "Facile Synthesis of Sulfonyl Chlorides/Bromides from Sulfonyl Hydrazides" Molecules 26, no. 18: 5551. https://doi.org/10.3390/molecules26185551
APA StyleChen, R., Xu, S., Shen, F., Xu, C., Wang, K., Wang, Z., & Liu, L. (2021). Facile Synthesis of Sulfonyl Chlorides/Bromides from Sulfonyl Hydrazides. Molecules, 26(18), 5551. https://doi.org/10.3390/molecules26185551






